Abstract
Immunosuppression associated with human immunodeficiency virus (HIV) infection impacts all components of host defense against pulmonary infection. Cells within the lung have altered immune function and are important reservoirs for HIV infection. The host immune response to infected lung cells further compromises responses to a secondary pathogenic insult. In the upper respiratory tract, mucociliary function is impaired and there are decreased levels of salivary immunoglobulin A. Host defenses in the lower respiratory tract are controlled by alveolar macrophages, lymphocytes, and polymorphonuclear leukocytes. As HIV progresses, lung CD4 T cells are reduced in number causing a lack of activation signals from CD4 T cells and impaired defense by macrophages. CD8 T cells, on the other hand, are increased in number and cause lymphocytic alveolitis. Specific antibody responses by B lymphocytes are decreased and opsonization of microorganisms is impaired. These observed defects in host defense of the respiratory tract explain the susceptibility of HIV-infected persons for oropharyngeal candidiasis, bacterial pneumonia, Pneumocystis pneumonia, and other opportunistic infections.
Keywords: HIV infections, Host defense against infection, T-lymphocytes, B-lymphocytes, Lung diseases
INTRODUCTION
Approximately 1.2 million people in the United States are living with HIV infection.1 Although antibiotic and antiviral therapies have decreased the incidence of pulmonary infection in HIV-infected patients, this population remains at risk for opportunistic infections, such as Pneumocystis pneumonia. The high frequency of pulmonary infections in HIV-infected individuals suggests a disruption of important pulmonary host defenses.
A number of mechanisms explaining host susceptibility to lung infections during HIV, have been suggested.2,3 First, HIV can directly infect and kill host cells, leading to a decline in the number of cells that can participate in host defense against pathogens. Second, HIV can impair the secretory or cytokine functions of host immune cells (i.e. shift from Th1 cytokines to Th2 cytokines). Third, HIV infection can interfere with the ability of circulating immune cells to migrate into the lungs to clear pathogens. Lastly, coinfection of a pathogen may further impair host defense.
Abnormalities in host defense, caused by HIV, are dependent upon the duration or extent of HIV infection. Generally, bacterial pneumonia, tuberculosis, and oral thrush are commonly seen early in HIV infection whereas Pneumocystis pneumonia, atypical mycobacterial infections, toxoplasmosis, and cryptococcal infections are found later when CD4 T cell counts are low.4 In terms of pulmonary host defense, HIV infection may be seen as a cycle, with HIV infection causing defective host defense leading to pulmonary infection that further exacerbates the HIV infection.5
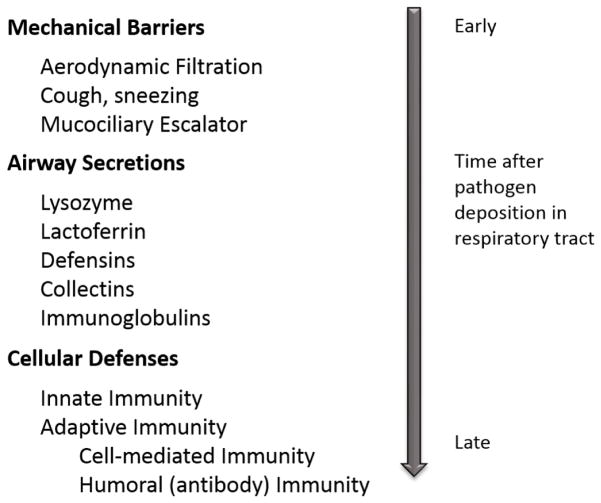
The lungs are protected against infection by defense systems operative at different anatomic levels and at different times after an infectious challenge. The first line of defense is provided by mechanical barriers and airway secretions (Figure 1).5 The earliest cellular response is provided by innate immunity followed by adaptive immunity. Prior reviews have been published that summarize defective host defense against pulmonary infections seen in HIV-infected individuals. This review will outline more recent knowledge, from 2012 to present, on host defense defects associated with HIV in the lung.
Figure 1.
Pulmonary host defense mechanisms.
PATHOGENESIS OF HIV INFECTION
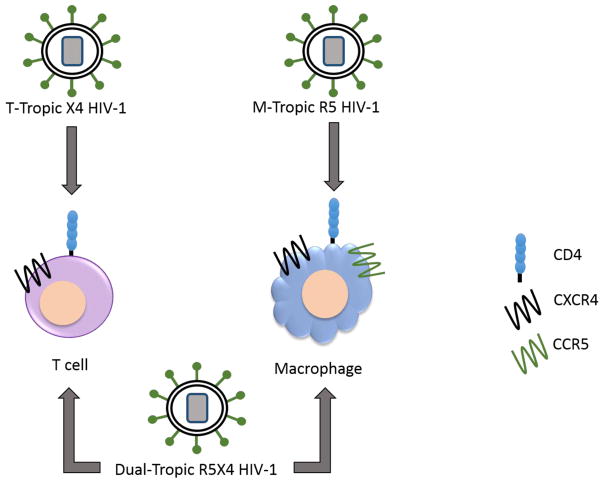
HIV infection can alter host defense by directly infecting pulmonary cells. Various strains of HIV demonstrate cellular tropism, based on the phenotype and receptor/co-receptors required for entry of the virus into host cells.2 The primary receptor for HIV is the CD4 receptor, found in humans on the surface of monocytes/macrophages and lymphocytes. HIV is divided into three groups based on cellular tropism. The macrophage-tropic (M-tropic) strain, which interacts with the chemokine coreceptor CCR5 (R5), infects peripheral blood mononuclear cells, monocytes, macrophages and T lymphocytes. The T-cell tropic (T-tropic) strain, which interacts with the chemokine coreceptor CXCR4 (X4), infects T lymphocytes and T cell lines but not monocytes or macrophages. Lastly, the dual tropic strain (R5X4), which interacts with both chemokine receptors, infects both monocytes/macrophages and T cell lines.6 It can be envisioned that cellular tropism of HIV might result in differential effects on pulmonary host defense and host susceptibility to specific infections, but the in vivo correlates of HIV tropism are unknown.
UPPER RESPIRATORY TRACT AND CONDUCTING AIRWAYS
Mechanical barriers provide the initial defense of the respiratory tract by filtering microorganisms from the air stream through nasal hairs and branching airways that serve to prevent HIV-associated pathogens from entering the lower respiratory tract.5 The initial mechanical barriers encountered by microorganisms are ciliated epithelial cells and airway secretions, which contain molecules that provide protection against infection of the respiratory tract. Ciliated epithelial cells line the upper respiratory tract and conducting airways and function as a mucociliary ladder, which sweeps foreign objects and infectious agents from the upper respiratory tract to be swallowed or expectorated. Decreased ciliary transport may explain the high rate of sinusitis seen in HIV-infected patients.7 Airway secretions contain protective molecules (i.e., lysozyme, lactoferrin, defensins, collectins, and immunoglobulin A (IgA)), which are secreted by cells of the immune system. Although changes in the concentration of these molecules in airway secretions have been observed during HIV infection, the correlation between changes and disease progression have not been determined. There are several HIV-associated upper respiratory tract infections, but the most frequent opportunistic infection is oropharyngeal candiasis (OPC), also known as thrush.8 Oral thrush is seen in later stages of HIV and is often used as a predictor of disease progression to AIDS, independent of CD4 T cell count.
DEFECTS IN INNATE IMMUNITY
The innate defense system consists of phagocytic cells and natural killer cells, which are able to neutralize bacteria and virus-infected cells through pattern recognition receptors. These receptors recognize conserved bacterial products and viral motifs. Innate immune cells also produce a wide range of pro-inflammatory cytokines and chemokines to facilitate an inflammatory response to infection. Many innate immune cells can also process and present antigen to lymphocytes.
Alveolar Macrophages
The primary reservoir for HIV in the lung is thought to be macrophages. All macrophages originate from precursor cells in hematopoietic organs and gain access to the respiratory tract via the blood and lymph. Alveolar macrophages (AM) represent 95% of the cells in bronchoalveolar lavage (BAL) fluid.9 AMs are resident lung phagocytes which express high densities of immunoglobulin receptor, complement receptor, mannose receptor and several types of scavenger receptors that aid in phagocytosis of opsonized and non-opsonized particles.10 AMs are active producers of cytokines/chemokines, and have important pro- and anti-inflammatory roles in the alveolus. The number of macrophages in BAL from AIDS patients have been reported as normal however the overall percent of AM in the BAL is decreased, which is thought to be caused by an increase in other cells, particularly lymphocytes.2
HIV-1 infection is characterized by sustained activation of the immune system. HIV can infect several types of immune cells, though macrophages and CD4 T cells are the principal targets of the virus.11–13 Macrophages are terminally differentiated and play an important role in the clearance of pathogens and cellular debris by phagocytosis. Macrophages also act as antigen presenting cells, causing an exchange of information between themselves and CD4 T cells. This exchange of information is important in the transmission of HIV-1 from macrophages to CD4 T cells.14–16 Unlike T cells, macrophages are less prone to the cytopathic effect of HIV.17,18 Once HIV enters the macrophage, the viral ribonucleoprotein complex is released into the cytoplasm and viral DNA is synthesized.12,18 The rate of reverse transcription is slower in macrophages, compared to T cells, because they are non-dividing cells that have limited dNTP pools.19,20 In addition, macrophages possess inhibitory factors, called host restriction factors, which interfere with the viral life cycle. The sterile alpha motif domain and HD domain containing protein 1 (SAMHD1) is a macrophage specific host restriction factor that has trophosphohydrolase activity.21–23 This causes hydrolysis of dNTPs and thus reduces the overall dNTP pool in macrophages resulting in the inefficient reverse transcription of the HIV-1 genomic RNA.24
Several HIV-1 proteins may modulate the macrophage signaling pathway resulting in T lymphocyte depletion and viral cellular reservoir formation, especially in macrophages. These soluble HIV proteins, such as Nef, Tat, and Vpr have been detected in the serum of HIV-1 infected patients.25 Soluble proteins are able to enter macrophages and modulate both cellular machinery and viral transcription. Nef, a 27 kDa protein, is expressed early in the virus life cycle and down-regulates cell surface receptors.26,27 Nef-expressing macrophages enhance resting CD4 T cell permissiveness through a complex cellular and soluble interaction involving macrophages, B cells, and CD4 T cells.28,29 Nef plays a dual role in the pathogenesis of HIV by protecting HIV-infected cells from cell death and inducing apoptosis in bystander CD4 T cells. HIV-1 Tat has a critical role in viral replication and is detected in the serum of HIV-infected patients. Tat protein is essential for efficient transcription of viral genes and for viral replication. Tat also regulates the expression of cellular genes and interferes with intracellular signaling.30,31 Functional consequences of Tat activation include TNFα release from macrophages and monocytes.32 Lastly, Vpr, a virion-associated protein, is critical for HIV replication in non-dividing cells, such as macrophages.33–38 In vitro studies have also demonstrated that high concentrations of recombinant Vpr (rVpr) is cytotoxic to macrophages. However, at a low concentration rVpr enhances the activity of several macrophage transcription factors resulting in increased viral replication.39
Cell death occurs after intracellular infection and many pathogens target cell death pathways as a virulence strategy.40 As cells undergo apoptosis they emit signals that allow macrophages, and other phagocytes, to respond and engulf the apoptotic cell (efferocytosis).41–43 Efferocytosis is crucial to macrophage function and is required to control certain infections. Attenuated strains of Mycobacterium tuberculosis (Mtb) have been shown to stimulate apoptosis causing efferocytosis to occur by binding of the phagosome to a lysosome causing killing of Mtb in vitro and in vivo.44 Macrophages from HIV-infected individuals have a reduced ability to phagocytose Candida albicans, and there is a significant decrease in oxidative processes for the intracellular killing. Impaired macrophage phagocytosis of the apoptotic bodies of neutrophils may contribute to the persistence of the inflammatory state in HIV-infected individuals.45
Infection with HIV alters the function of alveolar macrophages. HIV-infected macrophages release a variety of proinflammatory cytokines, including tumor necrosis factor alpha (TNFα), interleukin (IL)-1, IL-6, interferon α (IFN-α), and chemokines. Many of the cytokines and chemokines secreted induce T cell proliferation and viral replication. AMs also require a host of activating cytokines, like interferon-γ, to maximize their ability to detect and clear pathogens such as P. jirovecii.46 HIV infection depletes important immune effector cells and therefore diminishes a major source of cytokines that activate AMs in host defense. In addition, this phenomenon indirectly impairs the function of alveolar macrophages in responding to pathogens. Mwandumba et al. showed that alveolar macrophages from HIV-infected patients with Mtb had an enhanced expression of TNFα and IL-6, which suggested that alveolar macrophages, from HIV-infected individuals with Mtb, retained their capacity to mediate inflammatory responses. This further suggests that the increased susceptibility to Mtb, which occurs during HIV infection, may not be due to impairment of the alveolar macrophage innate inflammatory response. Mannose receptors, found on the surface of alveolar macrophages, have also been shown to be impaired during HIV infection. Mannose receptors permit binding and internalization of glycoconjugates containing mannose, fucose, N-acetylglucosamine, and galactose residues. Decreases in the mannose-receptor during HIV infection impairs clearance of pathogens (i.e. Pneumocystis jirovecii).47
Dendritic Cells
Many viruses target specialized cells of the respiratory tract for initial replication, thus causing disease that is restricted to the lungs and upper airways. Alternatively, viruses may infect mobile cellular populations, such as dendritic cells, that are resident in the respiratory tract and can carry the virus to a second target organ.48 Antigen processing, antigen presentation, and T cell activation are the primary roles of dendritic cells.48 Dendritic cells (DC) share a common precursor with macrophages in the bone marrow but differentiate in the tissues (including the airway submucosa) to form cells with distinct morphology, receptor expression, antigen processing capacity and function. Immature DCs (iDCs) are proficient in their ability to capture antigen. Once DCs process antigen in the lung and airways, they migrate to the regional lymph nodes and present the antigen to T cells. DCs are divided into two main groups: conventional myeloid DCs (cDC) and non-conventional plasmacytoid DCs (pDC).49 Plasmacytoid DCs, also referred to as type 1 IFN-producing dendritic cells, develop in the bone marrow and are found in blood, secondary lymphoid organs, as well as, peripheral tissues.50 Plasmacytoid DCs are specialized in producing massive amounts of IFNα and IFNβ during an HIV-1 infection.51–53
Conventional myeloid dendritic cells are central to the integration of nonspecific and specific immunity.54,55 These professional antigen presenting cells are located at sites of the body where maximal microbial encounter occurs, such as the skin, gut, and lung.56 Naïve T cells of the adaptive immune response need DCs to become fully activated. Innate immunity against viral infections depends upon type I interferons (interferons disrupt viral replication) from DC’s. IFN-α/β also increases MHC class I expression on virus infected cells and promotes the activation of NK cells.56
The interactions between DCs and CD4 T cells also represents a mechanism by which HIV spreads and infects new target cells. DCs express high levels of the HIV entry receptors CCR5 and CXCR4, as well as low levels of CD4.57 This suggest that DCs may be crucial for HIV transfer to uninfected CD4 T cells. Although DCs can be a vehicle for HIV transmission, DCs are themselves poorly infected compared to T cells. In vitro models have shown that DCs can sequester infectious HIV for several days and efficiently transfer intact virions to CD4 T cells for infection and viral replication.58–60 Several restriction factors have been shown to block HIV replication in DC’s at different stages of infection.61,62 Both cDCs and pDCs display reduced numbers in the blood early after infection and these levels persist in chronic infection.63,64 Due to poor infectivity, it is unlikely that infection by itself explains the reduced DC frequencies. DC numbers may be reduced due to acute and chronic type I IFN secreted upon HIV infection. IFNα can impair cDC differentiation and type I IFN has been shown to regulate pDC number negatively in vivo.65,66
Dendritic cells and their subsets (Table 1) have been shown to have a major role in immune defense against viral infection by generating innate and adaptive immune responses.67 HIV-1 infection mostly occurs through vaginal and rectal routes, which are also areas with high concentrations of DCs and their subsets. DCs capture and internalize invading pathogens, and subsequently process antigen through major histocompatibility complex class I and class II molecules for presentation to CD8 and CD4 T cells, respectively, allowing a bridge between the innate and adaptive immune system. Mucosal DCs allow for effective antigen capture and interaction with effector T cells in lymphoid tissues, facilitating spread of HIV infection to CD4 T cells.
Table 1.
Human dendritic cell (DC) subsets and functions during HIV infection.
| Human skin DC subsets | Human blood DC subsets (cDC) | Human blood DC subsets (pDC) | |||||
|---|---|---|---|---|---|---|---|
| DC subset | LC | CD14+DC | CD1a+DC | BDAC-1 | BDAC3− | BDAC3+ | CD123−pDC |
| Location | Epidermis Gut lumen |
Dermis | Dermis | Blood | Blood Secondary lymphoid organs |
Blood | Blood Secondary lymph organs Peripheral tissue (skin, lung, etc.) |
| Role in HIV infection | Degradation of HIV in Birbeck granules | HIV shuttle and transfer | HIV shuttle and transfer | HIV shuttle and transfer | Unknown | Unknown | Can be infected by HIV |
| Receptors and co-receptors | CD4, cc-chemokine receptor 5 (CCR5), and CXC-chemokine receptor (CXCR4) | ||||||
Upon pathogen encounter, DCs undergo maturation and upregulate molecules on their surface. Some of these molecules include: MHC class II, CD80, CD83, CD86, and CD40.68 Currently, two phases of HIV-1 viral transfer from DCs to T cells have been described.69,70 First, trans-infection where the virus is at or near the donor cell surface and transmitted to a different target cell via the infectious synapse.67,71,72 Secondly, cis-infection where HIV-1 can infect target cells and productively replicate, producing progeny virions. These virions subsequently infect new target cells.67
Neutrophils
Polymononuclear leukocytes (PMNs) are important in host defense during the early innate response against bacterial and fungal infections.73 During an infection, PMNs migrate from the blood to inflamed tissues and trigger the production of reactive oxygen species (ROS) as part of the oxidative burst.73 HIV does not directly infect PMNs but leads to impaired PMN responses, such as phagocytosis, oxidative burst, and bacterial killing. Once PMNs kill a microbe, they die spontaneously through apoptosis. Inappropriate survival of PMNs in HIV infection is thought to cause a chronic inflammatory state with ongoing release of inflammatory mediators.74
In the lung, neutrophils are the most important PMN and are recognized as essential effector cells of the innate immune system. Neutrophils migrate to the draining lymph nodes where they are involved in the induction and regulation of adaptive immune responses by exerting pro-inflammatory or anti-inflammatory functions. PMNs are important effectors of innate immunity. They recognize and phagocytose bacteria and other microorganisms, and additionally they can present antigen and mediate adaptive immune responses. In patients with HIV infection, neutrophil functions, such as chemotaxis, respiratory burst activity, bacterial killing and antibody-dependent cell-mediated cytotoxicity, are impaired.75–79 This suggests that these cells may be responsible for the increased frequency and severity of bacterial and opportunistic infections found in patients. It is still unclear whether these defects are caused directly from infection of these cells by HIV or are secondary to other events.
PMNs are a key component of the early innate response to fungal pathogens. In response to pathogen, PMNs rapidly migrate from the blood to inflamed tissues, where their activation triggers a microbicidal mechanism. Neutrophils have the shortest half-life of all circulating leukocytes. Pitrak et al. demonstrated that the rate of neutrophil apoptosis is accelerated in AIDS patients.80 Furthermore, it has been shown that phagocytosis of apoptotic neutrophils triggers the production of anti-inflammatory mediators from macrophages.81
T cell exhaustion during an HIV infection is associated with increased levels of programmed death- 1 (PD-1) on the surface of CD4 and CD8 T cells.82–85 Binding of PD-1 to its ligands, PD-L1, on cells of myeloid lineage negatively regulates T cell proliferation and production of cytokines.86–89 During HIV infection, neutrophils express increased levels of PD-L1, in addition to high levels of PD-1 on T cells, leading to suppression of T cell function via ROS and PD-1/PD-L1 signaling.90 This suggests that neutrophils contribute to T cell exhaustion and immune suppression during an HIV infection.
Natural Killer Cells
Natural killer (NK) cells are lymphocytes of the innate immune system that effect their activity through granule-mediated killing and cytokine production.91 NK cells are able to recognize various targets without specific sensitization and independent of major histocompatibility complex (MHC) expression. Bone-marrow derived NK cells undergo a maturation process that leads to the acquisition of their effector functions, changes in receptor repertoire, and migration from the bone marrow through the blood to the spleen, liver, lung, and many other organs.92 In healthy lungs, NK cells account for approximately 10% of all lymphocytes.93 NK cells can also be infected with HIV causing a decrease of NK cells in the blood. Decreased numbers of NK cells in blood have been associated with a more rapid progression of HIV. Kelly et al. recently identified a functional interaction between NK cells and CD4 T cells during murine Pneumocystis pneumonia.94 The investigators found that, both in vivo and in vitro, the addition of CD4 T cells to NK cells caused an increase in clearance of Pneumocystis pneumonia and activation of NK cells.94 This observation suggests that NK cells play an important role in pulmonary host defense against opportunistic pathogens and support an adaptive immune response.
Natural killer T (NKT cells) are a lineage of lymphocytes that share characteristics of both T cells and NK cells. NKT cells have been shown to play important roles in various immune responses, including antitumor, autoimmune, and antimicrobial immune responses. Human Vα24 natural killer T (NKT) cells are a CD1-d restricted T lymphocyte lineage and are characterized by co-expression of an invariant and conserved αβ T-cell receptor and the NK cell marker CD161.95 These NKT cells recognize endogenous and exogenous glycolipid antigens presented by CD1d. NKT cells have been reported to be targets of HIV infection and are reduced in peripheral blood mononuclear cell (PBMC) cultures infected with HIV in vitro.96–98 NKT cells are also mainly targeted by CCR5 HIV strains early during HIV infection, suggesting NKT cells may play an important role in establishing HIV infection.95–97 However, in a longitudinal study there was no evidence to support an important role of NKT cells in determining the rate of progression during HIV-1 infection.98 Currently it is still unknown how lung NK cells change during the course of HIV infection and the role of NKT cells in the progression of HIV infection.
DEFECTS IN ADAPTIVE IMMUNITY
Cell-Mediated Immunity
HIV weakens cell mediated immunity by destroying CD4 T cells and impairing the production of new T cells. HIV-1 infection is further characterized by immune cell dysfunctions driven by chronic immune activation. Plasma viral load and CD4 T cell count are two surrogate markers of HIV-1 disease progression and immunologic health. The status of immunologic health is routinely assessed by several quantitative traits that center on CD4 T cells, including absolute CD4 count, CD4 percentage, CD4 counts over time, and thresholds of severe CD4 deficiency. Among immunologic markers of HIV-1 pathogenesis, CD8 activation, CD8 exhaustion, CD4:CD8 ratio, and delayed-type hypersensitivity to recall antigens can also serve as outcome measures that reflect immunologic health.99–104 The blood CD4:CD8 ratio is rarely measured below 1.0 in healthy patients, so an inverted CD4:CD8 ratio is often viewed as clinically relevant.105
Studies have shown an increased percentage of lymphocytes in BAL from HIV-infected individuals compared to uninfected individuals. In the BAL, there are decreased numbers of CD4 lymphocytes and increased numbers of CD8 lymphocytes resulting in a decreased CD4:CD8 lymphocyte ratio.106 An excess of pulmonary lymphocytes (lymphocytic alveolitis) in HIV-infected individuals is associated with pulmonary complications. HIV specific T cells in BAL have impaired proliferative capacity and increased expression of the programmed cell death marker, PD-1.107 Increased PD-1 expression on the HIV-specific T cells was associated with reduced proliferation, which was shown to be reversed with PD-1 blockade.107 This suggests that the HIV-specific T cells in the BAL are exhausted, which is often seen in other chronic viral infections. The lymphocytic alveolitis seen in HIV infection likely represents an influx of dysfunctional HIV-specific CD8 cells. In the peripheral blood higher numbers of lymphocytes are observed during the middle phase of an HIV infection, when CD4 counts are 200 to 500 cells/μL, with lower lymphocyte numbers observed during early and advanced HIV infection.108 Ho et al. have previously shown that the CD4 T cell depletion seen in AIDS is primarily a consequence of the destruction of CD4 T cells and not a lack of their production.109
During infection, CD4 T cells undergo phenotypic and functional impairments, causing an increase in HIV pathogenesis. Viral load correlates with disease progression and the level of immune activation. Changes include elevated expression of activation, exhaustion, and senescent markers. Studies reveal that DNA methylation at the IL2 locus in CD4 T cells plays a role in the dysregulation seen during HIV-1 infection.110 Nakayama and colleagues revealed that the persistent presence of HIV-1 directly affected the ability of memory T cells to produce Th1- and Th17-related cytokines during chronic HIV-1 infection.111 Th17 cells are a subset of CD4 T cells and are characterized by IL-17 production, which is crucial for protection against bacteria and fungi. Studies have shown that Th17 cells are depleted in the blood and lymphoid tissues during an HIV infection. A link between Th17 and Th17/Treg ratio with key HIV-specific CD8 T cell responses against the infection has been identified. NKG2D, an activating receptor of natural killer and CD8 T cells, plays an important role in immune responses against HIV-1.112 NKG2D delivers activating and co-stimulatory signals resulting in cytotoxicity and release of cytokines from NK and CD8 T cells. Recently, lower NKG2D expression was found on both NK and CD8 T cells in HIV-1 infected patients, which suggests that NKG2D may be involved in the control of HIV-1 infection.112
Interleukin 7 is important in early T cell development and homeostatic proliferation of naïve and memory CD8 T cells.113–116 Signaling via the IL7-receptor is mediated through alterations in CD127 expression levels, which are present in high levels on naïve and memory T cells. Reduced expression of CD127 is associated with HIV and other viral infections. Decreases in CD127 expression have been correlated with reduced CD4 T cell numbers, increased viral replication, and immune activation.117–120 In addition to increased plasma IL-7 in HIV infections, the production of IL4 is also increased, which has been shown to decrease CD127 expression on CD8 T cells and thymocytes.121 More research must be done to address the effect of HIV infection on IL-7 levels in the lung.
CD8 and CD4 T cells play a central role in controlling HIV -1 replication and progression to AIDS. However, HIV-1 is associated with a progressive loss of T cell functional capacity including decreased responsiveness to antigenic stimuli, lowered capacity to produce cytokines, and reduced proliferative and cytotoxic activity. Loss of CD4 T cells and functional impairment of CD8 T cells eventually results in a failure of host immune system to maintain control of HIV-1 leading to an accelerated disease progression. HIV-1 specific T cells from rapidly progressing patients exert decreased cytotoxic and proliferative activity and produce reduced levels of TNFα, IL-2, IFNγ, and CD107 compared to T cells from non-progressors.
Humoral Immunity
In healthy individuals, most B cells in peripheral blood are either resting naïve B cells or memory B cells. On the other hand, in HIV-infected individuals, additional populations have been observed, such as: immature transitional B cells, exhausted B cells, activated mature B cells and plasmablasts.122 The increase in these non-traditional B cell populations reflects the non-specific immune activation associated with HIV infection. Infection of individuals with HIV leads to progressive loss of immune functions and defects in humoral immune responses are clearly present. In vitro it has been observed that local immunoglobulin production by B cells depends on stimulation by T cell cytokines secreted as a result of interactions with antigen-primed accessory cells. Since opsonizing antibody activity resides primarily within the IgG isotype, it is possible that increased susceptibility to pulmonary infections caused by encapsulated organisms in HIV-infected patients might be secondary to decreased local IgG concentrations in the lung.123 It has been observed that BAL fluid from HIV-infected subjects contains significantly less IgG than normal BAL. In contrast, IgA and IgM concentrations are relatively unaffected in HIV BAL. BAL fluid from HIV-infected individuals also contains lower concentrations of antigen specific IgG than normal BAL. This defect is due to an impaired ability of HIV-infected AM to induce IgG secretion from B cells, likely a result of enhanced TGF-β secretion by HIV-infected AM.124 While an increase in total B cell numbers is observed, antibodies generated to specific antigens are impaired. B cells from AIDS patients spontaneously secrete immunoglobulin, have impaired proliferation in response to mitogens and do not initiate normal antibody synthesis in response to newly encountered antigens.125
THE MICROBIOME AND HIV INFECTION
The lungs of healthy individuals were previously believed to be sterile, however, a recent study revealed communities of bacteria that were diverse and few in number.126,127 Lozupone et al. showed that the lung of subjects infected with HIV harbored a different community of bacteria, compared to HIV-negative individuals, with Tropheryma whipplei being a dominant species.128 T. whipplei is the causative agent of Whipple’s disease and is often associated with defects in innate immune activation. In this study it was also observed that HIV-infected individuals have a reduced ability to clear T. whipplei once it enters the lung. Characterization of the lung microbiome may provide important pathogenic insights into respiratory illnesses and host defense in HIV-infected patients.
SUMMARY
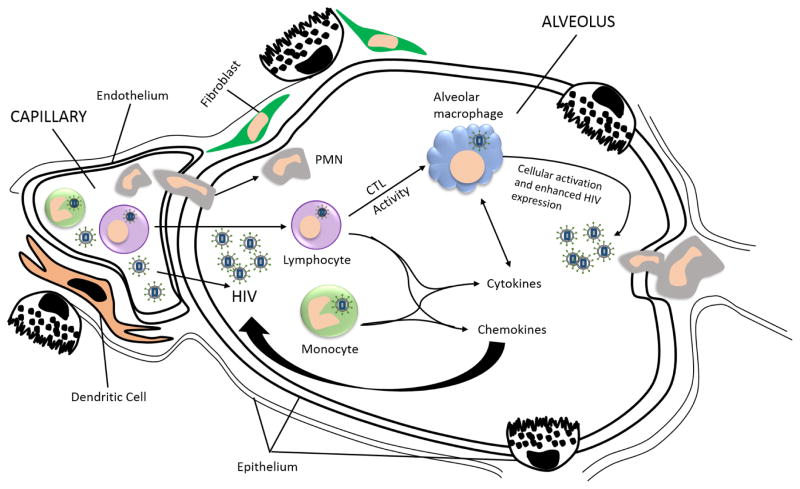
Although the fundamental pathogenic process in HIV infection is depletion of CD4 T cells, multiple components of the pulmonary host defense network are impaired. The observations above suggest that the alveolar space in an HIV-infected individual can be viewed as an environment of inflammatory cellular recruitment, active HIV infection, cytokine release, and cellular activation (Figure 3).5 Once a pathogen enters the HIV+ alveolar space, alveolar macrophages are unable to recognize the pathogen and the host is unable to mount a sufficient cell-mediated or humoral response to prevent infection. Although much is known about how HIV infection alters immune function, more work is required to understand how HIV infection alters specific defense mechanisms of the respiratory tract. In the future, improved understanding of these lung impairments will lead to the development of more effective therapies for opportunistic pulmonary infections.
Figure 3.
Immune reactions to HIV in the alveolar space. The presence of HIV-infected cells, or free virus, in lung tissue stimulates an adaptive immune response and the recruitment of HIV-specific cytotoxic T cells (CTLs). These CTLs accumulate in the alveolar space and release pro-inflammatory cytokines that further activate alveolar macrophages to release additional cytokines leading to further inflammatory cell recruitment. When infected with HIV, alveolar macrophages are compromised in binding and recognizing pathogen and the level of CD4 T cells is decreased.
Figure 2.
Tropism of HIV strains for lung cells. Coreceptors determine viral entry into different cell types. Macrophages express CCR5 and CXCR4, whereas T cell lines only express CXCR4. M-tropic strains infect macrophages using CD4 as the main receptor and the coreceptor CCR5. T tropic stains infect T cells using CXCR4 as the coreceptor. The dual tropic strain is able to use either coreceptor and is therefore able to infect both cell types.
Acknowledgments
The authors thank Derrick Samuelson for review of this manuscript. This work was supported by HL076100 (JES) and UG54-GM104940 (LACaTS Center)
References
- 1.HIV in the United States | Statistics Overview | Statistics Center | HIV/AIDS | CDC. CDC. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. HIVSurveillance Supplemental Report. 2012;17(4) Subpopulations representing 2% or less are not reflected in thischart. Abbreviations: MSM, men who have sex with men; IDU, injection drug user., 2015. at http://www.cdc.gov/hiv/statistics/basics/ataglance.html.) [Google Scholar]
- 2.Beck JM. Abnormalities in host defense associated with HIV infection. Clin Chest Med. 2013;34:143–53. doi: 10.1016/j.ccm.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White NC, Agostini C, Israel-Biet D, Semenzato G, Clarke JR. The growth and the control of human immunodeficiency virus in the lung: implications for highly active antiretroviral therapy. Eur J Clin Invest. 1999;29:964–72. doi: 10.1046/j.1365-2362.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 4.Crowe SM, Carlin JB, Stewart KI, Lucas CR, Hoy JF. Predictive value of CD4 lymphocyte numbers for the development of opportunistic infections and malignancies in HIV-infected persons. J Acquir Immune Defic Syndr. 1991;4:770–6. [PubMed] [Google Scholar]
- 5.Shellito JE. Failure of host defenses in human immunodeficiency virus. Semin Respir Crit Care Med. 2004;25:73–84. doi: 10.1055/s-2004-822307. [DOI] [PubMed] [Google Scholar]
- 6.Naif HM. Pathogenesis of HIV Infection. Infect Dis Rep. 2013:5. doi: 10.4081/idr.2013.s1.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milgrim LM, Rubin JS, Small CB. Mucociliary clearance abnormalities in the HIV-infected patient: a precursor to acute sinusitis. Laryngoscope. 1995;105:1202–8. doi: 10.1288/00005537-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Montella F, Pezzotti P, Di Sora F, Recchia O, Lauria F, Rezza G. Improving the prognostic value of CD4+ count using IgA and clinical signs in HIV-seropositive i.v. drug users. Infection. 1997;25:117–20. doi: 10.1007/BF02113591. [DOI] [PubMed] [Google Scholar]
- 9.Gordon SB, Read RC. Macrophage defences against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Mosser DM. Receptors on phagocytic cells involved in microbial recognition. Immunol Ser. 1994;60:99–114. [PubMed] [Google Scholar]
- 11.Iordanskiy S, Santos S, Bukrinsky M. Nature, nurture and HIV: The effect of producer cell on viral physiology. Virology. 2013;443:208–13. doi: 10.1016/j.virol.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas W, Herbein G. T-Cell Signaling in HIV-1 Infection. Open Virol J. 2013;7:57–71. doi: 10.2174/1874357920130621001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A, Herbein G. The macrophage: a therapeutic target in HIV-1 infection. Mol Cell Ther. 2014;2:10. doi: 10.1186/2052-8426-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe SM, Mills J, Kirihara J, Boothman J, Marshall JA, McGrath MS. Full-length recombinant CD4 and recombinant gp120 inhibit fusion between HIV infected macrophages and uninfected CD4-expressing T-lymphoblastoid cells. AIDS Res Hum Retroviruses. 1990;6:1031–7. doi: 10.1089/aid.1990.6.1031. [DOI] [PubMed] [Google Scholar]
- 15.Crowe SM, Mills J, Elbeik T, et al. Human immunodeficiency virus-infected monocyte-derived macrophages express surface gp120 and fuse with CD4 lymphoid cells in vitro: a possible mechanism of T lymphocyte depletion in vivo. Clin Immunol Immunopathol. 1992;65:143–51. doi: 10.1016/0090-1229(92)90217-c. [DOI] [PubMed] [Google Scholar]
- 16.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–3. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 17.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–41. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter CA, Ehrlich LS. Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008;62:425–43. doi: 10.1146/annurev.micro.62.081307.162758. [DOI] [PubMed] [Google Scholar]
- 19.Gavegnano C, Kennedy EM, Kim B, Schinazi RF. The Impact of Macrophage Nucleotide Pools on HIV-1 Reverse Transcription, Viral Replication, and the Development of Novel Antiviral Agents. Mol Biol Int. 2012;2012:625983. doi: 10.1155/2012/625983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–42. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 22.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 23.Hrecka K, Hao C, Gierszewska M, et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–61. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahouassa H, Daddacha W, Hofmann H, et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13:223–8. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbein G, Gras G, Khan KA, Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das SR, Jameel S. Biology of the HIV Nef protein. Indian J Med Res. 2005;121:315–32. [PubMed] [Google Scholar]
- 27.Lamers SL, Fogel GB, Singer EJ, et al. HIV-1 Nef in macrophage-mediated disease pathogenesis. Int Rev Immunol. 2012;31:432–50. doi: 10.3109/08830185.2012.737073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–31. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- 29.Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noonan D, Albini A. From the outside in: extracellular activities of HIV Tat. Adv Pharmacol. 2000;48:229–50. doi: 10.1016/s1054-3589(00)48008-7. [DOI] [PubMed] [Google Scholar]
- 31.Gautier VW, Gu L, O’Donoghue N, Pennington S, Sheehy N, Hall WW. In vitro nuclear interactome of the HIV-1 Tat protein. Retrovirology. 2009;6:47. doi: 10.1186/1742-4690-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 33.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–52. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 34.Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 35.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 36.Subbramanian RA, Kessous-Elbaz A, Lodge R, et al. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J Exp Med. 1998;187:1103–11. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–85. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacquot G, Le Rouzic E, David A, et al. Localization of HIV-1 Vpr to the nuclear envelope: impact on Vpr functions and virus replication in macrophages. Retrovirology. 2007;4:84. doi: 10.1186/1742-4690-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varin A, Decrion AZ, Sabbah E, et al. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J Biol Chem. 2005;280:42557–67. doi: 10.1074/jbc.M502211200. [DOI] [PubMed] [Google Scholar]
- 40.Faherty CS, Maurelli AT. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008;16:173–80. doi: 10.1016/j.tim.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hume DA. Bring out your dead. Nat Immunol. United States. 2008:12–4. doi: 10.1038/ni0108-12. [DOI] [PubMed] [Google Scholar]
- 43.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin CJ, Booty MG, Rosebrock TR, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torre D, Gennero L, Baccino FM, Speranza F, Biondi G, Pugliese A. Impaired macrophage phagocytosis of apoptotic neutrophils in patients with human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 2002;9:983–6. doi: 10.1128/CDLI.9.5.983-986.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin WJ, 2nd, Pasula R. Role of alveolar macrophages in host defense against Pneumocystis carinii. Am J Respir Cell Mol Biol. 2000;23:434–5. doi: 10.1165/ajrcmb.23.4.f203. [DOI] [PubMed] [Google Scholar]
- 47.Koziel H, Li X, Armstrong MY, Richards FF, Rose RM. Alveolar macrophages from human immunodeficiency virus-infected persons demonstrate impaired oxidative burst response to Pneumocystis carinii in vitro. Am J Respir Cell Mol Biol. 2000;23:452–9. doi: 10.1165/ajrcmb.23.4.4084. [DOI] [PubMed] [Google Scholar]
- 48.Peebles RS, Jr, Graham BS. Viruses, dendritic cells and the lung. Respir Res. 2001;2:245–9. doi: 10.1186/rr63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilfried Posch CL-FraDW. Current Perspectives in HIV Infection. 2013. [Google Scholar]
- 50.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 51.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 52.Fanning SL, George TC, Feng D, et al. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J Immunol. 2006;177:5829–39. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- 53.Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fazekas de St Groth B. The evolution of self-tolerance: a new cell arises to meet the challenge of self-reactivity. Immunol Today. 1998;19:448–54. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- 55.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 56.Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur Respir J. 2001;18:692–704. [PubMed] [Google Scholar]
- 57.Manches O, Frleta D, Bhardwaj N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. 2014;35:114–22. doi: 10.1016/j.it.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 59.Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–7. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 60.Pope M, Betjes MG, Romani N, et al. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–98. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 61.Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–7. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- 62.Grutter MG, Luban J. TRIM5 structure, HIV-1 capsid recognition, and innate immune signaling. Curr Opin Virol. 2012;2:142–50. doi: 10.1016/j.coviro.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabado RL, O’Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–52. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 65.Kodama A, Tanaka R, Zhang LF, et al. Impairment of in vitro generation of monocyte-derived human dendritic cells by inactivated human immunodeficiency virus-1: Involvement of type I interferon produced from plasmacytoid dendritc cells. Hum Immunol. 2010;71:541–50. doi: 10.1016/j.humimm.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011;208:2367–74. doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed Z, Kawamura T, Shimada S, Piguet V. The role of human dendritic cells in HIV-1 infection. J Invest Dermatol. 2015;135:1225–33. doi: 10.1038/jid.2014.490. [DOI] [PubMed] [Google Scholar]
- 68.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 69.Turville SG, Santos JJ, Frank I, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–9. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 70.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–68. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia E, Pion M, Pelchen-Matthews A, et al. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic. 2005;6:488–501. doi: 10.1111/j.1600-0854.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 72.Choudhuri K, Llodra J, Roth EW, et al. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–23. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Gabelloni ML, Trevani AS, Sabatte J, Geffner J. Mechanisms regulating neutrophil survival and cell death. Semin Immunopathol. 2013;35:423–37. doi: 10.1007/s00281-013-0364-x. [DOI] [PubMed] [Google Scholar]
- 75.Ellis M, Gupta S, Galant S, et al. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. J Infect Dis. 1988;158:1268–76. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- 76.Lazzarin A, Uberti Foppa C, Galli M, et al. Impairment of polymorphonuclear leucocyte function in patients with acquired immunodeficiency syndrome and with lymphadenopathy syndrome. Clin Exp Immunol. 1986;65:105–11. [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy PM, Lane HC, Fauci AS, Gallin JI. Impairment of neutrophil bactericidal capacity in patients with AIDS. J Infect Dis. 1988;158:627–30. doi: 10.1093/infdis/158.3.627. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen H, Kharazmi A, Faber V. Blood monocyte and neutrophil functions in the acquired immune deficiency syndrome. Scand J Immunol. 1986;24:291–6. doi: 10.1111/j.1365-3083.1986.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 79.Pitrak DL, Bak PM, DeMarais P, Novak RM, Andersen BR. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406–10. doi: 10.1093/infdis/167.6.1406. [DOI] [PubMed] [Google Scholar]
- 80.Pitrak DL, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996;98:2714–9. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fichtenbaum CJ, Woeltje KF, Powderly WG. Serious Pseudomonas aeruginosa infections in patients infected with human immunodeficiency virus: a case-control study. Clin Infect Dis. 1994;19:417–22. doi: 10.1093/clinids/19.3.417. [DOI] [PubMed] [Google Scholar]
- 82.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 83.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 84.El-Far M, Halwani R, Said E, et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5:13–9. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 85.Rosignoli G, Cranage A, Burton C, et al. Expression of PD-L1, a marker of disease status, is not reduced by HAART in aviraemic patients. Aids. 2007;21:1379–81. doi: 10.1097/QAD.0b013e3281de7296. [DOI] [PubMed] [Google Scholar]
- 86.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carter L, Fouser LA, Jussif J, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–43. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 88.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wherry EJ, Ha SJ, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–84. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Bowers NL, Helton ES, Huijbregts RP, Goepfert PA, Heath SL, Hel Z. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog. 2014;10:e1003993. doi: 10.1371/journal.ppat.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mortha A, Diefenbach A. Natural killer cell receptor-expressing innate lymphocytes: more than just NK cells. Cell Mol Life Sci. 2011;68:3541–55. doi: 10.1007/s00018-011-0803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi F-D, Ljunggren H-G, Cava AL, Kaer LV. Organ-specific features of natural killer cells. Nature Reviews Immunology. 2011;11:658–71. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stegmann KA, Bjorkstrom NK, Veber H, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–97. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 94.Kelly MN, Zheng M, Ruan S, Kolls J, D’Souza A, Shellito JE. Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J Immunol. 2013;190:285–95. doi: 10.4049/jimmunol.1200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moll M, Snyder-Cappione J, Spotts G, Hecht FM, Sandberg JK, Nixon DF. Expansion of CD1d-restricted NKT cells in patients with primary HIV-1 infection treated with interleukin-2. Blood. 2006:3081–3. doi: 10.1182/blood-2005-09-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandberg JK, Fast NM, Palacios EH, et al. Selective Loss of Innate CD4+ Vα24 Natural Killer T Cells in Human Immunodeficiency Virus Infection. J Virol. 2002:7528–34. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted Human Natural Killer T Cells Are Highly Susceptible to Human Immunodeficiency Virus 1 Infection. J Exp Med. 2002:869–79. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hans JJ, van der Vliet BMEvB, Mette D, Hazenberg, Nishi Nobusuke, Otto Sigrid A, van Benthem Birgit H, Prins Maria, Claessen Frans A, van den Eertwegh Alfons JM, Giaccone Giuseppe, Miedema Frank, Scheper Rik J, Pinedo Herbert M. Selective decrease in circulating Vα24 Vβ11 NKT cells during HIV type 1 infection. Journal of immunology (Baltimore, Md: 1950) 2002:168. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 99.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 100.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–6. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 101.Taylor JM, Fahey JL, Detels R, Giorgi JV. CD4 percentage, CD4 number, and CD4:CD8 ratio in HIV infection: which to choose and how to use. J Acquir Immune Defic Syndr. 1989;2:114–24. [PubMed] [Google Scholar]
- 102.Zaman MM, Recco RA, Raguthu L, Likki S, Reddy S. Characteristics of HIV-1-infected patients with CD4:CD8 lymphocyte ratio normalization on antiretroviral therapy. AIDS Patient Care STDS. 2000;14:647–9. doi: 10.1089/10872910050206568. [DOI] [PubMed] [Google Scholar]
- 103.Margolick JB, Gange SJ, Detels R, O’Gorman MR, Rinaldo CR, Jr, Lai S. Impact of inversion of the CD4/CD8 ratio on the natural history of HIV-1 infection. J Acquir Immune Defic Syndr. 2006;42:620–6. doi: 10.1097/01.qai.0000223028.55080.9d. [DOI] [PubMed] [Google Scholar]
- 104.Pahwa S, Read JS, Yin W, et al. CD4+/CD8+ T cell ratio for diagnosis of HIV-1 infection in infants: Women and Infants Transmission Study. Pediatrics. 2008;122:331–9. doi: 10.1542/peds.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–83. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 106.Tang J, Li X, Price MA, et al. CD4:CD8 lymphocyte ratio as a quantitative measure of immunologic health in HIV-1 infection: findings from an African cohort with prospective data. Front Microbiol. 2015;6:670. doi: 10.3389/fmicb.2015.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neff CP, Chain JL, MaWhinney S, et al. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am J Respir Crit Care Med. 2015;191:464–73. doi: 10.1164/rccm.201408-1521OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Twigg HL, Soliman DM, Day RB, et al. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;159:1439–44. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 109.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 110.Nakayama-Hosoya K, Ishida T, Youngblood B, et al. Epigenetic repression of interleukin 2 expression in senescent CD4+ T cells during chronic HIV type 1 infection. J Infect Dis. 2015;211:28–39. doi: 10.1093/infdis/jiu376. [DOI] [PubMed] [Google Scholar]
- 111.Nakayama K, Nakamura H, Koga M, et al. Imbalanced production of cytokines by T cells associates with the activation/exhaustion status of memory T cells in chronic HIV type 1 infection. AIDS Res Hum Retroviruses. 2012;28:702–14. doi: 10.1089/aid.2011.0073. [DOI] [PubMed] [Google Scholar]
- 112.Giuliani E, Vassena L, Cerboni C, Doria M. Release of Soluble Ligands for the Activating NKG2D Receptor: One More Immune Evasion Strategy Evolved by HIV-1 ? Curr Drug Targets. 2015 doi: 10.2174/1389450116666150630110329. [DOI] [PubMed] [Google Scholar]
- 113.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 114.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 115.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lang KS, Recher M, Navarini AA, et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–45. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 118.Koesters SA, Alimonti JB, Wachihi C, et al. IL-7Ralpha expression on CD4+ T lymphocytes decreases with HIV disease progression and inversely correlates with immune activation. Eur J Immunol. 2006;36:336–44. doi: 10.1002/eji.200535111. [DOI] [PubMed] [Google Scholar]
- 119.Boutboul F, Puthier D, Appay V, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. Aids. 2005;19:1981–6. doi: 10.1097/01.aids.0000191919.24185.46. [DOI] [PubMed] [Google Scholar]
- 120.Golden-Mason L, Burton JR, Jr, Castelblanco N, et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098–109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 121.Crawley AM, Angel JB. The influence of HIV on CD127 expression and its potential implications for IL-7 therapy. Semin Immunol. 2012;24:231–40. doi: 10.1016/j.smim.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 122.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–45. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wilkes DS, Weissler JC. Alloantigen-induced immunoglobulin production in human lung: differential effects of accessory cell populations on IgG synthesis. Am J Respir Cell Mol Biol. 1994;10:339–46. doi: 10.1165/ajrcmb.10.3.8117452. [DOI] [PubMed] [Google Scholar]
- 124.Twigg HL, 3rd, Spain BA, Soliman DM, Bowen LK, Heidler KM, Wilkes DS. Impaired IgG production in the lungs of HIV-infected individuals. Cell Immunol. 1996;170:127–33. doi: 10.1006/cimm.1996.0142. [DOI] [PubMed] [Google Scholar]
- 125.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 126.Baughman RP, Thorpe JE, Staneck J, Rashkin M, Frame PT. Use of the protected specimen brush in patients with endotracheal or tracheostomy tubes. Chest. 1987;91:233–6. doi: 10.1378/chest.91.2.233. [DOI] [PubMed] [Google Scholar]
- 127.Thorpe JE, Baughman RP, Frame PT, Wesseler TA, Staneck JL. Bronchoalveolar lavage for diagnosing acute bacterial pneumonia. J Infect Dis. 1987;155:855–61. doi: 10.1093/infdis/155.5.855. [DOI] [PubMed] [Google Scholar]
- 128.Lozupone C, Cota-Gomez A, Palmer BE, et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187:1110–7. doi: 10.1164/rccm.201211-2145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]