Abstract
Background
Antiprogrammed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) therapies have demonstrated promising activity in advanced head and neck squamous cell carcinoma (HNSCC), with overall response rates of approximately 20% in unselected populations and survival benefit. Whether induction docetaxel, platinum and fluorouracil (TPF) modifies PD-L1 expression or tumour immune infiltrates is unknown.
Patients and methods
Patients with locally advanced HNSCC treated at Gustave Roussy (Villejuif, France) between 2006 and 2013 by induction TPF followed by surgery were retrospectively considered. Patients with paired samples (pre-TPF and post-TPF) were kept for further analysis. PD-L1 expression was quantified by immunohistochemistry according to a validated protocol. The objective of the study was to compare PD-L1 expression on tumour cells (TC) and immune cells (IC) (positivity threshold of ≥5%) before and after TPF. CD8+ and Foxp3+ lymphocytes densities before and after TPF were also quantified.
Results
Out of 313 patients receiving induction TPF, 86 underwent surgery; paired samples were available for 21 of them. Baseline PD-L1 expression was ≥5% in two and five samples for TC and IC, respectively. A significant increase of PD-L1 expression was observed after TPF, with 15 samples (71%) presenting a positive staining in IC after induction chemotherapy (P=0.003; Wilcoxon rank-sum test) and eight samples (38%) in TC (P=0.005; Wilcoxon rank-sum test). Tumour-infiltrating CD8+ mean densities also significantly increased post-TPF (P=0.01). There was no significant difference in Foxp3+ expression, CD8/Foxp3 ratio or correlation with outcome.
Conclusion
TPF induction chemotherapy in advanced HNSCC increases PD-L1 positivity on tumour-infiltrating ICs, as well as CD8+ lymphocytes density. These results warrant independent validation on larger datasets and might help therapeutic strategy in advanced HNSCC.
Keywords: head and neck squamous cell carcinoma, pd-l1, immunotherapy, induction chemotherapy, immune checkpoint blockers
Key questions.
What is already known about this subject?
Head and neck squamous cell carcinoma is associated with poor outcomes and better therapeutic strategies are needed.
Antiprogrammed cell death-1/programmed cell death-ligand 1 (PD-1/PD-L1) have shown efficacy in head and neck squamous cell carcinoma (HNSCC), with a 20% response rate in unselected populations and overall survival benefit.
PD-L1 expression and CD8+ T cell density are predictive biomarkers for anti-PD-1/PD-L1 efficacy.
Some cytotoxic chemotherapies have immunomodulatory actions.
What does this study add?
Docetaxel, platinum and fluorouracil (TPF) induction chemotherapy increases PD-L1 expression in tumour-infiltrating immune cells and in tumour cells.
TPF induction chemotherapy increases density of tumour-infiltrating CD8+ T cells.
TPF induction chemotherapy does not significantly modify density of tumour-infiltrating Foxp3+ regulatory T cells.
How might this impact on clinical practice?
Optimal therapeutic sequence between conventional cytotoxic chemotherapy and novel immune therapies still has to be defined.
Upregulation of PD-L1 and CD8+ T cell by TPF induction chemotherapy suggests that combination strategies of concomitant cytotoxic therapies and anti-PD-1/PD-L1 therapies might be relevant for HNSCC.
This approach may warrant clinical evaluation in dedicated trials.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the fifth most common cancer worldwide. Approximately 60% of patients present with advanced disease at diagnosis, and the 5-year survival rate is approximately 30%.1 One of the therapeutic strategies for locally advanced HNSCC consists in a combination of induction chemotherapy, surgery and adjuvant chemoradiotherapy. Docetaxel, platinum and fluorouracil (TPF) is the standard induction regimen, allowing organ preservation, reduction of distant metastases and time to treatment failure.2 3 However, it failed to improve overall survival (OS) in randomised phase III trials and was associated with higher toxicity.4 Alternative strategies are therefore urgently needed.
Programmed cell death-1 (PD-1) is an immune checkpoint that is expressed primarily on activated T cells. PD-1 binds to two ligands, PD-L1 (B7-H1 and CD274) and PD-L2 (B7-H2),5 that are expressed on tumour cells (TCs) as well as stromal immune cells (ICs), including lymphocytes and macrophages. The interaction between PD-1 and PD-L1 downregulates cytokine production and T cell activation, thereby promoting tumour immune escape. Blockade of immune checkpoints can restore antitumour immunity, notably by enhancing T cell response. Over the last 2 years, the advent of immune checkpoint blockers (ICB) targeting notably PD-1 or PD-L1 has revolutionised cancer treatment by leading to prolonged tumour response and OS improvements in several histologies. This led notably to the approval of Opdivo (Nivolumab, Bristol-Meyers Squibb) in non-small cell lung cancer (NSCLC), melanoma and renal cell carcinoma,6–9 as well as Keytruda (pembrolizumab, Merck Sharp & Dohme) in melanoma and PD-L1 positive NSCLC10 11 and Imfinzi (Durvalumab, Astrazeneca) in urothelial bladder cancer and NSCLC.12 13 The T cell inflamed phenotype of HNSCC (PD-L1 expression and tumour infiltrating lymphocytes) favours immune escape.14 There is robust evidence for a role of the PD-1/PD-L1 pathway in immune escape of HNSCC,15 and efficacy of ICB in these tumours is promising, with objective response rates of approximately 20% in unselected pretreated patients16 17 and a longer OS compared with standard chemotherapy in recurrent HNSCC.18
Although there is currently no validated biomarker for predicting activity of PD-L1 ICB in HNSCC, PD-L1 positivity on tumour or ICs as well as the nature and density of the immune infiltrates (notably CD8+ lymphocytes) are the most robustly validated predictive biomarkers of anti-PD-1/PD-L1 inhibitors efficacy. Recent publications describe a potential interplay between the action of conventional cytotoxic chemotherapy and modulation of immune tolerance. Notably, some cytotoxic agents such as paclitaxel, etoposide and 5-fluorouracil have been reported to induce PD-L1 surface expression in breast cancer cells and have the ability to activate cell autonomous immunogenic pathways.19 Cisplatin,20 BRAF inhibitors21 or EGFR inhibitors22 have also been described as being able to promote PD-L1 expression in various tumour types.
In this context, we aimed at assessing whether administering TPF induction chemotherapy in locally advanced HNSCC would modulate PD-L1 expression on tumour and tumour-infiltrating ICs, as well as the density of the CD8+ and Foxp3+ immune infiltrates.
Materials and methods
Patients’ selection and sample collection
We retrospectively analysed all patients with locally advanced larynx and oral cavity HNSCC treated by induction chemotherapy and followed by surgery at Gustave Roussy Cancer Centre (Villejuif, France) between 2006 and 2013. All patients were treatment naïve before induction. For each patient, we selected two paired tumour samples: one representative biopsy of the primary tumour before treatment and one representative block of the resected primary tumour after three cycles of TPF. There was no metastatic lesion in samples. All samples were fixed in 10% neutral buffered formalin and embedded in paraffin (FFPE).
Immunohistochemistry (IHC) for evaluation of PD-L1, CD8 and Foxp3 expression
IHC was performed on 4 µm thick sections from formalin-fixed parafin-embedded (FFPE) blocks using a validated standard protocol on a Ventana Discovery Ultra autostainer (Ventana Medical Systems, Roche Tissue Diagnostics, Tucson, Arizona, USA). Briefly, sections were deparaffinised, and epitope retrieval was performed in CC1 buffer for 32–60 min depending on the antibodies. Primary anti-PD-L1 (clone E1L3N, dilution 1 µg/mL, Cell Signalling Technology), anti-CD8 (clone SP16, dilution 1 µg/mL, Spring Bioscience) or anti-Foxp3 (clone SP97, dilution 1 µg/mL, Spring Bioscience) antibodies were incubated for 1 hour at room temperature. Detection was performed using either an Ultra-MAP kit (Ventana) for CD8 and Foxp3 stainings or an HQ-amplification kit (Ventana) for PD-L1 staining, both coupled with horseradish peroxidase. 3,3′-Diaminobenzidine (DAB) was used as a chromogen, and slides were counterstained with haematoxylin. An experimented pathologist assessed quantitatively PD-L1 expression in TC and IC as the percentage of cells with moderate to strong membranous staining. Following recommendations described in Herbst et al23 a ≥5% staining was considered as positive for TC and IC. Surface ulcerations or foreign body type giant cell reaction associated with stromal keratin depositions were excluded from IC evaluation.
Image analysis
Image acquisition was performed with a Virtual Slides VS120-SL microscope (Olympus, Tokyo, Japan), 20× air objective (0.75 NA). CD8 lymphocytes were detected using an algorithm created in Definiens Developer software. Image analysis was performed after manual selection of the regions of interest by the senior pathologist. As these regions were too large to be assessed in totality, they were divided into blocks of pixels that were processed individually and finally stitched. The method combined a watershed segmentation of DAB staining together with colour and morphological characteristics to automatically retrieve the CD8 stained cells. The number of CD8 stained lymphocytes per µm2 was retrieved for each analysed tissue. Foxp3-positive lymphocytes were detected using an algorithm for nuclei detection in Tissue Studio (Definiens) software. Briefly, nuclei stained with DAB were automatically detected according to their IHC staining spectral properties in the regions of interest already defined for CD8 analysis. The number of Foxp3 stained nuclei per µm2 was retrieved for each analysed tissue.
Statistical analysis
Statistical significance of PD-L1, CD8 and Foxp3 expression was evaluated using Wilcoxon rank-sum test (with exact P value). OS was calculated from the start of treatment with TPF to the date of death or the last follow-up (censored data). Progression-free survival (PFS) was calculated from the start of TPF induction to the date of disease progression, death or the last follow-up (censored data). Survival curves were estimated using Kaplan-Meier methods, and 95% CI for point survival estimates were calculated according to Rothman. Median follow-up was estimated using inverse Kaplan-Meier method. The prognosis role of PDL1 in PFS and OS was evaluated with log-rank test. Correlation between PDL1 and response to treatment were evaluated with χ2 test. All tests were two sided, and a P value <0.05 was considered statistically significant. Statistical analyses were performed using SAS software (V.9.1).
Results
Patient characteristics
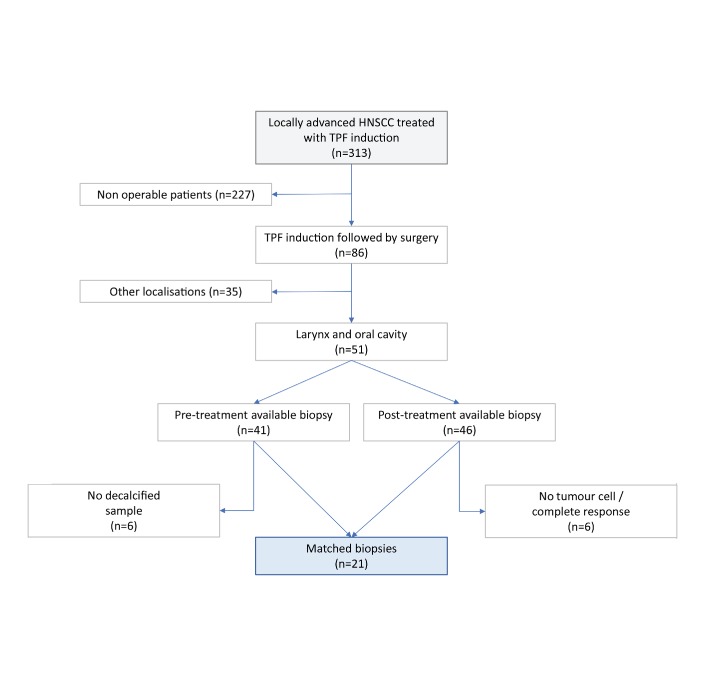
Between 2006 and 2013, 313 patients diagnosed with locally advanced HNSCC were treated with TPF induction at Gustave Roussy. Eighty-six (27%) of them had surgery after CT, including 51 with laryngeal and oral cavity tumours. Paired biopsies (pre-TPF and post-TPF) were available for 21 patients and selected for further analysis (figure 1). Fourteen patients (67%) were men (14 patients; 67%) and 20 patients (95%) had an Eastern Cooperative Oncology Group performance status of 0–1. The median age was 54 (range: 37–84). All patients but one had alcohol drinking and/or smoking history. Thirteen patients (62%) had an oral primary site, and eight patients (38%) had a laryngeal primary site. Others characteristics are summarised in table 1. All patient had a history of heavy drinking and smoking; HPV status was unavailable.
Figure 1.
Flow chart of the study. Description of the process that was used for sample selection; out of 313 patients, matched biopsies were available for 21 of them. HNSCC, head and neck squamous cell carcinoma; TPF, docetaxel, platinum and fluorouracil.
Table 1.
Clinicopathological characteristics of patients with HNSCC included in the study (n=21)
| n | % | |
| Sex | ||
| Men | 14 | 67 |
| Women | 7 | 33 |
| Smoking status | ||
| No | 4 | 19 |
| Yes | 17 | 81 |
| Alcohol | ||
| No | 12 | 57 |
| Yes | 9 | 43 |
| Age (years) (median) | 54 | (37–84) |
| ECOG performance status | ||
| 0 | 8 | 38 |
| 1 | 12 | 57 |
| 2 | 1 | 5 |
| Tumour site | ||
| Oral cavity | 13 | 62 |
| Larynx | 8 | 38 |
| Tumour size | ||
| T1 | 1 | 5 |
| T2 | 2 | 10 |
| T3 | 7 | 33 |
| T4 | 11 | 52 |
| Node involvement | ||
| N0 | 7 | 33 |
| N1 | 1 | 5 |
| N2 | 12 | 57 |
| N3 | 1 | 5 |
ECOG, Eastern Cooperative Oncology Group.
TPF induction chemotherapy increases PD-L1 expression in tumour-infiltrating ICs and in tumour cells
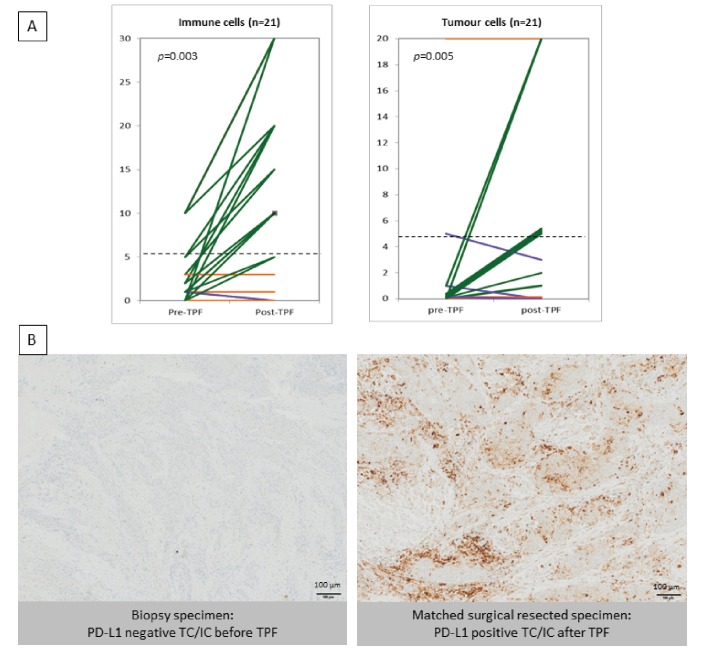
When assessing PD-L1 expression using the 5% cut-off for positivity,23 five samples (24%) were positive in IC at baseline. A significant increase of PD-L1 expression in IC was observed after TPF, with 15 samples (71%) being positive after induction chemotherapy (P=0.003, Wilcoxon rank-sum test) (figure 2A,B, table 2). At baseline, PD-L1 expression was infrequent in TC, with only two positive samples (9.5%). After induction chemotherapy, eight samples (38%) were positive in TC (P=0.005; Wilcoxon rank-sum test) (figure 2A,B, table 2). Overall, a significant increase in PD-L1 expression was observed in both TC and IC post-TPF induction chemotherapy.
Figure 2.
Expression of PD-L1 (%) in immune cells (ICs) and tumour cells before (pre-TPF) and after (post-TPF) induction chemotherapy. In the right panel, 10 samples are in the superimposed lines. (A) PD-L1 expression in stromal ICs and tumour cells (TC) in 21 patients, before and after taxotere, cisplatin and fluorouracile (TPF) triplet induction chemotherapy. The cut-off for positivity was 5% of positive cells (dotted line). Patients’ tumours with increased expression of PD-L1 after TPF are depicted in green, whereas tumours with stable and decreased expression are depicted in orange and purple, respectively. TPF increased PD-L1 expression in IC (P=0.003) and in TC (P=0.005). (B) Representative images of paired cases between biopsy specimen pre-TPF and matched surgical resected specimen post-TPF. (Virtual Slide VS120-SL microscope (Olympus, Tokyo, Japan), 20× air objective (0.75 NA)). PD-L1, programmed cell death-ligand 1; TPF, docetaxel, platinum and fluorouracil.
Table 2.
IHC staining results before and after TPF induction chemotherapy
| Paired samples | Pre-TPF | Post-TPF | P (Wilcoxon) | |||||
| Positive ≥1% (n (%)) |
Positive ≥5% (n (%)) |
Mean intensity (% (range)) |
Positive ≥1% (n (%)) |
Positive ≥5% (n (%)) |
Mean intensity (% (range)) |
|||
| IC PD-L1 expression | 21 | 15 (4) | 5 (24) | 3 (0–10) | 17 (80) | 15 (71) | 12 (0–30) | 0.003 |
| TC PD-L1 expression | 4 (19) | 2 (9.5) | 1 (0–20) | 13 (61) | 8 (38) | 4 (0–20) | 0.005 | |
| Mean density (n/mm2) (range) | Mean density (n/mm2) (range) | |||||||
| CD8+ cell density | 17 | 237 (4–779) | 512 (53–1190) | 0.01 | ||||
| Foxp3 cell density | 84 (26–180) | 164 (4–467) | 0.09 | |||||
| CD8+/ Foxp 3 ratio | 2.48 (0.03–6.27) | 6.23 (0.52–41.3) | 0.127 | |||||
ICs, immune cells; IHC, immunohistochemistry; PD-L1, programmed cell death-ligand 1; TC, tumour cells; TPF, docetaxel, platinum and fluorouracil.
TPF induction chemotherapy increases density of CD8+ lymphocytes
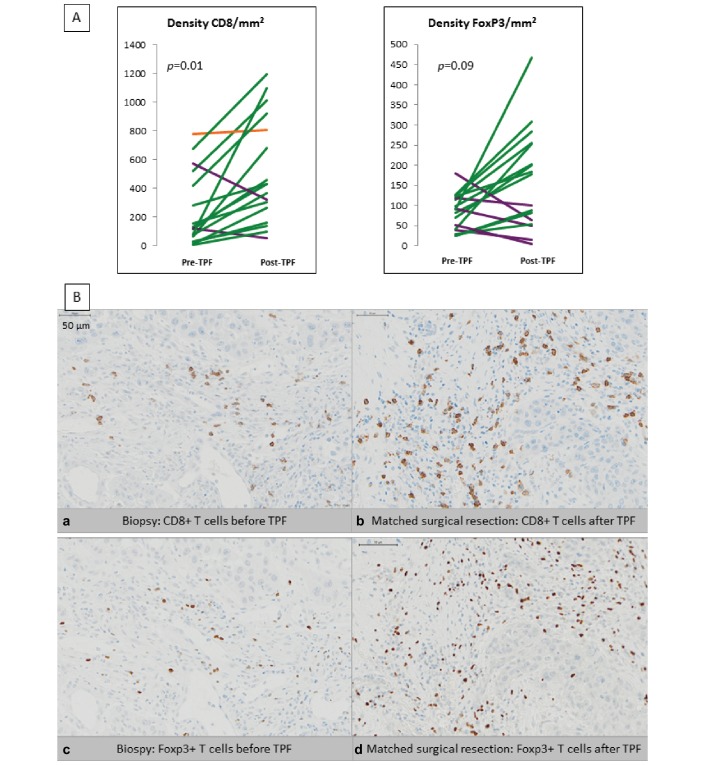
CD8 is a marker of cytotoxic lymphocytes, which have been involved in response to PD-1/PD-L1 inhibitors.24 Foxp3 is a marker of regulatory T cells, the role of which within the tumour bed is still debated.25 CD8 and Foxp3 IHC staining was analysed on 17 of the 21 patients included, as tumour material was insufficient for four patients (figure 3A,B). A statistically significant increase of the median density of CD8+ lymphocytes was observed after TPF induction therapy (237 cells/mm2 vs 512 cell/mm2; P=0.01); a trend to increase in Foxp3+ lymphocytes was noted as well (84 cells/mm2 vs 164 cell/mm2; t-test: P=0.09), although without reaching statistical significance (table 2). The CD8+/Foxp3+ lymphocytes ratio—an indicator of favourable outcome and sensitivity to anti-PD-1/PD-L1 in solid tumours26 27 was determined for each tumour. The mean CD8+/Foxp3+ ratio increased from 2.48 pre-TPF to 6.23 post-TPF (NS; P=0.127) (table 2). No significant correlation could be found between PD-L1 expression and immune infiltrate at baseline, or between the increase in PD-L1 expression in tumour-infiltrating ICs and the increase in CD8+ and Foxp3+ populations (data not shown).
Figure 3.
Mean density of CD8+ and Foxp3+ lymphocytes in tumour infiltrate before (pre-TPF) and after (post-TPF) induction chemotherapy, (A) Patients’ tumours with increased expression of CD8+/Foxp3+ lymphocytes after TPF are represented in green; patient’s tumours with stable and decreased expression are depicted in orange and purple, respectively. At baseline, mean CD8+ lymphocytes density was 237/mm2 and increased after TPF (512/mm2) (P=0.01). At baseline, mean Foxp3+ lymphocytes mean density was 84/mm2 and increased after TPF (164/mm2) (P=0.09). (B) Representative staining images: (a and b) CD8+ lymphocytes before induction chemotherapy (a) increased after induction chemotherapy (b). (c and d) Foxp3+ lymphocytes before induction chemotherapy (c) increased after induction chemotherapy (d). (Virtual Slide VS120-SL microscope (Olympus, Tokyo, Japan), 20× air objective (0.75 NA)). TPF, docetaxel, platinum and fluorouracil.
Association between PD-L1 expression and patient outcome
The median follow-up was 6.4 years (range: 1.9–8.2). At the time of the analysis, 11 patients were still alive (52%). Median OS was 3.4 years (95% CI 2.3–not reached). Median PFS was 2.9 years (95% CI 1.1–not reached). After TPF induction, 1 patient (5%) had a complete response, 14 (67%) a partial response (PR) and 6 (29%) a stable disease. No statistically significant correlation could be found between PD-L1 expression at baseline and survival (log-rank test; P=0.26 for OS and P=0.45 for PFS), PD-L1 expression after TPF and survival (log-rank test; P=0.94 for OS and P=0.72 for PFS) or PD-L1 induction after chemotherapy and survival (log-rank test; P=0.67 for OS and P=0.83 for PFS) (online supplementary figure 1). PD-L1 expression at baseline, PD-L1 expression after TPF or PD-L1 induction were neither correlated to response to treatment (χ2 test; P=0.45, P=0.29 and P=0.52, respectively). Interestingly, within the 14 (70%) patients with PR after TPF induction, an increase in PD-L1 expression was observed on 80% (12 patients) of them (vs 17% in patients that presented with stable disease as best response after TPF). Only three tumours of patients with response to induction therapy did not present any increase in PD-L1 expression, whereas PD-L1 expression was not increased in five of the six patients who presented stable disease as best response (online supplementary table 1).
esmoopen-2017-000257supp001.jpg (76.9KB, jpg)
esmoopen-2017-000257supp002.pdf (353.1KB, pdf)
Discussion
The most important current challenges in the development of immuno-oncology therapies in HNSCC are (1) the identification of the population who is the most likely to respond to ICB and (2) the definition of the optimal therapeutic sequence between conventional cytotoxic chemotherapy and novel immune therapies. Our study suggests, subject to the small number of patients, that TPF induction chemotherapy increases PD-L1 expression in tumour cells and tumour-infiltrating ICs, as well as CD8+ lymphocytes density in locally advanced HNSCC. An increase in the CD8+/Foxp3+ lymphocytes density is also observed, although not reaching significance.
To our knowledge, this is the first study evaluating PD-L1 expression and ICs infiltrates in paired samples before and after induction chemotherapy in HNSCC. Due to tumour heterogeneity, initial biopsy could be not representative of the real expression of PD-L1 in the whole tumour, as previously shown in NSCLC,28 but is the only way to asses PD-L1 expression before chemotherapy. With the exception of the constitutional high-level microsatellite instability status,29 PD-L1 expression remains the most robust predictive biomarker for response to anti-PD-1/PD-L1 therapy in various tumour types.17 Our anti-PD-L1 clone, staining and scoring method used had been previously validated and displayed comparable results as other staining methodologies (notably 22C3 and 28–8 antibodies) in harmonisation studies for PD-L1 IHC testing.30 31 In HNSCC, a high PD-L1 expression was identified as a strong prognostic factor of patient’s outcome.32 PD-L1 expression on ICs has also been identified as a relevant predictive biomarker for the response to pembrolizumab16 23 and durvalumab (MEDI4736, Medimmune), with response rates of 11% (95% CI 5% to 22%) in all evaluable patients (n=62) and 18% (95% CI 5% to 40%) in patients with PD-L1-high tumours (n=22).33 34 Assessing PD-L1 expression prior to treatment with anti-PD-1/PD-L1 therapy is therefore of clinical relevance.
In our series, a significant increase in PD-L1 expression was observed both on tumour and on tumour-infiltrating cells post-TPF chemotherapy. This is in line with recent findings in preclinical models and clinical samples of breast tumours, where S-phase-dependent DNA damage caused cell cycle-dependent chemotherapies—such as platinum salts—result in increased PD-L1 expression in tumour cells in a cell-autonomous fashion, following activation of the cGAS/STING pathway. The increased PD-L1 expression on both IC and TC might also represent a potential immune-mediated resistance mechanism to induction chemotherapy, via increased activation of the PD-1/PD-L1 axis through a similar mechanism as what has been described with poly(ADPribose) polymerase inhibitors.35 This suggests that the concomitant administration of cytotoxic therapy and ICBs targeting the PD-1/PD-L1 axis would be a relevant strategy for patients with HNSCC. Results obtained in NSCLC36 37 support such therapeutic approach, which is even more relevant for patients with HNSCC who tend to deteriorate rapidly and for whom sequential approaches of cytotoxic therapy followed by immune therapy might be suboptimal. However, considering the limited size and heterogeneity in tumour sampling of the current dataset, prospective validation in larger cohorts is required. Phase II and III studies evaluating anti-PD-1/PD-L1 agents in recurrent or metastatic HNSCC who have failed to platinum regimen are ongoing (NCT02255097, NCT02207530 and NCT02252042), and may bring interesting additional information.
PD-L1 has not been described as a prognostic biomarker, and no correlation could indeed be found between PD-L1 pre-TPF or post-TPF expression and patient outcome in our series. However, we observed that 80% of the patients’ tumours that responded to induction chemotherapy did express PD-L1 post-TPF, whereas only 17% of tumours that did not respond to therapy were PD-L1 positive post-TPF. Similarly to what has been reported by Parkes et al19 this suggests that tumours that are sensitive to induction chemotherapy, which are usually DNA repair-deficient tumours, potentially activate immunomodulating pathways that lead to PD-L1 expression and lack of T cell-mediated cytotoxicity.19
The nature (in quantity and quality) of the immune infiltrate is also important in response to ICB, as illustrated by the possibility of immunological ignorance, non-functional immune response and excluded infiltrate described in Herbst et al.23 Density of CD8+ and Foxp3+ cell populations have been studied in various tumours.38 CD8+ cytotoxic T lymphocytes, which directly kill cancer cells when activated, are associated with better outcome, whereas the protumourigenic or antitumourigenic role of Foxp3+ T lymphocytes (also called regulatory T lymphocytes) is still debated.25 39 40 Overall, a high CD8+/Foxp3+ ratio is classically associated with favourable clinical outcomes in solid tumours,41 and the presence of tumour-infiltrating lymphocytes is usually associated with PD-L1 expression.24 42 In our series, we could not correlate the presence of TILs with outcome or with the level of PD-L1 expression, potentially as a result of the limited number of samples. Further studies on larger cohorts might allow to better address this question.
To conclude, PD-L1 positivity on tumour cells and tumour-infiltrating ICs is significantly increased after TPF induction chemotherapy, as well as CD8+ T cell density within the tumour. As higher PD-L1 expression as well as higher CD8+ tumour immune infiltrates have been correlated with better response to immune checkpoints inhibitors, our results may suggest that combination strategies of concomitant administration of cytotoxic therapies and anti-PD-1/PD-L1 therapies might be relevant for HNSCC. This approach, if supported by similar results emerging from larger and independent series, may warrant clinical evaluation in dedicated trials.
Acknowledgments
We would like to thank Professor Fabrice André, MD, PhD, director of U981 INSERM.
Footnotes
CL and JA contributed equally.
Contributors: CL and SP-V: manuscript redaction. CL, KAO and SP-V: study conception and planning. CL, JA, EL, TS, ND, MB, EM, LF, M-SC-K, EB, M-CD, AR, OC, CE and ST: study conduct. CL and JA: IHC assessment. CL, JA, EL and SP-V: results interpretation.
Funding: CL was funded by the Fondation pour la Recherche Medicale (DEA20140630162) grant. U981 INSERM is funded by SIRIC SOCRATE, supported by INCa-DGOS-INSERM 6043.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Monnerat C, Faivre S, Temam S, et al. End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol 2002;13:995–1006. 10.1093/annonc/mdf172 [DOI] [PubMed] [Google Scholar]
- 2.Hitt R, López-Pousa A, Martínez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 2005;23:8636–45. 10.1200/JCO.2004.00.1990 [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007;357:1695–704. 10.1056/NEJMoa071028 [DOI] [PubMed] [Google Scholar]
- 4.Blanchard P, Bourhis J, Lacas B, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 2013;31:2854–60. 10.1200/JCO.2012.47.7802 [DOI] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–34. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 8.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 11.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 12.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (medi4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016;34:3119–25. 10.1200/JCO.2016.67.9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 14.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res 2009;15:6348–57. 10.1158/1078-0432.CCR-09-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badoual C, Bouchaud G, Agueznay NH, et al. The soluble alpha chain of interleukin-15 receptor: a proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Res 2008;68:3907–14. 10.1158/0008-5472.CAN-07-6842 [DOI] [PubMed] [Google Scholar]
- 16.Chow LQ, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016:3838–45. 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–65. 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 18.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkes EE, Walker SM, Taggart LE, et al. Activation of sting-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst 2017;109:djw199 10.1093/jnci/djw199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin X, Liu C, Zhou Y, et al. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk /MAPK signaling pathway. Cell Mol Biol 2010;56:OL1366-72. [PubMed] [Google Scholar]
- 21.Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013;19:598–609. 10.1158/1078-0432.CCR-12-2731 [DOI] [PubMed] [Google Scholar]
- 22.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355–63. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano E, Romero P. The therapeutic promise of disrupting the PD-1/PD-L1 immune checkpoint in cancer: unleashing the CD8 T cell mediated anti-tumor activity results in significant, unprecedented clinical efficacy in various solid tumors. J Immunother Cancer 2015;3:15 10.1186/s40425-015-0059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Näsman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One 2012;7:e38711 10.1371/journal.pone.0038711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azzimonti B, Zavattaro E, Provasi M, et al. Intense Foxp3+ CD25+ regulatory T-cell infiltration is associated with high-grade cutaneous squamous cell carcinoma and counterbalanced by CD8+/Foxp3+ CD25+ ratio. Br J Dermatol 2015;172:64–73. 10.1111/bjd.13172 [DOI] [PubMed] [Google Scholar]
- 27.Miyashita M, Sasano H, Tamaki K, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res 2015;17:124 10.1186/s13058-015-0632-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol 2016;2:46–54. 10.1001/jamaoncol.2015.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 2017;3:1051–8. 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adam J, 2016. Multicentric French Harmonization Study for PD-L1 IHC Testing in NSCLC. Proceedings of the IASLC 17th World Conference on Lung Cancer, Vienna, Austria;4-7 December 2016;Abstract PR04.04. [Google Scholar]
- 32.Müller T, Braun M, Dietrich D, et al. PD-L1: a novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget 2017;8 10.18632/oncotarget.17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segal NH, Ou S-HI, Balmanoukian AS, et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Annals of Oncology 2016;27:328–50. 10.1093/annonc/mdw376.01 [DOI] [Google Scholar]
- 34.Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol 2016;11:95 10.1186/s13000-016-0545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao S, Xia W, Yamaguchi H, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 2017;23:3711–20. 10.1158/1078-0432.CCR-16-3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497–508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2969–79. 10.1200/JCO.2016.66.9861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538–43. 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrade MC, Ferreira SB, Gonçalves LC, et al. Cell surface markers for T and B lymphocytes activation and adhesion as putative prognostic biomarkers for head and neck squamous cell carcinoma. Hum Immunol 2013;74:1563–74. 10.1016/j.humimm.2013.08.272 [DOI] [PubMed] [Google Scholar]
- 41.Fritzsching B, Fellenberg J, Moskovszky L, et al. CD8+/FOXP3+-ratio in osteosarcoma microenvironment separates survivors from non-survivors: a multicenter validated retrospective study. Oncoimmunology 2015;4:e990800 10.4161/2162402X.2014.990800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho YA, Yoon HJ, Lee JI, et al. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol 2011;47:1148–53. 10.1016/j.oraloncology.2011.08.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000257supp001.jpg (76.9KB, jpg)
esmoopen-2017-000257supp002.pdf (353.1KB, pdf)