Abstract
Objective:
To retrospectively evaluate the safety and technical efficacy of percutaneous radiofrequency ablation (RFA) of surface hepatocellular carcinoma (HCC) in comparison to intraparenchymal HCC in cirrhotic patients.
Materials and Methods:
Surface lesions were defined as tumours located or reaching within 1cm of liver capsule including exophytic lesions. Seventy-four surface HCC including 21 exophytic in 58 patients (surface group) and 60 intraparenchymal HCC in 54 patients (intraparenchymal group) measuring up to 4 cm in maximum extent underwent percutaneous [ultrasound (US) or computed tomography-guided (CT-guided)] RFA. The response to the treatment was assessed by contrast enhanced CT/magnetic resonance imaging (MRI) done at 1, 3, 6, 9, and 12 months of RFA and thereafter every 4–6 months. In case of features suggesting residual disease, a repeat RFA was performed. The technical success after single-session RFA, complications and disease recurrence rates were calculated and compared between two groups.
Results:
Technical success achieved after first session of RFA in surface HCC was 95% (70/74) and intraparenchymal HCC was 97% (58/60). Hundred percent secondary success rate was achieved in both groups after second repeat RFA in residual lesion. No major difference in complication and local recurrence rate in both group on follow-up in surface HCC and intraparenchymal HCC. No case of needle track, peritoneal seeding, and treatment mortality was found.
Conclusions:
The complication rate and efficacy of RFA for surface and exophytic HCC's were comparable to that of intraparenchymal HCC. Hence surface and exophytic lesions should not be considered a contraindication for RFA in cirrhotic patients.
Keywords: Cirrhotic patients, hepatocellular carcinoma, intraparenchymal, radiofrequency ablation, surface
Introduction
Hepatocellular carcinoma (HCC) is a prevalent disease in Asia and one of the most common malignancies in the world. Although hepatic resection and liver transplantation are considered the best treatment options for HCC, only 20–30% of patients with HCC are appropriate candidates for such treatments.[1] When contraindicated, various locoregional therapies can be used for patients with localized HCC.[2] The percutaneous radiofrequency ablation (RFA) is arguably the best treatment option available for patients with Child-A/B cirrhosis and single nodular type HCC <5 cm or < three HCCs each smaller than 3 cm, not amenable to resection or transplantation.[3] Traditionally, surface or exophytic tumours were considered as relative contraindication due to high incidence of local tumour recurrence,[4,5] risk of intraperitoneal bleeding and subcapsular hematoma, high rate of needle track seeding[6,7] along with extra hepatic/peritoneal dissemination[8] and an increased rate of major complications in subcapsular tumours abutting hollow viscera. So, subcapsular tumour location is considered a contraindication to RFA for HCC by some groups while some do not exclude subcapsular HCC from RFA. However, technology has improved the safety and efficacy of RFA and this concept of high risk tumour location has been challenged and therefore, it remains controversial whether the subcapsular location of HCC is an unfavourable factor for percutaneous RFA of HCC or not. The RFA still widely used in developing countries and we are doing ample of radiofrequency procedure so we did retrospective analysis of our result to evaluate the safety and effectiveness of percutaneous RFA of surface/subcapsular HCC in comparison to intraparenchymal HCC. Also, the rate of local tumor recurrence and recurrence free survival was evaluated between the two groups.
Materials and Methods
Patients and groups
Institutional review board approval was obtained for this retrospective analysis of all patients who underwent RFA for HCC between January 2010 and December 2015. A total of 112 patients who underwent percutaneous RFA for inoperable HCC were assigned to one of two groups: Group 1 (surface group)—included patients with one or more surface or subcapsular lesions which in turn defined as any lesion which is located or reaching at a distance of 10 mm or less from the liver capsule including exophytic lesions (lesion originated from within liver and extending outside the margins of liver) and nodules near the stomach, bowel, liver dome, diaphragm, and/or abdominal wall. The patients in group 2 (intraparenchymal group) included patients with nonsubcapsular nodules only and those with intraparenchymal lesions as well as subcapsular nodules were excluded from the study.
The diagnosis of HCC was based on imaging [triple-phase computed tomography (CT) or dynamic magnetic resonance imaging (MRI)] or histopathologically by fine-needle aspiration (FNA)/biopsy in patients with elevated alpha-fetoprotein (AFP) but having atypical radiological features.
The criteria for RFA eligibility were: Child-pugh status class A or B; up to three HCCs each ≤4 cm in size; no evidence of vascular or extra hepatic spread; patient must not have undergone any kind of prior interventional treatment; lesion not amenable to resection and not willing for liver transplantation; age younger than 85 years; and written informed consent for RFA. The patient outside these criteria were excluded from this study. All these cases were discussed in a multidisciplinary meeting prior to RFA. Our study was approved by the local ethics committee and conducted according to the standards of the declaration of Helsinki.
Radiofrequency ablation
All RFA procedures were performed percutaneously using combined ultrasound (US) and CT guidance with multi-tined expandable RFA electrode (RITA Starburst XL electrode, AngioDynamics) and the RF generator (RITA 1500X RF generator, AngioDynamics, Manchester, Georgia). All the procedures were performed under local anesthesia and conscious sedation. The direct puncture of lesion was avoided and an oblique pathway was taken so as to get a rim of non-tumorous liver tissue while placing the electrode [Figures 1 and 2]. Once the needle was positioned in the lesion, set the target temperature at 105°C (we use the automatic temperature control mode) and the power at 150 W and pressed the start button. Set timer after target temperature reached was according to size of tumor (5 min for 2 cm diameter, 6 min for 3 cm diameter, and 7 min for 4 cm diameter tumor. If at the end of the cool-down mode the temperatures are above 60°C, this is a good indication of complete tumor ablation. To ablate the needle track we retract completely the tines of the device and press the track ablation start button and then the needle was slowly withdrawn. All procedures were performed by one of two radiologists having experience of 10 years and 9 years in tumor ablation. Only patients with nodules accessible through intervening nontumorous liver tissue were selected; tumors with a significant exophytic component were avoided (if more than half of tumor volume were exophytic). Post-lesion ablation needle tract ablation was performed for each session.
Figure 1(A-I).
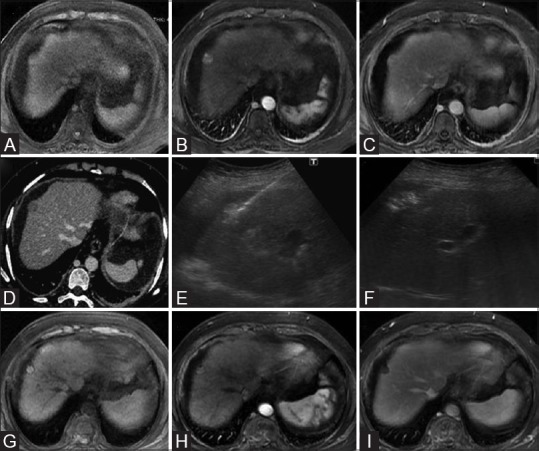
Baseline contrast MRI of liver showing a T1 isointense (A) solitary arterial enhancing surface nodule (B) which subsequently shows washout in venous phase image (C) consistent with HCC. Axial CT scan image in venous phase also confirm the washout in this nodule (D). Grey scale ultrasound image (E) showing RFA electrode needle entering the tumor nodule and multitinned electrode prongs after deployment. Ultrasound image showing hyperechoic ablated area (F) covering the entire tumor nodule. Follow-up multiphase contrast MRI images taken 1 month after ablation [unenhanced (G), arterial (H), and delayed (I)] shows no enhancing component within the targeted lesion in keeping with complete response according to m-RECIST criteria
Figure 2(A-F).
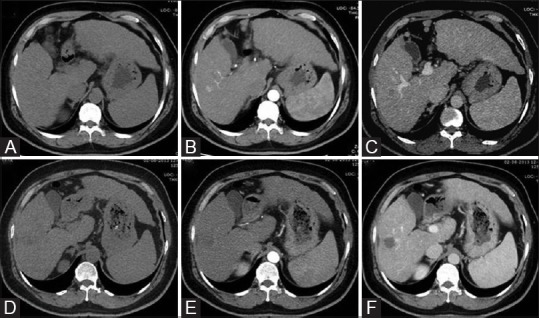
Axial triple phase CT scan images [unenhanced (A), arterial (B), and delayed (C)] shows an intraparenchymal nodule with imaging features consistent with HCC, i.e., arterial enhancement with washout. Post-RFA follow-up triple phase CT scan images 1 month after ablation [unenhanced (D), arterial (E), and delayed (F)] shows no enhancement in treated nodule, most appreciable on venous phase suggestive of complete response
Post-treatment assessment and follow-up
After RFA, all patients were screened with US examination of abdomen and pleural space to identify any immediate complication and then remained in the hospital overnight with vital signs monitoring and pain management. The complete blood cell count, liver function test (LFT), and US examination of the abdomen were performed next day, and if the patient's condition was considered to be satisfactory, patient was discharged by the afternoon.
All patients were followed up until death or time of data analysis (August 2016). The follow-up included assessment of serum AFP levels, liver function test, and a contrast-enhanced CT/MRI scan 1 month after RFA. The imaging evaluation was done using m-RECIST (response evaluation criteria in solid tumors) criteria. A repeat RFA was performed for residual disease while periodic follow-up was planned for patients having complete response. The imaging follow-up was repeated quarterly till 1 year after RFA and thereafter every 4–6 months for assessment of local tumor progression (LTP) and distant intra- and extrahepatic recurrences. The distant intra- or extrahepatic recurrence that occurred without evidence of local progression were treated with RFA or other therapies, and monitoring of baseline lesion was continued according to the study design, until documentation of local progression or death.
Outcome measures
The technical success (complete ablation) after first session and second session; LTP rates; distant intra- and extrahepatic recurrences and major complications were recorded. The definitions are based on the standardization by the International Working Group on Image-Guided Tumor Ablation.[9] The achievement of technical success was defined when the tumor was treated according to the protocol and was completely replaced by ablated tissue as evident on the 1-month follow-up examination. The LTP was diagnosed when a follow-up examination demonstrated findings of interval development/growth of the tumor along the margin of the ablation zone where the RFA had been considered to be technically effective on first follow-up scan. The recurrence free survival was defined as the time from the first RFA to either the earliest event (i.e., LTP, liver transplantation, or death) or the last follow-up date without an event. The distant intra- and extrahepatic recurrence was defined as a new lesion with similar characteristics, but not contacting the original ablation zone in the liver or extrahepatic in location and time period between first RFA date, and appearance of new lesion is defined as time to distant intrahepatic and extrahepatic new lesion. While LTP and recurrence free interval is related to the technical aspect of RFA procedure and adequacy of ablation, the appearance of distant new lesion is not related to RFA, but is attributable to the internal disease process of the liver. The event free survival refers to the time period between first RFA date and appearance of an event which includes local recurrence and distant intra- and extrahepatic new lesion. The major complication was defined as an event that leads to substantial morbidity and disability, increasing the level of care, or results in hospital admission or substantially lengthened the hospital stay. All other complications were regarded as minor.
Statistical analysis
The comparisons were made by considering either the number of patients or the number of nodules. The comparison between the two groups characteristics were made either by using Fisher's exact test if data is categorically variable or by Mann-Whitney test if data is continuous and characteristics were expressed as medians and ranges. The local therapeutic efficacy in terms of technique effectiveness, complication, and LTP was assessed on a tumor basis. All outcome measures were compared between the two groups by using the X2 test or Fisher's exact test, where appropriate. The recurrence free survival and appearance of new lesion was evaluated on a per-patient basis. The event free survival was computed by using the Kaplan-Meier method and compared between the two groups by using the log-rank test. All statistical analyses were performed by using a statistical Software program (SPSS10; SPSS, Chicago, Ill). P ≤.05 was considered to indicate a significant difference.
Results
Characteristics of patients
Total 112 patients with 134 nodules were enrolled in the study and all patients had underlying cirrhosis. Fifty-eight patients with 74 nodules were included in group 1, and 54 patients with 60 nodules were included in group 2. The patient and tumor characteristics are detailed in Table 1.
Table 1.
Comparison of baseline characteristics of patients with surface tumors (Group 1) and those with intraparenchymal tumors (Group 2)
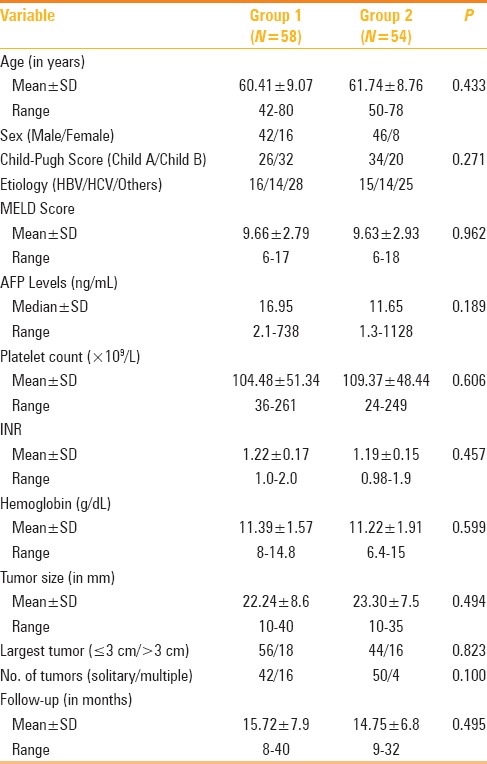
Ninety-two patients (82.14%) underwent RFA of a solitary tumor, 20 patients (17.85%) underwent RFA of mostly two and two three tumor nodules in a single session of RFA. The groups were similar with respect to most of the characteristics, including age, sex, and hepatitis viral markers, serum AFP, Child–Pugh class, and characteristic of HCC including size and proportions of solitary and multiple tumors. In one case, an artificial perihepatic ascites and in one case artificial right pleural effusion was created by instillation of 5% dextrose, due to close association of tumor nodule to the bowel and diaphragm respectively. The mean follow-up periods after initial RFA were 15.72 months (range, 8–40 months) for the group 1 and 14.75 months (range 9–32 months) for the group 2 (P = 0.495 Mann- Whitney test).
Complications
No significant differences were observed between the two groups in regards to complication and no procedure related death occurred in either group.
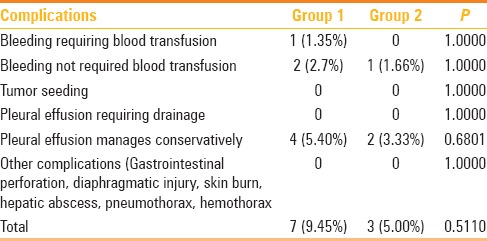
The few complications were seen in the group 1 patients (7 of 74, 9.45%): three cases of mild intraperitoneal bleeding, out of which one case required blood transfusion as he had low pre-procedure hemoglobin (8 g/dL), while other two were managed conservatively without transfusion. The procedural side effects of mild pleural effusion occurred in 4 (5.4%) patients in group 1 and 2 (3%) patient in group 2 and all cases were managed conservatively. One case of intraperitoneal bleed was noted in group 2, which managed conservatively. No needle track seeding, abscess formation, grounding pad burns, bowel perforation, and/or other complication were documented in either group as detailed in Table 2.
Table 2.
Comparison of complications in patients with subcapsular tumors (Group 1) and those without subcapsular tumors (Group 2)
Technical success
The technical success (complete ablation) for first session was achieved in 70 lesions (94.59%) out of 74 surface lesions in group 1 and 58 (96.66%) out of 60 intraparenchymal lesions in group 2. No statistically significant difference was found between the two groups (P > 0.05, Fisher's exact test). The overall technical success after second session was 100% in both the groups.
Local tumor progression
By the time of data analysis LTP was found in four treated HCC nodules out of 74 (5.40%) in group 1 having 2.3, 2.7, 3.1, and 3.8 cm diameter and six treated HCC nodules out of 60 (10.00%) in group 2 having 1.8, 2.2, 2.8 3.0, 3.3, and 3.5 cm diameter as detailed in Table 3. In 1 year, none in group 1 and 3.6% patients in group 2 and at 2 years 12.5% patients in group 1 and 16.2% patients in group 2 show LTP. The LTP-free survival was comparable and statistically not significant between the two groups as depicted by Kaplan-Meier graph analysis (P = 0.77, Figure 3).
Table 3.
Comparison of outcome measures of patients with surface tumors (Group 1) and those with intraparenchymal tumors (Group 2)
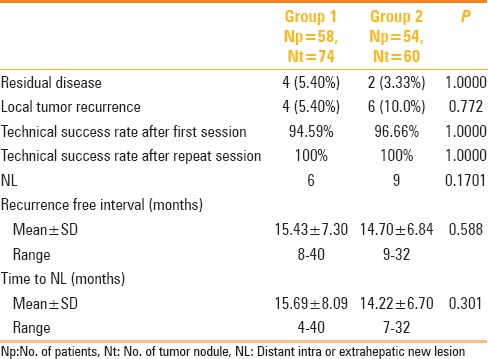
Figure 3.
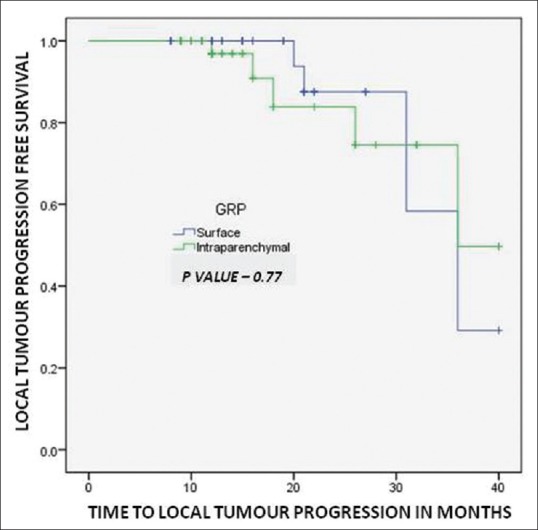
Kaplan–Meier analysis shows local tumor progression free survival for patients with surface tumors (Group 1) and those with intraparenchymal tumors (Group 2)
Event-free survival
No procedure related death occurred in the either group. During the follow-up period till data analysis, three patient (5%) in the group 1 and two patient (3.7%) in group 2 died (P > 0.05) due to progression of liver disease.
The mean time to distant intra- and extrahepatic progression or new lesion was 15.69 ± 8.09 (mean ± SD) months, range 4–40 month in group 1 and 14.22 ± 6.7 months, range 7–32 months in group 2 with P value of 0.30.
The event free survival as depicted by Kaplan-Meier graph analysis and compared between the two groups by using the log-rank test shows that event-free survival rates at 1, 2, and 3 years after RFA were 92.0%, 76.9%, and 64% respectively, in the group 1. While those for group 2 were 92.7%, 62.6%, and 41%, respectively, which were not significantly different between the two groups (P = 0.36, Figure 4).
Figure 4.
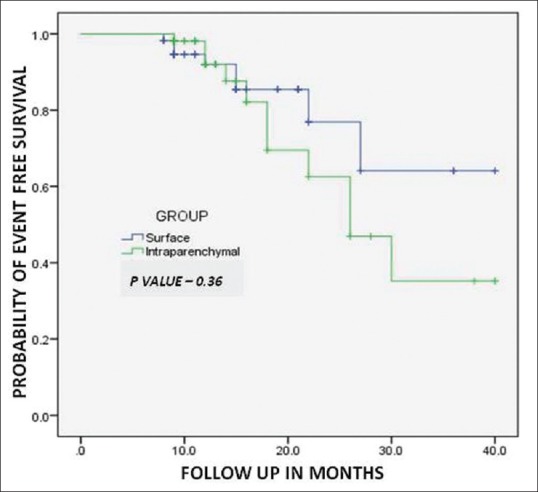
Kaplan–Meier analysis shows event free survival for patients with surface tumors (Group 1) and those with intraparenchymal tumors (Group 2)
Discussion
With improvement in imaging technologies, HCC is being increasingly diagnosed at earlier stages; hence many curative options like liver transplantation, resection, and percutaneous RFA are available. Liver transplantation is the best option for HCC; however, it is limited by the availability of organ, while resection is possible in only up to 10–20% of cases due to underlying comorbidities and advanced cirrhosis.[1] Thus, percutaneous ablation remains the only curative option for this large group of patients and RFA is one of the most popular local ablative therapies for inoperable HCC because of its efficacy and safety demonstrated in early studies.[2] Traditionally surface/subdiaphragmatic/exophytic tumors were considered as relative contraindication of RFA because of reported high incidence of needle tract seeding,[6,7] major complications,[10,11,12,13] and increased local recurrence rates.[4,5] This has important implication in the treatment of patients with HCC, because subcapsular location of HCC is quite common. The proportion of patient's with surface HCC was 52% (58/112) in our study and ranges from 15% to 60% in other studies.[7,14] Hence if surface location is considered as contraindication then a large number of patients will be denied of the benefits of this safe and effective procedure. The group 1 and group 2 had statistically similar patient and tumor nodule characteristics as shown in Table 1, and they underwent RFA during the same period, therefore supporting the validity of this comparative study.
The subcapsular tumors are considered to be associated with increased risk of complications especially in cirrhotic patients, mainly due to difficulty in electrode placement and thermal injury to hollow viscera, injury to diaphragmatic surface causing thoracic complications. The intraperitoneal or subcapsular bleeding and needle tract seeding are usually associated with inadequate or non-thermo coagulation of the needle tract. Major complication rates reportedly ranges from 2.2% to 10.6%, and mortality rates range from 0.09% to 1.45%.[10,11,12,13] There were no significant differences among two groups in post-RFA morbidity or mortality in our study. Our results show a major complication rate of 1.35% and a mortality rate of 0% in surface/subcapsular group which supports the good safety profile of RFA. The safety and effectiveness of RFA for surface lesions has been proven in many prospective and retrospective studies earlier.[2,3]
The reported rates of neoplastic seeding after RFA of hepatic tumors in general range from 0% to 4%.[15,16] To date, several articles have studied the relationship between subcapsular location of HCC and neoplastic seeding [Table 4]. Llovet et al.[6] and Jaskolka et al.[7] showed that needle track seeding were associated with subcapsular location of tumor, whereas other investigators did not find such an association.[14,17,18,19,20] In the former groups, direct puncture of the subcapsular tumor was performed and thermocoagulation of the needle track was not routinely performed. In contrast, in the latter groups, authors tried to avoid direct puncture of subcapsular HCCs—albeit some cases with direct puncture were included in the study and performed thermocoagulation of the needle track. At our institution, we think that bleeding and peritoneal tumor seeding likely occur because of puncture or rupture of the capsule of subcapsular tumor, and inability to thermocoagulate the needle track. Therefore, we always indirectly puncture the HCC by going through a normal liver parenchyma with needle tract ablation and this was our main concern at the time of booking, resulting in tract seeding in none of our patients. Our maximum tolerance for an exophytic component was half of the tumor volume (larger than Kim et al.[19]), and maximum tolerance for surface area contact was approximately half of the tumor surface (larger than Kim et al.[19]).
Table 4.
Various studies comparing radiofrequency ablation in patients with surface tumors and those with intraparenchymal tumors
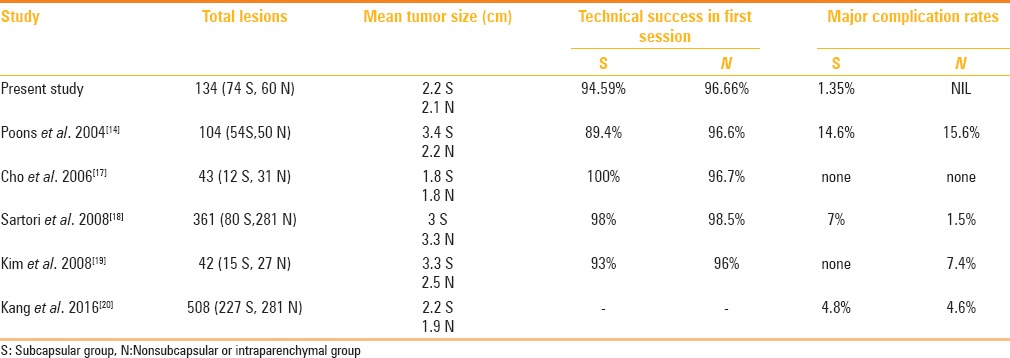
Another major concern of RFA of surface HCCs is the risk for local tumor recurrence, as Komorizono et al.[4] and Hori et al.[5] reported a higher local tumor recurrence after RFA of subcapsular HCCs. They argued the inability to achieve a 0.5–1.0 cm tumor free margin on the capsular side of a subcapsular lesion may explain the higher rate of local recurrence. However, it is unlikely and no definitive evidence currently available stating that the absence of a safety margin on the capsular side of a peripheral tumor increases the local progression rate after RF ablation. Our results indicated that there is no significant difference with regard to the rate of LTP between surface and intraparenchymal HCCs and our findings are comparable and consistent with studies by Poon et al.[14], Cho et al.[17], Sartori et al.[18], and Kang et al.[20], who found similar results. The possible reason behind higher recurrence rates reported by certain authors in surface HCC ablation was the increased technical difficulty of placing the radiofrequency electrode adequately for a subcapsular tumor as compared with a nonsubcapsular tumor, thus leading to incomplete ablation and local recurrence. The technical difficulty could be overcome in most cases by meticulous planning, advanced technique, and use of multi-tined electrode.
Our both the tumor groups are well-matched in the patient and tumor characteristics, with comparable number of ablation sessions and follow-up of 15.72 months in group 1 v/s 14.75 months in group 2. The first session ablation rate in group 1 was 95% vs. 97% rate in group 2 which seems slightly lower, but not statistically different. However, complete ablation rate was comparable between the two groups (100%). Lower first session ablation rate in group 1 is likely attributable to the technical difficulty in the electrode needle placement in the most desired position within the subcapsular HCC because of oblique pathway of needle insertion through a layer of nontumorous liver. In such cases, accurate puncture of subcapsular HCC can be achieved by laproscopic or open approach, but our institutional choice is percutaneous RFA. Our results indicated no significant difference in technical effectiveness between the subcapsular and nonsubcapsular HCC groups. This was in accordance with the previous reports by Poon et al.[14], Cho et al.[17] and Sartori et al.[18], Kim et al.[19], and Kang et al.[20]
There were few limitations of this study which include retrospective nature and small patient subset.
Conclusions
Our study shows that despite tumor being at surface location, a meticulous planning, and accurate placement of electrode through intervening normal liver parenchyma and effective needle track ablation lead to good ablation outcome which is comparable to intraparenchymal HCC ablation results in terms of effectiveness, safety, local tumor recurrence, and event-free survival. Hence, surface HCC in cirrhotic patients should not be considered a contradiction to percutaneous RF ablation and should be treated with RFA whenever feasible.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–9. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: A critical review from the surgeon's perspective. Ann Surg. 2002;235:466–86. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: A systematic Review. Ann Surg. 2009;249:20–5. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 4.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–62. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 5.Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Kato J, et al. Risk factor for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–81. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Vilana R, Bru C, Salmeron JM, Sala M, Sole M, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–9. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 7.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485–91. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 8.Livraghi T. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:608–9. doi: 10.1002/hep.510340325. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Leen E, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria-a 10-year update. Radiology. 2014;273:241–60. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radiofrequency ablation: Complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 11.Rhim H, Yoon KH, Lee JM, et al. Major complications after radiofrequency thermal ablation of hepatic tumors: Spectrum of imaging findings. Radiographics. 2003;23:123–34. doi: 10.1148/rg.231025054. [DOI] [PubMed] [Google Scholar]
- 12.Meloni MF, Goldberg SN, Moser V, Piazza G, Livraghi T. Colonic perforation and abscess following radiofrequency ablation treatment of hepatoma. Eur J Ultrasound. 2002;15:73–6. doi: 10.1016/s0929-8266(01)00171-9. [DOI] [PubMed] [Google Scholar]
- 13.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumors. Br J Surg. 2002;89:1206–22. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 14.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004;11:281–9. doi: 10.1245/aso.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SN, Solbiati L. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma [letter] Hepatology. 2001;34:609. doi: 10.1002/hep.510340327. [DOI] [PubMed] [Google Scholar]
- 16.De Sio I, Castellano L, De Girolamo V, di Santolo SS, Marone A, Marone G, et al. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma [letter] Hepatology. 2001;34:609–10. doi: 10.1002/hep.510340327. [DOI] [PubMed] [Google Scholar]
- 17.Cho YK, Rhim H, Ahn YS, Kim MY, Lim HK. Percutaneous radiofrequency ablation therapy of hepatocellular carcinoma using multitined expandable electrodes: Comparison of subcapsular and nonsubcapsular tumors. AJR Am J Roentgenol. 2006;186:S269–74. doi: 10.2214/AJR.04.1346. [DOI] [PubMed] [Google Scholar]
- 18.Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: A prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008;248:670–9. doi: 10.1148/radiol.2482071690. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Raman SS, Yu NC, Busuttil RW, Tong M, Lu DS. Radiofrequency ablation of hepatocellular carcinoma: Can subcapsular tumors be safely ablated? AJR Am J Roentgenol. 2008;190:1029–34. doi: 10.2214/AJR.07.2293. [DOI] [PubMed] [Google Scholar]
- 20.Kang TW, Lim KH, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long term therapeutic outcome of radiofrequency ablation of subcapsular versus nonsabcapsular heaptocellular carcinoma: A Propensity Score Matched Study. Radiology. 2016;280:300–12. doi: 10.1148/radiol.2016151243. [DOI] [PubMed] [Google Scholar]


