Abstract
A simple, rapid and sensitive method termed as magnetic solid phase extraction (MSPE) combined with high-performance liquid chromatography-ultraviolet detector (HPLC-UV) has been proposed for the determination of trace amounts of chlorpromazine (CPZ) in water, urine and plasma samples. The separation and determination was performed on a C18 column under the optimal chromatographic conditions. Several factors influencing the extraction efficiency of CPZ, such as pH, surfactant and adsorbent amounts, ionic strength, extraction time, sample volume and desorption conditions, were studied and optimized. Under the optimal MSPE conditions, the extraction percentage of CPZ was 74%, 27% and 16% in water, urine and plasma samples, respectively. The limits of detection (LODs) of the proposed approach were 0.1, 5.0 and 10 ng/mL in water, urine and plasma samples, respectively. The relative standard deviations (RSDs) based on five replicate determinations at 10 ng/mL level of CPZ was 1.2%. Good linear behaviors over the investigated concentration ranges (0.25–300 ng/mL) with good coefficient of determination, R2>0.9998, were obtained. Good spike recoveries with relative errors less than 9.0% were obtained when applying the proposed method to water, urine and plasma samples.
Keywords: Chlorpromazine, Magnetic solid phase extraction, Mixed-hemimicelles
1. Introduction
Chlorpromazine (CPZ) is the most important compound in the large group of phenothiazine derivatives. It is widely used as a therapeutic agent for controlling excitement, agitation and other psychomotor disturbances in schizophrenic patients and reduces the manic phase of manic-depressive conditions. It is used to treat hyperkinetic states and aggression and is sometimes given in other psychiatric conditions for the control of anxiety and tension [1].
Since the antipsychotic drugs are very active, they are usually administered at low daily dosages. In addition, CPZ is widely metabolized in the body. Therefore, the concentration of CPZ in plasma is low. From bioanalytical and clinical points of view, highly sensitive, selective and accurate bioanalytical methods are needed to determine CPZ in biological fluids for obtaining optimum therapeutic concentrations and controlling its side effects. Determination of CPZ in biological fluids has been extensively accomplished by high performance liquid chromatography (HPLC) with different detection systems [2], [3], [4], [5], [6]. However, in many cases, owing to matrix interference and insufficient instrumental detection limit for (ultra) trace level of analyte in real biological samples, direct chromatographic separation and determination of the species is difficult [7]. Therefore, in order to obtain accurate, reliable and sensitive results, a separation/preconcentration method is required prior to chromatographic separation and/or other determination techniques [7], [8]. Consequently, separation/preconcentration techniques such as solid phase microextraction [9], hollow fiber liquid phase microextraction (HF-LPME) [10] and molecularly imprinted polymer [11] have been reported for extraction of CPZ from biological fluids.
Recently, a new magnetic solid-phase extraction (MSPE) method based on mixed-hemimicelles assemblies (hemimicelles/admicelles) has been proposed for the preconcentration of a variety of inorganic and organic compounds from various matrices [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. In these SPE methods, the used adsorbents were produced by the adsorption of ionic surfactants such as sodium dodecyl sulfate (SDS) or cetyltrimethylammonium bromide (CTAB) on the surface of magnetite and/or modified magnetite nanoparticles (MNPs) with silica and alumina. Adopting the MNPs as adsorbent and mixed-hemimicelles led to overcoming the limitations of microparticle adsorbent such as a relatively low extraction capability in addition to being time-consuming when large volume samples were loaded. As a result, the use of mixed-hemimicelles assemblies in MSPE has a number of advantages, such as high extraction yields, high breakthrough volumes and easy elution of analytes and regeneration of adsorbent [12], [13], [15], [17], [20]. Moreover, due to high surface area and strong magnetism of MNPs [22] it can be assumed that using this type of adsorbent can improve the adsorption capacity of analytes and reduce the analysis time through the rapid isolation of MNPs with a strong magnet from large volumes of the sample solution. Therefore, compared with the traditional SPE method, the novel MSPE method based on Fe3O4 NPs would have higher extraction efficiency and enrichment factor.
The aim of the present study was to develop a simple and reliable MSPE method based on mixed hemimicelles assemblies for the preconcentration and determination of trace amounts of CPZ in biological fluids. The method is based on extraction of CPZ as ionic species on negatively charged SDS coated Fe3O4 NPs. Affecting factors of the formation of mixed hemimicelles and the extraction efficiency of CPZ were investigated and optimized. This methodology has not been employed previously for the extraction and determination of trace amounts of CPZ from biological fluids.
2. Experimental
2.1. Chemicals
All the reagents used were of analytical grade. Chlorpromazine hydrochloride with minimum purity >99.5% was kindly donated by the Department of Pharmacology, Tehran University (Tehran, Iran). The chemical structure of chlorpromazine hydrochloride is shown in Fig. 1. HPLC-grade acetonitrile, ferric chloride (FeCl3·6H2O), ferrous chloride (FeCl2·4H2O), sodium hydroxide, sodium dodecyl sulfate (SDS), sodium acetate, methanol, acetone, ethanol, hydrochloric acid and NaCl were all purchased from Merck (Darmstadt, Germany). The reagent water used was purified with a Milli-Q system from Millipore (Bedford, MA, USA). Stock standard solution of CPZ (1000 mg/L) was prepared by dissolving its hydrochloride salt in methanol. All the standard solutions were stored at 4 °C in the refrigerator. The working solutions were prepared by proper dilution of the standard solution in the reagent water.
Fig. 1.
Chemical structure of CPZ.
2.2. Apparatus
Chromatographic separations were carried out on a Varian HPLC containing a 9012 HPLC pump (Mulgrave Victoria, Australia), a six-port Cheminert HPLC valve from Valco Instruments (Houston, TX,USA) with a 20 µL sample loop and a Varian 9050 UV–vis detector. Chromatographic data were recorded and analyzed using Chromana software (version 3.6.4). The separations were carried out on an ODS-3 column (150 mm×4.0 mm, with 3 µm particle size) from MZ-Analysentechnik (Mainz, Germany). Mixture of sodium acetate (50 mM, pH=4.6) and acetonitrile (70:30, v/v) was used under isocratic elution condition as mobile phase at flow rate 0.8 mL/min. The injection volume was 20 µL for all of the samples and the detection was performed at the wavelength of 254 nm. A Cesil CE-7200 UV–vis spectrophotometer (Cambridge, England) was applied for the absorbance measurements of the solutions. All of the pH measurements were performed with a WTW Inolab pH meter (Weilheim, Germany).
2.3. Synthesis of Fe3O4 NPs
Fe3O4 NPs were prepared by chemical co-precipitation method via a reactor which had been designed in our previous work [21]. Briefly, 10.4 g of FeCl3·6H2O, 4.0 g of FeCl2·4H2O and 1.7 mL of HCl (12 M) were dissolved in 50 mL of deionized water in order to prepare stock solution of ferrous and ferric chloride in a beaker which was then degassed with nitrogen gas for 20 min. Simultaneously, 500 mL of 1.5 M NaOH solution was degassed (for 15 min) and heated to 80 °C in the reactor. Then, the stock solution was added dropwise using the dropping funnel during 30 min under nitrogen gas protection and vigorous stirring (1000 rpm) by a glassware stirrer. During the whole process, the solution temperature was maintained at 80 °C and nitrogen gas was purged to remove the dissolved oxygen. After completion of the reaction, the obtained Fe3O4 NPs precipitate was separated from the reaction medium by the magnetic field, and then washed with 500 mL doubly distilled water four times. Finally, the obtained Fe3O4 NPs were re-suspended in 500 mL of degassed deionized water and the concentration of the generated MNPs in suspension was estimated to be about 10 mg/mL. The MNPs were characterized using IR, XRD and SEM instruments in our previous work [21].
2.4. MSPE procedure
50 milliliter of aqueous sample containing CPZ with concentration of 100 ng/mL (pH=3.0) was transferred to 100 mL glassware beaker. Then, 1.0 mL of the suspension of Fe3O4 NPs and 0.5 mL of the SDS solution (10 mg/mL) were sequentially added together and thoroughly mixed with the sample solution. The mixture was shacked and allowed to complete the extraction process for 2 min. Subsequently, an Nd-Fe-B strong magnet (10 cm×5 cm×4 cm, 1.4 T) was placed at the bottom of the beaker, and the SDS-coated Fe3O4 NPs were isolated from the solution. After about 1 min, the solution became limpid and supernatant solution was decanted. Finally, the preconcentrated CPZ was eluted from the adsorbent using 300 µL of a mixture of ethanol:NaOH (0.01 M) (75:25, v/v) and 20 µL of this solution was injected into the 20 µL HPLC loop for analysis.
2.5. Extraction of CPZ from water, urine and plasma samples
In order to study the feasibility of the proposed MSPE method for extraction and determination of CPZ in the real samples, the developed technique was applied for the extraction of CPZ from the tap, plasma and urine samples. Tap water sample was collected freshly from our laboratory (Tehran, Iran) and human urine samples were obtained from three healthy males. Iranian Blood Transfusion Organization (Tehran, Iran) was the supplier of the plasma sample as well. Standard addition method was applied for the measurement of CPZ in all of the real samples. In order to reduce the matrix effect, the spiked urine and plasma samples were diluted 1:10 using ultra-pure water. Moreover, for plasma samples an extra preparation step was done in order to remove plasma׳s proteins by addition of 0.5 g of trichloroacetic acid to 50 mL of the diluted sample followed by centrifugation of the mixture to isolate precipitated proteins. After isolation of proteins, the extraction steps were performed on clear supernatant solution according to the MSPE procedure.
3. Results and discussion
3.1. Optimization of the MSPE technique
Optimization of parameters affecting the MSPE method was done by using spectrophotometer and HPLC–UV instruments. Except the volume of eluent, other parameters were optimized by spectrophotometer due to simplicity, rapidity and low cost of the instrument. On the other hand, validation of the method and real sample analysis were done by HPLC–UV due to sensitivity and ability of HPLC in separation of complex samples.
A solution containing 100 ng/mL of CPZ was used in the optimization experiments. All quantifications were done based on the measurement of absorption and peak area in spectrophotometry and HPLC–UV analysis, respectively. Final elution of CPZ was done in conical glassware tube (10 mL) for easy collection of the adsorbent in these tubes. Also, by using these tubes, the preconcentrated CPZ can be eluted using small volume of the eluent to obtain higher enrichment factors or sensitivity.
3.2. Selection of proper extraction mechanism
The amino group on molecule of CPZ is ionizable (Fig. 1). Based on pKa value of CPZ (9.3), extraction of CPZ with MSPE technique can be performed via two different mechanisms. First, based on this fact that at acidic pHs, CPZ is positively charged (due to protonation of amino group), it can be extracted by SDS-coated MNPs mixed-hemimicelles. Under this circumstance, extraction can be mostly driven by electrostatic attraction. Second, CPZ can be extracted at alkaline condition (pH~12) as its neutral form by CTAB-coated MNPs hemimicelles which under this circumstance extraction is driven only by hydrophobic attractions. The results showed that the first mechanism was more efficient than the second one. The probable reason might be concerned with driven forces of extraction in which electrostatic attraction is drastically more efficient than hydrophobic attraction. Therefore, the first mechanism was selected for further investigations.
3.3. Effect of sample׳s pH
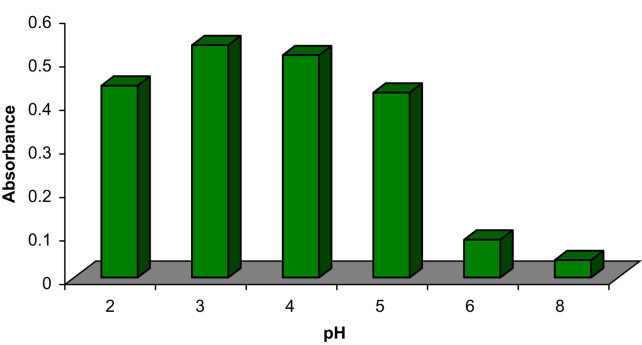
It is well known that the pH of the sample solution is one of the most important factors affecting the states of species (as ions or neutral forms). Moreover, pH is one of the factors influencing the adsorption behavior of mixed-hemimicelles system due to the change of the charge density on the MNPs surface [12], [13], [14], [15], [16], [17]. As shown in Fig. 2, maximum adsorption of CPZ was obtained when pH was 3.0. The adsorption amount decreased when the pH increased from 3.0 to 8.0. This can be attributed to the fact that the positively charged surface of the MNPs was favorable for the adsorption of anionic surfactants. When pH value increased, the positive charge density on the MNPs surface decreased [15], [20]. The electrostatic attraction between negative charges of SDS and positive charges of the surface of MNPs was not strong enough to produce hemimicelles, which made against the great adsorption of CPZ as positively charged form (pH=2.0–7.0 based on pKa=9.3). However, CPZ adsorption decreased slightly when pH was 2.0, which may be explained by the reduced adsorption of SDS ion, probably due to protonation of their sulfate groups [23]. Thus pH of 3.0 was selected for subsequent experiments.
Fig. 2.
Effect of pH on extraction efficiency. Conditions: sample volume is 50 mL; concentration of CPZ is 100 ng/mL; 10 mg Fe3O4 NPs; 2.5 mg SDS; stirring time is 5 min; elution with 1.0 mL methanol; desorption time is 5 min.
3.4. Effect of the amount of SDS on adsorption of CPZ
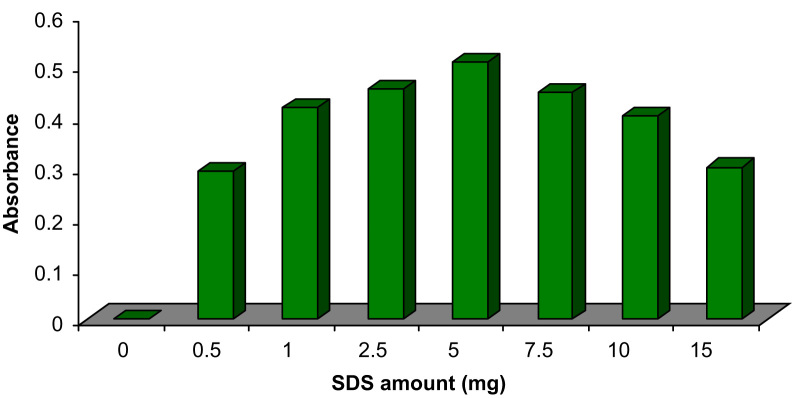
The outer surface of hemimicelles is hydrophobic whereas that of admicelles is ionic, which provides different mechanisms for retention of organic compounds and both are suitable for the SPE method. In mixed-hemimicelles phase, the adsorption is driven by both hydrophobic interactions and electrostatic attraction because of the formation of hemimicelles and admicelles on the surface of mineral oxides [12], [13], [15], [17]. One can see in Fig. 3 that in the absence of SDS, CPZ was hardly adsorbed onto the surface of MNPs. The adsorption amount of CPZ increased remarkably by increasing amount of SDS. Maximum adsorption was obtained when SDS amount was 5 mg. When SDS amount was above 5 mg, the adsorption of CPZ decreased gradually, which may be attributed to the formation of SDS micelles in the bulk of aqueous solution and the micelles caused CPZ to redistribute into the solution again. Based on the obtained results, 5 mg of SDS was added into the solution in the further studies.
Fig. 3.
Effect of SDS amount on extraction efficiency. Conditions: sample volume is 50 mL; concentration of CPZ is 100 ng/mL; sample׳s pH is 3.0; 10 mg Fe3O4 NPs; stirring time is 5 min; elution with 1.0 mL methanol; desorption time is 5 min.
3.5. The effect of ionic strength
The effect of ionic strength on adsorption of CPZ was investigated by addition of NaCl in the range of 0–10% (m/v). The results showed that as the NaCl concentration increased, adsorption capacity of the MNPs decreased significantly. The results can be justified by exchange reactions in the solution during adsorption as shown below [24]
| (1) |
According to Eq. (1), in the presence of large amounts of salt (Na+), decreases in adsorption of CPZ based on Le Chatelier׳s principle are expectable. So, all the subsequent experiments were performed in the absence of salt. It is worthy to note that in the biological samples due to existence of salts, lower extractions in comparison with the aqueous sample may be expected.
3.6. Effect of the adsorbent amount and extraction time
In order to study the effect of the adsorbent amount on the extraction efficiency, 0–20 mg of the MNPs was added to 50 mL of the sample solution. The obtained results showed that by increasing the adsorbent amounts up to 10 mg; due to the increase of accessible sites, extraction recovery slowly increased, and then remained constant. It can be attributed to higher surface area-to-volume ratio of the MNPs in comparison with traditional adsorbents (microsized adsorbents) [25], [26]. Therefore, satisfactory results could be achieved with fewer amounts of the MNPs (10 mg).
Also, the effect of the extraction time on the adsorption of CPZ was investigated in the range of 1–10 min. Based on the obtained results, adsorption of CPZ increased slightly up to 2 min and then remained constant. The results can be attributed to shorter diffusion route for MNPs [25], [26]. Meanwhile, in the experiment, SDS-coated MNPs possessed superparamagnetism properties and large saturation magnetization [12], [13], which enabled them to be completely isolated in a short time (less than 1 min) by a strong magnet. In other words, rapid extraction and desorption of the analytes from the MNPs could shorten the analysis time considerably. Therefore, 2 min was chosen as the optimum extraction time.
3.7. Desorption conditions
3.7.1. Selection of proper eluent
Organic solvents are known to disrupt mixed-hemimicelles [12], [13], [15], [17] and were used to elute analytes from the SDS-coated MNPs. Thus, desorption of CPZ from the SDS-coated MNPs mixed-hemimicelles was studied with different organic solvents (acetonitrile, methanol, and ethanol) and 0.01 M NaOH. Desorption ability of ethanol was found to be superior to that of other eluents. Further studies showed that by using a mixture of ethanol and 0.01 M NaOH (75:25, v/v) desorption efficiency could be improved. This could be attributed to the fact that when the pH level was higher or around its isoelectric pH, charge density in MNPs surface was weak and favorable for the disruption of mixed-hemimicelles [11]. So, basic solution of ethanol was selected for subsequent studies. Meanwhile, further studies showed that the adsorbent could be used for at least five successive extractions without a considerable change in its extraction efficiency.
3.7.2. Effect of eluent׳s volume and desorption time
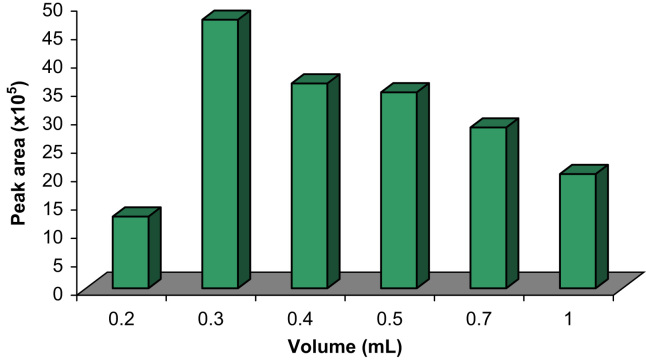
The effect of the volume of the basic solution of ethanol:0.01 M NaOH (75:25, v/v) on the desorption efficiency was studied in the range of 0.2–1.0 mL. The results are shown in Fig. 4. As can be seen, when the volume of eluent was increased, desorption efficiency of CPZ increased up to 0.3 mL; by further increasing the volume of the eluent, peak areas were decreased because of dilution effect. Therefore, 0.3 mL of the eluent was chosen as the optimum eluent volume. Moreover, in order to obtain maximum desorption efficiency, effect of desorption time was also investigated in the range of 1–10 min. Based on the obtained results, desorption time had no significant effect on the desorption efficiency of CPZ. Therefore, 1 min was chosen for further studies in order to reduce overall analysis time.
Fig. 4.
Effect of eluent׳s volume on extraction efficiency. Conditions: sample volume is 50 mL; concentration of CPZ is 100 ng/mL; sample׳s pH is 3.0; 10 mg Fe3O4 NPs; 5.0 mg SDS; stirring time is 2 min; elution with 1.0 mL basic ethanol (75:25, v/v); desorption time is 1 min.
3.7.3. Effect of sample volume
In order to obtain a higher enrichment factor, a large volume of sample solution is required. MNPs mixed hemimicelles SPE method based on magnetic carrier technology (MCT) avoids the time-consuming column passing and filtration steps and shows great analytical potential in preconcentration of large volume water samples [12], [13], [15], [17], [20]. The effect of sample volume on the enrichment of CPZ was investigated by extracting the drug from 50–200 mL aqueous solutions spiked with fixed 5 µg of CPZ. Under the optimal conditions, the recoveries for CPZ were still above 90% with sample volumes up to 150 mL. Although based on the obtained results volume of sample could be increased to 150 mL due to limited volumes of biological samples, further studies were done by extraction of CPZ from 50 mL sample solution. Also, this finding data could be used for dilution of the biological samples in order to reduce matrix effect.
3.8. Analytical performance
Table 1 summarizes quantitative parameters of the proposed method such as linearity, limit of detection (LOD), limit of quantification (LOQ), precision, enrichment factor (EF) and sensitivity for extraction of CPZ from 50 mL of three different aqueous solutions. Under the optimized conditions, the calibration graphs were linear in the range from 0.25 to 300, 5.0 to 300 and 10.0 to 300 ng/mL (n=8) for water, urine and plasma samples, respectively. LODs were 0.1, 0.5 and 1.0 and also LOQs were 0.25, 1.0 and 5.0 ng/mL for water, urine and plasma samples, respectively. LODs were calculated based on S/N=3. The EF of CPZ was obtained by dividing the slope of the calibration graph that was obtained after preconcentration and slope of calibration curve obtained from direct injection of standards of CPZ into HPLC column. The EFs of the proposed method were 106, 38 and 23 for water, urine and plasma samples, respectively. Also, relative standard deviations (RSDs) of intra-day (repeatability, n=5) and inter-day (reproducibility, n=5) were less than 9% at 10 µg/L of CPZ. Moreover, ANOVA one factor statistics showed no significant difference between inter-day peak areas obtained for CPZ.
Table 1.
Merits of the proposed method in water, urine and plasma matrices for extraction and determination of CPZ.
| Matrix | LOD (ng/mL) | LOQ (ng/mL) | RSD% (n=5, 10 ng/mL) |
Enrichment factor | Extraction percentage (%) | Linear range (ng/mL) | R2 | |
|---|---|---|---|---|---|---|---|---|
| Inter-day | Intra-day | |||||||
| Water | 0.1 | 0.25 | 1.2 | 3.1 | 106 | 74 | 0.25–300 | 0.9998 |
| Urine | 0.5 | 1.5 | 4.2 | 7.3 | 38 | 27 | 5.0–300 | 0.9986 |
| Plasma | 1.0 | 3.0 | 3.2 | 8.6 | 23 | 16 | 10.0–300 | 0.9936 |
3.9. Application of the MSPE for real samples
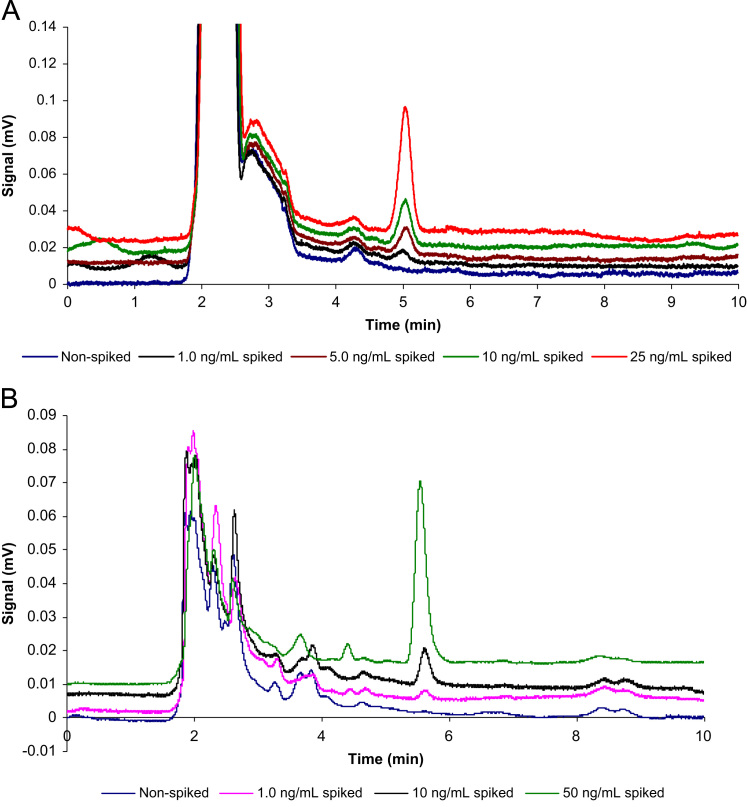
In order to assess the applicability of the newly developed extraction system for the analysis of the drug in real samples with complex matrices, the spiked tap water, urine and plasma samples were extracted and analyzed using the proposed method under optimum conditions. Since CPZ was not detected in the real samples, ng/mL amounts of CPZ were added into the real samples, and extraction and determination procedure was done based on the procedure. Table 2 shows that the results of three replicate analyses of each real sample obtained by the proposed method were in satisfactory agreement with the spiking amounts and relative errors <9.0% were obtained. Moreover, the proposed method displayed good reproducibility to determine the drug in the real samples with intra-day RSD% values in the range of 2.3–9.7. Fig. 5 depicts the MSPE–HPLC–UV chromatograms of CPZ in the diluted urine 3 (Fig. 5A) and plasma (Fig. 5B) samples, before and after spiking the samples with CPZ.
Table 2.
Determination of CPZ in different spiked samples.
| Samplea | Cadded (ng/mL) | Cfound (ng/mL) | RSD (%) (n=3) | Relative error (%) |
|---|---|---|---|---|
| Tap water | 5.0 | 4.9 | 2.3 | −2.0 |
| Urine 1 | 5.0 | 4.8 | 8.5 | −4.0 |
| 25.0 | 26.5 | 9.7 | 6.0 | |
| Urine 2 | 10.0 | 9.9 | 7.3 | −1.0 |
| 25.0 | 24.9 | 5.2 | −0.4 | |
| Urine 3 | 25.0 | 25.7 | 1.7 | 2.8 |
| Plasma | 25.0 | 27.2 | 3.2 | 8.8 |
| 50.0 | 48.9 | 4.2 | −2.2 |
All samples were diluted with dilution ratio of 1:10.
Fig. 5.
(A) The chromatograms of the spiked (at the concentration level of 1.0, 5.0, 10.0 and 25.0 ng/mL) and non-spiked urine sample 3 with chlorpromazine. (B) The chromatograms of the spiked (at the concentration level of 1.0, 10 and 50.0 ng/mL) and non-spiked plasma sample with CPZ.
3.10. Comparison of the proposed method with other reported methods
The proposed method was compared with a variety of methods that have been reported recently in the literature for preconcentration and determination of CPZ. The distinct features of the proposed method are summarized in Table 3. As can be seen from Table 3, it is evident that the proposed method has wide dynamic linear range and also lower LODs and RSDs are the other significant features of the method which are comparable to or even better than liquid–liquid extraction (LLE) methods which use very sensitive detection methods such as LC–ESI–MS/MS [6] and/or chemiluminescence techniques [27], [28], [29]. Also, due to large surface area and rapid extraction dynamics of the MNPs, extraction time of the proposed method is much shorter than that of the other reported methods, which leads to high sample throughout in comparison with the other reported methods [6], [10], [27].
Table 3.
Comparison of the proposed method with other developed methods to determine CPZ in aqueous solutions.
| Matrix | Extraction method | Detection system | Extraction time (min) | LOD (ng/mL) | Linear range (ng/mL) | RSD% (intra-day) | Ref. |
|---|---|---|---|---|---|---|---|
| Water | SPE | HPLC–UV | <2 | 0.1 | 0.25–300 | 1.2 | This work |
| Urine | 5 | 5.0–300 | 4.2 | ||||
| plasma | 10 | 10.0–300 | 3.2 | ||||
| Plasma | LLE | LC–ESI–MS/MS | >10 | 0.2 | 0.2–200 | <7.5 | [6] |
| Water | HF-LPME | HPLC–UV | 60 | 0.5 | 1.0–500 | <6.7 | [10] |
| Urine | MIP | HPLC–UV | a | 80 | 200–20,000 | <8.3 | [11] |
| Urine | CE – electro chemiluminescence | >10 | 1.5 | 5–800 | 3.6 | [27] | |
| Urine | LLE | Chemiluminescence | a | 6 | 50–10,000 | <2.6 | [28] |
| Urine | a | Chemiluminescence | a | 6.5 | 0–10,000 | 4.1 | [29] |
Data not reported.
4. Conclusion
A new SPE method based on the SDS-coated MNPs mixed-hemimicelles was developed for the preconcentration of CPZ in water, urine and plasma samples. The use of the MNPs endued the SPE method with high extraction capacity and preconcentration factors. Moreover, the magnetic separation greatly improved the separation rate while avoiding the time-consuming column passing or filtration operation. The strong electrostatic interactions between the mixed hemimicelles and protonated CPZ made this new developed SPE method possess high extraction efficiency and capacity. The adsorbed analyte was easily desorbed with the basic ethanol and no carry-over was observed in the next analysis. The established MSPE method proved to be effective for concentrating trace CPZ in different samples prior to HPLC analysis. Based on the obtained results, it is anticipated that the proposed method has a great analytical potential in preconcentration of drugs from real sample in the same way.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Seeman P. Brain dopamine receptors. Pharmacol. Rev. 1981;32:229–313. [PubMed] [Google Scholar]
- 2.Pistos C., Stewart J.T. Direct injection HPLC method for the determination of selected phenothiazines in plasma using a Hisep column. Biomed. Chromatogr. 2003;17:465–470. doi: 10.1002/bmc.275. [DOI] [PubMed] [Google Scholar]
- 3.Midha K.K., Cooper J.K., McGilveray I.J. High-performance liquid chromatographic assay for nanogram determination of chlorpromazine and its comparison with a radioimmunoassay. J. Pharm. Sci. 1981;70:1043–1046. doi: 10.1002/jps.2600700920. [DOI] [PubMed] [Google Scholar]
- 4.Chetty M., Miller R. Effect of storage on the plasma concentration of chlorpromazine and six of its metabolites. Ther. Drug Monit. 1991;13:350–355. doi: 10.1097/00007691-199107000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Hayen H., Karst U. Analysis of phenothiazine and its derivatives using LC/electrochemistry/MS and LC/electrochemistry/fluorescence. Anal. Chem. 2003;75:4833–4840. doi: 10.1021/ac0346050. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G., Terry J.A.V., Bartlett M.G. Sensitive liquid chromatography/tandem mass spectrometry method for the determination of the lipophilic antipsychotic drug chlorpromazine in rat plasma and brain tissue. J. Chromatogr. B. 2007;854:68–76. doi: 10.1016/j.jchromb.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Pizzolato T.M., de Alda M.J.L., Barceló D. LC-based analysis of drugs of abuse and their metabolites in urine. TrAC-Trends Anal. Chem. 2007;26:609–624. [Google Scholar]
- 8.Zhang L., Wu P., Zhang Y. A GC/MS method for the simultaneous determination and quantification of chlorpromazine and diazepam in pork samples. Anal. Methods. 2014;6:503–508. [Google Scholar]
- 9.Saraji M., Hajialiakbari Bidgoli A.A., Ensafi A.A. Highly sensitive determination of chlorpromazine by electrochemically treated pencil graphite fiber as both solid-phase microextraction fiber and working electrode for use in voltammetry method. Anal. Methods. 2013;5:5024–5030. [Google Scholar]
- 10.Sobhi H.R., Yamini Y., Haji Hosseini Baghdad Abadi R. Extraction and determination of trace amounts of chlorpromazine in biological fluids using hollow fiber liquid phase microextraction followed by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2007;45:769–774. doi: 10.1016/j.jpba.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Song S., Shi X., Li R. Extraction of chlorpromazine with a new molecularly imprinted polymer from pig urine. Process Biochem. 2008;43:1209–1214. [Google Scholar]
- 12.Zhao X.L., Shi Y.L., Cai Y.Q. Cetyltrimethylammonium bromide-coated magnetic nanoparticles for the preconcentration of phenolic compounds from environmental water samples. Environ. Sci. Technol. 2008;42:1201–1206. doi: 10.1021/es071817w. [DOI] [PubMed] [Google Scholar]
- 13.Zhao X., Shi Y., Wang T. Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J. Chromatogr. A. 2008;1188:140–147. doi: 10.1016/j.chroma.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 14.Zargar B., Parham H., Hatamie A. Modified iron oxide nanoparticles as solid phase extractor for spectrophotometeric determination and separation of basic fuchsin. Talanta. 2009;77:1328–1331. doi: 10.1016/j.talanta.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Sun L., Zhang C., Chen L. Preparation of alumina-coated magnetite nanoparticle for extraction of trimethoprim from environmental water samples based on mixed hemimicelles solid-phase extraction. Anal. Chim. Acta. 2009;638:162–168. doi: 10.1016/j.aca.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Faraji M., Yamini Y., Saleh A. A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal. Chim. Acta. 2010;659:172–177. doi: 10.1016/j.aca.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Zhao X., Shi Y. Mixed hemimicelles solid-phase extraction based on cetyltrimethylammonium bromide-coated nano-magnets Fe3O4 for the determination of chlorophenols in environmental water samples coupled with liquid chromatography/spectrophotometry detection. J. Chromatogr. A. 2008;1180:24–31. doi: 10.1016/j.chroma.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Shariati S., Faraji M., Yamini Y. Fe3O4 magnetic nanoparticles modified with sodium dodecyl sulfate for removal of safranin O dye from aqueous solutions. Desalination. 2011;270:160–165. [Google Scholar]
- 19.Parham H., Rahbar N. Solid phase extraction-spectrophotometric determination of salicylic acid using magnetic iron oxide nanoparticles as extractor. J. Pharm. Biomed. Anal. 2009;50:58–63. doi: 10.1016/j.jpba.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Faraji M., Yamini Y., Rezaee M. Extraction of trace amounts of mercury with sodium dodecyl sulphate-coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta. 2010;81:831–836. doi: 10.1016/j.talanta.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Faraji M., Yamini Y., Tahmasbi E. cetyltrimethylammonium bromide-coated magnetite nanoparticles as highly efficient adsorbent for rapid removal of reactive dyes from the textile companies׳ wastewaters. J. Iran. Chem. Soc. 2010;7:S130–S144. [Google Scholar]
- 22.Faraji M., Yamini Y., Rezaee M. Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J. Iran. Chem. Soc. 2010;7:1–37. [Google Scholar]
- 23.Saitoh T., Nakayama Y., Hiraide M. Concentration of chlorophenols in water with sodium dodecylsulfate-gamma-alumina admicelles for high-performance liquid chromatographic analysis. J. Chromatogr. A. 2002;972:205–209. doi: 10.1016/s0021-9673(02)01118-4. [DOI] [PubMed] [Google Scholar]
- 24.Lin S.-H., Juang R.-S. Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J. Hazard. Mater. 2002;92:315–326. doi: 10.1016/s0304-3894(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 25.Moeller K., Kobler J., Bein T. Colloidal suspensions of nanometer-sized mesoporous silica. Adv. Funct. Mater. 2007;17:605–612. [Google Scholar]
- 26.Klabunde K.J. Wiley-Interscience; New York: 2001. Nanoscale Material in Chemistry. [Google Scholar]
- 27.Li J., Zhao F., Ju H. Simultaneous determination of psychotropic drugs in human urine by capillary electrophoresis with electrochemiluminescence detection. Anal. Chim. Acta. 2006;575:57–61. doi: 10.1016/j.aca.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 28.Shi W., Yang J., Huang Y. Ion-pair complex-based solvent extraction combined with chemiluminescence determination of chlorpromazine hydrochloride with luminol in reverse micelles. J. Pharm. Biomed. Anal. 2004;36:197–203. doi: 10.1016/j.jpba.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y., Chen Z. Chemiluminescence of chlorpromazine hydrochloride based on cerium(IV) oxidation sensitized by rhodamine 6G. Talanta. 2002;57:953–959. doi: 10.1016/s0039-9140(02)00142-x. [DOI] [PubMed] [Google Scholar]