Abstract
Background
The results presented here are part of a five-year cluster-randomised intervention trial that was implemented to understand how best to gain and sustain control of schistosomiasis through different preventive chemotherapy strategies. This paper presents baseline data that were collected in ten districts of Cabo Delgado province, northern Mozambique, before treatment.
Methods
A cross-sectional study of 19,039 individuals was sampled from 144 villages from May to September 2011. In each village prevalence and intensity of S. haematobium were investigated in 100 children first-year students (aged 5–8 years), 100 school children aged 9–12 years (from classes 2 to 7) and 50 adults (20–55 years). Prevalence and intensity of S. haematobium infection were evaluated microscopically by two filtrations, each of 10 ml, from a single urine specimen. Given that individual and community perceptions of schistosomiasis influence control efforts, community knowledge and environmental risk factors were collected using a face-to-face interview. Data were entered onto mobile phones using EpiCollect. Data summary was made using descriptive statistics. Chi-square and logistic regression were used to determine the association between dependent and independent variables.
Results
The overall prevalence of urogenital schistosomiasis was 60.4% with an arithmetic mean intensity of infection of 55.8 eggs/10 ml of urine. Heavy infections were detected in 17.7%, of which 235 individuals (6.97%) had an egg count of 1000 eggs/10 ml or more. There was a significantly higher likelihood of males being infected than females across all ages (62% vs 58%; P < 0.0005). Adolescents aged 9–12 years had a higher prevalence (66.6%) and mean infection intensity (71.9 eggs/10 ml) than first-year students (63.1%; 58.2 eggs/10 ml). This is the first study in Mozambique looking at infection rates among adults. Although children had higher levels of infection, it was found here that adults had a high average prevalence and intensity of infection (44.5%; 23.9 eggs/10 ml). Awareness of schistosomiasis was relatively high (68.6%); however, correct knowledge of how schistosomiasis is acquired was low (23.2%) among those who had heard of the disease. Schistosomiasis risk behaviour such as washing (91.3%) and bathing (86.7%) in open water sources likely to be infested with host snails was high.
Conclusions
Urogenital schistosomiasis is widespread in Cabo Delgado. In addition, poor community knowledge about the causes of schistosomiasis and how to prevent it increases the significant public health challenge for the national control program. This was the first study in Mozambique that examined infection levels among adults, where results showed that S. haematobium infection was also extremely high. Given that this controlled trial aims to understand the impact of different combinations of schistosomiasis control through treatment of communities, schools, and treatment holidays over a five-year period, these findings highlight the importance of examining the impact of different treatment approaches also in adults.
Trial registration
The trials have been registered with the International Standard Randomised Controlled Trial registry under ISRCT 14117624 Mozambique (14 December 2015).
Keywords: Schistosomiasis, Schistosoma haematobium, School-based treatment, Community-wide treatment, Mozambique
Background
Schistosomiasis affects at least 240 million people worldwide, with an estimated 20 million suffering from severe and debilitating forms of the disease [1, 2]. The burden is concentrated in Africa, where more than 90% of the infections occur [3]. Schistosomiasis is strongly associated with poverty, where the disease delays the social and economic development of endemic countries [4, 5].
Schistosomiasis is a major public health problem in Mozambique, as shown by an epidemiological survey of schistosomiasis and soil-transmitted helminths (STH) among school children carried out between 2005 and 2007 [6]. The mean estimated prevalence of urogenital schistosomiasis, Schistosoma haematobium, was 47% but schistosomiasis is focal, and so prevalence varied dramatically across the country [6]. In Cabo Delgado Province, the chosen area for this study, the prevalence was 57.9% ranging from 8.8% on the coast to 93% inland [6]. In contrast, the prevalence of intestinal schistosomiasis, whose causative agent is S. mansoni, is extremely low in Mozambique, with an average prevalence of 1% [6–8].
Regular large-scale preventive chemotherapy with praziquantel is the current control strategy recommended by the World Health Organisation (WHO) that aims to alleviate subtle morbidity and prevent infected individuals from developing severe, late-stage morbidity due to schistosomiasis [9]. The current recommended WHO treatment strategy for schistosomiasis depends on the prevalence among school-aged children (SAC) aged 5–14 years, where if infection is higher than 50% the school-aged population should be treated once a year; if the prevalence is between 10 and 50% treatment is focused on SAC biennially; and if less than 10% SAC should be treated twice, once at school entry and once before finishing school [10]. Studies have found, however, a community-wide approach for schistosomiasis a very effective strategy for reaching children who do not attend school as well as potentially high-risk adults [11–13]. Recent models on STH have also demonstrated the potential cost and health benefits for expansion of drug coverage to the entire community [14, 15].
The Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) was established in 2008 to provide an evidence base for programme managers to address strategic questions about schistosomiasis control. It comprises of multi-country field studies including “Gaining control of schistosomiasis”, investigating the impact of different treatment strategies involving community-wide treatment (CWT), school-based treatment (SBT) and drug holidays (years in which a village did not receive praziquantel treatment) [16]. The gaining control study has been implemented in various African countries, including Tanzania, Kenya and Mozambique [16]. The SCORE study protocol and baseline characteristics have been described elsewhere [17, 18]. In the Mozambique study, the aim was to determine the strategy for the preventive chemotherapy that provides greatest reductions in prevalence and intensity of S. haematobium in school-aged children after four years of intervention in an area where baseline prevalence of infection was 25% or more. This article describes the baseline results pre-treatment and reports age, sex, prevalence and intensity of S. haematobium across all ages among 144 study sites selected for the study as well as potential environmental risk factors of local communities.
Methods
Study area and population
We selected 10 out of 17 districts of Mozambique’s northernmost province, Cabo Delgado, believed to be one of the least developed areas of the country. The province consists of lowlands adjacent to the Atlantic coast, with altitudes varying between 200 and 300 m above sea level. The climate is semi-humid with moderate rainfall of 800–1200 mm in the rainy season from November until April.
The area was chosen as the previous mapping had shown a high prevalence of S. haematobium and a low prevalence of S. mansoni (around 1%), which met one of the SCORE country selection criteria of not working in areas with mixed infections [6, 17, 18]. The ten out of 17 districts included in this study were Metuge, Mecufi, Macomia, Ancuabe, Meluco, Quissanga, Namuno, Montepuez, Chiúre and Balama.
Eligibility for communities to be included in the study was determined by selecting only villages that had a primary school with a minimum of 100 children aged 9–12 years who attend school, since several arms of the study are school-based, and each community was randomised to any arm of the study. A cross-sectional survey was carried out in May and June 2011 to identify communities eligible to participate in the SCORE study. In total, 155 villages were selected at random across the ten districts. In each school, 50 children aged 13–14 years were randomly selected and tested for haematuria with dipsticks. The rationale for using 13–14-year olds in the eligibility survey was that children who test positive must be treated, therefore testing children aged 9–12 years and treating those infected could affect the subsequent study results, especially if prevalence is high [17]. Only those villages that demonstrated a microhaematuria prevalence of ≥ 21% by reagent strip testing (equivalent to ≥25% microscopic examination of filtered urine) were included in the study [17]. Since the rainy season lasts from November to April, baseline data was collected between July and October every year, commencing in 2011, to ensure there was no seasonal variation across the five years of data collection. Additional criteria for eligibility were that if two nearby communities share water sources and schools have an overlapping catchment area they were not considered separate communities for the study, and therefore only one of the schools were chosen. Secondly, communities were only considered that had not previously received preventive chemotherapy against schistosomiasis. All communities in this study were treatment naïve.
Study arms
This study was a parallel cluster-randomised, intervention trial with six study arms [17, 18]. Communities received various combinations of CWT, SBT or drug holiday over a four-year period, with the final round of data collection carried out in Year 5. This paper only examines the baseline data pre-treatment.
Sample size
To provide the sample size needed to complete all the planned arms, the baseline survey was conducted in 144 schools found eligible from July to October 2011. Initially, the school provided a list of the names, ages and sex of all children enrolled in each class. Primary schools have pupils at seven-year stages, Year 1 to Year 7. A year stage can, therefore, be defined as a group of pupils entering primary education at a common date. In each school 100 children aged 9–12 years (Years 2–7) and 100 children in the first class (Year 1) were then randomly selected from the list to participate. Where there were insufficient numbers of children in the age range, children were selected from outside of the age range. Also, parasitological data and information on the occupation of each sample were collected from 50 adults (aged 20–55 years) from each community.
Urine examination
In each school, participants were given a wide-mouthed plastic bottle labelled with a specific identification number and asked to provide a single urine specimen. All samples were collected between 10 am and 2 pm and examined in the school by the field team straight away. Urine samples were vigorously shaken, 10 ml drawn into a syringe and pressed through a mesh nylon filter with a pore size of 20 μm (Sefar AG, Heiden, Switzerland). Two filtrations were carried out per urine sample. Both filters were placed on two separate microscope slides and, after adding a drop of Lugol, the filters were examined for S. haematobium eggs under a light microscope. All parasitological examinations were performed by experienced laboratory technicians. For quality control, around 10% of all microscope slides were re-examined by a senior technician. The intensity of infection was expressed as the number of eggs per 10 ml of urine filtered. For specimens of less than 10 ml, the volume of urine filtered was measured and the number of eggs per 10 ml calculated by extrapolation. If estimated counts were above 1000 eggs per 10 ml, they were truncated at 1000. The arithmetic mean of two filtrations taken from a single urine specimen was calculated to be the egg count of the child. A child was deemed egg positive if one or more eggs were found in any of the slides examined.
Village and household level data
In addition to the parasitological survey, a village level questionnaire was asked to the community leaders to collect information that might affect schistosomiasis transmission. The questionnaire had sections on (i) general information (village name, population of village, number of households); (ii) main occupations of people in the village; (iii) local health facility information and whether they stock praziquantel; (iv) water contact sites (standing and flowing water); (v) water sources (drinking, washing and bathing); (vi) sanitation facilities in the village; and (vii) knowledge about schistosomiasis (heard about schistosomiasis, how to catch schistosomiasis, symptoms of schistosomiasis, how to prevent schistosomiasis, and name of the treatment).
Data handling and analysis
Demographic data were collected on smartphones and uploaded to a dedicated database maintained on a central server (EpiCollect) hosted by Imperial College London. Laboratory data were collected on paper forms and entered into the smartphone retrospectively. Data cleaning and management were carried out by biostatisticians at SCI.
Data analysis was performed using R version 3.2 (URL https://www.r-project.org//) and STATA version 14 (StataCorp, College Station, TX, USA). The primary outcome of SCORE study is the change in prevalence and intensity of S. haematobium over the four years of intervention. Here, we present the findings of the baseline survey before treatment. Prevalence of S. haematobium infection from the eligibility survey was calculated from haematuria with dipsticks from a single urine sample from children aged 13–14 years.
From the baseline survey, infection categories for S. haematobium infection were defined per WHO thresholds: light infection (< 50 eggs/10 ml of urine) and heavy infection (≥ 50 eggs/10 ml of urine) [19]. Prevalence estimates were then calculated from the average egg count of two slides or egg count from one urine filtration (if only one 10 ml sample was taken) from three groups: (i) first-year students (aged 5–8 years); (ii) children aged 9–12 years; and (iii) adults (aged 20–55 years). Village-level prevalence was calculated for the prevalence of infection (0 for not infected, 1 for infected) and prevalence of heavy infection (0 for non-heavy infection, 1 for heavy infection). Prevalence of infection and heavy infection aggregated by other factors (e.g. age, sex) were calculated as arithmetic means of the infection categories. The Arithmetic mean of infection intensity was calculated for all subjects (including those with zero egg counts), which is a measure of community-level contamination potential. Although mean intensity was underestimated as egg counts were capped at 1000 eggs/10 ml, this was done consistently over the years, therefore, the trend in the change in intensity is believed to be valid. The association between sociodemographic characteristics (sexes, age groups, village and adult occupation) and infection was examined using Pearson χ2 tests, while one-way analysis of variance (ANOVA) was used to assess differences in intensities of infection. All tests and confidence intervals used the 5% level of significance.
Results
Baseline prevalence and intensity of Schistosoma haematobium infection
Village level prevalence varied from 14.2% to 92.5%, with heavy prevalence ranging from 0 to 60%, across the 10 districts surveyed in Cabo Delgado (Fig. 1). Figure 1 is a map of the prevalence of S. haematobium across each village included in the study, in the 10 districts surveyed. Table 1 summarises the S. haematobium prevalence and intensity of infection pre-intervention by age group and sex. In total 151 out of 155 villages that were selected for the eligibility survey fulfilled the target endemicity cut-offs defined by SCORE with a microscopic haematuria prevalence of ≥ 21% and were therefore selected for the study. One village was dropped at random to achieve the target 150 villages. Unfortunately, the time taken to carry out the census, eligibility and cross-sectional survey at baseline took longer than expected and the data collection occurred in the rainy season for some villages. Six villages were inaccessible due to the rains and data was only collected in 144 villages.
Fig. 1.
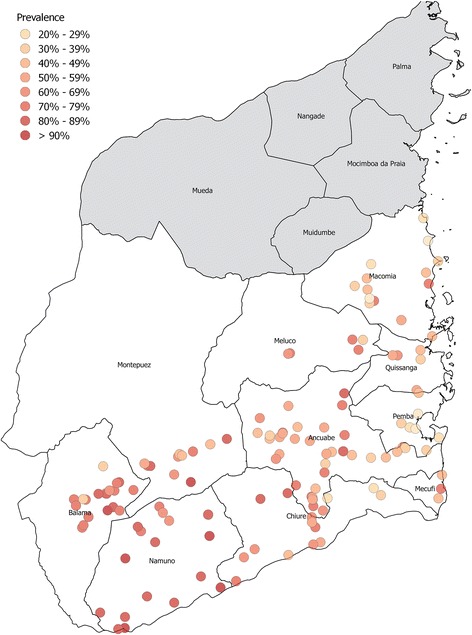
Prevalence of S. haematobium across all villages included in the SCORE study at baseline, in the ten districts of Cabo Delgado, Mozambique
Table 1.
Prevalence and intensity of S. haematobium in first-year students (aged 5–8 years), 9–12-year-olds, 13–14-year-olds, and adults (aged 20–55 years) at baseline of a SCORE study in northern Mozambique
| Age group (years) | No. of individuals examined | No. of individuals infected (%)a | Intensity of S. haematobium infection | |
|---|---|---|---|---|
| Arithmetic mean intensity (eggs/10 ml)b | No. of heavily infected individuals (%) | |||
| Both sexes | ||||
| 5–8 | 7463 | 4709 (63.1) | 58.2 | 1440 (33.8) |
| 9–12 | 7317 | 4873 (66.6) | 71.9 | 1633 (33.9) |
| 13–14c | 5429 | 4010 (73.9) | ||
| 20–55 | 4259 | 1910 (44.8) | 23.9 | 300 (15.8) |
| Totald | 19,039 | 11,492 (60.4) | 55.8 | 3373 (17.7) |
| Females | ||||
| 5–8 | 3196 | 1922 (60.1) | 44.7 | 517 (27.1) |
| 9–12 | 3013 | 1890 (62.7) | 54.4 | 547 (29.0) |
| 13–14c | 2276 | 1666 (73.2) | ||
| 20–55 | 1329 | 560 (42.1) | 22.0 | 88 (15.9) |
| Totald | 7538 | 4372 (58.0) | 44.6 | 1152 (23.8) |
| Males | ||||
| 5–8 | 4261 | 2784 (65.3) | 68.1 | 923 (33.2) |
| 9–12 | 4239 | 2946 (69.5) | 84.9 | 1086 (37.1) |
| 13–14c | 3153 | 2344 (74.3) | ||
| 20–55 | 2924 | 1349 (46.1) | 24.8 | 212 (15.8) |
| Totald | 11,424 | 7079 (62.0) | 63.12 | 2221 (26.7) |
aFor sexes combined, there was a significant difference in prevalence between age groups (P < 0.001) with the following sequence: 9–12 > 5–8 > 20–55 years (χ2 test). Within every age group, males had higher prevalence of infection than females in 5–8, 9–12 and 20–55 years (P < 0.001, P < 0.001, P < 0.01, respectively)
bFor sexes combined, there was no difference between the two groups of children (age group 5–8 and 9–12 years), but intensity of infection among children was significantly higher (P < 0.001) than adults (one-way ANOVA). There was no difference between genders among school children, but male adults had higher intensities than females (P < 0.02)
cEligibility results: prevalence of haematuria by dipstick from a single urine sample from children aged 13–14 years
dTotal only includes the microscopic urine filtration data from the cross-sectional analysis, and excludes eligibility data that was collected in 13–14 year-olds using reagent strips only
The overall S. haematobium prevalence based on microhaematuria was 73.9% in 5429 children aged 13–14 years who participated in the eligibility survey (Table 1). A total of 19,039 individuals then provided urine samples at baseline, of which a total of 60.4% were infected. Examination revealed that of the 7463 children in the first year of school, 63.1% were infected with S. haematobium with significantly more boys (68.1%) than girls (60.1%) (χ2 = 23.2, P < 0.001). Among 7317 children aged 9–12 years tested, S. haematobium infection was found in 66.6% where the infection was higher in boys (69.5%) than girls (62.7%) (χ2 = 34.5, P < 0.001). A total of 4259 adults aged 20–55 years participated where 44.8% were found to be infected with significantly more males (46.1%) infected than females (42.1%) (χ2 = 6.35, P < 0.01). For sexes combined, the different age groups had significantly different prevalence (χ2 = 8.21, P < 0.01) with 9–12 years being the most infected, followed by first-year students and then adults.
In all age groups, the majority had light infections. A total of 17.7% had heavy infections of which 235 individuals (6.97%) had an egg count of 1000 eggs/10 ml or more. For the sexes combined, there was no difference between the two groups of children (first-year students and 9–12 years), but the intensity of infection among children was significantly higher (one-way ANOVA: F(2,4154) = 8.013, P < 0.001) than adults. There was no statistically significant difference in intensity of infection between males and females among school children, but adult males had higher intensities than females (one-way ANOVA: F(2,4152) = 6.197, P < 0.02).
The age profile for prevalence and heavy intensity infection with S. haematobium infection in Fig. 2 shows the breakdown by age, with a peak in infection rates among school-aged children and then a decrease into adulthood.
Fig. 2.
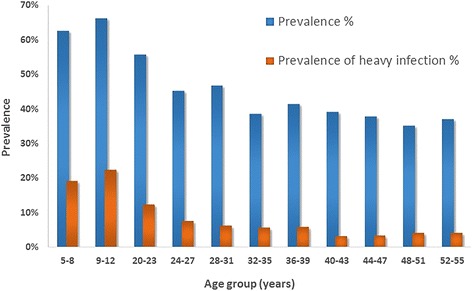
Prevalence of S. haematobium infection (dark blue) and heavy infection (red) by age group
Schistosoma haematobium infection and demographic and potential environmental risk factors of local communities
The prevalence and intensity of infection related to the main occupation among 4154 randomly selected adults is shown in Table 2. Most adults (95.6%) stated that farming was their main occupation. Overall there were no statistically significant differences between the prevalence of infection between professions. However, mean intensity of infection was higher among students although the numbers were not large enough to be significant (see Table 2).
Table 2.
Prevalence and intensity of S. haematobium by occupation adults aged 20–55 years
| Main occupation | No. of individuals examined | No. of individuals infected (%) | Intensity of S. haematobium infection | |
|---|---|---|---|---|
| Arithmetic mean intensity of infectiona | Heavy infection (%) | |||
| Farmer | 3971 | 1805 (45.5) | 46.7 | 276 (15.3) |
| Teacher | 73 | 31 (43.7) | 49.9 | 5 (16.1) |
| Student | 45 | 21 (46.7) | 103.4 | 12 (57.1) |
| Other | 66 | 21 (31.8) | 103.4 | 3 (14.3) |
| Total | 4154 | 1877 | 49.8 | 297 (15.8) |
aThere was no statistical difference between prevalence or intensity of infection by occupation in the univariate logistic regression, controlling for sex and age
Table 3 summarises the potential environmental determinants of schistosomiasis infection in the study area. A village-level questionnaire was asked to 144 community leaders to collect information on potential risk factors associated with urogenital schistosomiasis. “Agriculture” was the main reported occupation (75.3%), although this was not expanded on in the questionnaire, and rice cultivation was practised in 23 villages (15.3%). Sixty-two villages had a health centre, however, only one of these stocked praziquantel. A total of 20.7% and 11.3% villages had a permanent and seasonal stagnant water body, respectively. Schistosomiasis risk behaviour such as washing (91.3%) and bathing (86.7%) in open water sources was high. Open urination was reported in most villages (83.3%), and use of pit latrines was reported in nearly all (98%) communities. Knowledge of schistosomiasis was relatively high (68.6%), as defined by the question “Prior to today’s conversation, had you ever heard of [local term for schistosomiasis]?”, However, correct knowledge of how the disease is acquired was low (23.2%) among those who had heard of the disease. Misconceptions such as the belief that schistosomiasis is transmitted through sexual contact were 12.3%. Most interviewees reported symptoms of schistosomiasis as pain on urination (76.5%), increased need to urinate (8.72%) and abdominal pain (3.36%). Knowledge about the existence of treatment against schistosomiasis was high (69.7%), although nearly all the respondents did not know the name of the drug (97.4%).
Table 3.
Frequency of potential demographic, health-system related, and environmental risk factors associated with urogenital schistosomiasis reported by 144 communities in Cabo Delgado, northern Mozambique
| Variable | Frequency | Percentage |
|---|---|---|
| Main occupation of inhabitants in the village | ||
| Agriculture | 113 | 75.3 |
| Rice farming | 23 | 15.3 |
| Irrigation-based farming | 7 | 4.7 |
| Fishing | 5 | 3.3 |
| Local health facility | ||
| Health facility open regularly | 62 | 41.3 |
| Does the health facility dispense PZQ | 1 | 0.7 |
| Water contact sites | ||
| No. of villages with seasonal rivers | 85 | 56.7 |
| No. of villages with permanent rivers | 76 | 51.0 |
| No. of villages with permanent standing water body | 31 | 20.7 |
| No. of villages with seasonal standing water body | 17 | 11.3 |
| Water sources for drinking | ||
| Well or borehole | 133 | 88.7 |
| Open surface water | 125 | 83.3 |
| Tap water | 8 | 5.3 |
| Water sources for washing/bathing | ||
| Open surface water | 137 | 91.3 |
| Well or borehole | 131 | 87.3 |
| Tap water | 9 | 6.0 |
| Sanitation facilities | ||
| Pit latrine | 147 | 98.0 |
| Bush/field | 125 | 83.3 |
| Improved latrine | 10 | 6.0 |
| Toilet | 10 | 6.0 |
| Heard about schistosomiasis | 155 | 68.6 |
| How do you catch schistosomiasis?a | ||
| Do not know | 79 | 50.1 |
| Bathing in open water sources | 36 | 23.2 |
| Sexual | 19 | 12.3 |
| Drinking water | 18 | 11.6 |
| Symptoms of schistosomiasisa | ||
| Pain on urination | 114 | 76.5 |
| Do not know | 17 | 11.4 |
| Increase need to urinate | 13 | 8.7 |
| Abdominal pain | 5 | 3.4 |
| How do you prevent schistosomiasis? | ||
| Treatment | 108 | 69.7 |
| Do not know | 31 | 20.0 |
| Education | 31 | 20.0 |
| Other | 12 | 7.7 |
| What is the name of the treatment for schistosomiasis? | ||
| Do not know | 151 | 97.4 |
| Praziquantel | 4 | 2.6 |
aKnowledge among a total of 155 participants who had heard of urogenital schistosomiasis
Discussion
Preventive chemotherapy with praziquantel is the current mainstay of schistosomiasis control, specifically the treatment of school-aged children as a function of prevalence level. To determine the best strategy to gain and sustain the control of S. haematobium in highly endemic areas, communities were selected to participate in a four-year cluster randomised intervention trial if there was a haematuria prevalence of ≥ 21% in 13–14-year-olds [16–18]. Here, we report the baseline parasitological situation in northern Mozambique before the onset of the study to assess the differential impact of school-based and community-based treatment.
Overall microhaematuria was high among the 144 villages examined, with 73.9% among 13–14 years by urine dipstick. When first-year students (aged 5–8 years) and 9–12-year-old children were examined with double filtration on a single urine sample in the baseline survey, the overall prevalence of S. haematobium was 63.1% and 66.6%, respectively. These findings were consistent with a national mapping survey carried out between 2005 and 2007 where schistosomiasis was found to be highly endemic among school children (aged 7–22 years) in Cabo Delgado province, with an overall prevalence of 57.9% of S. haematobium. The study highlights urine examination with reagent strips as a rapid diagnostic tool to identify high-risk areas for urogenital schistosomiasis consistent with previous studies [20–22].
As shown in other studies both gender and age had a significant effect on infection, with the highest prevalence and proportion of those heavily infected occurring in males and among 9–12-year age group with those same factors decreasing into adulthood [1, 23–28]. This finding may be explained by socio-cultural factors where an increased predisposition in boys to behaviours that expose them to the risk of infection such as swimming or bathing. This group will, therefore, be the focus for the monitoring and evaluating disease transmission from baseline through to Year 5 of this SCORE study.
This is the first study in Mozambique looking at infection rates among adults. Although children had higher levels of infection than adults, it was found here that adults had a high average infection of 44.5%. Per current WHO guidelines, children aged 5–14 years should be treated once every year in such high-risk communities (prevalence ≥50% measured by parasitological methods), as well as adults, considered “at-risk”. Occupation was recorded in two places in the baseline survey. When parasitological samples were taken from adults, they were asked their profession. Most adults (95.6%) stated that “farming” was their main occupation; however, there was no significant association between occupation and prevalence of infection. In addition, the village inventory questionnaire asked for village leaders about the main occupations of people in the village. Here, fishing and rice farming, which are widely shown to be a determinant of S. haematobium, were reported by 2% and 15.3%, respectively [29–32]. Since there is no single major cash crop in Cabo Delgado, agriculture has a more subsistence character with farmers selling small surpluses of crops such as cashew, groundnuts, cassava and maize.
The importance of contact with snail infested water and therefore with cercariae through bathing, combined with open urination, is acknowledged for governing the transmission of urogenital schistosomiasis [33, 34]. The community questionnaires were important in highlighting that knowledge of disease transmission is rudimentary, which has also been highlighted in knowledge, attitude and practices schistosomiasis survey that was conducted simultaneously to the SCORE study in the neighbouring province of Nampula in Mozambique [35]. Among our study population, use of open surface water was widely reported for washing and bathing, basic sanitation facilities such as pit latrines were reported to exist in most communities, yet open urination was widely practised. Furthermore, many villagers spend months at a time sleeping in tented accommodation when they were working in the ‘machambas’ (fields) with no access to sanitation. Although knowledge of the existence of schistosomiasis (translated into various local languages) was high, few understood the transmission cycle associated with spending time in open water sources. Misconceptions such as drinking dirty water and sexual intercourse were also described as ways to get schistosomiasis. Safe water, adequate hygiene and good sanitation interventions have shown to be successful in preventing schistosomiasis transmission elsewhere, and it would be recommended to complement the praziquantel treatment with supplementary measures such as health education and sanitation facilities [36, 37].
Conclusions
In conclusion, the baseline results showed that Cabo Delgado Province in northern Mozambique is hyperendemic for urogenital schistosomiasis, particularly among school-aged children, and this risk appears to have remained unchanged over time [6, 7]. Prevalence rates were found to be greater than 50% in all districts, which highlights the need for annual praziquantel treatment in accordance with WHO guidelines. It also shows that prevalence differs by age and sex where the infection was also high among adults, which needs to be considered when planning programmatic schistosomiasis control efforts. In addition to preventive chemotherapy, access to safe water for washing and bathing, improved sanitation facilities and developing suitable health education tools could help tackle the burden of schistosomiasis. The aim of the SCORE project in Mozambique is to provide an evidence base for the national control programme to make informed decisions about preventive chemotherapy strategies to gain and sustain the control of S. haematobium infection. The results after a five-year period will demonstrate the cost-benefit impact of alternative approaches to schistosomiasis control through treatment of communities, schools, and treatment holidays.
Acknowledgements
We are grateful to the members of SCORE secretariat and advisory committee for reviewing our study, their advice, input and support of our work. We are grateful to the Schistosomiasis Control Initiative for providing praziquantel for the study, through the donation to the Ministry of Health in Mozambique. We are grateful to Dr. Ben Styles from SCI for the randomisation of the schools. We thank the National Neglected Tropical Disease Manager at the time of the study, Dr. Olga Amiel, for her support in putting the PZQ in place for the treatment. We thank the technicians from different institutions of Mozambique for their support in the field and the laboratory, particularly Mr. Almeida da Ilda Ernesto and Olivio Cuambe. We are grateful to the health, education and village authorities of all districts for their contribution. Finally, we thank all the children, parents, teachers and village leaders who participated in this study.
Funding
This research was financially supported by the Bill & Melinda Gates Foundation through the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) based at the University of Georgia in Athens, United States of America, Grant RR374–053/4893196. Praziquantel tablets for schistosomiasis treatment are donated by the Schistosomiasis Control Initiative (SCI) based at Imperial College London, United Kingdom.
Availability of data and materials
The datasets used and analysed during the current study are not publicly available as per the data sharing agreement in place with the Mozambique Ministry of Health, but are available from the corresponding author on reasonable request.
Abbreviations
- CWT
Community-wide treatment
- SAC
school-aged children
- SBT
school-based treatment
- SCORE
Schistosomiasis Consortium for Operational Research and Evaluation
- STH
soil-transmitted helminths
- WHO
World Health Organisation
Authors’ contributions
AP was responsible for the formulation of the overarching research aim of the manuscript. AP and ND were responsible for the data curation and analysis. AP and AF were responsible for the funding acquisition. AP, PGG, HOA and AD were responsible for the investigation and data collection. AP, PGG, HOA, RN and JF provided the resources for the study and were involved in the overall administration of the project. AP wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
National and local health, educational and administrative authorities were comprehensively informed of the study. Prior to the start of the study in each village, open public meetings were carried out in the local language followed by question and answer sessions with the survey team. The purpose of the study was explained to all schoolchildren, and verbal consent was obtained from the children themselves. It was explained that any child had the chance to withdraw from the study at any point, without any consequence. Written informed consent was also obtained from school headmasters. Due to the high rate of illiteracy among these communities, finger-print consent was obtained from all participants and parents or legal guardians of the children. Ethical review of the protocol was obtained from the National Bio-ethical Committee for Health of Mozambique (NBCHM), and the survey was conducted according to NBCHM guidelines (ref: IRB00002657). The study protocol was also approved by Imperial College London (ref: ICREC_10_8_2). In addition to these, the University of Georgia institutional review board IRB implemented an administrative human subjects review and issued additional approval 10533-0 for Mozambique.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna E. Phillips, Email: a.phillips05@imperial.ac.uk
Pedro H. Gazzinelli-Guimarães, Email: pedro.gazzinelliguimaraes@nih.gov
Herminio O. Aurelio, Email: valdoaurelio1@yahoo.com.br
Neerav Dhanani, Email: n.dhanani@imperial.ac.uk.
Josefo Ferro, Email: josefoferro@yahoo.com.br.
Rassul Nala, Email: rassulmn@gmail.com.
Arminder Deol, Email: arminder.deol@imperial.ac.uk.
Alan Fenwick, Email: a.fenwick@imperial.ac.uk.
References
- 1.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utzinger J, Raso G, Brooker S, de Savigny D, Tanner M, Ørnbjerg N, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859–1874. doi: 10.1017/S0031182009991600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 5.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augusto G, Nala R, Casmo V, Sabonete A, Mapaco L, Monteiro J. Geographic distribution and prevalence of schistosomiasis and soil-transmitted helminths among schoolchildren in Mozambique. Am J Trop Med Hyg. 2009;81(5):799–803. doi: 10.4269/ajtmh.2009.08-0344. [DOI] [PubMed] [Google Scholar]
- 7.Traquinho GA, Quintó Ll, Nalá RM, Gama Vaz G, Corachan M. Schistosomiasis in northern Mozambique. Trans R Soc Trop Med Hyg 1998;92:279–281. [DOI] [PubMed]
- 8.Schur N, Hurlimann E, Stensgaard AS, Chimfwembe K, Mushinge G, Simoonga C, et al. Spatially explicit Schistosoma infection risk in eastern Africa using Bayesian geostatistical modelling. Acta Trop. 2013;128(2):365–377. doi: 10.1016/j.actatropica.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation. Schistosomiasis: Progress Report 2001–2011 and Strategic Plan 2012–2020. WHO/HTM/NTD/PCT/2013.2. Geneva, Switzerland: World Health Organization; 2013.
- 10.World Health Organisation . Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organisation; 2006. [Google Scholar]
- 11.Gabrielli AF, Toure S, Sellin B, Sellin E, Ky C, Ouedraogo H, et al. A combined school- and community-based campaign targeting all school-age children of Burkina Faso against schistosomiasis and soil-transmitted helminthiasis: performance, financial costs and implications for sustainability. Acta Trop. 2006;99(2–3):234–242. doi: 10.1016/j.actatropica.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Lo NC, Bogoch II, Blackburn BG, Raso G, N'Goran EK, Coulibaly JT, et al. Comparison of community-wide, integrated mass drug administration strategies for schistosomiasis and soil-transmitted helminthiasis: a cost-effectiveness modelling study. Lancet Glob Health. 2015;3(10):629–638. doi: 10.1016/S2214-109X(15)00047-9. [DOI] [PubMed] [Google Scholar]
- 13.Gurarie D, Yoon N, Li E, Ndeffo-Mbah M, Durham D, Phillips AE, et al. Modelling control of Schistosoma haematobium infection: predictions of the long-term impact of mass drug administration in Africa. Parasit Vectors. 2015;8:529. doi: 10.1186/s13071-015-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson R, Truscott J, Hollingsworth TD. The coverage and frequency of mass drug administration required to eliminate persistent transmission of soil-transmitted helminths. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369:20130435. doi: 10.1098/rstb.2013.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson RM, Truscott JE, Pullan RL, Brooker SJ, Hollingsworth TD. How effective is school-based deworming for the community-wide control of soil-transmitted helminths? PLoS Negl Trop Dis. 2013;7(2):e2027. doi: 10.1371/journal.pntd.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colley DG. Morbidity control of schistosomiasis by mass drug administration: how can we do it best and what will it take to move on to elimination? Trop Med Health. 2014;42(Suppl. 2):25–32. [DOI] [PMC free article] [PubMed]
- 17.Ezeamama AE, He C-L, Shen Y, Yin X-P, Binder SC, Campbell CH, et al. Gaining and sustaining control of schistosomiasis: study protocol and baseline data before implementation of cluster randomized trials with different treatment schemes in five African countries. BMC Infect Dis. 2016;16:229. doi: 10.1186/s12879-016-1575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips AE, Gazzinelli-Guimaraes PH, Aurelio HO, Ferro J, Nala R, Clements M, et al. Assessing the benefits of five years of different approaches to treatment of urogenital schistosomiasis: a SCORE project in northern Mozambique. PLoS Negl Trop Dis. (In press) [DOI] [PMC free article] [PubMed]
- 19.World Health Organisation Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 20.Knopp S, Person B, Ame SM, Mohammed KA, Ali SM, Khamis S, et al. Elimination of schistosomiasis transmission in Zanzibar: baseline findings before the onset of a randomized intervention trial. PLoS Negl Trop Dis. 2013;7(10):e2474. doi: 10.1371/journal.pntd.0002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor P, Chandiwana SK, Matanhire D. Evaluation of the reagent strip test for haematuria in the control of Schistosoma haematobium infection in schoolchildren. Acta Trop. 1990;47:91–100. doi: 10.1016/0001-706X(90)90071-7. [DOI] [PubMed] [Google Scholar]
- 22.Emukah E, Gutman J, Eguagie J, Miri ES, Yinkore P, Okocha N, et al. Urine heme dipsticks are useful in monitoring the impact of praziquantel treatment on Schistosoma haematobium in sentinel communities of Delta state, Nigeria. Acta Trop. 2012;122:126–131. doi: 10.1016/j.actatropica.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolhouse MEJ. Patterns in parasite epidemiology: the peak shift. Parasitol Today. 1998;14:428–434. doi: 10.1016/S0169-4758(98)01318-0. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt H, Brooker S, Lwambo NJ, Siza JE, Bundy DA. The performance of school-based questionnaires of reported blood in urine in diagnosing Schistosoma haematobium infection: patterns by age and sex. Tropical Med Int Health. 1999;4(11):751–757. doi: 10.1046/j.1365-3156.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 25.Senghor B, Diallo A, Sylla SN, Doucouré S, Ndiath MO, Gaayeb L, et al. Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasit Vectors. 2014;7:5. doi: 10.1186/1756-3305-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudge JW, Stothard JR, Basáñez MG, Mgeni AF, Khamis IS, Khamis AN, Rollinson D. Micro-epidemiology of urinary schistosomiasis in Zanzibar: local risk factors associated with the distribution of infections among schoolchildren and relevance for control. Acta Trop. 2008;105(1):45–54. doi: 10.1016/j.actatropica.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 27.King CH, Keating CE, Muruka JF, Ouma JH, Siongok TK, Mahmoud AA. Urinary tract morbidity in schistosomiasis haematobia: associations with age and intensity of infection in an endemic area of Coast Province, Kenya. Am J Trop Med Hyg. 1988;39:361–368. doi: 10.4269/ajtmh.1988.39.361. [DOI] [PubMed] [Google Scholar]
- 28.Njunda AL, Ndzi EN, Assob JCN, Kamga HF, Kwenti ET. Prevalence and factors associated with urogenital schistosomiasis among primary school children in the barrage, Magba sub-division of Cameroon. BMC Public Health. 2017;17(1):618. doi: 10.1186/s12889-017-4539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geleta S, Alemu A, Getie S, Mekonnen Z, Erko B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo Primary School children in Gambella Regional State, southwestern Ethiopia: a cross-sectional study. Parasit Vectors. 2015;10:8:215. [DOI] [PMC free article] [PubMed]
- 30.Mboera LE, Senkoro KP, Rumisha SF, Mayala BK, Shayo EH, Mlozi MR. Plasmodium falciparum and helminth coinfections among schoolchildren in relation to agro-ecosystems in Mvomero District, Tanzania. Acta Trop. 2011;120(1–2):95–102. doi: 10.1016/j.actatropica.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Yapi YG, Briët OJ, Diabate S, Vounatsou P, Akodo E, Tanner M, Teuscher T. Rice irrigation and schistosomiasis in savannah and forest areas of Côte d'Ivoire. Acta Trop. 2005;93(2):201–211. doi: 10.1016/j.actatropica.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Fournet F, N'Guessan NA, Cadot E. Land-use and urinary schistosomiasis in Daloa (Côte d'Ivoire) Bull Soc Pathol Exot. 2004;97(1):33–36. [PubMed] [Google Scholar]
- 33.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(12):1–12. doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YAL, Mahmud R, Surin J. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. 2013;7(8):e2377. doi: 10.1371/journal.pntd.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rassi C, Kajungu D, Martin S, Arroz J, Tallant J, Zegers de Beyl C, et al. Have you heard of schistosomiasis? Knowledge, attitudes and practices in Nampula Province, Mozambique. PLoS Negl Trop Dis. 2016;10(3):e4504. doi: 10.1371/journal.pntd.0004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton M. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8:e3296. doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are not publicly available as per the data sharing agreement in place with the Mozambique Ministry of Health, but are available from the corresponding author on reasonable request.


