Abstract
Forced degradation is a degradation of new drug substance and drug product at conditions more severe than accelerated conditions. It is required to demonstrate specificity of stability indicating methods and also provides an insight into degradation pathways and degradation products of the drug substance and helps in elucidation of the structure of the degradation products. Forced degradation studies show the chemical behavior of the molecule which in turn helps in the development of formulation and package. In addition, the regulatory guidance is very general and does not explain about the performance of forced degradation studies. Thus, this review discusses the current trends in performance of forced degradation studies by providing a strategy for conducting studies on degradation mechanisms and also describes the analytical methods helpful for development of stability indicating method.
Keywords: Degradation conditions, Degradation product, Forced degradation, Stability indicating method, Stress testing
1. Introduction
Chemical stability of pharmaceutical molecules is a matter of great concern as it affects the safety and efficacy of the drug product. The FDA and ICH guidances state the requirement of stability testing data to understand how the quality of a drug substance and drug product changes with time under the influence of various environmental factors. Knowledge of the stability of molecule helps in selecting proper formulation and package as well as providing proper storage conditions and shelf life, which is essential for regulatory documentation. Forced degradation is a process that involves degradation of drug products and drug substances at conditions more severe than accelerated conditions and thus generates degradation products that can be studied to determine the stability of the molecule. The ICH guideline states that stress testing is intended to identify the likely degradation products which further helps in determination of the intrinsic stability of the molecule and establishing degradation pathways, and to validate the stability indicating procedures used [1]. But these guidelines are very general in conduct of forced degradation and do not provide details about the practical approach towards stress testing. Although forced degradation studies are a regulatory requirement and scientific necessity during drug development, it is not considered as a requirement for formal stability program.
It has become mandatory to perform stability studies of new drug moiety before filing in registration dossier. The stability studies include long term studies (12 months) and accelerated stability studies (6 months). But intermediate studies (6 months) can be performed at conditions milder than that used in accelerated studies. So the study of degradation products like separation, identification and quantitation would take even more time. As compared to stability studies, forced degradation studies help in generating degradants in much shorter span of time, mostly a few weeks. The samples generated from forced degradation can be used to develop the stability indicating method which can be applied latter for the analysis of samples generated from accelerated and long term stability studies. This review provides a proposal on the practical performance of forced degradation and its application for the development of stability indicating method.
2. Objective of forced degradation studies
Forced degradation studies are carried out to achieve the following purposes:
-
1.
To establish degradation pathways of drug substances and drug products.
-
2.
To differentiate degradation products that are related to drug products from those that are generated from non-drug product in a formulation.
-
3.
To elucidate the structure of degradation products.
-
4.
To determine the intrinsic stability of a drug substance in formulation.
-
5.
To reveal the degradation mechanisms such as hydrolysis, oxidation, thermolysis or photolysis of the drug substance and drug product [1], [2].
-
6.
To establish stability indicating nature of a developed method.
-
7.
To understand the chemical properties of drug molecules.
-
8.
To generate more stable formulations.
-
9.
To produce a degradation profile similar to that of what would be observed in a formal stability study under ICH conditions.
-
10.
To solve stability-related problems [3].
3. Time to perform forced degradation
It is very important to know when to perform forced degradation studies for the development of new drug substance and new drug product. FDA guidance states that stress testing should be performed in phase III of regulatory submission process. Stress studies should be done in different pH solutions, in the presence of oxygen and light, and at elevated temperatures and humidity levels to determine the stability of the drug substance. These stress studies are conducted on a single batch. The results should be summarized and submitted in an annual report [4]. However, starting stress testing early in preclinical phase or phase I of clinical trials is highly encouraged and should be conducted on drug substance to obtain sufficient time for identifying degradation products and structure elucidation as well as optimizing the stress conditions. An early stress study also gives timely recommendations for making improvements in the manufacturing process and proper selection of stability-indicating analytical procedures [5], [6].
4. Limits for degradation
The question of how much degradation is sufficient has been the topic of many discussions amongst pharmaceutical scientists. Degradation of drug substances between 5% and 20% has been accepted as reasonable for validation of chromatographic assays [7], [8]. Some pharmaceutical scientists think 10% degradation is optimal for use in analytical validation for small pharmaceutical molecules for which acceptable stability limits of 90% of label claim is common [9]. Others suggested that drug substance spiked with a mixture of known degradation products can be used to challenge the methods employed for monitoring stability of drug product [2]. No such limits for physiochemical changes, loss of activity or degradation during shelf life have been established for individual types or groups of biological products [10].
It is not necessary that forced degradation would result in a degradation product. The study can be terminated if no degradation is seen after drug substance or drug product has been exposed to stress conditions than those conditions mentioned in an accelerated stability protocol [11]. This is indicative of the stability of the molecule under test. Over-stressing a sample may lead to the formation of a secondary degradation product that would not be seen in formal shelf-life stability studies and under-stressing may not generate sufficient degradation products [12]. Protocols for generation of product-related degradation may differ for drug substance and drug product due to differences in matrices and concentrations. It is recommended that maximum of 14 days for stress testing in solution (a maximum of 24 h for oxidative tests) to provide stressed samples for methods development [13].
5. Strategy for selection of degradation conditions
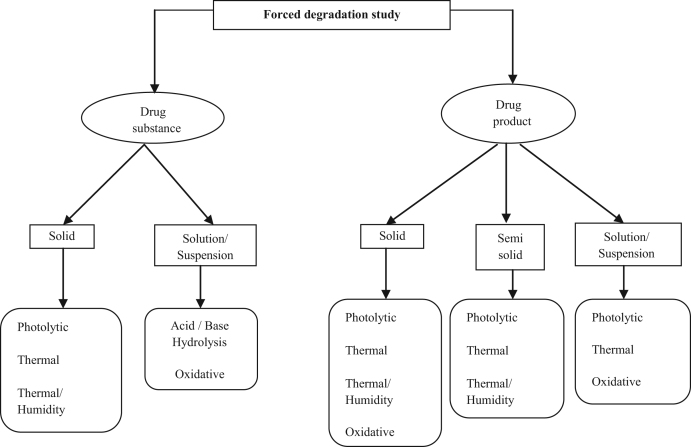
Forced degradation is carried out to produce representative samples for developing stability-indicating methods for drug substances and drug products. The choice of stress conditions should be consistent with the product's decomposition under normal manufacturing, storage, and use conditions which are specific in each case [9]. A general protocol of degradation conditions used for drug substance and drug product is shown in Scheme 1.
Scheme 1.
An illustrative flowchart describing various stress conditions used for degradation of drug substance and drug product.
A minimal list of stress factors suggested for forced degradation studies must include acid and base hydrolysis, thermal degradation, photolysis, oxidation [5], [14], [15], [16] and may include freeze–thaw cycles and shear [10]. There is no specification in regulatory guidelines about the conditions of pH, temperature and specific oxidizing agents to be used. The design of photolysis studies is left to the applicant's discretion although Q1B specifies that the light source should produce combined visible and ultraviolet (UV, 320–400 nm) outputs, and that exposure levels should be justified [11]. The initial trial should have the aim to come upon the conditions that degrade the drug by approximately 10%. Some conditions mostly used for forced degradation studies are presented in Table 1 [17].
Table 1.
Conditions mostly used for forced degradation studies.
| Degradation type | Experimental conditions | Storage conditions | Sampling time (days) |
|---|---|---|---|
| Hydrolysis | Control API (no acid or base) | 40 °C, 60 °C | 1,3,5 |
| 0.1 M HCl | 40 °C, 60 °C | 1,3,5 | |
| 0.1 M NaOH | 40 °C, 60 °C | 1,3,5 | |
| Acid control (no API) | 40 °C, 60 °C | 1,3,5 | |
| Base control (no API) | 40 °C, 60 °C | 1,3,5 | |
| pH: 2,4,6,8 | 40 °C, 60 °C | 1,3,5 | |
| Oxidation | 3% H2O2 | 25 °C, 60 °C | 1,3,5 |
| Peroxide control | 25 °C, 60 °C | 1,3,5 | |
| Azobisisobutyronitrile (AIBN) | 40 °C, 60 °C | 1,3,5 | |
| AIBN control | 40 °C, 60 °C | 1,3,5 | |
| Photolytic | Light 1× ICH | NA | 1,3,5 |
| Light 3× ICH | NA | 1,3,5 | |
| Light control | NA | 1,3,5 | |
| Thermal | Heat chamber | 60 °C | 1,3,5 |
| Heat chamber | 60 °C/75% RH | 1,3,5 | |
| Heat chamber | 80 °C | 1,3,5 | |
| Heat chamber | 80 °C/75% RH | 1,3,5 | |
| Heat control | Room temp. | 1,3,5 | |
Ref.: [17].
NA: Not applicable.
Some scientists have found it practical to begin with extreme conditions such as 80 °C or even higher temperatures and testing at shorter (2, 5, 8, 24 h, etc.) multiple time points, so that the rate of degradation can be evaluated [18]. The primary degradants and their secondary degradations products can be distinguished by testing at early time points and thus help in a better degradation pathway determination. In another approach degradation is started by considering the drug substance to be labile and doing degradation at the conditions mentioned in Table 1. Then stress would be increased or decreased to obtain sufficient degradation. As compared to harsher conditions and less time approach, this strategy is better due to the following reasons: (i) there may be a change in mechanism of reaction when a harsh condition is used, and (ii) there is a practical problem in neutralizing or diluting every sample, when it contains a high concentration of reactants, e.g., acid or base, before an injection can be made on the HPLC column. Both these reasons are strong enough to suggest that as normal as possible conditions should be used for causing the decomposition of the drug [19]. Studies should be repeated when formulations or methods change because the change may lead to the production of new degradation products.
6. Selection of drug concentration
Which concentration of drug should be used for degradation study has not been specified in regulatory guidance. It is recommended that the studies should be initiated at a concentration of 1 mg/mL [20]. By using drug concentration of 1 mg/mL, it is usually possible to get even minor decomposition products in the range of detection. It is suggested that some degradation studies should also be done at a concentration which the drug is expected to be present in the final formulations [19]. The reason for proposing this is the examples of aminopenicillins and aminocephalosporins where a range of polymeric products have been found to be formed in commercial preparations containing drug in high concentrations [21].
7. Degradation conditions
7.1. Hydrolytic conditions
Hydrolysis is one of the most common degradation chemical reactions over a wide range of pH. Hydrolysis is a chemical process that includes decomposition of a chemical compound by reaction with water. Hydrolytic study under acidic and basic condition involves catalysis of ionizable functional groups present in the molecule. Acid or base stress testing involves forced degradation of a drug substance by exposure to acidic or basic conditions which generates primary degradants in desirable range. The selection of the type and concentrations of acid or base depends on the stability of the drug substance. Hydrochloric acid or sulfuric acids (0.1–1 M) for acid hydrolysis and sodium hydroxide or potassium hydroxide (0.1–1 M) for base hydrolysis are suggested as suitable reagents for hydrolysis [20], [22]. If the compounds for stress testing are poorly soluble in water, then co-solvents can be used to dissolve them in HCl or NaOH. The selection of co-solvent is based on the drug substance structure. Stress testing trial is normally started at room temperature and if there is no degradation, elevated temperature (50–70 °C) is applied. Stress testing should not exceed more than 7 days. The degraded sample is then neutralized using suitable acid, base or buffer, to avoid further decomposition.
7.2. Oxidation conditions
Hydrogen peroxide is widely used for oxidation of drug substances in forced degradation studies but other oxidizing agents such as metal ions, oxygen, and radical initiators (e.g., azobisisobutyronitrile, AIBN) can also be used. Selection of an oxidizing agent, its concentration, and conditions depends on the drug substance. It is reported that subjecting the solutions to 0.1–3% hydrogen peroxide at neutral pH and room temperature for seven days or up to a maximum 20% degradation could potentially generate relevant degradation products [22]. The oxidative degradation of drug substance involves an electron transfer mechanism to form reactive anions and cations. Amines, sulfides and phenols are susceptible to electron transfer oxidation to give N-oxides, hydroxylamine, sulfones and sulfoxide [23]. The functional group with labile hydrogen like benzylic carbon, allylic carbon, and tertiary carbon or α-positions with respect to hetro atom is susceptible to oxidation to form hydro peroxides, hydroxide or ketone [24], [25].
7.3. Photolytic conditions
The photo stability testing of drug substances must be evaluated to demonstrate that a light exposure does not result in unacceptable change. Photo stability studies are performed to generate primary degradants of drug substance by exposure to UV or fluorescent conditions. Some recommended conditions for photostability testing are described in ICH guidelines [11]. Samples of drug substance and solid/liquid drug product should be exposed to a minimum of 1.2 million lx h and 200 W h/m2 light. The most commonly accepted wavelength of light is in the range of 300–800 nm to cause the photolytic degradation [26], [27]. The maximum illumination recommended is 6 million lx h [25]. Light stress conditions can induce photo oxidation by free radical mechanism. Functional groups like carbonyls, nitro aromatic, N-oxide, alkenes, aryl chlorides, weak C—H and O—H bonds, sulfides and polyenes are likely to introduce drug photosensitivity [28].
7.4. Thermal conditions
Thermal degradation (e.g., dry heat and wet heat) should be carried out at more strenuous conditions than recommended ICH Q1A accelerated testing conditions. Samples of solid-state drug substances and drug products should be exposed to dry and wet heat, while liquid drug products should be exposed to dry heat. Studies may be conducted at higher temperatures for a shorter period [22]. Effect of temperature on thermal degradation of a substance is studied through the Arrhenius equation:
where k is specific reaction rate, A is frequency factor, Ea is energy of activation, R is gas constant (1.987 cal/deg mole) and T is absolute temperature [25], [29], [30]. Thermal degradation study is carried out at 40–80 °C.
8. Stability indicating method
A stability indicating method (SIM) is an analytical procedure used to quantitate the decrease in the amount of the active pharmaceutical ingredient (API) in drug product due to degradation. According to an FDA guidance document, a stability-indicating method is a validated quantitative analytical procedure that can be used to detect how the stability of the drug substances and drug products changes with time. A stability-indicating method accurately measures the changes in active ingredients concentration without interference from other degradation products, impurities and excipients [14]. Stress testing is carried out to demonstrate specificity of the developed method to measure the changes in concentration of drug substance when little information is available about potential degradation product. The development of a suitable stability indicating method provides a background for the pre-formulation studies, stability studies and the development of proper storage requirements. Bakshi and Singh [19] discussed some critical issues about developing stability indicating methods. Dolan [31] made comments and suggestions on stability indicating assays. Smela [32] discussed from a regulatory point of view about stability indicating analytical methods. The RP-HPLC is a most widely used analytical tool for separation and quantifying the impurities and it is most frequently coupled with a UV detector [29]. The following are the steps involved for development of SIM on HPLC which meets the regulatory requirements.
8.1. Sample generation
For generating samples for SIM the API is force degraded at conditions more severe than accelerated degradation conditions. It involves degradation of drug at hydrolytic, oxidative, photolytic and thermal conditions as discussed earlier. The forced degradation of API in solid state and solution form is carried out with an aim to generate degradation products which are likely to be formed in realistic storage conditions [33]. This sample is then used to develop an SIM.
8.2. Method development and optimization
Before starting the method development, various physiochemical properties like pKa value, log P, solubility and absorption maximum of the drug must be known, for it lays a foundation for HPLC method development. Log P and solubility helps select mobile phase and sample solvent while pKa value helps determine the pH of the mobile phase [19].
Reverse phase column is a preferred choice to start the separation of sample components as the degradation is carried out in aqueous solution. Methanol, water and acetonitrile can be used as mobile phase in various ratios for the initial stages of separation. Selection between methanol and acetonitrile for organic phase is based on the solubility of the analyte. Initially the water: organic phase ratio can be kept at 50:50 and suitable modifications can be made as trials proceed to obtain a good separation of peaks. Latter buffer can be added if it is required to obtain better peak separation and peak symmetry. If the method is to be extended to liquid chromatography–mass spectrometry (LC–MS), then mobile phase buffer should be MS compatible like triflouroacetic acid and ammonium formate. Variation in column temperature affects the selectivity of the method as analytes respond differently to temperature changes. A temperature in the range of 30–40 °C is suitable to obtain good reproducibility [34]. It is better to push the drug peak further in chromatogram as it results in separation of all degradation products. Also a sufficient run time after the drug peak is to be allowed to obtain the degradants peak eluting after the drug peak [19].
During the method development it may happen that the drug peak may hide an impurity or degradant peak that co-elutes with the drug. This requires peak purity analysis which determines the specificity of the method. Direct analysis can be done on line by using photo diode array (PDA) detection. PDA provides information of the homogeneity of the spectral peak but it is not applicable for the degradants that have the similar UV spectrum to the drug. Indirect method involves change in the chromatographic conditions like mobile phase ratio, column, etc. which will affect the peak separation. The spectrum of altered chromatographic condition is then compared with the original spectra. If the degradant peaks and area percentage of the drug peak remain same, then it can be confirmed that the drug peak is homogeneous [35]. The degradant that co-elutes with the drug would be acceptable if it is not found to be formed in accelerated and long term storage conditions [1]. The method is then optimized for separating closely eluting peaks by changing flow rate, injection volume, column type and mobile phase ratio.
8.3. Method validation
The developed SIM is then validated according to USP/ICH guideline for linearity, accuracy, precision, specificity, quantitation limit, detection limit, ruggedness and robustness of the method. It is required to isolate, identify and quantitate the degradants found to be above identification threshold (usually 0.1%) [36], [37]. If the method does not fall within the acceptance criteria for validation, the method is modified and revalidated [35].
9. Other analytical methods for developing SIM
Stability-indicating methods will be characterized by potency, purity and biological activity [38]. The selection of tests is product specific. Stability indicating methods may include various methods like electrophoresis (SDS-PAGE, immunoelectrophoresis, Western blot, isoelectrofoccusing), high-resolution chromatography (e.g., reversed phase chromatography, SEC, gel filtration, ion exchange, and affinity chromatography) and peptide mapping [39]. The analytical method of choice should be sensitive enough to detect impurities at low levels (i.e., 0.05% of the analyte of interest or lower) and the peak responses should fall within the range of detector's linearity. The analytical method should be capable of capturing all the impurities formed during a formal stability study at or below ICH threshold limits [40], [41]. Degradation product identification and characterization are to be performed based on formal stability results in accordance with ICH requirements. Conventional methods (e.g., column chromatography) or hyphenated techniques (e.g., LC–MS, LC–nuclear magnetic resonance (NMR)) can be used in the identification and characterization of the degradation products. Use of these techniques can provide a better insight into the structure of the impurities that could add to the knowledge space of potential structural alerts for genotoxicity and the control of such impurities with tighter limits [36], [39], [40], [41], [42], [43]. It should be noted that structural characterization of degradation products is necessary for those impurities formed during formal shelf-life stability studies and above the qualification threshold limit [40].
New analytical technologies that are continuously being developed can also be used when it is appropriate to develop stability indicating method [44]. The unknown impurity, which is observed during the analysis, pharmaceutical development, stress studies and formal stability studies of the drug substances and drug product, can be separated and analyzed by using various chromatographic techniques like reversed phase high performance liquid chromatography (RP-HPLC), thin layer chromatography (TLC), gas chromatography (GC), capillary electrophoresis (CE), capillary electrophoresis chromatography (CEC) and super critical fluid chromatography (SFC). An excellent combination of hyphenated chromatographic and spectroscopic technique such as HPLC-photodiode array ultraviolet detector (DAD), LC–MS, LC–NMR and GC–MS are used when degradants cannot be isolated in pure form. HPLC-DAD and LC–MS are used to compare the relative retention time (RRT), UV spectra, mass spectra (MS/MS or MSN) [29]. Singh and Rehman [45] discussed the role of hyphenated systems for the isolation of degradants and impurities.
10. Conclusion
Forced degradation studies provide knowledge about possible degradation pathways and degradation products of the active ingredients and help elucidate the structure of the degradants. Degradation products generated from forced degradation studies are potential degradation products that may or may not be formed under relevant storage conditions but they assist in the developing stability indicating method. It is better to start degradation studies earlier in the drug development process to have sufficient time to gain more information about the stability of the molecule. This information will in turn help improve the formulation manufacturing process and determine the storage conditions. As no specific set of conditions is applicable to all drug products and drug substances and the regulatory guidance does not specify about the conditions to be used, this study requires the experimenter to use common sense. The aim of any strategy used for forced degradation is to produce the desired amount of degradation i.e., 5–20%. A properly designed and executed forced degradation study would generate an appropriate sample for development of stability indicating method.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.ICH guidelines, Q1A (R2): Stability Testing of New Drug Substances and Products (revision 2), International Conference on Harmonization. Available from: 〈http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm128204.pdf〉, 2003.
- 2.Reynolds D.W., Facchine K.L., Mullaney J.F. Available guidance and best practices for conducting forced degradation studies. Pharm. Technol. 2002;26(2):48–56. [Google Scholar]
- 3.Brummer H. How to approach a forced degradation study. Life Sci. Technol. Bull. 2011;31:1–4. [Google Scholar]
- 4.FDA Guidance for Industry, INDs for Phase II and III Studies—Chemistry, Manufacturing, and Controls Information, Food and Drug Administration. Available from: 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070567.pdf〉, 2003.
- 5.FDA Guidance for Industry, INDs for Phase 2 and 3 Studies of Drugs, Including Specified Therapeutic Biotechnology-Derived Products, Draft Guidance, Food and Drug Administration. Available from: 〈http://www.fda.gov/ohrms/dockets/98fr/990674gd.pdf〉, 1999.
- 6.Kats M. Forced degradation studies: regulatory considerations and implementation. BioPharm Int. 2005;18:1–7. [Google Scholar]
- 7.Szepesi G. Selection of high-performance liquid chromatographic methods in pharmaceutical analysis. III. Method validation. J. Chromatogr. 1989;464:265–278. doi: 10.1016/s0021-9673(00)94245-6. [DOI] [PubMed] [Google Scholar]
- 8.Carr G.P., Wahlich J.C. A practical approach to method validation in pharmaceutical analysis. J. Pharm. Biomed. Anal. 1990;86:613–618. doi: 10.1016/0731-7085(90)80090-c. [DOI] [PubMed] [Google Scholar]
- 9.Jenke D.R. Chromatographic method validation: a review of common practices and procedures II. J. Liq. Chromatogr. 1996;19:737–757. [Google Scholar]
- 10.ICH, Final Guidance on Stability Testing of Biotechnological/Biological Products Availability, International Conference on Harmonization. Available from: 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073466.pdf〉, 1996.
- 11.ICH Guidance for Industry, Q1B: Photo stability Testing of New Drug Substances and Product, International Conference on Harmonization. Available from: 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073373.pdf〉, 1996.
- 12.Maheswaran R. FDA perspectives: scientific considerations of forced degradation studies in ANDA submissions. Pharm. Technol. 2012;36(5):73–80. [Google Scholar]
- 13.Klick S., Pim G.M., Waterval J. Toward a generic approach for stress testing of drug substances and drug products. Pharm. Technol. 2005;29(2):48–66. [Google Scholar]
- 14.FDA Guidance for Industry, Analytical Procedures and Methods Validation: Chemistry, Manufacturing, and Controls Documentation, Draft Guidance, Food and Drug Administration. Available from: 〈http://www.fda.gov/downloads/Drugs/…/Guidances/ucm122858.pdf〉, 2000.
- 15.CDER, Reviewer Guidance: Validation of Chromatographic Methods, Centre for Drug Evaluation and Research. Available from: 〈http://www.fda.gov/downloads/Drugs/Guidances/UCM134409.pdf〉, 1994.
- 16.ICH Guidance for Industry, Q2B: Validation of Analytical Procedures: Methodology, International Conference on Harmonization. Available from: 〈http://permanent.access.gpo.gov/LPS113764/LPS113764/www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM128049.pdf〉, 1996.
- 17.Ngwa G. Forced degradation studies as an integral part of HPLC stability indicating method development. Drug Deliv. Technol. 2010;10(5):56–59. [Google Scholar]
- 18.Banker G.S., Rhodes C.T. fourth ed. Marcel Dekker, Inc.; New York: 2002. Modern Pharmaceutics. [Google Scholar]
- 19.Bakshi M., Singh S. Development of validated stability-indicating assay methods—critical review. J. Pharm. Biomed. Anal. 2002;28(6):1011–1040. doi: 10.1016/s0731-7085(02)00047-x. [DOI] [PubMed] [Google Scholar]
- 20.Singh S., Bakshi M. Guidance on conduct of stress tests to determine inherent stability of drugs. Pharm. Technol. 2000;24:1–14. [Google Scholar]
- 21.Larsen C., Bundgaard H. Polymerization of Penicillins: V. Separation, identification and quantitative determination of Antigenic Polymerization products in Ampicillin Sodium preparations by high-performance liquid chromatography. J. Chromatogr. 1978;A147:143–150. [Google Scholar]
- 22.Alsante K.M., Ando A., Brown R. The role of degradant profiling in active pharmaceutical ingredients and drug products. Adv. Drug Deliv. Rev. 2007;59(1):29–37. doi: 10.1016/j.addr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A., Yadav J.S., Rawat S. Method development and hydrolytic degradation study of Doxofylline by RP HPLC and LC–MS/MS. Asian J. Pharm. Anal. 2011;1:14–18. [Google Scholar]
- 24.Boccardi G. Oxidative susceptibility testing. In: Baertschi S.W., editor. Pharmaceutical Stress Testing-Predicting Drug Degradation. Taylor and Francis; New York: 2005. p. 220. [Google Scholar]
- 25.Alsante K.M., Hatajik T.D., Lohr L.L. Solving impurity/degradation problems: case studies. In: Ahuja S., Alsante K.M., editors. Handbook of Isolation and Characterization of Impurities in Pharmaceutical. Academics Press; New York: 2003. p. 380. [Google Scholar]
- 26.Baertschi S.W., Thatcher S.R. Sample presentation for photo stability studies: problems and solutions. In: Piechocki J., editor. Pharmaceutical Photostability and Stabilization Technology. Taylor & Francis; New York: 2006. p. 445. [Google Scholar]
- 27.Allwood M., Plane J. The wavelength-dependent degradation of vitamin A exposed to ultraviolet radiation. Int. J. Pharm. 1986;31:1–7. [Google Scholar]
- 28.Ahuja S., Scypinski S. first ed. Academic Press; New York: 2001. Handbook of Modern Pharmaceutical Analysis. [Google Scholar]
- 29.Qiu F., Norwood D.L. Identification of pharmaceutical impurities. J. Liq. Chromatogr. Relat. Technol. 2007;30:877–935. [Google Scholar]
- 30.Trabelsi H., Hassen I.E., Bouabdallah S. Stability indicating LC method for determination of Pipamperone. J. Pharm. Biomed. Anal. 2005;39:914–919. doi: 10.1016/j.jpba.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Dolan J.W. Stability-indicating assays: LC troubleshooting. LC-GC N. Am. 2002;20(4):346–349. [Google Scholar]
- 32.Smela J.W. Regulatory considerations for stability indicating analytical methods in drug substance and drug product testing. Am. Pharm. Rev. 2005;8(3):51–54. [Google Scholar]
- 33.Annapurna M.M., Mohapatro C., Narendra A. Stability-indicating liquid chromatographic method for the determination of Letrozole in pharmaceutical formulation. J. Pharm. Anal. 2012;2(4):298–305. doi: 10.1016/j.jpha.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Synder L.R., Glajch J.L., Kirkland J.J. second ed. Wiley; New York: 1997. Practical HPLC Method Development. [Google Scholar]
- 35.Riddhiben M.P., Piyushbhai M.P., Natubhai M.P. Stability indicating HPLC method development—a review. Int. Res. J. Pharm. 2011;2(5):79–87. [Google Scholar]
- 36.ICH, Q6A: Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances, International Conference on Harmonization, Geneva. Available from: 〈http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002823.pdf〉, 2000. [PubMed]
- 37.Ali N.W., Abbas S.S., Zaazaa H.E. Validated stability indicating methods for determination of Nitazoxanide in presence of its degradation products. J. Pharm. Anal. 2012;2(2):105–116. doi: 10.1016/j.jpha.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bichsel V.E., Curcio V., Gassmann R. Requirements for the quality control of chemically synthesized peptides and biotechnologically produced proteins. Pharm. Acta Helv. 1996;71:439–446. [Google Scholar]
- 39.EMA Guideline on the Limits of Genotoxic Impurities, Committee for Medical Products for Human Use (CHMP). Available from: 〈http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002903.pdf〉, 2007.
- 40.ICH, Q3A (R2): Impurities in New Drug Substances, International Conference on Harmonization, Geneva. Available from: 〈http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3A_R2/Step4/Q3A_R2__Guideline.pdf〉, 2006.
- 41.ICH, Q3B (R2): Impurities in New Drug Products, International Conference on Harmonization, Geneva. Available from: 〈http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm128033.pdf〉, 2006.
- 42.FDA Guidance for Industry, ANDAs: Impurities in Drug Substances (draft), Food and Drug Administration, Rockville, MD. Available from: 〈http://www.fda.gov/OHRMS/DOCKETS/98fr/1998d-0514-gdl0003.pdf〉, 2005.
- 43.FDA Guidance for Industry, ANDAs: Impurities in Drug Products (draft), Food and Drug Administration, Rockville, MD. Available from: 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072861.pdf〉, 2010.
- 44.ICH Guidance for Industry, Q6B: Specifications: Test Procedures and Acceptance Criteria for Bio-technological/Biological Products, International Conference on Harmonization. Available from: 〈http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073488.pdf〉, 1999.
- 45.Singh R., Rehman Z. Current trends in forced degradation study for pharmaceutical product development. J. Pharm. Educ. Res. 2012;3(1):54–63. [Google Scholar]