Abstract
Background
Mentha piperita L. is a flowering plant belonging to the Lamiaceae family. Mentha plants constitute one of the main valuable sources of essential oil used in foods and for medicinal purposes.
Methods
The present study aimed to investigate the composition and in vitro antioxidant activity of Mentha piperita leaf essential oil (MpEO). A single dose of CCl4 was used to induce oxidative stress in rats, which was demonstrated by a significant rise of serum enzyme markers. MpEO was administrated for 7 consecutive days (5, 15, 40 mg/kg body weight) to Wistar rats prior to CCl4 treatment and the effects on serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and γ -glutamyl transpeptidase (γ-GT) levels, as well as the liver and kidney superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activity and thiobarbituric acid reactive substances (TBARS) levels were evaluated. In addition, histopathological examinations of livers and kidneys was performed.
Results
The in vitro antioxidant activity of MpEO was lower than that of silymarin. Pretreatment of animals with MpEO at a dose of 5 mg/kg did not have a significant effect on ALT, AST, ALP, LDH, γGT, urea or creatinine levels in CCl4-induced stress. Whereas pretreatment with MpEO at doses of 15 and 40 mg/kg prior to CCl4, significantly reduced stress parameters (ALT, AST, ALP, LDH, γGT, urea and creatinine) compared to the CCl4-only group. Moreover, a significant reduction in hepatic and kidney lipid peroxidation (TBARS) and an increase in antioxidant enzymes SOD, CAT and GPx was also observed after treatment with MpEO (40 mg/kg) compared to CCl4-treated rats. Furthermore, pretreatment with MpEO at 40 mg/kg can also markedly ameliorate the histopathological hepatic and kidney lesions induced by administration of CCl4.
Conclusions
We could demonstrate with this study that MpEO protects liver and kidney from CCl4-induced oxidative stress and thus substantiate the beneficial effects attributed traditionally to this plant.
Keywords: M. piperita, Essential oil, Liver, Kidney, Oxidative stress, Histopathological
Background
Reactive oxygen species (ROSs) are various forms of activated oxygen. A disproportion of the reactive oxygen species and the absence of their scavenge systems in cells lead to oxidative stress and increases the risk of several human chronic diseases [1]. ROS contributes to the development of various diseases such as diabetes, atherosclerosis, cancer, neurodegenerative diseases, liver cirrhosis and the aging process [2]. The liver plays a central role in the maintenance of systemic lipid homeostasis and is especially susceptible to ROS damage. CCl4 is now of greatest concern as an environmental contaminant [3]. It was reported that CCl4 was one of the most commonly used toxins in the experimental study of liver diseases [4]. Abraham et al. [5] showed that the nephrotoxic effects of CCl4 were also associated with free radical production.
To prevent the damage caused by ROS, living organisms have developed an antioxidant defense system that includes the presence of non-enzymatic antioxidants and enzymes such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) [6]. It has been anticipated that in addition to these natural antioxidants, other synthetic or natural ROS scavengers may reduce the incidence of free radical-mediated diseases. The use of antioxidants in the prevention and cure of various diseases is intensifying, and there is considerable interest in the study of the antioxidant activities of molecules such as plant polyphenolic and carotenoid components [6, 7]. Antioxidants appear to act against disease processes by increasing the levels of endogenous antioxidant enzymes and decreasing lipid peroxidation [8].
A number of studies showed that various herbal extracts could protect liver and kidney against CCl4-induced oxidative stress by inhibiting lipid peroxidation and enhancing antioxidant enzyme activity [9]. Silymarin, a flavonolignan mixture of milk thistle (Silybum marianum), is one such important herbal hepatoprotective drug. Silymarin exhibits hepatoprotective effects by altering cytoplasmic membrane architecture and, in turn, preventing the penetration of hepatotoxic substances, such as carbon tetrachloride (CCl4), thioacetamide and D-galactosamine [10].
The well-known and widely used peppermint (Mentha piperita L.) (Lamiaceae) is a cultivated natural hybrid of Mentha aquatica L. (water mint) and Mentha spicata L. (spearmint). Although a native genus of the Mediterranean region, it is cultivated all over the world for its use in flavor, fragrance, medicinal, and pharmaceutical applications. Peppermint oil is one of the most widely produced and consumed essential oils [11, 12]. Besides its uses in food, herbal tea preparations, and confectioneries, the medicinal uses of mint, which date back to ancient times, include carminative, anti-inflammatory, antispasmodic, antiemetic, diaphoretic, analgesic, stimulant, emmenagogue, and anticatharrhal application. It is also used against nausea, bronchitis, flatulence, anorexia, ulcerative colitis, and liver complaints. Mint essential oils are generally used externally for antipruritic, astringent, rubefacient, antiseptic, and antimicrobial purposes, and for treating neuralgia, myalgia, headaches, and migraines [13, 14].
From the experimental and clinical studies performed on Mentha piperita leaf essential oil (MpEO), it seems that most of its pharmacological actions are due to its antioxidant activity which is mainly due to its ability to scavenge free radicals and/or inhibit lipid peroxidation [15, 16]. Antioxidants are substances that delay or prevent the oxidation of inter- or intra-cellular oxidizable substrates from oxidative stress. In this study, we report the chemical composition and antioxidant effects of MpEO in several in vitro systems (DPPH and superoxide scavenging activities). Besides, we are interested in determining the possible protective effects of MpEO against oxidative damage of the liver and kidney following an intraperitoneal administration of CCl4, by assessing the oxidative stress profile and some serum biochemical parameters.
Methods
Plant material
Fresh leaves of M. piperita L. samples were harvested from the local market at Sfax (Tunisia) (N: 34.4426°, E: 10.4537°) during the vegetative stage in June 2013. The samples were identified and authenticated by a senior botanist, Pr. Ferjani Ben Abdallah, at the Faculty of Science of Sfax, University of Sfax (Tunisia). From 50 individual M. piperita L. plants each, a total of 80–100 leaves (≈ 12 cm2 in size) were randomly collected from the base to the apex. The fresh leaves were mixed and immediately dried in the shade away from light at room temperature. After drying, the samples were grounded to a fine powder that was used for the extraction of essential oil.
Essential oil preparation
MpEO was extracted by the steam distillation method. A mass of 3 kg of dry plant material was hydrodistillated for 2 h in a Clevenger-type apparatus. The recovered (0.47%) essential oil was dried with anhydrous Na2SO4, and stored at 4 °C.
Mentha piperita essential oil composition
MpEO compositional analysis of the volatile constituents was performed on a Hewlett-Packard gas chromatograph GC: 5890 series II. The fused HP-Innowax capillary column (polyethylene glycol, 30 m, 0.25 μm, ID, 0.25 mm film thickness) was directly connected to the mass spectrometer. Nitrogen was used as a carrier gas at a flow rate of 1.2 ml/min. Oven temperature was initially set at 50 °C (1 min) and gradually raised to 250 °C (5 min) at 7 °C/min. The temperatures of the injection port and detector were maintained at 250 and 280 °C, respectively. The mass spectrometer was operated (full scan-mode) in the EI-mode at 70 eV.
Component identification
The essential oil components were identified based on their mass spectra and computer matching with the data available in the Wiley 275 library (Wiley, New York).
In vitro antioxidant activities test
The antioxidant activity of the MpEO was determined by two methods and compared with the activity of silymarin, a standardized extract of the milk thistle seeds that containes a mixture of flavonolignans. Silymarin has a number of potential mechanisms including chemoprotective effects from environmental toxins and anti-inflammatory activity and is used as a drug.
Measurement of free radical-scavenging action
2,2-Diphenyl picrylhydrazyl (DPPH) free radicals scavenging activity was assessed according to Blois [17], with a slight modification. Different concentrations of the MpEO and silymarin (5–100 μg/ml) were mixed with 1 ml of 0.1 mM DPPH in ethanol solution and 450 μL of 50 mM Tris-HCl buffer (pH 7.4) was added. The solution was incubated at 37 °C for 30 min and the reduction of DPPH free radicals was measured by reading the absorbance at ʎ = 517 nm. Silymarin was used as reference standard. The activity is given as % DPPH scavenging and calculated according to the following equation:
The antioxidant activity of MpEO is expressed as IC50, defined as the concentration of MpEO required to cause a 50% decrease in initial DPPH concentration. Each sample was analyzed six times.
Scavenging of superoxide anion
The influence of MpEO on the generation of superoxide anion was measured according to the method described by Yen & Chen, 1995 [18]. Superoxide anion was generated in a non-enzymatic system and determined by spectrophotometric measurement for the reduction of nitroblue tetrazolium. The reaction mixture, which contained 100 μL of essential oil in ethanol, 800 μL of 1 M phosphate buffer (pH 7.4), 400 μL of distilled water, 100 μL of 0.1 M Na4EDTA, 100 μL of 1.5 mM NBT and 50 μL of 0.12 mM riboflavin was incubated at ambient temperature for 5 min, and the color was read at ʎ = 560 nm against blank samples.
Where blank OD is the absorbance of the control reaction and sample OD is the absorbance in the presence of MpEO. The IC50 was calculated from the plot of the inhibition percentage against the essential oil concentration. Each sample was analyzed six times.
In vivo antioxidant properties
Animal
Male Wistar rats, weighing about 200–220 g, were purchased from the Central Pharmacy of Tunisia (SIPHAT, Tunisia). They were housed at 22 ± 3 °C with light/dark periods of 12 h and a minimum relative humidity of 40%. The animals had free access to commercial pellet diet (SICO, Sfax, Tunisia) and water ad libitum. The general guidelines for the use and care of living animals in scientific investigations were followed [19]. The handling of the animals was approved by the Tunisian Ethical Committee for the Care and Use of laboratory animals.
Experimental design
After acclimatizing to the laboratory conditions for 1 week, 70 rats were divided into 7 groups of 10 animals and treated for 7 days as follow [20]:
The rats of group 1 served as normal control and received saline orally daily for 7 days and were injected with 1 ml/kg BW of just olive oil (the solvent of CCl4) on the 7 day. The rats of group 2 served as CCl4-hepato and renotoxicity control and were received saline orally daily for 7 days and were injected with 1 ml/kg BW of CCl4 and olive oil mixture on the 7 day (a single intraperitoneal injection). The CCl4 dose was selected according to the reference dose for chronic oral exposure (RFD) as recommended for CCl4 (CASRN 56–23-5) [21].
The rats of group 3 were pretreated orally seven times with a dose of 50 mg/kg BW of reference drug silymarin with an interval of 24 h [22].
The rats of groups 4, 5, 6 and 7 were pretreated orally seven times with doses of 5, 15 and 40 mg MpEO /kg BW, respectively with an interval of 24 h [23].
After pretreatment with either silymarin or MpEO for 7 days, the rats of groups 3, 4, 5 and 6 received a single intraperitoneal injection of CCl4 (1 ml/kg BW) on the 7 day.
Rats were killed 24 h after vehicle or CCl4 single injection. The animals in the different groups were killed by cervical decapitation to avoid stress conditions.
Sample collection
Serum was prepared by centrifugation (1500×g, 15 min, 4 °C; Beckman-Coulter, Marseille, France) and stored at −80 °C for further biochemical assays. The liver and kidney tissues were immediately removed and dissected over ice-cold glass slides and a part was homogenized (10% w/v) with an Ultra Turrax homogenizer in ice-cold, 1.15% KCl-0.01 M sodium, potassium phosphate buffer. Homogenates were centrifuged at 10000×g for 20 min at 4 °C. The resulting supernatants were used for immediate lipid peroxidation and protein oxidation determination. Homogenate aliquots were stored at −80 °C for further biochemical assays. Other parts of these livers and kidney tissues were fixed in 10% formaldehyde solution and processed for paraffin sectioning and histological studies.
Biochemical assays
Biochemical markers in plasma
Plasma levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), creatinine and urea rates were measured in plasma samples by standardized enzymatic procedures using commercial kits from (Biolabo, Maizy, France) on an automatic biochemistry analyzer (Vitalab Flexor E, Diamond Diagnostics, Holliston, MA).
Protein quantification
Protein content in liver and kidney tissues were determined according to the method of Lowry et al. [24] using bovine serum albumin as a standard.
Lipid peroxidation
Malondialdehyde concentrations (marker for lipid peroxidation) in liver and kidney tissues were determined spectrophotometrically according to Draper & Hadley [25]. Briefly, an aliquot of liver and kidney extracts supernatant was mixed with 1 ml of 5% trichloroacetic acid and centrifuged at 2500×g for 10 min. One ml of thiobarbituric acid reagent (0.67%) was added to 500 μl of supernatant and heated at 90 °C for 15 min. The mixture was cooled and the absorbance measured at 532 nm using a spectrophotometer (Jenway UV-6305, Essex, England). The malondialdehyde values were calculated using 1,1,3,3-tetraethoxypropane as standard and expressed as nmol of malondialdehyde/mg of protein.
Determination antioxidant enzyme activities in liver and kidney tissue
Catalase (CAT) activity was measured according to Aebi [26]. A total of 20 μL tissue homogenate (about 1.5 mg proteins) was added to 1 ml phosphate buffer (0.1 M, pH 7) containing 100 mM H2O2. Rate of H2O2 decomposition was followed by measuring the decrease in absorbance at 240 nm for 1 min. The enzyme activity was calculated using an extinction coefficient of 0.043 mM−1 cm−1 and expressed in international units (I.U.), i.e. in μmol H2O2 destroyed/min/ mg protein, at 25 °C.
Superoxide dismutase (SOD) activity was estimated according to Beyer and Fridovich [27]. The reaction mixture contained 50 mM of tissue homogenates in potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 mM L-methionine, 2 mM riboflavin and 75 mM nitro blue tetrazolium (NBT). The developed blue color in the reaction was measured at 560 nm. Units of SOD activity were expressed as the amount of enzyme required to inhibit the reduction of NBT by 50% and the activity was expressed as units/mg of protein, at 25 °C. Glutathione peroxidase (GPx) activity was measured by the procedure of Flohe and Gunzler [28]. One milliliter of reaction mixture containing 0.3 ml of phosphate buffer (0.1 M, pH 7.4), 0.2 ml of 2 mM glutathione (GSH), 0.1 ml of sodium azide (10 mM), 0.1 ml of H2O2 (1 mM) and 0.3 ml of liver and kidney supernatant were prepared. After incubation at 37 °C for 15 min, the reaction was terminated by adding 0.5 ml 5% TCA. Tubes were centrifuged at 1500×g for 10 min and the supernatant was collected. To 0.1 ml of this reaction supernatant, 0.2 ml of (0.1 M pH 7.4) and 0.7 mL of 5,5 dithiobis-(2-Nitrobenzoic acid) (DTNB, 0.4 mg/ml) were added. After mixing, absorbance was recorded at 420 nm and the enzyme activity was calculated as μmol of GSH oxidized/min/mg protein.
Histopathological studies
At the time of sacrifice, the liver and kidney tissues were removed and fixed in 10% formaldehyde solution and washed. The tissues were dehydrated in increasing gradient of ethanol, finally cleared in toluene and embedded in molten paraffin wax. Sections were cut at 4–5 μm thickness and stained with hematoxylin and eosin (H&E). The slides were photographed with an Olympus UTU1X-2 camera connected to an Olympus CX41 microscope (Tokyo, Japan).
The histological damage in liver was quantified by measuring the index of tissue large numbers of inflammatory cells such as lymphocytes together with hepatic sinusoidal inflammation, hepatocyte necrosis and devastating liver architecture. Moreover, the histological damage in kidney was quantified by measuring the index of tissue the glomerular and tubular necrosis. To evaluate the severity of lesions, the degree of liver and kidney damage was graded according to a zero to four-point scoring system [29], where 0 indicates no damage, I indicates slight damage (1–25%), II indicates discrete damage (26–50%), III indicates moderate damage (51–75%) and IV indicates severe damage (76–100%).The tabulation of data and the statistical analysis were made in accordance with the number of animals with established scores. All the parameters were quantified by a single observer who was not aware of the treatment groups.
Statistical analysis
All values are expressed as mean ± SE for continues variables or as median with inter quartile range [25%, 75%] where appropriate. The results were analyzed by One-Way Analysis of Variance (ANOVA) followed by Tukey test for multiple comparisons using SPSS for Windows (version. 12) or ANOVA-on-ranks with Dunn’s correction. Differences were considered significant at p < 0.05.
Results
Chemical constitution of Mentha piperita L. leaf essential oil
Chemical composition of MpEO was determined by GC/MS analysis. The compounds, their percentages as well as their retention indices are listed in Table 1. MpEO is a mixture with 26 compounds representing 98.17% of the total oil composition. The most abundant chemicals categories for MpEO are oxygenated monoterpenes (79.50%), followed by monoterpene hydrocarbons (16.23%) and sesquiterpene hydrocarbons (2.44%). The major components of MpEO are menthol (33.59%) and iso-menthone (33.00%). In lower amounts we found a variety of compounds including limonene (8.00%), piperitone (3.20%), 1,8-cineole (2.80%), linalool (2.64%), iso-pulegol (2.40%), caryophyllene (1.95%) and pulegone (1.60%).
Table 1.
Chemical composition (%) of leaves essential oil from Tunisian M.piperita as identified by GC/MS analysis
| Peak | Compounds | Retention Time (min) | Percentage (%) |
|---|---|---|---|
| 1 | α-pinene | 4.42 | 1.80 |
| 2 | β-pinene | 6.07 | 0.14 |
| 3 | Sabinene | 6.42 | 0.25 |
| 4 | Myrcene | 7.81 | 1.30 |
| 5 | Limonene | 8.30 | 8.0 |
| 6 | 1,8-cineole | 8.48 | 2.80 |
| 7 | 3-octanone | 10.13 | 0.45 |
| 8 | 3-octanol | 10.41 | 0.53 |
| 9 | Limonene oxide | 13.44 | 0.59 |
| 10 | α-terpineol | 18.62 | 0.37 |
| 11 | Linalool | 15.73 | 2.64 |
| 12 | iso-menthone | 15.14 | 33.00 |
| 13 | Menthyl acetate | 16.35 | 0.68 |
| 14 | Iso-pulegol | 16.71 | 2.40 |
| 15 | Isomenthol | 17.29 | 0.28 |
| 16 | Neo-iso-menthol | 17.90 | 0.45 |
| 17 | Menthol | 18.25 | 33.59 |
| 18 | Pulegone | 18.39 | 1.6 |
| 19 | Neryl acetate | 18.45 | 0.8 |
| 20 | Piperitone | 19.09 | 3.2 |
| 21 | Myrtenol | 19.56 | 0.55 |
| 22 | Carveol | 19.99 | 0.31 |
| 23 | Caryophyllene | 17.33 | 1.95 |
| 24 | Caryophyllene oxide | 20.10 | 0.11 |
| 25 | Germacrene D | 20.80 | 0.11 |
| 26 | Δ –Cadinene | 21.92 | 0.27 |
| Monoterpene hydrocarbons (%) | 16.23 | ||
| Oxygenated monoterpenes (%) | 79.5 | ||
| Sesquiterpene hydrocarbons (%) | 2.44 | ||
| Total (%) | 98.17 | ||
Essential oil antioxidant activity
The antioxidant activity of MpEO was compared to that of silymarin, a well-known antioxidant, using two different assays, namely DPPH and superoxide oxygen radicals inhibition, the results are reported in Table 2. DPPH showed for MpEO an IC50 value around 3 times higher than the one recorded for silymarin indicating that antioxidant activity of MpEO was lower than that of silymarin.
Table 2.
MpEO effects and positive controls on the in vitro free radical(DPPH and superoxide)
| MpEO | Silymarin | |
|---|---|---|
| 50% scavenging concentration (μg/ml) on DPPH radical | 61.28 ± 0.02* | 21.25 ± 0.13 |
| 50% scavenging concentration (μg/ml) on superoxide anion | 356.45 ± 0.35* | 39.04 ± 1.02 |
Values are represented as mean ± SEM of six different experiments
*p < 0.05 versus silymarin
Serum biochemical parameters
The results of biochemical indicators of liver and kidney function are summarized in Tables 3, 4 and 5. The administration of CCl4 caused severe hepato and reno-toxicity in the treated rats, as evidenced by the significant elevations of serum ALT, AST, ALP, LDH, γGT, total cholesterol, triglycerides, LDL urea and creatinine levels, while HDL level was decreased compared to control animals.
Table 3.
Effects of CCl4, MpEO and their combination MpEO/CCl4 on hepatic markers in serum of control and experimental rats
| Treatment and parameters | AST (U/L) | ALT (U/L) | ALP (U/L) | LDH (U/L) | γ-GT (U/L) |
|---|---|---|---|---|---|
| Control | 164.82 ± 1.28 | 65.88 ± 1.44 | 149.61 ± 2.19 | 20.81 ± 0.52 | 3.17 ± 0.39 |
| CCl4 | 524.12 ± 5.11*** | 179.23 ± 3.39*** | 217.47 ± 8.55*** | 40.66 ± 0.81*** | 5.43 ± 0.21*** |
| SL/CCl4 | 191.15 ± 2.14### | 87.82 ± 7.61### | 157.50 ± 5.12## | 28.62 ± 0.62### | 3.47 ± 0.06### |
| MpEOa/CCl4 | 479.89 ± 36.41 | 162.02 ± 9.18 | 202.42 ± 7.62 | 37.75 ± 1.35 | 4.45 ± 0.25 |
| MpEOb/CCl4 | 356.82 ± 35.77## | 147.35 ± 5.67## | 186.74 ± 3.98# | 34.16 ± 1.35## | 4.06 ± 0.17## |
| MpEOc/CCl4 | 216.80 ± 7.26### | 97.82 ± 6.98### | 165.96 ± 5.12### | 31.64 ± 5.12### | 3.51 ± 0.20### |
| MpEOc | 160.80 ± 2.47 | 63.08 ± 1.16 | 139.77 ± 7.27 | 19.89 ± 0.18 | 2.81 ± 0.24 |
AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, LDH lacatate dehydrogenase and γGT gamma glutamyl transferase.aMpEO(5 mg/kg BW), bMpEO(15 mg/kg BW), cMpEO(40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Values are mean ± SEM for eight rats in each group. CCl4, MpEO and MpEO/CCl4 treated groups vs control group; ** p < 0.01, *** p < 0.001, CCl4 group vs MpEO/CCl4 group; # p < 0.05, # # p < 0.01, # # # p < 0.001
Table 4.
Effects of CCl4,MpEO and their combination MpEO/CCl4 on lipid profile in serum of control and experimental rats
| Treatment and parameters | T-Cholesterol (mmol/l) | T-Triglycerides (mmol/l) | HDL (mmol/L) | LDL (mmol/l) |
|---|---|---|---|---|
| Control | 1.133 ± 0.033 | 0.736 ± 0.075 | 1.413 ± 0.023 | 0.123 ± 0.014 |
| CCl4 | 1.750 ± 0.028*** | 1.743 ± 0.078*** | 0.446 ± 0.074*** | 0.726 ± 0.053*** |
| SL/CCl4 | 0.966 ± 0.033### | 1.043 ± 0.012### | 1.240 ± 0.030### | 0.176 ± 0.008### |
| MpEOa/CCl4 | 1.606 ± 0.063 | 1.610 ± 0.041 | 0.690 ± 0.135 | 0.640 ± 0.066 |
| MpEOb/CCl4 | 1.376 ± 0.076## | 1.196 ± 0.039## | 0.873 ± 0.053## | 0.253 ± 0.024## |
| MpEOc/CCl4 | 1.033 ± 0.033### | 1.050 ± 0.010### | 1.296 ± 0.039### | 0.186 ± 0.017### |
| MpEOc | 1.100 ± 0.115 | 0.763 ± 0.069 | 1.210 ± 0.106 | 0.113 ± 0.006 |
TC Total cholesterol (mmol/l), HDL high density lipoprotein (mmol/l), TG triglyceride (mmol/l) and LDL low density lipoprotein (mmol/l). aMpEO (5 mg/kg BW), bMpEO (15 mg/kg BW), cMpEO (40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Values are mean ± SEM for eight rats in each group. CCl4, MpEO and MpEO/CCl4 treated groups vs control group; ** p < 0.01, *** p < 0.001, CCl4 group vs MpEO/CCl4 group; # p < 0.05, # # p < 0.01, # # # p < 0.001
Table 5.
Effects of CCl4, MpEO and their combination MpEO/CCl4 on kidney markers in serum of control and experimental rats
| Treatment | Urea (mmol/l) |
Creatinine (μmol /l) |
|---|---|---|
| Control | 7.53 ± 0.59 | 11.12 ± 0.30 |
| CCl4 | 15.41 ± 1.57** | 12.79 ± 0.15** |
| SL/CCl4 | 8.04 ± 0.32# # | 11.19 ± 0.27# # |
| MpEOa/CCl4 | 12.52 ± 0.32 | 11.94 ± 0.47 |
| MpEOb/CCl4 | 10.50 ± 0.29# | 11.45 ± 0.40# |
| MpEOc/CCl4 | 9.44 ± 0.67# # | 11.39 ± 0.30# # |
| MpEOc | 7.34 ± 0.38 | 11.27 ± 0.35 |
aMpEO(5 mg/kg BW), bMpEO(15 mg/kg BW), cMpEO(40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Values are mean ± SEM for ten rats in each group. CCl4, MpEO and MpEO/CCl4 treated groups vs control group; ** p < 0.01, *** p < 0.001, CCl4 group vs MpEO/CCl4group; # p < 0.05, # # p < 0.01, # # # p < 0.001
Pretreatment with the MpEO at doses of 15 or 40 mg/kg significantly reduced levels of ALT, AST, ALP, LDH, γGT, total cholesterol, triglycerides, LDL urea and creatinine and increased the level of HDL compared to the CCl4 group. It is worth noting that the treatment with 5 mg/kg MpEO did not induce any significant changes in the biochemical parameters (ALT, AST, ALP, LDH, γGT, total cholesterol, triglycerides, LDL, urea, creatinine or HDL) when compared to the CCl4 group. Treatment of rats with only MpEO (40 mg/kg BW) did not result in significant alterations in biochemical parameters compared to control rats.
Pretreatment with silymarin (50 mg/kg), used as positive control, significantly decreased the elevated levels of ALT, AST, ALP, LDH, γGT, total cholesterol, triglycerides, LDL urea and creatinine and increased of HDL level as compared to CCl4 group. Its effect was comparable in reducing of liver and kidney damage induced by CCl4 with that observed for the highest dose of MpEO (40 mg/kg).
Effects on lipid peroxidation
TBARS level is widely used as a marker for free radical mediated lipid peroxidation injury. We determined TBARS levels in the liver and kidney tissues of the investigated animals and our results are shown in Fig. 1a, b. The levels of TBARS were significantly increased in both liver and kidney tissues of CCl4-treated animals when compared to control untreated rats.
Fig. 1.
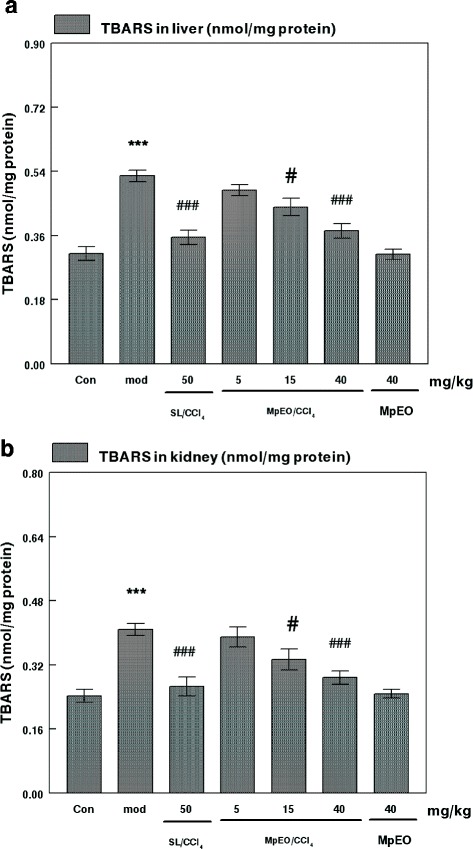
a Effects of CCl4, MpEO and their combinations MpEO/CCl4 on hepatic TBARS of control (Con) and experimental rats. Con, control group; mod, CCl4-model group; SL/CCl4, silymarin 50 mg/kg + CCl4; MpEO/CCl4 5 mg/kg + CCl4 group; MpEO/CCl4 15 mg/kg + CCl4 group; MpEO/CCl4 40 mg/kg + CCl4 group; MpEO 40 mg/kg group. Values are mean ± SEM for ten rats in each group. CCl4, MpEO, MpEO/CCl4 treated groups vs control group; *p < 0.05, **p < 0.01, *** p < 0.001, CCl4 group vs (MpEO/CCl4) group; #p < 0.05, ##p < 0.01, ###p < 0.001. b. Effects of CCl4, MpEO and their combinations (MpEO/CCl4) on kidney TBARS of control (Con) and experimental rats. Con, control group; mod, CCl4-model group; SL/CCl4, silymarin 50 mg/kg + CCl4; MpEO/CCl4 5 mg/kg + CCl4 group; MpEO/CCl4 15 mg/kg + CCl4 group; MpEO/CCl4 40 mg/kg + CCl4 group; MpEO 40 mg/kg. Values are mean ± SEM for ten rats in each group. CCl4, MpEO, MpEO/CCl4 treated groups vs control group; *p < 0.05, **p < 0.01, *** p < 0.001, CCl4 group vs (MpEO/CCl4) group; #p < 0.05, ##p < 0.01, ###p < 0.001
Pre-treatment with the MpEO at doses of 15 and 40 mg/kg BW significantly reduced levels of TBARS in liver and kidney tissues as compared to CCl4 group. There was a dose effect; treatment with MpEO at 5 mg/kg BW did not induce any significant decrease in the levels of TBARS in liver and kidney as compared to CCl4 group. When rats were treated with only MpEO (40 mg/kg BW), no significant differences in the TBARS values was observed compared to control rat. Pretreatment with silymarin (50 mg/kg) significantly decreased the elevated levels of TBARS in both liver and kidney compared to CCl4 control. Moreover, the effect of silymarin (50 mg/kg) in attenuation of TBARS levels in liver and kidney was comparable with highest dose of MpEO (40 mg/kg).
Effects on antioxidant enzymes
Results presented in Tables 6 and 7 showed a significant decrease in the levels of CAT, SOD, GPx in liver and kidney in CCl4-treated group when compared to control group. The decrease in hepatic and kidney CAT, SOD and GPx levels induced by CCl4 injection were significantly restored (elevated) in the MpEO and silymarin groups, and this effect was more pronounced with the increase of essential oil concentration. Pretreatment with MpEO at doses of 15 and 40 mg/kg significantly increased levels of hepatic and kidney CAT, SOD and GPx as compared to CCl4 group. It is worth noting that the treatment with MpEO at a dose 5 mg/kg did not induce any significant increase in the levels of hepatic and kidney CAT, SOD and GPx as compared to CCl4 group. No significant differences in the values were observed in rats treated with MpEO only (40 mg/kg) compared to control rat values. Moreover, the effect of silymarin (50 mg/kg) was comparable in attenuation of levels of hepatic and kidney CAT, SOD and GPx with highest dose of MpEO (40 mg/kg).
Table 6.
Effects of CCl4, MpEO and their combination MpEO/CCl4 on the activities of enzymatic antioxidants in liver of control and experimental rats
| Treatment | SOD (Units/mg protein) |
CAT (μmol H2O2/min/mg protein) |
GPx (μmol GSH/min/mg protein) |
|---|---|---|---|
| Control | 16.04 ± 0.11 | 14.03 ± 0.29 | 7.66 ± 0.51 |
| CCl4 | 13.60 ± 0.50*** | 11.13 ± 0.37*** | 5.25 ± 0.25** |
| SL/CCl4 | 15.80 ± 0.50## | 13.48 ± 0.25### | 7.41 ± 0.16### |
| MpEOa/CCl4 | 13.92 ± 0.40 | 11.34 ± 0.41 | 5.41 ± 0.31 |
| MpEOb/CCl4 | 14.98 ± 0.19# | 12.32 ± 0.32# | 6.04 ± 0.17# |
| MpEOc/CCl4 | 15.65 ± 0.44## | 13.20 ± 0.19### | 7.26 ± 0.22### |
| MpEOc | 15.83 ± 0.31 | 13.85 ± 0.33 | 7.45 ± 0.33 |
aMpEO(5 mg/kg BW), bMpEO (15 mg/kg BW), cMpEO(40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Values are mean ± SEM for ten rats in each group. CCl4, MpEO and MpEO/CCl4 treated groups vs control group; ** p < 0.01, *** p < 0.001, CCl4 group vs MpEO/CCl4 group; # p < 0.05, # # p < 0.01, # # # p < 0.001
Table 7.
Effects of CCl4, MpEO and their combination MpEO/CCl4 on the activities of enzymatic antioxidants in kidney of control and experimental rats
| Treatment | SOD (Units/mg protein) |
CAT (μmol H2O2/min/mg protein) |
GPx (μmol GSH/min/mg protein) |
|---|---|---|---|
| Control | 15.13 ± 0.22 | 12.90 ± 0.15 | 5.68 ± 0.27 |
| CCl4 | 12.12 ± 0.50*** | 10.05 ± 0.34*** | 4.06 ± 0.28** |
| SL/CCl4 | 14.52 ± 0.35## | 12.23 ± 0.24### | 5.56 ± 0.14## |
| MpEOa/CCl4 | 12.92 ± 0.20 | 10.80 ± 0.22 | 4.46 ± 0.33 |
| MpEOb/CCl4 | 13.69 ± 0.10# | 11.02 ± 0.22# | 5.10 ± 0.28# |
| MpEOc/CCl4 | 14.20 ± 0.35## | 12.04 ± 0.19### | 5.32 ± 0.06## |
| MpEOc | 14.91 ± 0.21 | 12.62 ± 0.33 | 5.59 ± 0.27 |
aMpEO(5 mg/kg BW), bMpEO(15 mg/kg BW), cMpEO(40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Values are mean ± SEM for ten rats in each group. CCl4, MpEO and MpEO/CCl4 treated groups vs control group; ** p < 0.01, *** p < 0.001, CCl4 group vs MpEO/CCl4 group; # p < 0.05, # # p < 0.01, # # # p < 0.001
Histopathological findings
The normal liver architecture was observed in liver histology of control group (Fig. 2a). Large numbers of inflammatory cells such as lymphocytes together with hepatic sinusoidal inflammation, hepatocyte necrosis and devastating liver architecture were observed in the CCl4 group (Fig. 2b). However, pretreatment with MpEO (40 mg/kg, Fig. 2f) can remarkably ameliorate the histopathological hepatic lesions induced by administration of CCl4. MpEO 15 mg/kg showed very few inflammatory cells along with prominent nucleolus (Fig. 2e). The highest dose of MpEO (40 mg/kg) and silymarin (50 mg/kg) significantly attenuated the damaged liver depicting marked focal regenerative changes which are illustrated by presence of actively dividing cells with a prominent nucleolus (Fig. 2f). In addition, silymarin at the dose of 50 mg/kg has shown to produce hepatoprotection evidenced by area of regeneration and dark nucleus (Fig. 2c). The histological pattern was almost normal in rats treated with MpEO oil alone. By analyzing the histopathological scoring attributed to the liver tissues it is possible to note that the highest dose of MpEO (40 mg/kg) or silymarin (50 mg/kg) pretreatment just conferred good protection on CCl4-induced liver damage (Table 8).
Fig. 2.
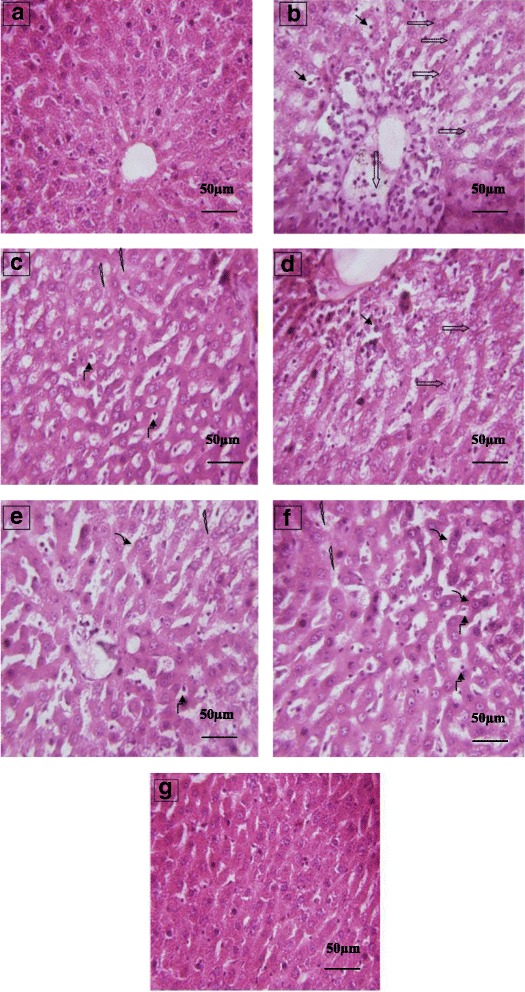
Effect of MpEO on CCl4-induced liver damage. a Control group; (b) CCl4-model group showing marked inflammatory cells, necrosis and reduced lesions of necrosis; (c) Silymarin 50 mg/kg + CCl4 group; (d) MpEO 5 mg/kg + CCl4 group (e); MpEO 15 mg/kg + CCl4 group; (f) MpEO 40 mg/kg + CCl4 group; (g) MpEO 40 mg/kg group. Hematoxylin/eosin staining; magnification ×400.  : Marked inflammatory cells;
: Marked inflammatory cells;  : Necrosis cells;
: Necrosis cells;  : Reduced lesions of necrosis;
: Reduced lesions of necrosis;  : Regeneration area;
: Regeneration area;  : Prominent nucleolus;
: Prominent nucleolus;  : Mild inflammatory cells
: Mild inflammatory cells
Table 8.
Grades of inflammatory cells and cellular necrosis in rat liver
| Pathologic grading of inflammatory cells and cellular necrosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Zero | I | II | III | IV | N | P value vs Control | P value vs CCl4 |
| Control | 10 | 0 | 0 | 0 | 0 | 10 | – | |
| CCl4*** | 0 | 1 | 3 | 3 | 3 | 10 | P < 0.001 | – |
| SL/CCl4### | 8 | 2 | 0 | 0 | 0 | 10 | ns | P < 0.001 |
| MpEOa/CCl4 | 0 | 2 | 6 | 1 | 1 | 10 | P < 0.05 | ns |
| MpEOb/CCl4# | 6 | 4 | 0 | 0 | 0 | 10 | ns | P < 0.05 |
| MpEOc/CCl4### | 7 | 3 | 0 | 0 | 0 | 10 | ns | P < 0.001 |
| MpEOc | 9 | 1 | 0 | 0 | 0 | 10 | ns | P < 0.001 |
aMpEO (5 mg/kg BW), bMpEO (15 mg/kg BW), cMpEO (40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Kidney sections of normal histological appearance (Fig. 3a) and the CCl4 control group showed some nephrotoxic lesions, as evidenced by the glomerular and tubular necrosis (Fig. 3b). However, pretreatment with MpEO (40 mg/kg, Fig. 3f) can remarkably ameliorate the histopathological kidney lesions induced by administration of CCl4 (Fig. 3f). In addition, silymarin at the dose of 50 mg/kg has shown to produce renoprotection evidenced by amelioration the histopathological kidney lesions induced by injection of CCl4 (Fig. 3c). The histological pattern in kidney was almost normal in rats treated with MpEO alone. By analyzing the histopathological scoring attributed to the kidney tissues it is possible to note that the highest dose of MpEO (40 mg/kg) or silymarin (50 mg/kg) pretreatment just conferred good protection on CCl4-induced kidney damage (Table 9).
Fig. 3.
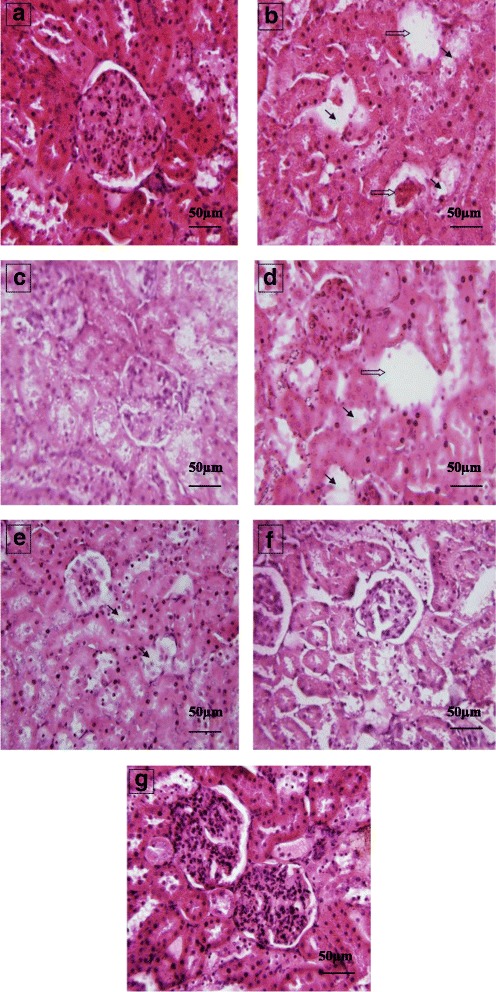
Effect of MpEO on CCl4-induced kidney damage. a Control group; (b) CCl4-model group showing some nephrotoxic lesions, as evidenced by the glomerular and tubular necrosis; (c) Silymarin 50 mg/kg + CCl4 group; (d) MpEO 5 mg/kg + CCl4 group (e); MpEO 15 mg/kg + CCl4 group; (f) MpEO 40 mg/kg + CCl4 group; (g) MpEO 40 mg/kg group. Hematoxylin/eosin staining; magnification ×400.  : glomerular necrosis;
: glomerular necrosis;  : necrosis in epithelial cells of the proximal tubules
: necrosis in epithelial cells of the proximal tubules
Table 9.
Grades of glomerular and epithelial cells of the proximal tubules necrosis in rat kidney
| Pathologic grading of glomerular and epithelial cells of the proximal tubules necrosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Zero | I | II | III | IV | N | P value vs Control | P value vs CCl4 |
| Control | 10 | 0 | 0 | 0 | 0 | 10 | – | |
| CCl4*** | 0 | 2 | 2 | 3 | 3 | 10 | P < 0.001 | – |
| SL/CCl4### | 8 | 2 | 0 | 0 | 0 | 10 | ns | P < 0.001 |
| MpEOa/CCl4 | 0 | 2 | 5 | 3 | 0 | 10 | P < 0.05 | ns |
| MpEOb/CCl4# | 6 | 4 | 0 | 0 | 0 | 10 | ns | P < 0.05 |
| MpEOc/CCl4### | 7 | 3 | 0 | 0 | 0 | 10 | ns | P < 0.001 |
| MpEOc | 9 | 1 | 0 | 0 | 0 | 10 | ns | P < 0.001 |
aMpEO (5 mg/kg BW), bMpEO (15 mg/kg BW), cMpEO (40 mg/kg BW), SL: Silymarin (50 mg/kg BW)
Discussion
Chemical composition of the essential oil obtained from MpEO was determined by GC-MS analysis. The compounds, their percentages as well as the retention indices are listed in Table 1. The essential oil is a complex mixture with 26 compounds representing 98.17% of the total oil composition. The major component of the essential oil is menthol (33.59%) followed by iso-menthone (33.00%). In lower amounts we found a variety of compounds including limonene (8.00%), piperitone (3.20%), 1,8-cineole (2.80%), linalool (2.64%), iso-pulegol (2.40%), caryophyllene (1.95%) and pulegone (1.60%). The obtained results are in accordance with previous studies of M. piperita oils from Turkey, Spain (Barcelona), Norway and Poland that also had menthone and menthol as their most important components [30–32]. On the other hand, the composition of the essential oil from Iran is totally different, with α-terpinene (19.70%), isomenthone (10.30%), trans-carveol (14.50%), pipertitinone oxide (19.30%) and β-caryophyllene (7.60%) as the major compounds, and also the oil from the Girona region (Spain) is different, where limonene and 1.8-cineole, eucalyptol are the main compounds (33.37% and 30.75%, respectively) [33, 34].
These studies showed variable chemotypes of M. piperita L. extracts with various major oil components. Differences in chemical composition observed for essential oils is likely related to abiotic factors such as soil type and climate specific regions of provenance samples and geographical factors [35]. Furthermore, menthol and iso-menthone, found at relatively high concentrations in the MpEO used in the present study, have been reported to exhibit anti-inflammatory activity [14], making MpEO use a promising candidate against oxidative damage of the liver and kidney following an intraperitoneal administration of CCl4. To be noted: working with natural extracts, the antioxidant activity is considered to be primary related to the major active compounds in the essential oil such as menthol and its derivatives [15]. However the antioxidant activity could also come from a minor compound interacting in a synergistic or antagonistic way, to create an effective system against free radicals [36, 37], this has to be realized when evaluating different MpEO preparations.
Liver injury after CCl4 exposure is characterized by the elevated levels of serum hepatic marker enzymes indicating the cellular leakage and loss of functional integrity of hepatic membrane architecture. High levels of ALT, AST, ALP, LDH and γGT activities are sensitive indicators of liver cell injury and are most helpful in recognizing hepatic diseases [38]. CCl4-treated rats show increased activities of these enzymes, reflecting damage to the liver cells or changes in the cell membrane permeability leading to leakage of enzymes from cells to the circulation [39]. In the present study increased levels of serum hepatic markers suggested that an extensive liver injury was occasioned by CCl4 due to increased lipid peroxidation which had the ability to cause membrane damage. It is now generally accepted that CCl4 hepatotoxicity is the result of reductive dehalogenation, which is catalyzed by its specific isoenzyme of cytochrome P450 2E1, and which forms the highly reactive free radical. Hence, the suppression of P450 2E1 could result in reduced levels of reactive metabolites, and thus decreased tissue damage [40].
The liver plays a fundamental role in the metabolism of lipids. Injection of CCl4 caused a significant increase in the triglyceride, total cholesterol, and LDL levels and decrease in HDL level. Increase in the cholesterol levels might be due to the increased esterification of fatty acids, inhibition of fatty acid β-oxidation, and decreased excretion of cellular lipids [41]. CCl4 stimulates the transfer of acetate into liver cells (probably by increasing access to acetate) and leads to an increase in cholesterol synthesis. It also increases the synthesis of fatty acids and triglyceride from acetate and enhances lipid esterification [42]. The accumulation of triglyceride in liver might occur due to the inhibition of lysosomal lipase activity and VLDL secretion [43].
The administration of CCl4 induced also renal toxicity evidenced by an elevation of serum creatinine and urea [44, 45]. These pathological changes can also be attributed to damages touching the structural integrity of nephrons [46], which is consistent with reports confirming that the level of serum creatinine increases only if at least half of the kidney nephrons are already damaged [47].
Treatment of rats with MpEO prior to CCl4 exposure resulted in a dramatically protective effect against acute hepato and renotoxicity and oxidative stress, which was further also confirmed by the hepatic histopathological examinations. The stimulation of hepatic regeneration makes the liver more resistant to damage by the toxin [48]. Treatment with silymarin (50 mg/kg) or MpEO (40 mg/kg) significantly decreased the elevated levels of ALT, AST, ALP, LDH, γGT, total cholesterol, triglycerides, LDL urea and creatinine and increased of HDL level as compared to CCl4 group. Pharmacological studies have shown that essential oil derived from various plant materials possesses anti-inflammatory activities [49, 50] Knowing that sesquiterpenes have excellent anti-inflammatory activities [51], the anti-inflammatory activity of M. piperita L. leaf essential oil could be partly explained by the presence of sesquiterpenes, such as spathulenol, cadinene, caryophyllene and caryophyllene oxide. The ethanolic extract of parsley leaves also showed significant anti-inflammatory [52] and antioxidant activities [53, 54] which may contribute to its hepatoprotective action. Furthermore, menthol and iso-menthone, found at relatively high concentrations in the MpEO used in the present study, have been reported to exhibit anti-inflammatory activity [14].
In the present study, the two fold increase in the TBARS levels and reduce activity of SOD, CAT and GPx observed in liver and kidney homogenate of CCl4-intoxicated rats. Silymarin significantly reversed CCl4-induced TBARS levels elevation but values obtained with MpEO at the highest dose was comparable in attenuation of TBARS levels in liver and kidney. Silymarin reversed CCl4-induced SOD, CAT and GPx activities decrease but values obtained with MpEO at the highest dose (40 mg/kg) was comparable in attenuation of SOD, CAT and GPx activities in liver and kidney.
These results suggested that MpEO could exert its antioxidant and/or radical scavenging activities thus preventing the formation of the carbon free radicals originated from CCl4 metabolism as well as ROS and peroxidation products. This hypothesis is supported by the recent findings on the in vitro antiradical and antioxidative activities of MpEO [16]. Previous studies showed that menthol and its derivatives were the major compounds responsible for antioxidant activity of MpEO [15].
In the present study, the rats of group 2 served as CCl4-hepato and renotoxicity control and rats of groups 3, 4, 5 and 6 were injected with 1 ml/kg BW of CCl4 and olive oil mixture on day seven (a single intraperitoneal injection). It has already been shown that a single dose of CCl4 initiates lipid peroxidation [55–58] that results in the disruption of cellular and organelle membrane integrity and subsequent leakage of cellular contents into the blood [59, 60]. CCl4 is further well known to induce fibrosis of the hepatic tissue that may further progress to cirrhosis if the stimuli persists [61–65]. Thus, a single CCl4 injection in mice can be used as an attractive and highly reproducible model of liver regeneration after toxic injury. The first appearance of histological fibrosis and scarring fibers is usually observed after repeated CCl4 treatment for 2 to 3 weeks, depending on the dosage and mouse strains used [66]. In the present work, the hepatic histoarchitecture of the CCl4-treated rats resulted large numbers of inflammatory cells such as lymphocytes along with hepatic sinusoidal inflammation, hepatocyte necrosis and devastating liver architecture The highest dose of MpEO (40 mg/kg) or silymarin (50 mg/kg) significantly attenuated the damaged liver depicting marked focal regenerative changes which are illustrated by presence of actively dividing cells with a prominent nucleolus. The administration of MpEO reducing the histological alterations in liver provoked by CCl4 was quite noticeable. In fact, the histological changes seen in the kidney of rats treated with CCl4 were characterized by some nephrotoxic lesions, as evidenced by the glomerular and tubular necrosis. Our results confirmed previous findings of Ozturk et al. [67] who had found degenerative changes in kidney of rats exposed to CCl4. The results suggest that MpEO treatment prior to CCl4 intoxication could prevent the CCl4-induced alterations in kidney tissues of treated animals.
Conclusions
The contents of MpEO not only protect the integrity of plasma membrane but, at the same time, increased the regenerative and reparative capacity of the liver and kidney. These results suggest that the compound present in MpEO has hepatorenal protective effects against CCl4 induced oxidative stress in rats. Further investigations are essential to elucidate the precise mechanism of active agents of MpEO protection against CCl4-induced hepatotoxicity and nephrotoxicity and it has to be tested against other biological parameters.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included within the article.
Abbreviations
- BHT
Butylated hydroxytoluene
- CAT
Catalase
- DPPH
1-Diphenyl-2-picrylhydrazyl
- DTNB
5,5′-Dithio-bis(2-nitrobenzoic acid)
- EDTA
Ethylenediaminetetraacetic acid
- GPX
Glutathione peroxidase
- GSH
Glutathione
- M. piperita
Mentha piperita
- MpEO
Mentha piperita essential oil
- NBT
Nitroblue Tetrazolium
- ROS
Reactive oxygen species
- SD
Standard deviation
- SOD
Superoxide dismutase
- TBA
Thiobarbituric Acid
- TBARS
Thiobarbituric acid reactive substances
- TBS
Tris Buffered Saline
- TCA
Trichloroacetic Acid
- TRIS
Trishydroxymethyl aminomethane
Authors’ contributions
KB, ABH, KA, JvP, FMA, TR and AE designed and wrote the paper. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- 2.Basaga HS. Biochemical aspects of free radicals. Biochem Cell Biol. 1990;68:989–998. doi: 10.1139/o90-146. [DOI] [PubMed] [Google Scholar]
- 3.ATSDR "Agency for Toxic Substance and Diseases Registry". Toxicological profile for carbon tetrachloride, U.S. Department of Health and Human Services, Public Health Service, TP-39/02. Atlanta, CA, 1994.
- 4.Wang CY, Ma FL, Liu JT, Tian JW, Fu FH. Protective effect of salvianic acid a on acute liver injury induced by carbon tetrachloride in rats. Biol Pharm Bull. 2007;30:44–47. doi: 10.1248/bpb.30.44. [DOI] [PubMed] [Google Scholar]
- 5.Abraham P, Wilfred G, Cathrine SP. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clin Chim Acta. 1999;289:177–190. doi: 10.1016/S0009-8981(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Fang YZ, Yang S, Wu G. Free radicals antioxidants and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 8.Bansal AK, Bansal M, Soni G, Bhatnagar D. N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156:101–111. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Shahjahan M, Sabitha KE, Jainu M, Shyamala Devi CS. Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res. 2004;120:194–198. [PubMed] [Google Scholar]
- 10.Li CC, Hsiang CY, Wu CL, Ho TY. Identification of novel mechanisms of silymarin on the carbontetrachloride-induced liver fibrosis in mice by nuclear factor-KB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxicol. 2012;50:1568–1575. doi: 10.1016/j.fct.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Heywood VH. Flowering plants of the world. Oxford, U.K: Oxford University Press; 1979. [Google Scholar]
- 12.Brown D. Encyclopaedia of herbs and their uses. London, U.K: Dorling Kindersley; 1995. [Google Scholar]
- 13.Foster S. Peppermint, Mentha piperita, In Botanical Series, American Botanical Council: Austin, Texas, no 306, 1990.
- 14.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CP, Shih PH, Hsu CL, Yen GC. Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol. 2007;45:888–895. doi: 10.1016/j.fct.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Wang H, Wang J, Zhou L, Yang P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China, Plos One. 10.1371/journal.pone.0114767, (2014) 1–15. [DOI] [PMC free article] [PubMed]
- 17.Blois MS. Antioxidant determinations by the use of stable free radical. Nature. 1958;26:199–200. [Google Scholar]
- 18.Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agr Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- 19.Council of European Communities. Council instructions about the protection of living animals used in scientific investigations. Off J Euro Commun. 2010; 276:1-33.
- 20.Mihailović V, Mihailović M, Uskoković A, Arambašić J, Mišić D, Stanković V, Katanić J, Mladenović M, Solujić S, Matić S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem Toxicol. 2013;52:83–90. doi: 10.1016/j.fct.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 21.Bruckner JV, MacKenzie WF, Muralidhara S, Luthra R, Kyle M, Acosta D. Oral toxicity of carbon tetrachloride: acute, subacute and subchronic studies in rates. Fundam Appl Toxicol. 1986;6:16–34. doi: 10.1016/0272-0590(86)90260-5. [DOI] [PubMed] [Google Scholar]
- 22.Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. Complement Altern Med. 2011;1:1–9. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spindler P, Madsen C. Subchronic toxicity study of peppermint oil in rats. Toxicol letter. 1992;62:215–220. doi: 10.1016/0378-4274(92)90024-E. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;2:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- 26.Aebi H. Catalase in vitro. Methods Enzymol. 1984;2:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 27.Beyer JWF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;113:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 28.Flohé L, Günzler WA. Assays for glutathione peroxidase. Methods Enzymol. 1984;105:114-21. [DOI] [PubMed]
- 29.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-O. [DOI] [PubMed] [Google Scholar]
- 30.Kim NS, Lee DS. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography-mass spectrometry. J Chromatogr. 2002;22:31–47. doi: 10.1016/S0021-9673(02)01445-0. [DOI] [PubMed] [Google Scholar]
- 31.Rohloff J, Dragland S, Mordal R, Iversen TH. Effect of harvest time and drying method on biomass production, essential oil yield, and quality of peppermint (Mentha×piperita L.) J Agric Food Chem. 2005;53:4143–4148. doi: 10.1021/jf047998s. [DOI] [PubMed] [Google Scholar]
- 32.Skalicka-Wozniak K, Walasek M. Preparative separation of menthol and pulegone from peppermint oil (Mentha piperita L.) by high-performance counter-current chromatography. Phytochem Lett. 2014;728: 10.1016/j.phytol.06.007.
- 33.Yadegarinia D, Gachkar L, Rezaei MB, Taghizadeh M, Alipoor-Astaneh S, Rasooli I. Biochemical activities of Iranian Mentha piperita L. and Myrtus communis L. essential oils. Phytochemistry. 2006;60:1249–1255. doi: 10.1016/j.phytochem.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz Del Castillo ML, Blanch GP, Herraiz M. Natural variability of the enantiomeric composition of bioactive chiral terpenes in Mentha piperita. J Chromatogr A. 2004;1054:87–93. doi: 10.1016/j.chroma.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 35.Orav A, Raal A, Arak E. Comparative chemical composition of the essential oil of Mentha piperita L. from various geographical sources. Proc Estonian Acad Sci Chem. 2004;53:174–181. [Google Scholar]
- 36.Lu Y, Foo LY. Antioxidant activities of polyphenols from sage (Salvia officinalis) Food Chem. 2001;75:197–202. doi: 10.1016/S0308-8146(01)00198-4. [DOI] [Google Scholar]
- 37.Singh G, Marimuthu P, De Heluani CS, Catalan CAN. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J Agric Food Chem. 2006;54:174–181. doi: 10.1021/jf0518610. [DOI] [PubMed] [Google Scholar]
- 38.Pradeep K, Victor Raj Mohan C, Gobianand K, Karthikeyan S. Protective effect of Cassia fistula Linn. On diethylnitrosamine induced hepatocellular damage and oxidative stress in ethanol pretreated rats. Biol Res. 2010;43:113–125. doi: 10.4067/S0716-97602010000100013. [DOI] [PubMed] [Google Scholar]
- 39.Botsoglou NA, Taitzoglou IA, Botsoglou E, Lavrentiadou SN, Kokoli AN, Roubies N. Effect of long-term dietary administration of oregano on the alleviation of carbon tetrachloride-induced oxidative stress in rats. J AgricFood Chem. 2008;56:6287–6293. doi: 10.1021/jf8003652. [DOI] [PubMed] [Google Scholar]
- 40.Zangar RC, Benson JM, Burnett VL, Springer DL. Cytochrome P4502E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact. 2000;125:233–243. doi: 10.1016/S0009-2797(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr. 2005;135:2075–2078. doi: 10.1093/jn/135.9.2075. [DOI] [PubMed] [Google Scholar]
- 42.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003; 33: 10.1080/713611034. [DOI] [PubMed]
- 43.Marimuthu S, Adluri RS, Rajagopalan R, Menon VP. Protective role of ferulic acid on carbon tetrachloride-induced hyperlipidemia and histological alterations in experimental rats. J Basic Clin Physiol Pharmacol. 2013;24:59–66. doi: 10.1515/jbcpp-2012-0053. [DOI] [PubMed] [Google Scholar]
- 44.Abdel Moneim AE, El-Deib KM. The possible protective effects of Physalis peruviana on carbon tetrachloride-induced nephrotoxicity in male albino rats. Life Sci J. 2012;9:1038–1052. [Google Scholar]
- 45.Al-Yahya M, Mothana R, Al-Said M, Al-Dosari M, Al-Musayeib N, Al-Sohaibani M, Parvez MK, Rafatullah S. Attenuation of CCl4-Induced Oxidative Stress and Hepatonephrotoxicity by Saudi Sidr Honey in Rats, Evidence-Based Complementary and Alternative Medicine. 2013; 10.1155/2013/569037. [DOI] [PMC free article] [PubMed]
- 46.Khan RA, Khan MR, Sahreen S, Bokhari J. Prevention of CCl4-induced nephrotoxicity with Sonchus Asper in rat. Food Chem Toxicol. 2010;48:2469–2476. doi: 10.1016/j.fct.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Bhattacharya H, Lun L, Gomez R. Biochemical Effects to Toxicity of CCl4 on Rosy Barbs (Puntius conchonius). Our Nat. 2005;3:20-25.
- 48.Sadasivan S, Latha PG, Sasikumar JM, Rajashekaran S, Shymal S, Shine VJ. Hepatoprotective studies on Hedyotis Corymbosa (L.) lam. J Ethnopharmcol. 2006;106:25–249. doi: 10.1016/j.jep.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Martins FT, Doriguetto AC, De Souza TC, De Souza KR, Moreira ME, Barbosa LC. Composition, and Antiinflammatory and antioxidant activities of the volatile oil from the fruit peel of Garcinia brasiliensis. Chem Biodivers. 2008;5:251–258. doi: 10.1002/cbdv.200890022. [DOI] [PubMed] [Google Scholar]
- 50.Kim JY, Oh TH, Kim BJ, Kim SS, Lee NH, Hyun CG. Chemical composition and anti-inflammatory effects of essential oil from Farfugium japonicum flower. J Oleo Sci. 2008;5:623–628. doi: 10.5650/jos.57.623. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Sun Z, Zhang M, Meng X, Xia X, Yua NW, Xue F, Liu C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicas. Carbohydr Polym. 2012;90:1664–1670. doi: 10.1016/j.carbpol.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 52.Al-Howiriny T, Al-Sohaibani M, El-Tahir K, Rafatullah S. Prevention of experimentally-induced gastric ulcers in rats by an ethanolic extract of "parsley" Petroselinum crispum. Am J Chin Med. 2003;31:699–711. doi: 10.1142/S0192415X03001405. [DOI] [PubMed] [Google Scholar]
- 53.Wong PYY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505–515. doi: 10.1016/j.foodchem.2005.05.031. [DOI] [Google Scholar]
- 54.Zhang H, Chen F, Wang X, Yao HY. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res Int. 2006;39:833–839. doi: 10.1016/j.foodres.2006.03.007. [DOI] [Google Scholar]
- 55.Hafeman DG, Hoekstra WG. Protection against carbon tetrachloride-induced lipid peroxidation in the rat by dietary vitamin E, selenium, and methionine as measured by ethane evolution. J Nutr. 1977;107:656–665. doi: 10.1093/jn/107.4.656. [DOI] [PubMed] [Google Scholar]
- 56.Lee PY, Mccay PB, Hornbrook KR. Evidence for carbon tetrachloride-induced lipid peroxidation in mouse liver. Biochem Pharmacol. 1982;31:405–409. doi: 10.1016/0006-2952(82)90189-7. [DOI] [PubMed] [Google Scholar]
- 57.Burk RF, Lane JM, Patel K. Relationship of oxygen and glutathione in protection against carbon tetrachloride-induced hepatic microsomal lipid peroxidation and covalent binding in the rat. Rationale for the use of hyperbaric oxygen to treat carbon tetrachloride ingestion. J Clin Invest. 1984;74:1996–2001. doi: 10.1172/JCI111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 59.Recknagel RO. A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983;33:401–408. doi: 10.1016/0024-3205(83)90787-7. [DOI] [PubMed] [Google Scholar]
- 60.Brattin WJ, Glende EA, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 61.Post J, Earle DP, Patek AJ, Victor J. Effects of yeast and food intake on experimental carbon tetrachloride cirrhosis of the liver in the rat. Am J Pathol. 1942;18:661–673. [PMC free article] [PubMed] [Google Scholar]
- 62.Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med. 2005;117:237–250. doi: 10.1385/1-59259-940-0:237. [DOI] [PubMed] [Google Scholar]
- 63.Smyth R, Munday MR, York MJ, Clarke CJ, Dare T, Turton JA. Comprehensive characterization of serum clinical chemistry parameters and the identification of urinary superoxide dismutase in a carbon tetrachloride-induced model of hepatic fibrosis in the female Hanover Wistar rat. Int J Exp Pathol. 2007;88:361–376. doi: 10.1111/j.1365-2613.2007.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smyth R, Lane CS, Ashiq R, Turton JA, Clarke CJ, Dare TO, York MJ, Griffiths W, Munday MR. Proteomic investigation of urinary markers of carbon-tetrachloride-induced hepatic fibrosis in the Hanover Wistar rat. Cell Biol Toxicol. 2009;25:499–512. doi: 10.1007/s10565-008-9104-8. [DOI] [PubMed] [Google Scholar]
- 65.Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, Lanuti M, TANABE KK. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6:19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Iinduced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology. 2003;62:353–356. doi: 10.1016/S0090-4295(03)00255-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included within the article.


