Abstract
A novel method for analysis of three active components curcumin, demethoxycurcumin and bisdemethoxycurcumin in Curcuma longa L. was developed by HPLC coupled with electrochemical detection. Three curcuminoids were well separated on a C18 column and detected with high sensitivity. A mobile phase containing acetonitrile and 10 mM Na2HPO4–H3PO4 (pH 5.0) (50:50, v/v) was used. Good linearity was obtained in the range of 0.208–41.6, 0.197–39.4, and 0.227–114 μM for curcumin, demethoxycurcumin and bisdemethoxycurcumin respectively. The limit of detection reached up to 10−8 M, which was lower than that by UV detection. The relative standard deviations (RSDs) ranged from 1.06% to 1.88% for intra-day precision and from 4.30% to 5.79% for inter-day precision, respectively. The proposed method has been applied in real herb sample and recoveries ranging from 86.3% to 111% were obtained.
Keywords: Curcumin, High performance liquid chromatography, Electrochemical detection, Curcuma longa L.
1. Introduction
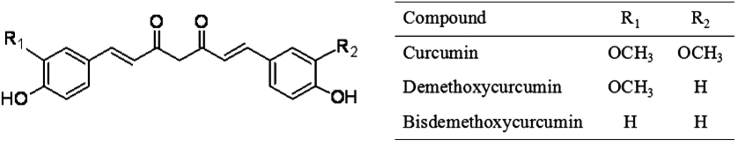
Curcuma longa L. is a representative of traditional Chinese medicine and widely used as carminative, laxative, anthelmintic and a cure for liver ailment. It can also be used as spice and approved colorants in food [1]. The main active components include curcuminoids, monoterpenoids and sesquiterpenoids [2]. Curcumin, demethoxycurcumin and bisdemethoxycurcumin belong to curcuminoids as the pharmacologically significant compounds and their chemical structures are shown in Fig. 1. These three curcuminoids have been proved to show strong anti-oxidant [3], anti-inflammatory [4], anti-bacterial [5], and anti-carcinogenic activities [6], [7].
Fig. 1.
Structures of the three curcuminoids.
Among them, curcumin has been found to exert the best inhibitory effects for various cancers by preventing the activation and proliferation of carcinogen and inducting apoptosis of the tumor cells [8]. Moreover, it can enhance the effect of existing chemotherapeutic drugs as well. In recent years, several reports have revealed the potential therapeutic activities against breast cancer through multiple signaling pathways [9], [10], [11]. Ide and Killian et al. have investigated its activity on prostate cancer [12], [13], which is the second leading cause of death in men and the research results proved to be positive. Some researchers have concentrated on lung cancer [14], [15], liver cancer [16], skin cancer [17], oral cancer [18] and obesity-related cancers [19]. Rencently, curcumin has been discovered to demonstrate beneficial properties against diabetes and related chronic metabolic diseases [20] and attenuates diabetic neuropathic pain [21]. It also has renoprotective effects on diabetic nephropathy, which is associated with its inhibitory effects on the sphingosine kinase 1-sphingosine 1-phosphate (SphK1-S1P) signaling pathway [22]. Moreover, curcumin may interfere with the replication of human immuno-deficiency virus (HIV) and as a promising anti-HIV agent [23]. Its potential applications in clinical treatment attract great interest from researchers, which calls for effective methods of quality control. However, the main problem existing in the analysis of pharmacologically active components in herbal medicines is low content and the complexity of the matrix. Therefore, it is essential to develop a method of high sensitivity and selectivity.
The main methods for the quantitative determination of curcumin include UV spectrophotometry, HPLC [24], [25], [26], [27] and capillary electrophoresis (CE) [28]. HPLC is known for its high efficiency and rapid separation. HPLC–UV is the most widely used method without considering its limited sensitivity and low selectivity. HPLC–MS is very sensitive and has advantages over other methods in obtaining structure information of analytes. However, it requires nebulization and vaporization steps, ionization of sample, and the expensive instruments, which makes it unavailable in many laboratories. Therefore, a simple method with high sensitivity and low cost is still needed for the determination of these curcuminoids.
Electrochemical detection (ECD) is a promising analytical method as it is sensitive, economical and easy to be miniaturized. It has been widely applied in the fields of pharmaceutical analysis and food quality control. The ECD method has also been reported for curcuminoid detection [29]. HPLC coupled with ECD is a highly sensitive and selective technique for the determination of redox substances. In recent years, our group has made great progress in the development of HPLC–ECD for analysis of active components in the traditional Chinese medicine [30], [31], [32], [33]. The achievement in sensitivity and selectivity for analysis of electro-active components in Chinese medicines by LC–ECD encourages us to further develop other new methods for the analysis of Chinese medicine.
In this work, we aimed to develop a novel method for analysis of curcuminoids by HPLC coupled with electrochemical detection, which integrates the good separation efficiency of HPLC and high sensitivity of electrochemical detection. Parameters that affected separation and chemical detection were investigated and the method has been successfully applied to analysis of curcumin, demethoxycurcumin and bisdemethoxycurcumin in C. longa L.
2. Experimental
2.1. Chemicals and reagents
Standards of curcumin, demethoxycurcumin and bisdemethoxycurcumin were purchased from Chengdu Herbpurify Co., Ltd. (HPLC grade, ≥98%, Chengdu, China). C. longa L. was purchased from local pharmacy. Acetonitrile (HPLC grade) was obtained from Tedia (OH, USA). Sodium dihydrogen phosphate and disodium hydrogen phosphate were supplied by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All the other chemicals were of analytical grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Instrumentation
The HPLC system consisted of an LC-20AD from Shimadzu (Kyoto, Japan) and a C18 column (Wondasil™, 4.6 mm×250 mm, 5 μm, GL Sciences Inc., Made in Japan). The CHI842B electrochemical workstation was supplied by CH Instrument Company (Shanghai, China). The electrochemical detection cell was composed of a three-electrode system: a 3 mm diameter glassy carbon electrode, an Ag/AgCl reference electrode and a stainless steel tube used as the counter electrode which also served as the tube for the outlet.
2.3. Chromatography and detection conditions
The mobile phase was composed of acetonitrile and 10 mM Na2HPO4–H3PO4 (pH 5.0) with a proportion of 50:50 (v/v). The flow rate was 1.0 mL/min and the injection volume was 20 μL. The mobile phase was filtered through a 0.22 μm membrane filter before use. The column temperature was maintained at 40 °C. The detection potential was set at 0.9 V.
2.4. Solution preparations
The stock solutions were prepared with acetonitrile and stored in a refrigerator. The concentration of curcumin, demethoxycurcumin and bisdemethoxycurcumin was 0.23 mg/mL, 0.20 mg/mL, and 0.21 mg/mL, respectively. The mixed stock solution of these three curcuminoids was diluted for about 100 times, after which the concentrations of curcumin, demethoxycurcumin and bisdemethoxycurcumin were 2.08 μM, 1.97 μM and 2.27 μM, respectively. This solution was used for the method validation, as well as comparison of the selectivity.
2.5. Pretreatment of C. longa L. sample
The C. longa L. sample was ground into powder and then 0.1 g sample was weighed into a 10 mL centrifugation tube. The sample was soaked and extracted with 5 mL methanol in an ultrasonic apparatus for 30 min. After centrifugation at 10,000 rpm for 15 min, the supernatant liquid was filtered through a 0.22 μm membrane filter and used for subsequent chromatography analysis.
3. Results and discussion
3.1. Electrochemical behavior of curcumin
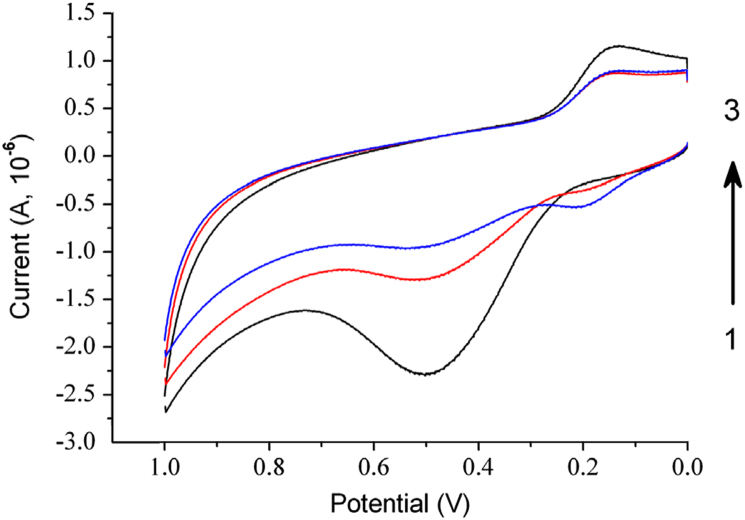
As it is not easy to predict whether a molecule has good electrochemical activity just by the chemical structure or functional group, research on electroactivity of curcumin was performed. Fig. 2 shows the electrochemical behavior of curcumin. Three consecutive cycles of cyclic voltammetry scanning were conducted; the current of the oxidation peak near 0.5 V and reduction peak near 0.15 V decreased gradually, indicating that the reaction products might be absorbed on the surface of the glassy carbon electrode, thus hindering the electron transfer of redox reaction on the electrode surface. As for the oxidation peak near 0.2 V, the response current became higher with increased scan cycles, probably it was because the products were further oxidized.
Fig. 2.
Cyclic voltammograms for curcumin. 0.1 M KH2PO4–Na2HPO4 (pH 7.0).
3.2. Effect of pH
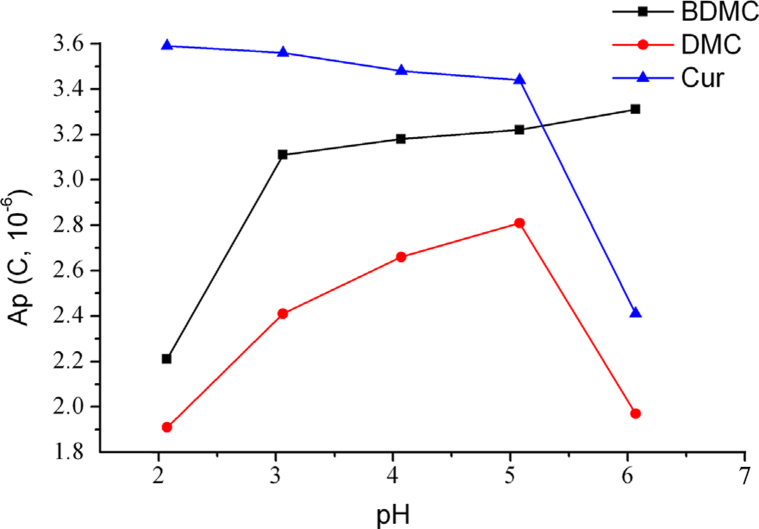
The acetic system aroused large background interference and led to low ratio of signal to noise, while the inorganic phosphate solutions system as the most frequently used buffer could well solve the problem. The effect of pH value was investigated since it could influence ionization and charging of the analyte, and further affect the retention on the sorbent or stationary phase [34], [35]. The pH of the phosphate buffer was adjusted by H3PO4 to 8.0, 7.0, 6.0, 5.0, 4.0, 3.0, and 2.0. It was found that the resolution of three compounds was greatly influenced by pH. When pH values were 8.0 and 7.0, baseline separation was not achieved. A slightly acidic condition could improve separation and the shape of peaks, for the reason that three curcuminoids belong to polyphenols and the ionization of phenolic hydroxyl functionalities was inhibited when the buffer was acidic, thus enhancing their retention on the column. The effects on signal are shown in Fig. 3. Under the condition of pH 5.0, demethoxycurcumin obtained the maximum peak area, while curcumin and bisdemethoxycurcumin also got good responses. Therefore, pH 5.0 was used for further study.
Fig. 3.
Effect of pH on the electrochemical responses of three curcuminoids.
3.3. Effect of buffer concentration
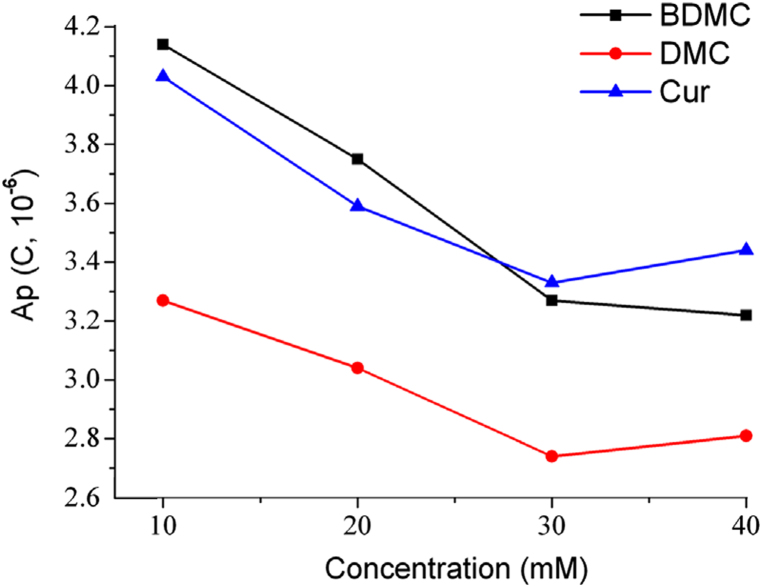
In electrochemical analysis, salts are often added in order to increase ionic activity, reduce the resistance of the solution and accelerate the electron transfer rate, which results in improving the sensitivity of electrochemical detection to a certain extent. As can be seen in Fig. 4, the concentration of phosphate did not influence the peak area a lot. When the buffer concentration was increased from 10 mM to 40 mM, the electrochemical response of these three curcuminoids decreased instead. The results showed that the highest sensitivity was achieved when 10 mM phosphate buffer was used.
Fig. 4.
Effect of buffer concentration on the electrochemical responses of three curcuminoids.
3.4. Effect of detection potential
Compounds with electrochemical activity need a certain potential to conduct the redox reaction in the process of electrochemical analysis. If the potential is too low, the analyte would not be completely oxidized or reduced, thus resulting in low sensitivity. When the potential increases, the detection signal enhances; however, high potential could lead to large background noise and cause the loss of signal to noise ratio and low selectivity. What is more, the electrode would also be damaged at a high potential. As a result, an appropriate detection potential is needed to ensure good selectivity and sensitivity.
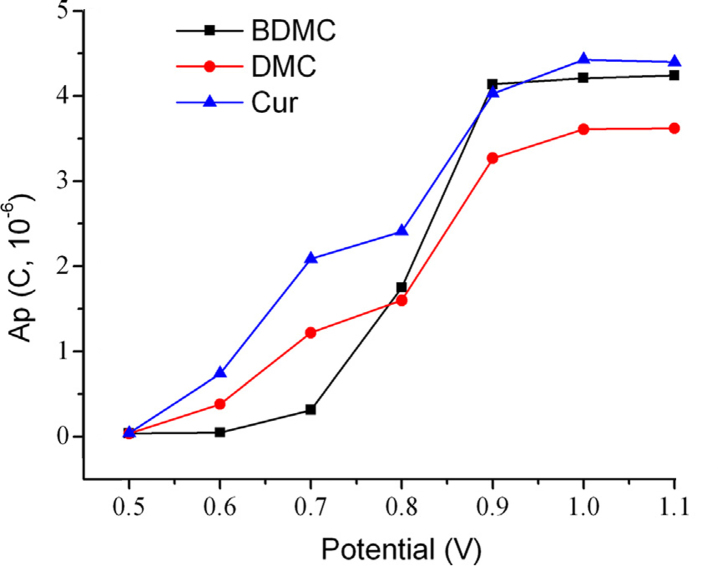
To determine the optimum potential, the relationship of applied working electrode potential between responses of the analytes was investigated by applying a series of detection potential of 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, and 1.1 V. The relationship of peak areas with detection potential is shown in Fig. 5. The response of all these three curcuminoids increased along with the potential and reached a platform when the potential was 0.9 V. Therefore, 0.9 V was selected as the detection potential.
Fig. 5.
Effect of detection potential on the electrochemical responses of three curcuminoids.
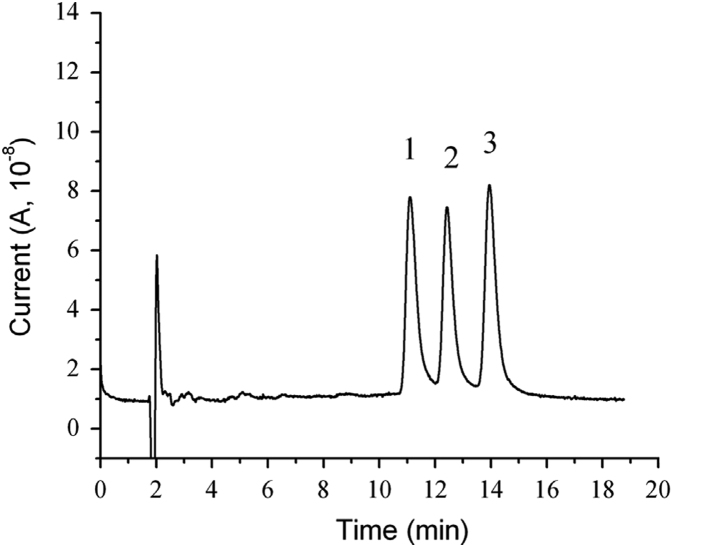
Analysis of these three curcuminoids standards was made (Fig. 6) and the baseline separation of these three polyphenolic compounds was completed within 15 min with resolutions larger than 2.0.
Fig. 6.
Chromatogram of standards. Peak identification: 1: bisdemethoxycurcumin, 2: demethoxycurcumin, and 3: curcumin.
3.5. Method validation
In order to make sure that the method developed was feasible, method validation including linearity, limit of detection, precision and recovery was studied.
Stock solutions were diluted with acetonitrile to obtain the standard solutions with different dilutions for calibration purposes. The subsequent experiments all conducted under the optimum conditions. The results are listed in Table 1. Regression analysis of the data showed a linear relationship with excellent correlation coefficients of curcumin, demethoxycurcumin and bisdemethoxycurcumin of 0.9985, 0.9993 and 0.9999, respectively. The limits of detection (LODs) were determined, and electrochemical detection exhibited better sensitivity than that by UV detection, which was in accordance with the results reported previously [36].
Table 1.
Linearity and limit of detection.
| Compound | Linear range (μM) | Equation | r | LOQ (μM) | LOD (μM) |
|---|---|---|---|---|---|
| Curcumin | 0.208–41.6 | Ap=2.46E−7+0.187c | 0.9985 | 0.208 | 0.0416 |
| Demethoxycurcumin | 0.197–39.4 | Ap=1.53E−7+0.170c | 0.9993 | 0.197 | 0.0394 |
| Bisdemethoxycurcumin | 0.227–114 | Ap=8.51E−8+0.175c | 0.9999 | 0.227 | 0.0494 |
The repeatability experiment was conducted by triple repetitive analysis of the standards per day for three continuous days. As listed in Table 2, the intra-day RSD values of these three curcuminoids were lower than 1.88% and the inter-day RSD values were lower than 5.79%, which suggested a good reproducibility of the method.
Table 2.
The intra-day and inter-day precisions of three curcuminoids.
| Compound | Precision (RSD%) |
|
|---|---|---|
| Intra-daya | Inter-dayb | |
| Curcumin | 1.06 | 5.79 |
| Demethoxycurcumin | 1.87 | 4.30 |
| Bisdemethoxycurcumin | 1.88 | 5.77 |
n=6.
Triple injections for 3 days.
3.6. Determination of three curcuminoids in C. longa L.
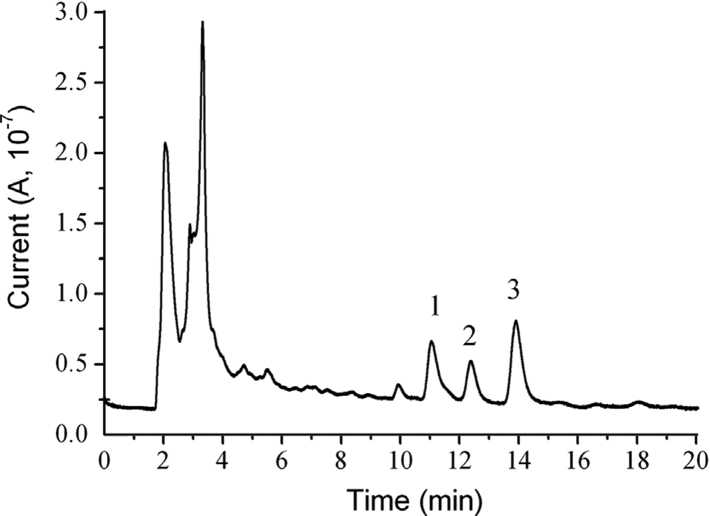
The C. longa L. was extracted by the ultrasonication method [37]. Three main active compounds (curcumin, demethoxycurcumin and bisdemethoxycurcumin) and other coexisting compounds in C. longa L. were completely separated with a running time of 15 min under the optimum conditions described above. As shown in Fig. 7, peaks for curcumin, demethoxycurcumin and bisdemethoxycurcumin were observed and identified in the chromatogram. From the obtained peak areas, the contents of curcumin, demethoxycurcumin and bisdemethoxycurcumin were found to be 0.275, 0.118, and 0.200 mg/g, respectively (n=3).
Fig. 7.
Chromatogram of Curcuma longa L. extract. Peak identification: 1: bisdemethoxycurcumin, 2: demethoxycurcumin, and 3: curcumin.
Recovery experiment was performed in the C. longa L. extract by the method of standard addition, and calculated by the equation
As listed in Table 3, recoveries in the range of 86.3–111% and RSD values below 10% were obtained, indicating that the proposed method is accurate for simutaneous determination of curcumin, demethoxycurcumin and bisdemethoxycurcumin in real herb samples.
Table 3.
The recoveries of three curcuminoids standards in Curcuma longa L. extract (n=3).
| Compound | Added (μM) | Found (μM) | Recovery (%) | RSD (%, n=3) |
|---|---|---|---|---|
| Curcumin | 2.97 | 3.30 | 111.0 | 4.44 |
| 5.20 | 5.07 | 97.4 | 7.94 | |
| Demethoxycurcumin | 2.81 | 2.43 | 86.3 | 4.16 |
| 4.93 | 4.66 | 94.4 | 2.06 | |
| Bisdemethoxycurcumin | 3.24 | 3.42 | 105.0 | 6.85 |
| 5.68 | 5.59 | 98.3 | 9.73 |
4. Conclusion
A novel method has been developed for simultaneous determination of curcumin, demethoxycurcumin and bisdemethoxycurcumin by HPLC coupled with electrochemical detection with high sensitivity. Curcuminoids could be well separated and detected within 15 min. Good selectivity and sensitivity were achived by the electrochemical detection. This method also showed wide linear range, good linearity and precision. The proposed method is simple, rapid and exhibits better sensitivity by comparing with the commonly used HPLC–UV method. Application in C. longa L. also showed good analytical performance of the method, and the proposed method has the potential of being used for qualitative analysis in real herb and medical samples.
Acknowledgments
This work was supported by the National Scientific Foundation of China (Grant nos. 21375101, 90817103, and 30973672), Doctroral Fund of Ministry of Education of China (No. 20110141110024), Innovation Seed Fund and Translational Medical Research Fund of Wuhan University School of Medicine.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
References
- 1.Srimal R.C. Turmeric: a brief review of medicinal properties. Fitoterapia. 1997;68:483–493. [Google Scholar]
- 2.Li R., Xiang C., Zhang X. Chemical analysis of the Chinese herbal medicine turmeric (Curcuma longa L.) Curr. Pharm. Anal. 2010;6:256–268. [Google Scholar]
- 3.Choi H.Y. Antioxidant activity of Curcuma longa L., novel foodstuff. Mol. Cell. Toxicol. 2009;5:237–242. [Google Scholar]
- 4.Lantz R.C., Chen G.J., Solyom A.M. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 2005;12:445–452. doi: 10.1016/j.phymed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Park B.S., Kim J.G., Kim M.R. Curcuma longa L. constituents inhibit sortase A and staphylococcus aureus cell adhesion to fibronectin. J. Agr. Food Chem. 2005;53:9005–9009. doi: 10.1021/jf051765z. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008;52:S103–S127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 7.Das L., Vinayak M. Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-kappa B signalling in lymphoma-bearing mice. Biosci. Rep. 2012;32:161–170. doi: 10.1042/BSR20110043. [DOI] [PubMed] [Google Scholar]
- 8.Basnet P., Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M., Huang O., Zhang X. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules. 2013;18(1):701–720. doi: 10.3390/molecules18010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha D., Biswas J., Sung B. Chemopreventive and chemotherapeutic potential of curcumin in breast cancer. Curr. Drug Targets. 2012;13:1799–1819. doi: 10.2174/138945012804545632. [DOI] [PubMed] [Google Scholar]
- 11.Huang T., Chen Z.J., Fang L.P. Curcumin inhibits LPS-induced EMT through downregulation of NF-kappa B-Snail signaling in breast cancer cells. Oncol. Rep. 2013;29:117–124. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- 12.Ide H. Prostate cancer and curcumin: current application and future prospect. Int. J. Urol. 2012;191:SI59. [Google Scholar]
- 13.Killian P.H., Kronski E., Michalik K.M. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis. 2012;33:2507–2519. doi: 10.1093/carcin/bgs312. [DOI] [PubMed] [Google Scholar]
- 14.Alexandrow M.G., Song L.J., Altiok S. Curcumin: a novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur. J. Cancer Prev. 2012;21:407–412. doi: 10.1097/CEJ.0b013e32834ef194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y., Zhang J.J., Han J. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of alpha 1-antitrypsin in lung cancer. Mol. Oncol. 2012;6:405–417. doi: 10.1016/j.molonc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darvesh A.S., Aggarwal B.B., Bishayee A. Curcumin and liver cancer: a review. Curr. Pharm. Biotechnol. 2012;13:218–228. doi: 10.2174/138920112798868791. [DOI] [PubMed] [Google Scholar]
- 17.Tsai K.D., Lin J.C., Yang S.M. Curcumin protects against UVB-induced skin cancers in SKH-1 hairless mouse: analysis of early molecular markers in carcinogenesis. Evid. Based Complement. Altern. Med. 2012:593952. doi: 10.1155/2012/593952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlotogorski A., Dayan A., Dayan D. Nutraceuticals as new treatment approaches for oral cancer—I: curcumin. Oral Oncol. 2013;49:187–191. doi: 10.1016/j.oraloncology.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Shehzad A., Khan S., Lee Y.S. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol. 2012;8:490. doi: 10.2217/fon.11.145. [DOI] [PubMed] [Google Scholar]
- 20.Meng B., Li J., Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013;19:2101–2113. [PubMed] [Google Scholar]
- 21.Li Y., Zhang Y., Liu D.B. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-alpha in a rat model. Int. J. Med. Sci. 2013;10:377–381. doi: 10.7150/ijms.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J., Huang K.P., Lan T. Curcumin ameliorates diabetic nephropathy by inhibiting the activation of the SphK1-S1P signaling pathway. Mol. Cell. Endocrinol. 2013;365:231–240. doi: 10.1016/j.mce.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Hatcher H., Planalp R., Cho J. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lui S.Q., Liu Z.H., Huang J.N. Simultaneous determination of three main components in Curcuma longa L. by reversed-phase high performance liquid chromatography. Chin. J. Anal. Chem. 2005;33:309–312. [Google Scholar]
- 25.Jayaprakasha G.K., Rao L., Sakariah K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agr. Food Chem. 2002;50:3668–3672. doi: 10.1021/jf025506a. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez M., Gallego M., Valcarcel M. Determination of natural and synthetic colorants in prescreened dairy samples using liquid chromatography-diode array detection. Anal. Chem. 2003;75:685–693. doi: 10.1021/ac020468f. [DOI] [PubMed] [Google Scholar]
- 27.Koop H.S., de Freitas R.A., de Souza L.M. Development and validation of a RP-HPLC–PDA method for determination of curcuminoids in microemulsions. Chromatographia. 2013;76:15–16. [Google Scholar]
- 28.Lechtenberg M., Quandt B., Nahrstedt A. Quantitative determination of curcuminoids in curcuma rhizomes and rapid differentiation of Curcuma domestica Val. and Curcuma xanthorrhiza Roxb. by capillary electrophoresis. Phytochem. Anal. 2004;15:152–158. doi: 10.1002/pca.759. [DOI] [PubMed] [Google Scholar]
- 29.Wray D.M., Batchelor-McAuley C., Compton R.G. Selective curcuminoid separation and detection via nickel complexation and adsorptive stripping voltammetry. Electroanalysis. 2012;24:2244–2248. [Google Scholar]
- 30.Liu L., Chen Z. Analysis of four alkaloids of Coptis chinensis in rat plasma by high performance liquid chromatography with electrochemical detection. Anal. Chim. Acta. 2012;737:99–104. doi: 10.1016/j.aca.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 31.Liu L., Li S., Chen Z. Simultaneous determination of tetrandrine and fangchinoline in herbal medicine Stephania tetrandra S. Moore by liquid chromatography with electrochemical detection. J. Pharma. Biomed. Anal. 2012;61:251–255. doi: 10.1016/j.jpba.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Wang F., Chen Z. Determination of bavachin and isobavachalcone in Fructus psoraleae by high-performance liquid chromatography with electrochemical detection. J. Sep. Sci. 2011;34:514–519. doi: 10.1002/jssc.201000801. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Chen Y., Zhang Y. Determination of tryptophan and kynurenine in human plasma by liquid chromatography-electrochemical detection with multi-wall carbon nanotube-modified glassy carbon electrode. Biomed. Chromatogr. 2011;25:938–942. doi: 10.1002/bmc.1550. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Chen Z. Preparation of micropipette tip-based molecularly imprinted monolith for selective micro-solid phase extraction of berberine in plasma and urine samples. Talanta. 2013;103:103–109. doi: 10.1016/j.talanta.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W., Chen Z. Mussel inspired polydopamine functionalized poly(ether ether ketone) tube for online solid-phase microextraction-high performance liquid chromatography and its application in analysis of protoberberine alkaloids in rat plasma. J. Chromatogr. A. 2013;1278:29–36. doi: 10.1016/j.chroma.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q., Li Y., Chen Z. Separation, identification, and quantification of active constituents in Fructus psoraleae by high-performance liquid chromatography with UV, ion trap mass spectrometry, and electrochemical detection. J. Pharmaceut. Anal. 2012;2:143–151. doi: 10.1016/j.jpha.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Y.P. Cao, J.S. Xiao, D. Zhang, Ultrasound-assisted extraction of curcuminoids from turmeric (Curcuma longa L.), in: Proceedings of the 2nd Conference on Horticulture Science and Technology, Beijing, China, 2010. pp. 189–195.