Abstract
We review the evolution of the concept of informed consent from a radiology standpoint, the current international guidelines on the need for obtaining consent in diagnostic radiology practice, and the current Indian scenario, focusing on both practical and medicolegal aspects. We discuss the concept of patient information sheet with signature, a potential way forward benefiting both patients and radiologists.
Keywords: Anaphylaxis, informed consent, IV contrast, medicolegal
Introduction
In broad terms, consent is a method of effective mutual communication between the patient and the doctor, which leads to the patient giving (or withholding) permission from the doctor to act in a particular way.[1] Consent can either be implied or express. In the medical field, consent is often implied when the risk of adverse effects is low. Both verbal and written consent are accepted forms of express consent.[1,2] The traditional model of obtaining consent is based on the “prudent doctor” model, where the doctor weighs the risk–benefit ratio of any patient treatment/investigation and omits mentioning rare nonserious complications. Currently, the model slowly gaining ground in the Western countries, is the “prudent patient” model, which is based more on what the average judicious patient would want to know about a particular treatment/examination and its associated risks.[2,3] Ideally, both would be expected to reasonably coincide.
Along with the evolution in the concept of obtaining patient consent and its medicolegal implications, our own understanding of various diagnostic radiology associated complications and their management has also tremendously evolved. While obtaining written informed consent is the standard practice worldwide for interventional procedures, consent in diagnostic radiology practice remains a gray zone in India.[2,4,5] From personal communication with various institutes and radiology centers, we understand that current Indian practices in this regard are not congruent with international guidelines and academic practices.[2,4,5] Accordingly, we are writing this review to discuss the current international guidelines and their applicability in the Indian setting.
What are the Reasons to Obtain Consent before Computed Tomography/Magnetic Resonance Imaging?
Contrast-allergy and contrast-induced nephropathy are the two complications which possibly require obtaining patient consent.[6,7,8,9] Other potential consent-related issues include imaging pregnant patients and complications related to contrast extravasation.[6,7] There are recent debates in the radiology community on the need to inform all patients about risks associated with radiation exposure, particularly in children.[10]
What is the Risk of Developing a Serious Contrast-related Complication?
Contrast reaction
The risk of developing a contrast reaction after intravenous (IV) iodinated or gadolinium-based contrast is ≤0.7% as per multiple large recent studies.[11,12,13] However, majority of these reactions are mild, and the frequency of severe reactions is rare. In a large much cited Japanese study involving almost 170,000 patients who received nonionic contrast, the incidence of serious allergic reactions was 0.04% (4 in 10,000), with a single death, which was also not definitely attributable to the contrast injection.[14] Other studies have reported death rates ranging from 2.1–9 per million.[15,16]
Contrast-induced nephropathy
Recent studies have also shown that the incidence of contrast-induced nephropathy is significantly overestimated.[17] Most patients with renal dysfunction are anyway usually screened out from a contrast study as almost all practices ask for a baseline creatinine value prior to contrast administration.
Other risks
Contrast extravasation has an incidence of <1% and is usually mild.[4,13] The risk related to radiation exposure in a nonpregnant patient is still a matter of debate and research.[10]
Based on this data, the American College of Radiology (ACR), the Society of Pediatric Radiology (SPR), and the Society of Interventional Radiology (SIR) state that IV contrast has a relatively low incidence of adverse events.[4,5]
Given this low risk, do we still need to obtain consent?
Given that IV contrast has a low incidence of adverse reactions, the ACR, SPR, and SIR recommend that IV contrast injection may be exempted from the need for obtaining informed consent. They do, however, mention that the policy should be based on state law, institutional and departmental policies, and local community practices.[4,5,13] The Royal College of Radiology, on the other hand, states that potential risks and benefits still need to be explained to the patients in all cases, but implied consent should suffice in very low-risk procedures.[2] In practice, majority of the institutes in USA do take implied consent by providing a patient-information sheet explaining potential risks to the patient and clarifying that a radiologist is available to answer any queries.[6]
How Effective is a Patient Information Sheet?
A typical patient information sheet will ask questions, in the patient's preferred language to determine the patient's risk for developing a contrast related complication (history of prior contrast allergy, diabetes, renal disease etc.), explain the procedure, and the potential risks such as anaphylaxis and death, and their incidence. Importantly, the patient is informed that, if there are any queries, a radiologist is available to answer them.[6] A positive check mark in any of the questions (such as presence of history of contrast allergy) may trigger a doctor–patient conversation. If the patient undergoes the CT/MRI after having read the information sheet without asking for the radiologist, consent is implied. Private practices often play it safer by asking the patient to sign at the end of the information sheet while returning it back for legal documentation.
Various studies have shown that improving the content of informed consent forms/patient information sheets and providing information in clear and simple terms makes them more understandable and decreases patient anxiety.[18,19,20] The National Institutes of Health (NIH) and American Medical Association (AMA) state that the readability of the patient material should be at 3rd to 7th grade level to ensure that the average patient can read the study.[21] Hence, it is important to keep the language in the patient information sheet simple and to avoid uncommon medical terms as much as possible. Free online websites such as Readability Test Tool are available which test copied and pasted text (or a website link) for readability and assign a grade level, thus allowing us to create an appropriate level patient information sheet.[22]
Isn’t it safer instead talk to the patient, explain all the risks, and obtain express consent?
Radiologists, particularly private practice radiologists, may feel it more prudent to take express informed consent. However, it is often not feasible to talk to every patient personally prior to contrast injection given time constraints. The work often ends up being delegated to technicians or nurses or at times even clerical staff. Given their lack of training and competence in medical issues or of any certification courses which would stand legal validity, it is unclear how safe it is to rely on them for obtaining appropriate informed consent. Would they be able to accurately answer questions posed by the patients based on the current guidelines and literature rather than give arbitrary assurances? One of the biggest fears for which consent is obtained is to avoid medicolegal issues if there is a serious reaction/death. The authors feel that, in such cases, history of consent being obtained by a nonradiologist could potentially worsen the case for the radiologist.
Furthermore, if we look at the usual practices in other medical specialties, we do not find a parallel. For example, a drug like penicillin has an estimated rate of anaphylaxis of 1–5 per 10,000 injections.[23] However, no written consent is usually obtained while administering it; consent is implied. Another equivalent example would be administering IV Amphotericin-B, a toxic drug with an incidence of nephropathy as high as 14–65%, and an incidence of infusion reaction of 4–21%.[24,25] Although much more toxic than IV contrast, doctors again do not take written consent while administering it (personal communication with several institutes). While it is understood that there is a difference between a drug administered for treatment and one used for diagnostic purposes, there are parallels to be drawn here for radiologists.
What is the legal status on this issue in India?
To the best of the authors’ knowledge, there is no legal precedent specific to radiology on this issue. Singh et al., in an article on medicolegal issues in radiology, give a theoretical example of a radiologist being successfully sued for malpractice after the patients expires due to an anaphylactic reaction.[7] They do not mention any traceable details of the judgement (order date, court name etc.), and clarify in the next paragraph that the case is simply “an illustration.”
In a letter to the editor in IJRI on the topic of medical negligence, Sohoni states that his practice routinely takes consent before a CT/MRI due to risks associated with IV contrast administration.[8] He further goes on to equate radiological studies with surgical interventions and suggests that the scope of consent should be extended to all investigations such as mammography, sonography, and X-ray, and should include details on the limitations of the study, possibilities of intra and interobserver variations, and typographical errors, among others,[8] without any data or references to back these arguments. We do not concur with this view. Medical negligence is well established to represent lack of reasonable competence.
Why are Indian radiologists so hesitant then to do away with consent and use a patient information sheet?
From personal experience and communication with other institutes, we believe that majority of Indian radiology practices, both academic and private, obtain some form of express consent. The reasons for this are manifold. Radiology bodies such as the IRIA do not have any guidelines on this issue that would help the radiologist. Moreover, the fear of medicolegal claims persists.
The road ahead: What do we suggest
In essence, consent is about the patient having sufficient information to make an informed decision. Personally obtaining express consent is not possible in most practices, and delegating the task to relatively untrained paramedical or clerical staff is fraught with risk. We believe that a patient information sheet with a signature at the bottom is the best way forward for this to happen (an example is provided in Figure 1). The patient gets time to read the document, signs it, and returns it to the technician or nurse at the time of the study, who then countersign and keep the sheet in their records for proof. If well-constructed, a patient information sheet will supply more accurate and clear information to the patients to allow an informed decision. It is legally sound and will also improve workflow. In this age of Google and second opinions, it allows the patient sufficient time to read or talk to others regarding any apprehensions, think everything out, and approach the radiologist with genuine queries which we should be happy to answer. For example, a patient with an aneurysm coil would have more time confirming with his interventionist regarding the MRI-compatibility of the coil, rather than having this question thrust at him right before stepping into the scanner room, which will definitely make him lose his MRI slot, and create a backlog at the radiology center. Finally, there are certain high risk occasions when obtaining express written consent remain prudent, such as when imaging pregnant patients with studies involving radiation exposure, or giving IV contrast to a patient having prior history of contrast reaction. This should be done preferably by the radiologist.
Figure 1.
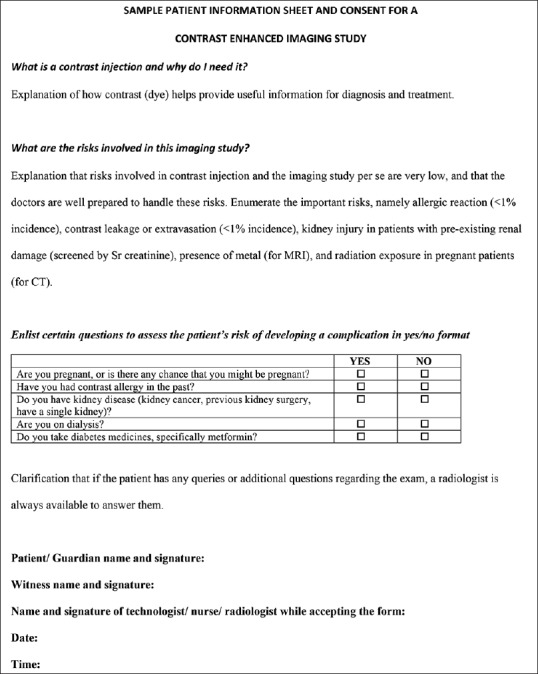
Sample format for a patient information sheet with signature for consent. Use of simple language and lay terms as much as possible (e.g. “dye” instead of “contrast,” or “leakage” instead of “extravasation”) as also having the sheet in local language would be ideal. Always clarify that a radiologist is available in case of any doubts. Please note that any positive history/check mark should always lead to a conversation between the patient and the care providers
Radiology organizations such as the IRIA need to give specific guidelines as also possibly a model patient information sheet with signature. This will help during legal cases, if any, and help change practice patterns for the better. Finally, it is important for the radiology community to ensure regular updates and CMEs on the management of contrast-related complications and exercise strict quality control and preparedness in managing them at their respective institutes.
We would once again emphasize that we do not suggest doing away with consent. What we are suggesting is that Indian radiologists should start following internationally accepted guidelines on this issue, and instead of express informed consent, we should obtain an equally legally valid consent through the patient information sheet with signature. This is indeed a complex topic, and differences of opinion are bound to be there among the radiology fraternity; ideally all such evidence-based opinions should be discussed. We have attempted to provide our viewpoint based on international norms and guidelines and the current standard of practice in other branches of medicine in India. We hope that this article kickstarts the process of debate and reaching some evidence-based consensus on this issue.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Satyanarayana Rao KH. Informed consent: An ethical obligation or legal compulsion? J Cutan Aesthet Surg. 2008;1:33–5. doi: 10.4103/0974-2077.41159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Standards for patient consent particular to radiology. Second edition. The Royal College of Radiologists; 2015. [Google Scholar]
- 3.Edozien LC. UK law on consent finally embraces the prudent patient standard. BMJ. 2015;350:h2877. doi: 10.1136/bmj.h2877. [DOI] [PubMed] [Google Scholar]
- 4.ACR Manual on Contrast Media. Version 10.2. ACR Committee on Drugs and Contrast Media. 2016 [Google Scholar]
- 5.ACR–SIR–SPR practice parameter on informed consent for image-guided procedures, Revised 2016 (Resolution 17) [Google Scholar]
- 6.Bettmann MA. Frequently asked questions: Iodinated contrast agents. Radiographics. 2004;24(Suppl 1):S3–10. doi: 10.1148/rg.24si045519. [DOI] [PubMed] [Google Scholar]
- 7.Singh SS, Jayaram N. Medico-legal issues in radiology: Indian context. J Med Soc. 2015;29:129–34. [Google Scholar]
- 8.Sohoni CA. Medical negligence: A difficult challenge for radiology. Indian J Radiol Imaging. 2013;23:110–2. doi: 10.4103/0971-3026.113631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berlin L. Informed consent for contrast media and gadolinium injections. Am J Roentgenol. 2011;197:359. doi: 10.2214/AJR.10.5551. [DOI] [PubMed] [Google Scholar]
- 10.Semelka RC, Armao DM, Elias J, Jr, Picano E. The information imperative: Is it time for an informed consent process explaining the risks of medical radiation? Radiology. 2012;262:15–8. doi: 10.1148/radiol.11110616. [DOI] [PubMed] [Google Scholar]
- 11.Callahan MJ, Poznauskis L, Zurakowski D, Taylor GA. Nonionic iodinated intravenous contrast material-related reactions: Incidence in large urban children's hospital-retrospective analysis of data in 12,494 patients. Radiology. 2009;250:674–81. doi: 10.1148/radiol.2503071577. [DOI] [PubMed] [Google Scholar]
- 12.Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001;176:1385–8. doi: 10.2214/ajr.176.6.1761385. [DOI] [PubMed] [Google Scholar]
- 13.Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008;191:409–15. doi: 10.2214/AJR.07.3421. [DOI] [PubMed] [Google Scholar]
- 14.Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990;175:621–8. doi: 10.1148/radiology.175.3.2343107. [DOI] [PubMed] [Google Scholar]
- 15.Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high- vs low-osmolality contrast media: A meta-analysis. AJR Am J Roentgenol. 1991;156:825–32. doi: 10.2214/ajr.156.4.1825900. [DOI] [PubMed] [Google Scholar]
- 16.Lasser EC, Lyon SG, Berry CC. Reports on contrast media reactions: Analysis of data from reports to the U.S. Food and Drug Administration. Radiology. 1997;203:605–10. doi: 10.1148/radiology.203.3.9169676. [DOI] [PubMed] [Google Scholar]
- 17.McDonald RJ, McDonald JS, Newhouse JH, Davenport MS. Controversies in Contrast Material-induced Acute Kidney Injury: Closing in on the Truth? Radiology. 2015;277:627–32. doi: 10.1148/radiol.2015151486. [DOI] [PubMed] [Google Scholar]
- 18.Coyne CA, Xu R, Raich P, Plomer K, Dignan M, Wenzel LB, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: A study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21:836–42. doi: 10.1200/JCO.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: A comparative study of standard versus simplified forms. J Natl Cancer Inst. 1998;90:668–74. doi: 10.1093/jnci/90.9.668. [DOI] [PubMed] [Google Scholar]
- 20.Pereira SP, Hussaini SH, Wilkinson ML. Informed consent for upper gastrointestinal endoscopy. Gut. 1995;37:151–3. doi: 10.1136/gut.37.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansberry DR, John A, John E, Agarwal N, Gonzales SF, Baker SR. A critical review of the readability of online patient education resources from RadiologyInfo.Org. AJR Am J Roentgenol. 2014;202:566–75. doi: 10.2214/AJR.13.11223. [DOI] [PubMed] [Google Scholar]
- 22.Readability Test Tool. [Last accessed on 2017 Jun 30]. https://www.webpagefx.com/tools/read-able/
- 23.Bhattacharya S. The facts about penicillin allergy: A review. J Adv Pharm Technol Res. 2010;1:11–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Deray G. Amphotericin B nephrotoxicity. J Antimicrob Chemother. 2002;49(Suppl 1):37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- 25.Liposomal Amphoterecin B. [Last accessed on 2016 Oct 22]. Available from http://reference.medscape.com/drug/ambisome-amphotericin-bliposomal-999576-4 .


