Abstract
A highly sensitive, selective, and precise ultra-performance liquid chromatography tandem mass spectrometry method was developed and validated for simultaneous quantification of itraconazole and hydroxy itraconazole in human plasma by a single liquid–liquid extraction step. The precursor to product ion transitions of m/z 705.3/392.3, m/z 721.2/408.3 and m/z 708.2/435.4 were used to detect and quantify itraconazole, hydroxy itraconazole and itraconazole-d3 respectively. The lower limit of quantitation was found to be 0.500 ng/mL for itraconazole and 1.00 ng/mL for hydroxy itraconazole. The mean recoveries for itraconazole and hydroxy itraconazole were found to be 100.045% and 100.021%, respectively. This developed method with a chromatographic run time of 2.0 min was successfully applied to a bioequivalence study of 100 mg itraconazole capsule.
Keywords: Itraconazole, Hydroxy itraconazole, Ultra-performance liquid chromatography, Human plasma, Simultaneous analysis
1. Introduction
Itraconazole [1], [2], [3], [4], [5] (R 51 211), (+-)-cis-4-[4-[4-[4-[[2-(2,4-dichlorophenyl)-2-(lH-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl] methoxy]phenyl]-1-piperazinyl]phenyl]-2,4-dihydro-2(1-methylpropyl)-3H-1,2,4-triazol-3-one, is an orally active triazole antifungal agent which demonstrates broad spectrum activity against a number of fungal species including dermatophytes, Malassesia furfur, Candida species, Aspergillus species, and Histoplasma capsulatum var. capsulatum [6]. It is widely used in the treatment and prophylaxis of a variety of fungal infections, especially aspergillus species which can cause a hypersensitivity and inflammatory reaction, called allergic bronchopulmonary aspergillosis (ABPA) that can result in damage to lung tissues. Itraconazole is a 1:1:1:1 racemic mixture of four diastereomers (two enantiomeric pairs), each possessing three chiral centers. The structures of itraconazole and hydroxy itraconazole are shown in Scheme 1.
Scheme 1.
Structures of itraconazole and hydroxy.
The mechanism of action for its antifungal activity is believed to involve efficient inhibition of the fungal-demethylase by itraconazole. Following oral absorption, it is extensively metabolized including side chain hydroxylation with CYP3A4 to form hydroxyl itraconazole which is the major metabolite. Hydroxy itraconazole is biologically active and its plasma concentration is two folds higher than itraconazole at steady state. Itraconazole is extensively metabolized in humans by the liver, yielding over 30 metabolites. It is given mainly by the oral route (as capsules), but also as an aqueous solution with a cyclodextrin as a solubilizer. In some countries it is also available as an injectable solution. The pharmacokinetics of orally administered itraconazole are of continuing interest, partly because of its very poor water solubility, which contributes to low, variable absorption, but also because it is both an inhibitor and substrate of the CYP3A4 and the P-glycoprotein transporter system. It has a pKa of 3.70 (based on extrapolation of values obtained from methanolic solutions) and a log(n-octanol/water) partition coefficient of 5.66 at pH 8.1. Itraconazole is over 99% protein bound and has virtually no penetration into cerebrospinal fluid. Therefore, it should never be used to treat meningitis or other central nervous system infections. The plasma protein binding of itraconazole is 99.8% and that of hydroxy itraconazole is 99.5%.
Identification and quantification of itraconazole and hydroxy itraconazole require a specific and sensitive method that is suitable for routine analysis of biological samples. Few methods have been reported in the literature for quantification of itraconazole and its hydroxy metabolite. The technique used in these includes high performance liquid chromatography (HPLC) method in human serum [7], [8], [9], [10] and in human plasma [11], [12], [13], [14], [15], [16], flourimetric method in human plasma [17], [18] and liquid chromatography tandem mass spectrometry (LC–MS/MS) method in human plasma. However, the HPLC methods are less selective and not reliable.
Several papers have been reported for the determination of itraconazole and hydroxy itraconazole in human plasma by LC–MS/MS [19], [20], [21], [22], [23]. However, their total analysis time per sample and sample volume requirement often do not suit the requirements for routine plasma sample analysis of itraconazole. No method was reported for quantification of itraconazole and its hydroxy metabolite in human plasma employing ultra-performance liquid chromatography/tandem mass spectrometry (UPLC–MS/MS). The high efficiency of sub-2-mm particle columns with UPLC conditions has made it the ideal partner for mass spectrometry. Further, this LC system having an ultra-low system volume; a fast, sensitive, low volume detector; intelligent software; negligible carry over; and the ability to reliably run at the higher backpressures generated by small particles improves results in analyte resolution, throughput, and sensitivity.
We now report a highly sensitive high throughput UPLC–MS/MS method for the quantification of itraconazole and its metabolite (hydroxy itraconazole) in human plasma using itraconazole D3 as an internal standard. The validated method has been successfully used to analyze samples obtained after the administration of a single dose of 100 mg itraconazole tablets to healthy volunteers participated in bioavailability study. The current method includes a very simple, economical and rapid sample preparation technique as well as significantly shorter analysis run time compared to previously published methods. Therefore, this easily applicable and low-cost method can be used for analysis of itraconazole and its metabolite from human plasma.
2. Materials and methods
2.1. Chemicals and reagents
Itraconazole and hydroxy itraconazole standards (purity>99.3%) were obtained from Vivan Life Sciences Pvt. Ltd. (India) and itraconazole D3 from Clearsynth Labs Pvt. Ltd. (India). Di-potassium salts of ethylene diamine tetra acetic acid (K2EDTA) plasma of healthy volunteers were obtained from white cross blood bank, Mumbai (India). Acetonitrile (HPLC grade) and methanol were obtained from JT Baker, Germany. A Milli-Q water (Millipore Co., MA, USA) purification system was used to obtain purified water for the UPLC analysis.
2.2. Instrumentation
Zorbax SB-C18, 50 mm×2.1 mm, 5 µm (Agilent) column was used for chromatographic separation with a flow rate of 0.400 mL/min. Chromatography was performed at ambient temperature, with the mobile phase consisting of acetonitrile and 2 mM ammonium acetate (pH 3.5 with formic acid) in the ratio of 80:20 (v/v). The mobile phase was delivered by the ultra-performance liquid chromatography (UPLC) solvent manager and the samples were injected by a UPLC sample manager (Waters® Corporation, Milford, MA, USA). Mobile phase was also used as wash solvent for strong needle wash and weak needle wash on UPLC system. Milli-Q water was used as seal wash solution on UPLC system. All the solutions used on UPLC system were filtered by using 0.22 µm membrane filters.
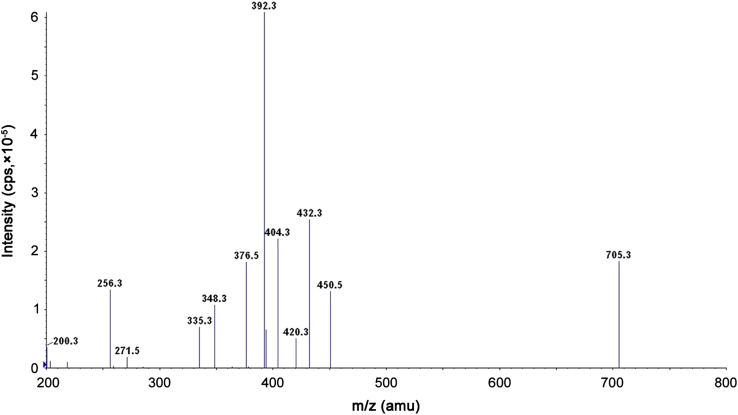
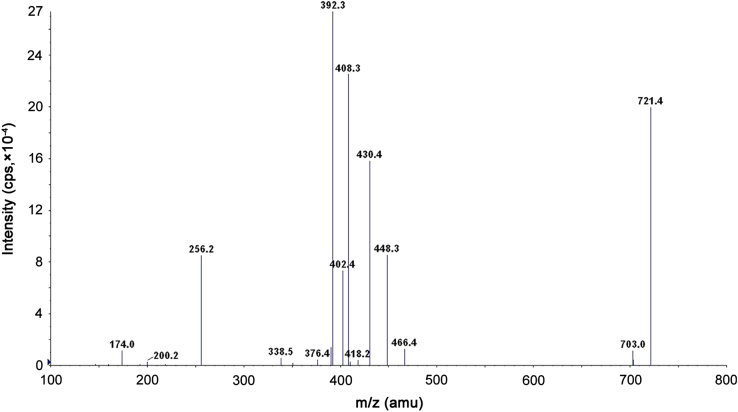
Ionization and quantification of analytes and IS were performed on a triple quadrupole mass spectrometer, API-4000 Q-Trap equipped with Turbo Ion spray®, from MDS SCIEX (Toronto, Canada) operated in the positive ion mode. Quantitation was done by using multiple reaction monitoring (MRM) mode to monitor protonated precursor→product ion transition of m/z 705.3→392.3, m/z 721.2→408.3 and m/z 708.2→435.4 for itraconazole, hydroxy itraconazole, and itraconazole D3 respectively. All the parameters of UPLC and MS were controlled by analyst software version 1.5.1. The source dependent parameters maintained for analytes and internal standard were Gas 1 (Nebulizer gas): 35 psi, Gas 2 (Heater gas): 65 psi, ion spray voltage (ISV): 4000 V, turbo heater temperature (TEM): 500 °C, entrance potential (EP): 10 V, collision activation dissociation (CAD): 6 psi, curtain gas (CUR): 30 psi. The compound dependent parameters like declustering potential (DP), collision energy (CE) and cell exit potential (CXP) were optimized at 130.00, 51.00 and 11.00 V for itraconazole, 94.00, 52.00 and 11.00 V for hydroxy itraconazole, 80.00, 51.00 and 11.00 V for itraconazole D3 respectively. Optimization of the triple quadrupole settings of the instrument for the detection of itraconazole, hydroxy itraconazole, and itraconazole D3 was performed by infusing a 5 ng/mL solution of each drug dissolved in methanol:water (50:50, v/v) solution at a constant flow rate of 10 µL/min. Fig. 1, Fig. 2 show a product ion mass spectrum of pure itraconazole and hydroxy itraconazole.
Fig. 1.
Product ion spectra of itraconazole.
Fig. 2.
Production spectra of hydroxy itraconazole.
2.3. Preparation of standards and quality control samples
The stock solutions of itraconazole, hydroxy itraconazole and itraconazole D3 were prepared by dissolving reference standards in methanol containing 8% formic acid. These stock solutions were later used to prepare the working solutions of itraconazole, hydroxy itraconazole and itraconazole D3 in methanol: Milli-Q/HPLC grade water mixture (50:50, v/v) by appropriate dilution. Calibration curve standards consisting of eight non-zero concentrations were prepared by spiking standards in 0.980 mL of blank human plasma, each with 0.010 mL working solution of itraconazole and hydroxy itraconazole in the concentration range of 0.500–402.406 ng/mL for itraconazole and 1.000–597.920 ng/mL for hydroxy itraconazole. Similarly, quality control (QC) standards were prepared for the lower limit of quantification (LLOQ QC), low quality control (LQC), medium quality control (MQC) and high quality control (HQC) levels for both the analytes within the calibration curve range. After bulk spiking, 600 µL of spiked plasma samples were pipetted out in pre-labeled polypropylene tubes. The calibration curve standards and QC samples were logged in ultra-low temperature deep freezer (temperature range: −55 °C to −75 °C) except 24 samples each of LQC and HQC which were transferred for storage in cell frost deep freezer (temperature range: −17 °C to −27 °C) for the generation of long term stability at –22±5 °C. These samples were used for performing the method validation. All the stock solutions were stored at 2–8 °C for further use.
2.4. Extraction procedure
A set of calibration curve standards and QC samples were withdrawn from the deep freezer and allowed to thaw at room temperature in water bath. Plasma samples (500 µL) were pipetted from the pre-labeled polypropylene tubes into prelabeled 5 mL ria vials followed by the addition of 50 µL of internal standard dilution (300 ng/mL). The samples were vortex-mixed for 10 s. 200 µL of 2 M ammonium acetate was then added and samples were again vortexed. Finally 2 mL methyl tert-butyl ether (TBME) was added for extraction and samples were vortexed for approximately 5 min. Once the extraction completed, samples were flash freezed for 1–2 min and supernatant was decanted off followed by evaporation to dryness at 40 °C at constant pressure in nitrogen evaporator. The resulted dried samples were reconstituted with 300 µL of mobile phase, vortexed and transferred into vials for analysis.
2.5. Validation
The selectivity, sensitivity, linearity, precision, accuracy, recovery, stabilities and dilution integrity of the method have been validated according to the US Food and Drug Administration guidance for the validation of bioanalytical methods [24], [25]. Selectivity and specificity were determined in six different lots of normal K2EDTA plasma and four different lots of lipemic K2EDTA and heamolysed K2EDTA plasma. Three precision and accuracy batches of spiked plasma calibration curve standard at eight different concentrations levels ranging from 0.500 ng/mL to 402.406 ng/mL for itraconazole and 1.000 ng/mL to 597.920 ng/mL for hydroxy itraconazole were prepared and analyzed. Weighted 1/(concentration)2 linear regression was used to construct the itraconazole and hydroxy itraconazole calibration curves. Spiked QC samples were processed in six replicates at four concentration levels (0.500, 1.425, 203.534 and 328.280 ng/mL for itraconazole and 1.000, 2.83, 297.96 and 480.58 ng/mL for hydroxy itraconazole) for three different analytical batches to evaluate the intra- and inter-batch assay accuracy and precision. Recovery of both the analytes were determined in normal K2EDTA plasma at three different concentrations (1.425, 203.534 and 328.280 ng/mL of itraconazole and 2.830, 297.960 and 480.580 ng/mL of hydroxy itraconazole) by comparing the analyte peak areas of the extracted QC samples with those of the non-extracted equivalent QC mixture representing 100% recovery.
The stability of drug and metabolites in human plasma was studied by subjecting them into different storage conditions at two different concentrations (LQC and HQC) levels. For the evaluation of bench top stability the required number of QC aliquots was kept at room temperature for 10 h, for evaluating auto-sampler stability the required number of QC's was processed, reconstituted and kept in autosampler for 79 h. After bulk spiking sufficient number of QC's was stored at −65 °C for a period of 306 days and −22 °C for 127 days to access the long term stability. Freeze/thaw stability was also evaluated after subjecting for three cycles of freezing and thawing. Dry extract stability was evaluated after storage of dry extract QC samples at −17 °C to −27 °C in deep freezer for a period of 47 h and wet extract stability was evaluated after storage of reconstituted QC samples at 2–8 °C in refrigerator for a period of 24 h. The stabilities were evaluated by comparing with freshly spiked calibration curve standards and QC samples in pooled plasma. The analytes were considered stable in human plasma when a percent change of ±15% of the initial concentration was found. Robustness was analyzed at low and high pH of buffer solution, at low and high flow rate (0.380 μL/min and 0.420 μL/min) of mobile phase and at low and high column oven temperature (38 °C and 42 °C). For ruggedness evaluation one precision and accuracy batch was processed by different analysts and analyzed by using different columns and different solutions.
3. Results and discussion
3.1. Method development
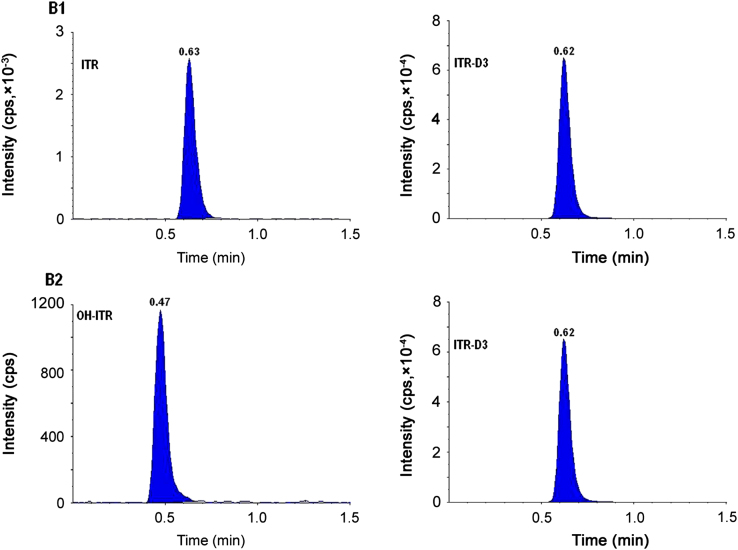
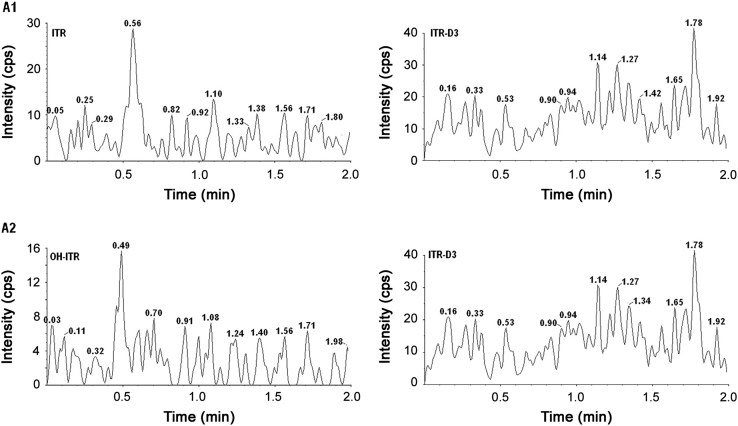
The higher sensitivity of MS/MS detector allowed the development of a sensitive and robust method for the determination of itraconazole and hydroxy itraconazole. Linearity was developed within the concentration range of 0.500–402.406 ng/mL for itraconazole and 1.000–597.920 ng/mL for hydroxy itraconazole. Results of the back-calculated concentrations from three calibration curves for itraconazole and hydroxy itraconazole using UPLC–MS/MS are tabulated in Table 1. The table also represents the precision and accuracy for each standard of the calibration curve. The results obtained were within the acceptance criteria of ±20% of the test concentration at the LLOQ level and ±15% for the standards above LLOQ. Blank plasma sample clean up was one of the most important point during the subject sample analysis using UPLC–MS/MS technique. Liquid–liquid extraction method was investigated with TBME as extraction solvent and found to be more rugged and robust enabling to enhance sensitivity and recovery compared to solid phase extraction and protein precipitation. No endogenous substances were detected, which significantly interfered with the quantification of itraconazole and hydroxy itraconazole. Even though there is an improvement in cost optimization due to the use of liquid–liquid extraction, still the aim of the researchers is to reduce the chromatographic run time so that more number of samples can be analyzed within a day. The retention times for itraconazole, hydroxy itraconazole and itraconazole D3 were found to be within a min. The total chromatography run time of 2.00 min made it possible to analyze a large number of samples within a day. Fig. 3 shows representative chromatograms of itraconazole and hydroxy itraconazole at concentrations of 0.500 ng/mL and 1.000 ng/mL in plasma along with the IS. A representative chromatogram of the extracted blank plasma of itraconazole, hydroxy itraconazole and itraconazole D3 is presented in Fig. 4. This analytical method was used to analyze plasma samples containing itraconazole, hydroxy itraconazole and itraconazole D3 from 60 healthy male volunteers after administering single doses of 100 mg itraconazole capsule.
Table 1.
Back calculated concentrations of itraconazole and hydroxy itraconazole (n=3).
| Analyte | Standard concentration (ng/mL) | Mean (ng/mL) | SD | CV (%) | Nominal (%) | Slope | Intercept | r2 |
|---|---|---|---|---|---|---|---|---|
| Itraconazole | 0.500 | 0.5047 | 0.00321 | 0.64 | 100.93 | 0.0716 | 0.00319 | 0.9981 |
| 1.000 | 0.9803 | 0.01286 | 1.31 | 98.03 | ||||
| 51.495 | 54.2123 | 0.38126 | 0.70 | 105.28 | ||||
| 100.773 | 104.7237 | 1.45349 | 1.39 | 103.92 | ||||
| 181.083 | 182.6683 | 0.80404 | 0.44 | 100.88 | ||||
| 258.335 | 240.7577 | 0.80453 | 0.33 | 93.20 | ||||
| 337.823 | 343.6347 | 2.56547 | 0.75 | 101.72 | ||||
| 402.406 | 386.5090 | 4.75955 | 1.23 | 96.05 | ||||
| Hydroxy itraconazole | 1.00 | 0.987 | 0.0451 | 4.57 | 98.67 | 0.0193 | 0.00202 | 0.9967 |
| 2.00 | 2.060 | 0.1700 | 8.25 | 103.00 | ||||
| 74.80 | 74.407 | 6.4174 | 8.62 | 99.47 | ||||
| 149.60 | 144.790 | 1.4839 | 1.02 | 96.78 | ||||
| 269.06 | 266.763 | 4.5265 | 1.70 | 99.15 | ||||
| 392.08 | 377.490 | 8.2378 | 2.18 | 96.28 | ||||
| 509.70 | 507.647 | 2.7153 | 0.53 | 99.60 | ||||
| 597.92 | 640.857 | 14.2873 | 2.23 | 107.18 | ||||
Fig. 3.
Representative chromatograms of itraconazole (B1) and hydroxy itraconazole (B2) at concentrations of 0.500 ng/mL and 1.000 ng/mL with internal standard.
Fig. 4.
Representative chromatogram of extracted blank plasma of itraconazole (A1), hydroxy itraconazole (A2) and itraconazole D3.
3.2. Precision and accuracy
The inter-batch precision and accuracy were calculated after analyzing three precision and accuracy batches with spiked QC samples. The intra-batch precision and accuracy were calculated after analyzing six spiked samples of itraconazole and hydroxy itraconazole at each QC level (0.500, 1.425, 203.534 and 328.280 ng/mL of itraconazole and 1.000, 2.830, 297.960 and 480.580 ng/mL of hydroxy itraconazole). Intra-day and inter-day precision ranged from 1.11% to 3.53% and 1.74% to 4.23% for itraconazole, 2.15% to 7.25% and 2.44% to 7.98% for hydroxy itraconazole while accuracy, expressed as % nominal was within 90.51–98.48% and 91.41–98.06% for itraconazole, 97.00–105.65% and 92.22–100.09% for hydroxy itraconazole respectively, as given in Table 2.
Table 2.
Intraday and interday accuracy of the method for itraconazole and hydroxy itraconazole.
| Analyte | Level | Concentration added (ng/mL) | Inter-day (n=6) |
Intra-day (n=18) |
||||
|---|---|---|---|---|---|---|---|---|
| Mean conc. found (ng/mL) | Nominal (%) | CV (%) | Mean conc. found (ng/mL) | Nominal (%) | CV (%) | |||
| Itraconazole | LLOQQC | 0.500 | 0.4867 | 97.33 | 3.53 | 0.4903 | 98.06 | 4.23 |
| LQC | 1.425 | 1.4033 | 98.48 | 1.34 | 1.394 | 97.86 | 1.99 | |
| MQC | 203.534 | 198.9537 | 97.75 | 3.40 | 198.8356 | 97.69 | 2.89 | |
| HQC | 328.280 | 297.1117 | 90.51 | 1.11 | 300.0714 | 91.41 | 1.74 | |
| Hydroxy itraconazole | LLOQQC | 1.00 | 0.97 | 97.00 | 6.90 | 0.922 | 92.22 | 7.89 |
| LQC | 2.83 | 2.99 | 105.65 | 7.25 | 2.831 | 100.04 | 7.98 | |
| MQC | 297.96 | 303.173 | 101.75 | 7.01 | 298.219 | 100.09 | 4.85 | |
| HQC | 480.58 | 483.708 | 100.65 | 2.15 | 480.206 | 99.92 | 2.44 | |
3.3. Recovery
The absolute recoveries were evaluated for itraconazole, hydroxy itraconazole and itraconazole D3 by comparing the peak areas response of the extracted samples with that of the unextracted stock solutions at the three QC concentration levels of 1.425, 203.534 and 328.280 ng/mL of itraconazole and 2.830, 297.960 and 480.580 ng/mL of hydroxy itraconazole. The absolute recovery determination for itraconazole and hydroxy itraconazole was shown to be consistent, precise and reproducible. The mean recovery for itraconazole, hydroxy itraconazole and itraconazole D3 were found to be 100.045%, 100.021% and 99.495%, respectively.
3.4. Stability
The stability of the analytes in human plasma under different temperatures and times as well as stability of the analytes in stock solution under different conditions were evaluated. Itraconazole, hydroxy itraconazole and itraconazole D3 stock solutions were found stable in 8% formic acid in methanol at 2–8 °C for 9 days. For bench top stability determination, the required QC aliquots were withdrawn from deep freezer and kept at room temperature for around 10 h. The samples were then extracted and analyzed according to the above-mentioned extraction procedure. No degradation occurred after leaving the QC aliquots on the bench at room temperature for a period of 10 h. The required QC aliquots were subjected for three freeze–thaw cycles to access the freeze–thaw stability. The freeze–thaw stability was evaluated at the end of the third cycle by analyzing with the freshly spiked calibration curve (CC) and QC samples in pooled plasma. The % change between the stability QC samples and comparison QC samples was observed within acceptance limit. To determine the autosampler stability, one PA batch was processed and kept in the autosampler with a set temperature of 5 °C for 79 h and compared with a freshly prepared calibration curve and QC samples. No significant difference was observed between the concentrations of freshly spiked QC samples and stability QC samples. For dry extract stability six sets of LQC and HQC samples were processed and kept at −17 °C to −27 °C after drying in deep freezer for a period of 47 h and for wet extract stability another six sets of LQC and HQC samples were processed and after reconstitution stored at 2–8 °C in refrigerator for a period of 24 h. The dry extract and wet extract stabilities were evaluated by analyzing the stored QC samples with the freshly spiked CC and QC samples in pooled plasma. The % change between the stability QC samples and comparison QC samples was observed within acceptance limit. The long-term stability was evaluated for QC samples kept at −22 °C for 127 days and at −65 °C for a period of 306 days at two different concentrations (LQC and HQC) and was found stable in plasma for both the analytes for 127 days when stored at −22 °C and for 306 days at −65 °C in polypropylene tubes. The results of all stabilities are given in Table 3.
Table 3.
Stability data of ITR and OH-ITR in processed QC samples for different stability activities in different conditions (n=6).
| Stability experiment | Analyte | Concentration added (ng/mL) | Mean concentration found in stability samples (ng/mL) | Nominal (%) | CV (%) | Mean concentration found in comparison samples (ng/mL) | Nominal (%) | CV (%) | Change (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bench top stability (10 h) | ITR | 1.425 | 1.4380 | 100.91 | 2.46 | 1.3918 | 98.02 | 2.88 | −2.95 |
| 328.280 | 317.9462 | 96.85 | 1.24 | 310.5540 | 94.93 | 0.96 | −2.03 | ||
| OH-ITR | 2.83 | 3.003 | 106.12 | 2.64 | 3.0133 | 107.24 | 3.83 | 1.04 | |
| 480.58 | 488.862 | 101.72 | 3.07 | 485.6383 | 100.84 | 3.94 | −0.88 | ||
| Autosampler stability (79 h) | ITR | 1.425 | 1.3073 | 91.74 | 2.67 | ||||
| 328.280 | 316.5477 | 96.43 | 2.65 | ||||||
| OH-ITR | 2.83 | 2.7302 | 96.47 | 5.76 | |||||
| 480.58 | 498.9805 | 103.83 | 7.19 | ||||||
| Freeze–thaw stability (3-cycles) | ITR | 1.425 | 1.5810 | 110.95 | 10.33 | 1.6070 | 112.77 | 11.11 | 1.62 |
| 328.280 | 297.8808 | 90.74 | 0.85 | 297.9278 | 90.76 | 0.99 | 0.02 | ||
| OH-ITR | 2.83 | 2.6167 | 92.46 | 5.23 | 2.6683 | 95.30 | 4.13 | 2.98 | |
| 480.58 | 421.4617 | 87.70 | 9.24 | 438.7617 | 91.32 | 4.73 | 3.96 | ||
| Dry extract stability (47 h) | ITR | 1.425 | 1.5043 | 105.57 | 7.38 | 1.6070 | 112.77 | 11.11 | 6.39 |
| 328.280 | 302.5957 | 92.18 | 0.58 | 297.9278 | 90.76 | 0.99 | −1.56 | ||
| OH-ITR | 2.83 | 2.703 | 95.52 | 4.23 | 2.668 | 95.30 | 4.13 | −0.24 | |
| 480.58 | 452.395 | 94.14 | 2.54 | 438.762 | 91.32 | 4.73 | −3.09 | ||
| Wet extract stability (24 h) | ITR | 1.425 | 1.4008 | 98.30 | 3.44 | 1.3918 | 98.02 | 2.88 | −0.29 |
| 328.280 | 313.7125 | 95.56 | 0.92 | 310.5540 | 94.93 | 0.96 | −0.67 | ||
| OH-ITR | 2.83 | 2.943 | 104.00 | 3.59 | 3.013 | 107.24 | 3.83 | 3.01 | |
| 480.58 | 484.422 | 100.80 | 5.15 | 485.638 | 100.84 | 3.94 | 0.04 | ||
| Long term stability at −65±10 °C (306 days) | ITR | 1.425 | 1.3792 | 99.87 | 3.24 | 1.4377 | 104.18 | 5.35 | 4.14 |
| 328.280 | 343.0008 | 103.67 | 2.71 | 357.3060 | 108.09 | 2.66 | 4.09 | ||
| OH-ITR | 2.83 | 2.8380 | 102.45 | 4.89 | 2.8740 | 103.94 | 6.26 | 1.43 | |
| 480.58 | 482.0488 | 97.93 | 3.07 | 486.3110 | 98.97 | 9.03 | 1.05 | ||
| Long term stability at −22±5 °C (127 days) | ITR | 1.425 | 1.3305 | 93.37 | 6.70 | 1.3197 | 92.67 | 6.15 | −0.75 |
| 328.280 | 305.4362 | 93.04 | 4.42 | 293.9437 | 89.59 | 2.97 | −3.85 | ||
| OH-ITR | 2.83 | 2.820 | 99.65 | 5.13 | 2.827 | 99.88 | 3.92 | 0.24 | |
| 480.58 | 446.580 | 92.93 | 2.89 | 434.613 | 90.66 | 1.74 | −2.49 | ||
3.5. Matrix effect
Matrix effect was determined by IS normalization matrix factor [26], [27] at LQC and HQC concentration levels by using six different plasma lots those passed the selectivity criteria. Blank plasma samples were processed in duplicate from all plasma lots and after drying these blank plasma samples were reconstituted in respective prepared neat sample of LQC and HQC concentrations. The percentage coefficient of variance of both the analytes and internal standard were within the acceptance range of ≤15%. Table 4 shows the data of the results for matrix factor.
Table 4.
Matrix effect evaluation for itraconazole and hydroxy itraconazole.
| Matrix factor |
||
|---|---|---|
| Analyte | LQC | HQC |
| Itraconazole | ||
| Mean | 1.033 | 0.973 |
| S.D.± | 0.064 | 0.065 |
| CV (%) | 6.20 | 6.68 |
| Matrix effect (%) | −3.30 | 2.72 |
| Hydroxy itraconazole | ||
| Mean | 1.119 | 0.999 |
| S.D.± | 0.090 | 0.026 |
| CV (%) | 7.99 | 2.61 |
| Matrix effect (%) | −11.93 | 0.08 |
3.6. Application of the method
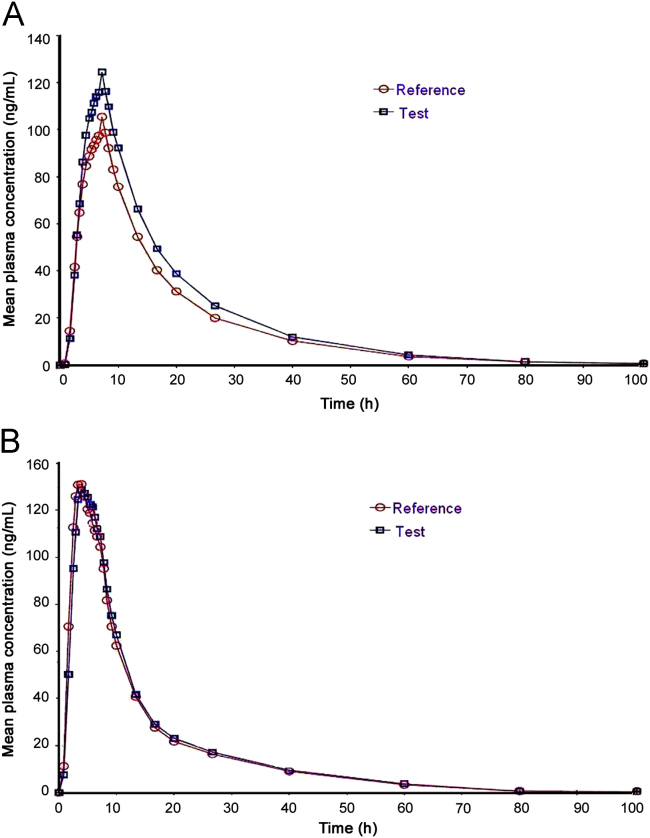
The developed and validated method was successfully applied for the analysis of plasma samples obtained from the pharmacokinetic study. The study was conducted as a randomized, open label, single-dose, two-treatment, two sequence, four-way replicate crossover in 60 Indian healthy, adult, male, human subjects under fed condition. Each subject received 100 mg capsule of itraconazole of test and reference. Blood samples were collected using K2EDTA vacutainers at the following times: pre-dose, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 8.0, 10.0, 12.0, 16.0, 24.00, 48.00, 72.00 and 96.0 h after administration. Pharmacokinetic parameters were calculated from the subjects who had successfully completed periods I and II of the study. Some of the main pharmacokinetic parameters are given in Table 5. The mean plasma concentration versus time profile is shown in Fig. 5.
Table 5.
Pharmacokinetics parameter.
| Analyte | Drug | Statistics | Cmax (ng/mL) | AUC0-t (ng/mL h) | AUC0-inf (ng/mL h) |
|---|---|---|---|---|---|
| Itraconazole | Test (T) | Mean | 116.076 | 1522.932 | 1613.547 |
| RSD% | 62.0924 | 638.2451 | 688.1134 | ||
| Reference (R) | Mean | 128.940 | 1662.532 | 1756.137 | |
| RSD% | 61.1698 | 684.1797 | 719.9309 | ||
| 90% Confidence interval for the ratio of the means T/R (%) | 78.540–97.380 | 82.597–100.300 | 82.734–100.432 | ||
| Hydroxy itraconazole | Test (T) | Mean | 159.303 | 3343.322 | 3398.416 |
| RSD% | 60.6879 | 1549.1508 | 1549.0455 | ||
| Reference (R) | Mean | 175.738 | 3660.083 | 3757.739 | |
| RSD% | 63.0287 | 1559.1787 | 1544.6742 | ||
| 90% Confidence interval for the ratio of the means T/R (%) | 81.383–96.583 | 79.051–99.268 | 78.372–97.669 | ||
Fig. 5.
Mean plasma concentration versus time profile of itraconazole (A) and hydroxy itraconazole (B).
4. Conclusions
A highly sensitive and selective method for the simultaneous determination of itraconazole and hydroxy itraconazole in human plasma was developed using UPLC–MS/MS with turbo ion spray in positive ion mode for the very first time with a total analysis run time of 2.0 min. This developed method was successfully used in a pharmacokinetic study in which 60 healthy male volunteers were given a 100 mg capsule of itraconazole in fed condition. The use of cost effective, time saving and easy to handle liquid–liquid extraction method made it possible to achieve the sensitivity for both the analytes. By the use of UPLC a shorter chromatographic run time of 2.0 min was achieved in this method and a large number of subject samples were easily analyzed on one system in a single day. Only one analytical column was used to chromatograph about 6000 subject samples analysis. This validated method is an excellent analytical option for the simultaneous, rapid quantification of itraconazole and hydroxy itraconazole in human plasma.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Beule K.D., Gestel J.V. Pharmacology of itraconazole. Drugs. 2001;61:27–37. doi: 10.2165/00003495-200161001-00003. [DOI] [PubMed] [Google Scholar]
- 2.Florea N.R., Capitano B., Nightingale C.H. Beneficial pharmacokinetic interaction between cyclosporine and itraconazole in renal transplant recipients. Transplant. Proc. 2003;35:2873–2877. doi: 10.1016/j.transproceed.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 3.Conway S.P., Etherington C., Peckham D.G. Pharmacokinetics and safety of itraconazole in patients with cystic fibrosis. J. Antimicrob. Chemother. 2004;53:841–847. doi: 10.1093/jac/dkh175. [DOI] [PubMed] [Google Scholar]
- 4.Janssen Pharmaceutica Products LP.Sporanox® (Itraconazole) Injection, US Food and Drug Administration, Washington, DC, 2002. Available from: 〈http://www.fda.gov/cder/foi/label/2002/20966s6lbl.pdf〉 (accessed 01.05.06).
- 5.Kramer M.R., Marshall S.E., Denning D.W. Cyclosporine and itraconazole interaction in heart and lung transplant recipients. Ann. Intern. Med. 1990;113:327–329. doi: 10.7326/0003-4819-113-4-327. [DOI] [PubMed] [Google Scholar]
- 6.Beule K.D., Van G. Pharmacology of itraconazole. Drugs. 2001;61:27–37. doi: 10.2165/00003495-200161001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ng T.K.C., Chan R.C.Y., Adeyemi-Doro F.A.B. Rapid high performance liquid chromatographic assay for antifungal agents in human serum. J. Antimicrob. Chemother. 1996;37:465–472. doi: 10.1093/jac/37.3.465. [DOI] [PubMed] [Google Scholar]
- 8.L. Chan, J.M. Potter, V. Miranda, et al., A simple high-performance liquid chromatography assay for quantitation of itraconazole and hydroxyitraconazole in serum, in: 8th International Congress of Therapeutic Itraconazole Monitoring and Clinical Toxicology, Basel, 2003.
- 9.Compas D., Touw D.J., Goede P. Rapid method for the analysis of itraconazole and hydroxyitraconazole in serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1996;687:453–456. doi: 10.1016/s0378-4347(96)00245-9. [DOI] [PubMed] [Google Scholar]
- 10.Gubbins P., Gurley B., Bowman J. Rapid and sensitive high performance liquid chromatographic method for the determination of itraconazole and its hydroxy-metabolite in human. J. Pharm. Biomed. Anal. 1998;16:1005–1012. doi: 10.1016/s0731-7085(97)00062-9. [DOI] [PubMed] [Google Scholar]
- 11.Badock N.R. Micro-scale method for itraconazole in plasma by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1990;525:478–483. doi: 10.1016/s0378-4347(00)83427-1. [DOI] [PubMed] [Google Scholar]
- 12.Brandsteterova E., Kubalec P., Rady A. Determination of itraconazole and its metabolite in plasma using SPE–HPLC. Pharmazie. 1995;50:597–599. [PubMed] [Google Scholar]
- 13.Koks C., Sparidans R., Lucassen G. Selective high-performance liquid chromatographic assay for itraconazole and hydroxyitraconazole in plasma from human immunodeficiency virus-infected patients, J. Chromatogr. B Biomed. Appl. 2002;767:103–110. doi: 10.1016/s0378-4347(01)00550-3. [DOI] [PubMed] [Google Scholar]
- 14.Poirier J.M., Cheymol G. A rapid and specific liquid chromatographic assay for the determination of itraconazole and hydroxyitraconazole in plasma. Ther. Drug. Monit. 1997;19:247–248. doi: 10.1097/00007691-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Poirier J.M., Lebot M., Descamps P. Determination of itraconazole and its active metabolite in plasma by column liquid chromatography. Ther. Drug. Monit. 1994;16:596–601. doi: 10.1097/00007691-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Cox S., Orosz S., Burnette J. Microassay for determination of itraconazole and hydroxyitraconazole in plasma and tissue biopsies. J. Chromatogr. B Biomed. Appl. 1997;702:175–180. doi: 10.1016/s0378-4347(97)00378-2. [DOI] [PubMed] [Google Scholar]
- 17.Redmann S., Charles B.G. A rapid HPLC method with fluorometric detection for determination of plasma itraconazole and hydroxy-itraconazole concentrations in cystic fibrosis children with allergic bronchopulmonary aspergillosis. Biomed. Chromatogr. 2006;20:343–348. doi: 10.1002/bmc.569. [DOI] [PubMed] [Google Scholar]
- 18.Al-Rawithi S., Hussein R., Al-Moshen I. This expedient and rugged method is being used to monitor therapeutic levels in bone marrow transplant recipients who are taking the drug for prophylaxis. Ther. Drug Monit. 2001;23:445–448. doi: 10.1097/00007691-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Vijaya Bharathi D., Hothvidya Sagar P.V., Kumar S.S. Development and validation of a highly sensitive and robust LC–MS/MS with electrospray ionization method for simultaneous quantitation of itraconazole and hydroxyitraconazole in human plasma: application to a bioequivalence study. J. Chromatogr. B Biomed. Appl. 2008;868:70–76. doi: 10.1016/j.jchromb.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Kousoulos C., Tsatsou G., Apostolou C. Development of a high-throughput method for the determination of itraconazole and its hydroxy metabolite in human plasma, employing automated liquid–liquid extraction based on 96-well format plates and LC/MS/MS. Anal. Bioanal. Chem. 2006;384:199–207. doi: 10.1007/s00216-005-0159-6. [DOI] [PubMed] [Google Scholar]
- 21.Yao M., Chen L., Srinivas N.R. Quantitation of itraconazole in rat heparinized plasma by liquid chromatography–mass spectrometry. J. Chromatogr. B Biomed. Appl. 2001;752:9–16. doi: 10.1016/s0378-4347(00)00505-3. [DOI] [PubMed] [Google Scholar]
- 22.Vogeser M., Spohrer U., Schiel X. Determination of itraconazole and hydroxyitraconazole in plasma by use of liquid chromatography–tandem mass spectrometry with on-line solid-phase extraction. Clin. Chem. Lab. Med. 2003;41:915–920. doi: 10.1515/CCLM.2003.139. [DOI] [PubMed] [Google Scholar]
- 23.Carrier A., Parent J. Liquid chromatographic–mass spectrometric determination of itraconazole and its major metabolite, hydroxyitraconazole, in dog plasma. J. Chromatogr. B Biomed. Appl. 2000;745:413–420. doi: 10.1016/s0378-4347(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 24.Guidance for industry, Bioanalytical Method Validation, US Department of Health and Human Service, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), 2001.
- 25.Zhang J.F., Sha C.J., Sun Y. Ultra-high-performance liquid chromatography for the determination of exenatide in monkey plasma by tandem quadrupole mass spectrometry. J. Pharm. Anal. 2013;3:235–240. doi: 10.1016/j.jpha.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isobe T., Shirashi H., Yasuda M. Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2003;984:195–202. doi: 10.1016/s0021-9673(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 27.B. Singh, R.S. Lokhandae, A.D wivedi, etal., Improved simultaneous quantitation of candesartan and hydrochlorthiazide in human plasma by UPLC–MS/MS and its application in bioequivalence studies, J. Pharm. Anal. 10.1016/j.jpha.2013.05.003, in press [DOI] [PMC free article] [PubMed]