Abstract
We conducted a systematic review and meta-analysis to determine the effectiveness of healthcare provider-led (HCPs) interventions to support medication adherence in patients with acute coronary syndrome (ACS). A systematic search of Cochrane Library, Medline, EMBASE, PsycINFO, Web of Science, IPA, CINAHL, ASSIA, OpenGrey, EthOS, WorldCat and PQDT was undertaken. Interventions were deemed eligible if they included adult ACS patients, were HCP-led, measured medication adherence and randomised participants to parallel groups. Intervention content was coded using the Behaviour Change Technique (BCT) Taxonomy and data were pooled for analysis using random-effects models. Our search identified 8870 records, of which 27 were eligible (23 primary studies). A meta-analysis (n=9735) revealed HCP-led interventions increased the odds of medication adherence by 54% compared to control interventions (k=23, OR 1.54, 95% CI 1.26 to 1.88, I2=57.5%). After removing outliers, there was a 41% increase in the odds of medication adherence with moderate heterogeneity (k=21, OR 1.41, 95% CI 1.21 to 1.65, I2=35.3%). Interventions that included phone contact yielded (k=12, OR 1.63, 95% CI 1.25 to 2.12, I2=32.0%) a larger effect compared to those delivered exclusively in person. A total of 32/93 BCTs were identified across interventions (mean=4.7, SD=2.2) with ‘information about health consequences’ (BCT 5.1) (19/23) the most common. HCP-led interventions for ACS patients appear to have a small positive impact on medication adherence. While we were able to identify BCTs among interventions, data were insufficient to determine the impact of particular BCTs on study effectiveness.
PROSPERO registration number
Keywords: acute coronary syndrome, medication adherence, meta-analysis, systematic review
Introduction
Pharmacological therapy is a key component of secondary prevention following acute coronary syndrome (ACS). Despite the effectiveness of such therapies, many patients do not follow their regimen as prescribed and are deemed non-adherent. It is estimated that approximately one-third of patients are non-adherent to cardiac medications following ACS.1 Non-adherence among cardiac patients presents a considerable clinical problem because of its association with poor outcomes that include mortality, morbidity and risk of rehospitalisation.2
Adherence is complex in nature and is driven by a myriad of patient-related (eg, beliefs about treatment), healthcare provider (HCP)-related (eg, communication) and healthcare system-wide factors (eg, treatment cost and access). A recent review of psychosocial factors found that depression and treatment beliefs were predictors of non-adherence following ACS.3 Identifying potentially modifiable factors is crucial for the design and implementation of evidence-based interventions to improve adherence.
There have been multiple attempts to synthesis the evidence base for adherence interventions in chronic disease,4 coronary artery disease (CAD)5 and cardiovascular disease.6 Moreover, there have been numerous reviews looking at interventions targeting adherence to specific medication classes including statins,7 antihypertensives8 and oral antiplatelet therapy.9 HCPs (ie, physicians, nurses, pharmacists) play a key role in supporting, promoting and monitoring adherence for chronic conditions. Previous reviews have reported the benefit of adherence interventions delivered by multiple HCPs,10 pharmacists11 and nurses.5 However, to date, the impact of these types of interventions for patients with ACS has yet to be systematically explored.
Interventions that target behaviours such as medication taking are often complex and comprise multiple components. In order to identify the specific strategies best suited to change specific behaviours, complex interventions need to be compartmentalised. Behaviour change frameworks such as the theoretical domains framework12 and behaviour change technique (BCT) taxonomy13 have been designed to aid this compartmentalisation process through specifying interventions into their ‘active content’. These types of models have been used across a range of health behaviours, and there is increasing application within medication adherence research.14
The primary objective of this systematic review and meta-analysis is to determine the effectiveness of HCP-led interventions to support medication adherence following ACS. Additionally, we aim to examine whether effectiveness is moderated by interventionist, delivery method and having a theory-based design. Finally, we aim use a behaviour change framework to identify the specific techniques used among adherence interventions.
Methodology
This review was conducted in accordance of the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines15 and was registered with PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016037706).
Eligibility criteria
Studies were included if they met the following criteria:
Participants: adults (>18 years of age) with a confirmed diagnosis of ACS.
Intervention: delivered by HCPs.
Comparator: parallel group design where treatment group is compared with a clearly defined control group.
Outcome: include a measurement or medication adherence as a primary or secondary outcome.
Setting: study group allocation determined by randomisation.
We defined an intervention as being HCP led if the primary method of delivery involved HCPs working therapeutically with patients in person and/or via phone.
Studies infrequently distinguish between the different types of non-adherence; therefore, we used a definition of medication adherence that includes treatment initiation, actual dosing and treatment persistence.16
Search strategy
A systematic search of the following electronic databases was conducted: The Cochrane Library, Medline, EMBASE, PsycINFO, Web of Science, International Pharmaceutical Abstracts, Cumulative Index to Nursing and Allied Health Literature and Applied Social Sciences Index and Abstracts. An additional grey literature search was also undertaken: OpenGrey, EthOS, WorldCat—Thesis and Dissertations and ProQuest Dissertations & Theses. Searches were limited to articles written in English with no timespan limits. Reference lists of relevant papers were also searched to identify any additional records.
Our search strategy was informed by previous review studies4 5 and comprised four search themes: condition; therapy type; adherence; study design (see table 1) (for full search strategy, see online supplementary material 1).
Table 1.
Search themes with example search terms
| Search theme | Examples of search terms |
|---|---|
| Condition | Acute coronary syndrome, myocardial infarction, unstable angina, coronary occlusion, coronary thrombosis |
| Therapy type | Treatment, medication, medicine, drug, pharmacotherapy, regimen, prescription, prescribed |
| Adherence | Compliance, non-compliance, concordance, adherence, non-adherence, discordance, persistence, non-persistence, discontinuation, drop-out, treatment refusal |
| Study design | Random, clinical, control, trial, intervention, outcome, treatment outcome |
openhrt-2017-000685supp001.pdf (516.4KB, pdf)
Data extraction
Records were imported into bibliographic software (EndNote X7) where duplicates were removed. All records were initially screened based on their title and abstract, and relevant articles were full-text screened using our eligibility criteria. All screening and data extraction was undertaken by a single researcher (JC) with experience conducting evidence syntheses. Two additional researchers (VA & JW) undertook partial screening using the eligibility criteria to validate the study selection and data extraction process. Any disagreements between raters (JC, VA and JW) were resolved by consensus. Data were extracted using a standardised data extraction form based on previous review studies4 5 17 (see table 2). Where necessary, study authors were contacted directly for additional information. We contacted 10 authors to clarify aspects of their methodology of which 80% responded.
Table 2.
Data extraction criteria
| Data category | Specific extraction |
|---|---|
| Study details | Author; title |
| Source attributes | Study type; funding details; year of distribution |
| Methodological features | Group assignment; allocation concealment; comparator group; blinding; attrition; intention to treat; study period; outcome measurement |
| Participant characteristics | Age; gender; ethnicity; diagnosis |
| Intervention features | Number of sessions; interventionist; length of delivery; theoretical basis; delivery method; targeting additional health behaviours |
| Intervention content | BCTs |
| Effect size determinations | Sample size; methods of analysis; means; main effects |
BCTs, behaviour change technique.
Risk of bias
Methodological quality was judged using A Cochrane Risk of Bias Assessment Tool (ACROBAT)18 where risk is rated as ‘high’, ‘unclear’ or ‘low’ among six domains of bias (Selection; Performance; Detection; Attrition; Reporting and Other Biases). ACROBAT has been used in previous systematic reviews looking at the effectiveness of adherence interventions.19 Risk of bias was assessed by a single researcher (JC).
Statistical analysis
Medication adherence was our target outcome, and the direction of effect was transformed for consistent reporting. Where studies reported adherence across multiple medications the data were pooled to provide an estimate of ‘overall adherence’. Effect size estimates are expressed in terms of ORs. Where data were originally expressed as means, standardised mean differences were calculated and then transformed to the OR metric using the probit method.20 These should be interpreted as standardised OR.
Random-effects models comparing HCP-led interventions with control interventions were used based on the assumption that there would be statistical heterogeneity from pooling primary study data. The I2 statistic was used to estimate statistical heterogeneity, and Cochrane guidelines were used for interpretation.21 Potential publication bias was determined using funnel plots and Egger’s test for small study effects. A critical value of. 1 was used for heterogeneity and small study effects significance testing. A study was deemed to be an outlier where the effect size was outside the pseudo 95% CI in the funnel plot as a means for detecting the potential impact of outliers on the pooled effect size.
Secondary studies (ie, primary study data with alternate end-points) were excluded from meta-analysis so as not to duplicate data. Prespecified subgroup analyses were conducted based on (1) type of interventionist, (2) delivery method and (3) theory-based design. Additional post hoc analyses were done based on adherence outcome and risk of bias. All analyses were done using Stata 14.1.
Coding intervention content
We used the BCT taxonomy13 to identify specific techniques used to change medication-taking behaviour among our intervention studies. The BCT taxonomy comprises 93 unique BCTs categorised into 16 clusters. A BCT is defined as an ‘active ingredient’ that can be used to alter or redirect behaviour. The BCT taxonomy includes a detailed description of each technique and provides specific examples (eg, ‘action planning’ (BCT 1.4): ‘prompt planning the performance of a particular physical activity at a particular time on certain days of the week’ (the numbers in parentheses refer to the BCT’s taxonomy cluster)). The BCT taxonomy has been used to code the content of interventions across a range of health behaviours including medication adherence.14
The BCT content of each intervention was rated by two researchers (JC and LA). Intervention data were sourced from each published manuscript and relevant supporting documents (ie, study protocols, intervention manuals). The researchers initially rated the interventions independently and then met to discuss. BCT content was scrutinised until consensus was met between researchers.
Results
Selection process
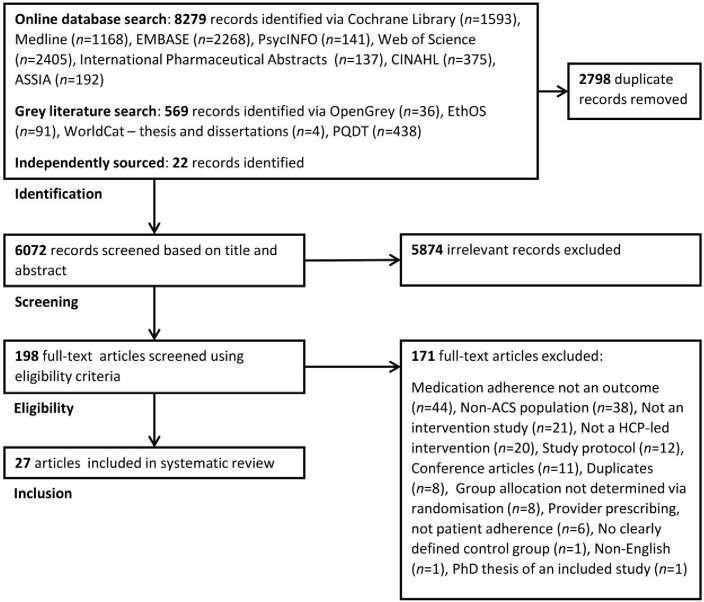
Our comprehensive search strategy identified 6072 records that were initially screened based on their title and abstract (see figure 1). A total of 5874 records were excluded, leaving 198 records to be full-text screened. Twenty-seven studies22–48 met our eligibility criteria, which comprised 23 primary studies (4 secondary studies37 38 43 47) (for full reason for exclusion list, see online supplementary material). Only primary study data (k=23, n=9735) will be discussed in the following sections.
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analysis flow diagram showing the study selection process. ACS, acute coronary syndrome; ASSIA, Applied Social Sciences Index and Abstracts; CINAHL, Cumulative Index to Nursing and Allied Health Literature; HCP, healthcare provider; PQDT, ProQuest Dissertations & Theses.
Study characteristics
Full details of the included studies can be found in table 3. The majority of interventions included nurses in their delivery (k=1323 24 26–28 31 33 35 36 40 42 45 48). Six interventions were led by pharmacists (k=622 29 30 32 41 48), and two were delivered by physicians (k=225 39). Physiotherapists,44 problem-solving therapists34 and community health workers46 acted as interventionists in singular trials. Nine studies were delivered exclusively in person (k=924 26 27 31 34 36 39 41 48), while 10 studies included both in person and phone contact (k=1022 23 29 30 32 33 42 44–46). Just four study interventions were delivered exclusively by phone (k=425 28 35 40), while six included a face-to-face predischarge component (k=622 23 28 30 39 41). The number of intervention sessions ranged from 128 32 48 to 2426 (k=21; median=4.0, SD=6.0). A total of 10 studies followed patients up for either 6 (k=522 24 32 34 39) or 12 months (k=529–31 46 48) (k=23; median=6.0 months, SD=10.3 months). Adherence to medication was a primary outcome in 14 studies (k=1422 24 28–30 32 34 36 39–42 45 46) and was measured exclusively by self-report in 16 studies (k=1623–28 31–34 36 39–41 44 45). Five studies used pharmacy data or pill counts (k=530 35 42 46 48), and just two studies used both self-report and pharmacy data to measure adherence (k=222 29).
Table 3.
Data extraction for all intervention studies identified in the systematic review process (k=27)
| Author | Study details | Participant characteristics | ||||
| Year | Country | Design, setting | Sample size | Sample characteristics | Control group | |
| Calvert et al 22 | 2012 | USA | RCT, multi-site (n=2) | 143 | Median age: IG=63, CG=62; male: IG=66%, CG=61%; White: IG=51%, CG=51% | Usual care: routine discharge counselling and discharge summary sent to community physician. |
| Cossette et al 23 | 2012 | Canada | RCT, single site | 242 | Mean age: IG=59, CG=59; male: IG=81%, CG=90% | Usual care: received standard predischarge care. Encouraged to use regular healthcare resources postdischarge. |
| Costa e Silva et al 24 | 2008 | Brazil | RCT, single site | 153 | Mean age: IG=58, CG=59; male: IG=63%, CG=64% | Usual care: standard outpatient follow-up with a cardiologist. |
| Du et al 25 | 2016 | China | RCT, single site | 979 | Mean age: IG=60, CG=62; male: IG=73%, CG=72% | Usual care: standard follow-up with research nurse. |
| Giallauria et al 26 | 2009 | Italy | RCT, single site | 52 | Mean age: IG=58, CG=57; male: IG=85%, CG=85% | Usual care: following standard 3-month cardiac rehab, patients were discharged with usual routine recommendations and were seen only at the 12-month and 24-month follow-up. |
| Giannuzzi et al 27 | 2008 | Italy | RCT, multi-site (n=78) | 3241 | Mean age: IG=58, CG=58; male: IG=86%, CG=87% | Usual care: a letter sent to the family physician recommending secondary prevention goals followed by standard cardiac rehab and follow-up. |
| Gould28 | 2011 | USA | RCT, single site | 129 | NR | Usual care: patients received routine discharge materials and usual care. |
| Gujral et al 29 | 2014 | Australia | RCT, single site | 200 | Mean age: IG=58, CG=60; male: IG=77%, CG=80% | Usual care: medication beliefs not communicated to their community pharmacist. The community pharmacists were asked to provide the patient with usual care when they collected their prescription medications. |
| Ho et al 30 | 2014 | USA | RCT, multi-site (n=4) | 253 | Mean age: IG=64, CG=64; male: IG=98%, CG=98%; White: IG=82%, CG=75% | Usual care: patients received standard ACS hospital discharge instructions, a discharge medication list and educational information about cardiac medications. A 12-month clinic visit was scheduled. |
| Jalal et al 32 | 2016 | UK | RCT, single site | 71 | Mean age=NR; male=76% | Usual care: following predischarge counselling from the hospital pharmacist, patients refilled their prescriptions at their usual pharmacies. |
| Jorstad et al 31 | 2013 | Netherlands | RCT, multi-site (n=11) | 733 | Mean age: IG=58, CG=58; male: IG=80%, CG=80% | Usual care: outpatient clinic visits to treating cardiologists and other relevant specialists. Patients were referred to cardiac rehab according to national guidelines. |
| Kotowycz et al 33 | 2010 | Canada | RCT, single site | 54 | Mean age: IG=56, CG=55; male: IG=81%, CG=70% | All discharge planning and follow-up were left to the treating physician and nursing team. |
| Kronish et al 34 | 2012 | USA | RCT, multi-site (n=5) | 177 | Mean age: IG=59, CG=61; male: IG=46%, CG=47% | Usual care: treating physicians notified about their patients’ depressive status. Patients given appropriate care for depressive symptoms. |
| Lapointe et al 35 | 2006 | Canada | RCT, single site | 127 | Mean age: IG=58, CG=57; male: IG=89%, CG=78% | Standard follow-up with patients’ regular physician. |
| Miller et al 36 | 1988 | USA | RCT, multi-site (n=3) | 103 | Mean age=NR (range 30 - 65); male: IG=73%, CG=89%; White: IG=98%, CG=87% | Usual care: all patients had received standard inpatient cardiac rehab. |
| Miller et al 37 | 1989 | USA | RCT, multi-site (n=3) | 81 | Mean age=54; male=81% | Usual care: all patients had received standard inpatient cardiac rehab. |
| Miller et al 38 | 1990 | USA | RCT, multi-site (n=3) | 51 | Mean age=55; male=76% | Usual care: all patients had received standard inpatient cardiac rehab. |
| Muñiz et al 39 | 2010 | Spain | RCT, multi-site (n=64) | 1757 | Mean age: IG=62, CG=64; male: IG=78%, CG=76% | Usual care. |
| Najafi et al 40 | 2016 | Iran | RCT, single site | 100 | Mean age: IG=59, CG=58; male: IG=54%, CG=38% | Routine care including check-ups with designated physician. |
| Polack et al 41 | 2008 | Canada | RCT, single site | 10 | Mean age: IG=59, CG=65; male: IG=80%, CG=100% | Usual care: standard predischarge nurse education. |
| Polsook et al 42 | 2016 | Thailand | RCT, single site | 44 | Mean age: IG=61, CG=63; male: IG=86%, CG=86% | Usual care in the cardiac inpatient department that included education about patients’ condition and treatment. |
| Redfern et al 44 | 2008 | Australia | RCT, single site | 144 | Mean age: IG=62, CG=67; male: IG=74%, CG=75% | Ongoing conventional care determined by patients’ family physician and cardiologist. |
| Redfern et al 43 | 2009 | Australia | RCT, single site | 144 | Mean age: IG=62, CG=67; male: IG=74%, CG=75% | Usual care: received medical treatment, including pharmacotherapy and lifestyle counselling, as determined by their usual doctors. |
| Uysal and Ozcan45 | 2015 | Turkey | RCT, multi-site (n=2) | 90 | Mean age=NR (47% between 45-54); male: IG=80%, CG=76% | Received home education kit comprised of brochures about healthy living post-MI. Not provided with telephone counselling and education. |
| Xavier et al 46 | 2016 | India | RCT, multi-site (n=14) | 806 | Mean age: IG=56, CG=57; male: IG=82%, CG=83% | Standard care: patients were asked to alert the research team to any hospital visits that they planned. |
| Sharma et al 47 | 2016 | India | RCT, single site | 100 | Mean age: IG=57, Con=61; Male total=84% | Usual care. |
| Yorio et al 48 | 2008 | USA | RCT, single site | 144 | Median age: IG=56, CG=56; male: IG=67%, CG=57%; White: IG=32%, CG=35% | Usual care: standard postdischarge care that included appointments with a cardiologist and family physician within 3 months. |
| Author | Methodological features | |||||
| Intention to treat | Follow-up | Adherence as an outcome | Primary outcome | Adherence measurement | Sessions | |
| Calvert et al 22 | Not stated | 6 months | Primary | Medication adherence | Self-report; MMAS-4; PDC | 4 |
| Cossette et al 23 | Not stated | 6 weeks | Secondary | Cardiac rehab attendance | MMAS-4 | 3 |
| Costa e Silva et al 24 | Yes | 6 months | Primary (one of) | Clinical improvement index (including medication adherence) | Self-report | 2 |
| Du et al 25 | Not stated | 36 months | Secondary | Mortality and MACE | MMAS-4 | 6 |
| Giallauria et al 26 | Not stated | 24 months | Secondary | Cardiopulmonary parameters and cardiovascular risk profile (including medication adherence) | Self-report | 24 |
| Giannuzzi et al 27 | Yes | 36 months | Secondary | MACE | Self-report | 11 |
| Gould28 | Not stated | 3 days | Primary (one of) | Medication adherence, use of urgent care, patient satisfaction and illness perceptions | MMAS-4 | 1 |
| Gujral et al 29 | Not stated | 12 months | Primary (one of) | Medication adherence and treatment beliefs | MARS; MPR | 2 |
| Ho et al 30 | Yes | 12 months | Primary | Medication adherence | PDC | 4 |
| Jalal et al 32 | Not stated | 6 months | Primary | Medication adherence | MMAS-8 | 1 |
| Jorstad et al 31 | Not stated | 12 months | Secondary | Lifestyle and biometric targets | Self-report | 4 |
| Kotowycz et al 33 | Yes | 6 weeks | Secondary | MACE | Self-report | 4 |
| Kronish et al 34 | Yes | 6 months | Primary (one of) | Adherence to medication, heart healthy diet, regular exercise and smoking cessation | Self-report | NR |
| Lapointe et al 35 | Not stated | 18 months | Secondary | LDL-C targets | Prescription refills | NR |
| Miller et al 36 | Not stated | 60 days | Primary | MRA | HBS | 3 |
| Miller et al 37 | Not stated | 12 months | Primary | MRA | HBS | 3 |
| Miller et al 38 | Not stated | 24 months | Primary | MRA | HBS | 3 |
| Muñiz et al 39 | Not stated | 6 months | Primary (one of) | Behavioural and clinical targets (including medication adherence) | Self-report | 2 |
| Najafi et al 40 | Not stated | 3 months | Primary | Medication adherence | MMAS-8 | 6 |
| Polack et al 41 | Not stated | 6 weeks | Primary (one of) | Medication adherence and knowledge retention | MMAS-4 | 2 |
| Polsook et al 42 | Not stated | 4 weeks | Primary (one of) | Medication adherence and self-efficacy | Pill count | 14 |
| Redfern et al 43 | Yes | 3 months | Secondary | Behavioural and clinical targets | Self-report | 5 |
| Redfern et al 44 | Yes | 12 months | Secondary | Behavioural and clinical targets | Self-report | 5 |
| Uysal and Ozcan45 | Not stated | 3 months | Primary (one of) | Physical activity, medication adherence, anginal symptoms | MMAS-4 | 3 |
| Xavier et al 46 | Yes | 12 months | Primary | Medication adherence | CMAS | 18 |
| Sharma et al 47 | Yes | 24 months | Primary | Mediation adherence | CMAS | 10 |
| Yorio et al 48 | Not stated | 12 months | Secondary | Improved LDL-C profile | Prescription refills | 1 |
| Author | Intervention features | |||
| Interventionist | Delivery method | Theoretical basis | Intervention summary | |
| Calvert et al 22 | Pharmacist | In person and phone | Not stated | Predischarge counselling covering the importance and purpose of medications and barriers to adherence. Pocket medication card, cheat sheet (tips for remembering) and pillbox also provided. Regular follow-up with community pharmacist to discuss adherence-related issues. |
| Cossette et al 23 | Nurse | In person and phone | CS-SRM | Predischarge counselling session: symptom and physical activity management, coherence around illness episode, concerns/worries. Postdischarge counselling sessions: disease management, concerns/worries and intentions about risk factor modification, problem solving. |
| Costa e Silva et al 24 | MDT (included nurse) | In person only | Not stated | Transdisciplinary outpatient care provided. Detailed treatment planning and follow-up with nurse, dietitian, endocrinologist and cardiologist. HCPs collaborated to reinforce lifestyle change and formulate a care plan. |
| Du et al 25 | Physician (cardiologist) | Phone only | Not stated | Physician-led intensive telephone follow-up over 36 months. Patients provided with additional health education, disease-prevention suggestions and consultations on medication usage. Face-to-face visits were scheduled if necessary. |
| Giallauria et al 26 | MDT (included nurse) | In person only | Not stated | Monthly hospital meetings to discuss lifestyle change and engage in exercise training. Received a booklet about lifestyle change and promoting patients’ role in their healthcare. Encouraged family support throughout. |
| Giannuzzi et al 27 | MDT (included nurse) | In person only | Not stated | Comprehensive cardiac rehab sessions that included exercise training and lifestyle and risk factor counselling. Encouraged family support throughout. Pharmacological treatments positively recommended to all patients. Received booklet to support lifestyle change and patient empowerment. |
| Gould28 | Nurse | Phone only | CS-SRM | Patients received written discharge materials, telephone follow-up by an expert, medication review materials, a medication pocket card and suggested websites. |
| Gujral et al 29 | Pharmacist | In person and phone | NCF | Tailored intervention targeting treatment beliefs. Beliefs and attitudes towards treatment elicited using repertory grid technique and then communicated to the community pharmacist. Information used to tailor their discussions with the patient during follow-up. Patient also reviewed monthly by community pharmacist to discuss adherence-related issues. |
| Ho et al 30 | Pharmacist | In person and phone | Not stated | Pharmacist-led postdischarge medication reconciliation and follow-up. Predischarge and postdischarge education sessions with pharmacist followed by automated educational voice messages. Use of pill boxes to organise medications. Increased communication between pharmacists and patients’ care team. Automated voice reminders to refill prescriptions. |
| Jalal et al 32 | Pharmacist | In person and phone | Not stated | Community pharmacist-led motivational interview aimed at improving protective cardiovascular medicine taking. Consultations were delivered as part of the New Medicine Service or a Medication Usage Review (established UK NHS pharmacy services). |
| Jorstad et al 31 | Nurse | In person only | Not stated | Outpatient visits with a nurse: educational sessions targeted lifestyle change and risk factor management. Lifestyle and risk factors reviewed and patients received individual counselling. Medication adherence encouraged and reasons for discontinuation discussed. |
| Kotowycz et al 33 | Nurse | In person and phone | Not stated | Nurse-led patient education about the nature and management of their cardiac disease, with a focus on medications and facilitation of discharge planning. |
| Kronish et al 34 | Other (problem-solving therapist) | In person only | Not stated | Patients given a choice of either PST and/or pharmacotherapy. Weekly PST sessions were brief, problem focused and designed to augment self-efficacy and address psychosocial issues. Focus also on the initiation of pleasant activities. Patients given choice of different pharmacotherapy. |
| Lapointe et al 35 | Nurse | Phone only | Not stated | Patients received postdischarge letter and phone call concerning risk factor education and management. Clinical goals (lipid profile) set and patients received additional intervention from their physician if goals not met. Compliance assessment with pharmacist conducted at 12 and 18 months. |
| Miller et al 36 | Nurse | In person only | TRA | Intervention included an assessment (addressing attitudes, beliefs and intentions), problem identification (coping and societal adjustment) and developing a detailed health plan. Spouses were encouraged to participate. |
| Miller et al 37 | Nurse | In person only | TRA | Intervention included an assessment (addressing attitudes, beliefs and intentions), problem identification (coping and societal adjustment) and developing a detailed health plan. Spouses were encouraged to participate. |
| Miller et al 38 | Nurse | In person only | TRA | Intervention included an assessment (addressing attitudes, beliefs and intentions), problem identification (coping and societal adjustment) and developing a detailed health plan. Spouses were encouraged to participate. |
| Muniz et al 39 | Physician | In person only | Not stated | Focused on the patient-provided relationship. Discharge interview included a signed agreement of secondary prevention care plan and comprehensive written material about risk factor management. During a follow-up session, agreement reviewed and adapted if necessary. |
| Najafi et al 40 | Researcher (nurse) | Phone only | Not stated | Nurse-led follow-up telephone calls based on lifestyle counselling and education. Agreed behavioural objectives were reviewed and barriers were addressed through problem solving. Family participation was encouraged throughout. |
| Polack et al 41 | Pharmacist | In person only | Not stated | Received predischarge pharmacist-led education around the benefits and risks of cardiac medications. Sessions included use of a patient education tool. |
| Polsook et al 42 | Researcher (nurse) | In person and phone | Not stated | Comprised a 4-week self-efficacy enhancement program that targeted patients’ motivation to be adherent, skills development and adherence self-monitoring. |
| Redfern et al 43 | Other (physiotherapist) | In person and phone | Not stated | Based around risk factor assessment and goal setting. Patients chose their risk factor module and self-committed to a written action plan. Received a resource pack that included information leaflets. Follow-up sessions tested patients’ knowledge of their risk factors. Personal goals were also identified and positive. Risk-lowering behaviour recorded. |
| Redfern et al 44 | Other (physiotherapist) | In person and phone | Not stated | Based around risk factor assessment and goal setting. Patients chose their risk factor module and self-committed to a written action plan. Received a resource pack that included information leaflets. Follow-up sessions tested patients’ knowledge of their risk factors. Personal goals were also identified and positive. Risk-lowering behaviour recorded. |
| Uysal and Ozcan45 | Researcher (nurse) | In person and phone | Not stated | Individualised education plans around lifestyle and risk factor management. Received access to a computer-based education along with brochures on lifestyle changes post-MI. Telephone counselling during follow-up addressing negative health behaviours, including treatment non-adherence. |
| Xavier et al 46 | Other: community health worker | In person and phone | Not stated | Involved personalised counselling to help overcome barriers to adherence and lifestyle modification. Also received an adherence calendar to record medication taking and were asked to complete diaries, which included information about their medications. Family participation encouraged. |
| Sharma et al 47 | Other: community health worker | In person and phone | Not stated | Involved personalised counselling to help overcome barriers to adherence and lifestyle modification. Also received an adherence calendar to record medication taking and were asked to complete diaries, which included information about their medications. Family participation encouraged. |
| Yorio et al 48 | Nurse or pharmacist | In person only | Not stated | Postdischarge session with nurse or pharmacist. Session included full medication review and titration, risk factor counselling and discussion/referral to cardiac rehab and other HCPs (dietician and/or smoking cessation service). |
ACS, acute coronary syndrome; CG, control group; CMAS, Composite Medication Adherence Score; CS-SRM, Common-Sense Model of Self-Regulation; HBS, Health Behaviour Scale; HCP, healthcare provider; IG, intervention group; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiac events; MARS, Medication Adherence Report Scale; MDT, multidisciplinary team; MI, myocardial infarction; MMAS-4, Morisky Medication Adherence Scale (4-item); MMAS-8, Morisky Medication Adherence Scale (8-item); MPR, medication possession ratio; MRA, medical regimen adherence; NCF, necessity concerns framework; NR, not reported; PDC, proportion of days covered; PST, problem-solving therapy; RCT, randomised controlled trial; TRA, theory of reasoned action.
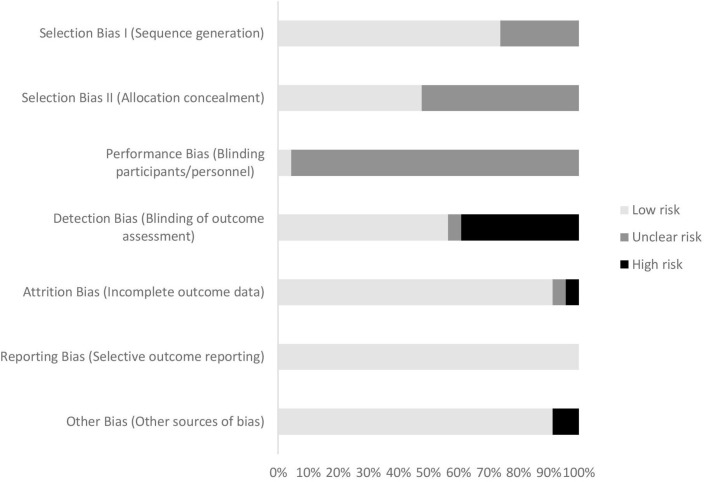
Risk of bias
A summary of the risk of bias assessment can be seen in figure 2. All but one of the studies31 were rated as having ‘unclear’ risk of performance bias due to the impracticality of blinding participants and personnel to group allocation during behavioural studies. ‘High’ risk of detection bias was judged in nine studies that did not adopt end-point blinding (k=924 26 28 36 39 40 44 45 48). After excluding performance bias ratings, six studies were judged to have ‘low’ risk of bias across all other domains (k=622 27 30 34 41 44). Three of these ‘low-risk’ studies were delivered by pharmacists (k=322 30 41), and the rest were led by nurses,27 physiotherapists44 or problem-solving therapists.34 Trials with the smallest41 and largest sample sizes27 were among the ‘low risk’-rated studies, and all six were either delivered exclusively in person (k=327 34 41) or in person with phone contact (k=322 30 44) (for complete risk of bias assessment, see online supplementary material).
Figure 2.
Risk of bias assessment.
BCT inclusion
Figure 3 shows the frequency of BCTs coded across studies. None of the studies referenced the BCT taxonomy in their intervention design. A total of 32 (34%) of the 93 BCTs listed in the taxonomy were identified among studies, ranging between 128 33 and 1044 (mean=4.7, SD=2.2). ‘Information about health consequences’ (BCT 5.1) was the most commonly identified BCT, coded in 19 of the 23 studies. ‘Social support (unspecified)’ (BCT 3.1) was coded in seven studies, and ‘action planning’ (BCT 1.4) was identified in just two studies.32 44 There were six instances of ‘goal setting (outcome)’ (BCT 1.3), ‘monitoring of behaviour by others without feedback’ (BCT 2.1), ‘feedback on outcome(s) of behaviour’ (BCT 2.7) and ‘instruction on how to perform the behaviour’ (BCT 4.1) across studies. Around two-thirds (67%) of the total number of BCTs coded were from just three taxonomy clusters: goals and planning (cluster 1, n=26 (24%)), natural consequences (cluster 5, n=25 (23%)) and feedback and monitoring (cluster 2, n=21 (20%)). There were no BCTs coded from three taxonomy clusters: reward and threat (cluster 10), scheduled consequences (cluster 14) and covert learning (cluster 16). There were no instances where every BCT in a cluster was coded (goals and planning: cluster 1, 8/9 BCTs coded; feedback and monitoring: cluster 2, 6/7 BCTs coded).
Figure 3.
Frequency of BCTs identified among interventions. BCT, behaviour change technique.
Meta-analysis
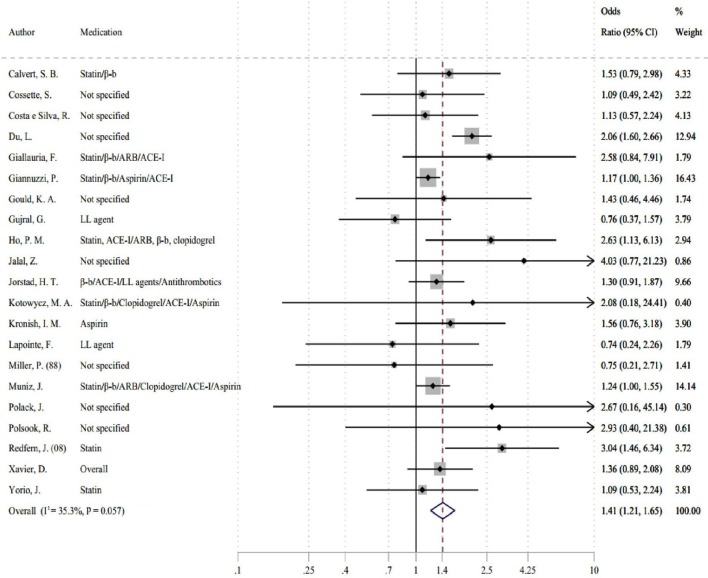
A random-effects meta-analysis of 23 primary studies (n=9735) revealed that HCP-led interventions increased the odds of medication adherence by 54% compared with control interventions with moderate to high statistical heterogeneity (k=23, OR 1.54, 95% CI 1.26 to 1.88 (I2=57.5%, P=0.001)) (see figure 4). After removing two outliers,40 45 a meta-analysis of 9545 patients indicated that HCP-led interventions increased the odds of medication adherence by 41% compared with control interventions with moderate statistical heterogeneity (k=21, OR 1.41, 95% CI 1.21 to 1.65 (I2=35.3%, P=0.057)) (see figure 5). While Egger’s test was non-significant (P=0.286), visual inspection of the funnel plot suggests a potential bias even after discounting outliers (for funnel plot, see online supplementary material).
Figure 4.
Forest plot showing pooled effects size for healthcare-provider-led interventions on medication adherence (k=23, includes outliers).
Figure 5.
Forest plot showing pooled effects size for healthcare-provider-led interventions on medication adherence (k=21, outliers removed).
Subgroup analyses
Table 4 shows the results of our prespecified (ie, interventionist, delivery method, theoretical basis) and post hoc (ie, adherence outcome, risk of bias) subgroup analyses. The largest effect sizes were for interventions that included phone contact (k=12, OR 1.63, 95% CI 1.25 to 2.12), and there was a trend for better-quality studies (ie, ‘low’ risk of bias) to increase the odds of adherence (k=6, OR 1.69, 95% CI 1.15 to 2.47). A negligible positive effect was found for interventions delivered by nurses (k=11, OR 1.19, 95% CI 1.04 to 1.36), and pharmacist-led interventions had a small though non-significant effect on medication adherence (k=6, OR 1.44, 95% CI 0.92 to 2.26). Studies led by HCPs other than nurses and pharmacists (ie, physicians,25 39 physiotherapists,44 problem-solving therapists,34 community health workers46) yielded a small positive effect on medication adherence (k=5, OR 1.66, 95% CI 1.22 to 2.24). We found no discernible differences in effect size between studies that included adherence as a primary or secondary outcome, and a small number of theoretically informed studies had a non-significant trend towards a negative effect on adherence (k=4, OR 0.94, 95% CI 0.60 to 1.49).
Table 4.
Overall effects and subgroup analyses for medication adherence interventions
| k | N | OR | CI | I² (%) | P heterogeneity | P bias | |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| All studies | 23 | 9735 | 1.54 | 1.26 to 1.88 | 57.5 | 0.001 | 0.066 |
| Excluding outliers | 21 | 9545 | 1.41 | 1.21 to 1.65 | 35.3 | 0.057 | 0.286 |
| Interventionist | |||||||
| Pharmacist | 6 | 813 | 1.44 | 0.92 to 2.26 | 30.0 | 0.210 | 0.309 |
| No pharmacist | 15 | 8732 | 1.41 | 1.19 to 1.68 | 41.0 | 0.049 | 0.439 |
| Nurse | 11 | 5030 | 1.19 | 1.04 to 1.36 | 0 | 0.920 | 0.501 |
| No nurse | 10 | 4515 | 1.63 | 1.26 to 2.10 | 52.1 | 0.027 | 0.454 |
| Other HCPs | 5 | 3842 | 1.66 | 1.22 to 2.24 | 67.1 | 0.012 | 0.550 |
| Nurse or pharmacists | 16 | 5703 | 1.21 | 1.07 to 1.38 | 0 | 0.663 | 0.167 |
| Delivery method | |||||||
| In person only | 9 | 6358 | 1.21 | 1.08 to 1.36 | 0 | 0.890 | 0.305 |
| Included phone contact | 12 | 3187 | 1.63 | 1.25 to 2.12 | 32.0 | 0.135 | 0.629 |
| Theoretical basis | |||||||
| Theory based | 4 | 686 | 0.94 | 0.60 to 1.49 | 0 | 0.781 | 0.692 |
| Not theory based | 17 | 8859 | 1.48 | 1.25 to 1.76 | 41.4 | 0.038 | 0.094 |
| Outcome | |||||||
| Primary | 12 | 3833 | 1.31 | 1.11 to 1.54 | 0 | 0.622 | 0.227 |
| Secondary | 9 | 5712 | 1.48 | 1.12 to 1.96 | 63.1 | 0.006 | 0.548 |
| Risk of bias | |||||||
| Low risk | 6 | 3948 | 1.69 | 1.15 to 2.47 | 51.4 | 0.068 | 0.042 |
| Higher risk | 15 | 5597 | 1.36 | 1.13 to 1.64 | 28.1 | 0.147 | 0.658 |
HCP, healthcare provider; I², heterogeneity; k, number of studies; N, sample size; P bias, small study effects significance; P heterogeneity, heterogeneity significance.
Interventionist, delivery method and theoretical basis were prespecified subgroups, while outcome and risk of bias were determined post hoc. Outliers were excluded from subgroup analyses.40 45
Discussion
The primary objective of this study was to identify interventions led by HCPs to improve medication adherence following ACS. Meta-analysis revealed a small effect of HCP-led interventions on medication adherence. Our results are consistent with previous meta-analysis studies that have looked at the effectiveness of adherence interventions in other cardiac patient populations.5 17
In line with recent adherence literature,49 the majority of intervention studies identified were delivered by nurses or pharmacists. However, we found no indication that study effectiveness was moderated by the HCP delivering the intervention. Studies that included nurses in their delivery had a negligible effect towards better medication adherence, which does not correspond to findings from another meta-analysis that found that nurse-led interventions had a small to medium effect on adherence in patients with CAD.5 Six pharmacist-led interventions had a small but non-significant effect on medication adherence, which is congruous with previous reviews across cardiac-related diseases.5 17 Objectively, pharmacists should be ideal candidates to deliver adherence interventions due to the necessary knowledge and skills they possess to promote and support medication-taking behaviour.50 A meta-analysis of 771 medication adherence intervention trials found that the most effective interventions were delivered by pharmacists,49 which suggests that pharmacists may be better utilsied in other patient populations. Our findings should, however, be interpreted with caution due to the small number of pharmacist-led studies included in our analyses.
In terms of delivery method, interventions that included phone contact had higher odds of medication adherence compared with interventions delivered exclusively in person. Phone-delivered interventions may be a more convenient method to reach patients after discharge to monitor and encourage good medication adherence over time. Half of the interventions that included phone contact also contained a face-to-face predischarge component. Cutrona et al 51 found that two-thirds of interventions delivered at discharge were effective at improving adherence to cardiovascular medicines. Periods of care transition such as during hospital discharge are ideal opportunities to discuss treatment to pre-empt potential barriers to regimen adherence. Moreover, the dynamic nature of adherence dictates that monitoring of medication-taking behaviour over time by both patients and/or HCPs is crucial to ensure therapy maintenance for long-term conditions such as ACS.
We expected to find a greater proportion of interventions that used theoretical approaches to change medication-taking behaviour. There were only four studies that reported a theoretical basis, of which just one was based on a model of medication-taking behaviour (necessity–concerns framework52). A review by Conn et al 53 found that theory-driven interventions had a significant but modest effect on medication adherence. Our findings suggest that theory-driven adherence interventions for ACS are lacking, thus highlighting an important avenue for future research.
Coding intervention content
To our knowledge, this review is the first to use the BCT taxonomy to code interventions that targeted adherence across all cardiac medications following ACS. The BCT taxonomy provided a useful tool to analyse the content of adherence interventions, and we found that one-third of all BCTs detailed in the taxonomy were identified in at least one intervention. This relatively small number of total BCTs identified was unsurprising as many were not applicable to medication-taking behaviour. It is likely that additional strategies may have been used among interventions but were not identified due to a lack of detail in the description of the intervention. A lack of transparency in study reporting is an issue that limits the usability and replicability of interventional research. Checklists such as TIDieR54 are becoming commonplace to improve the quality of intervention reporting.
Written, verbal or visual information provision about the consequences of adherence (BCT 5.1) was by far the most frequently used BCT among HCP-led interventions. Discussing the consequences of non-adherence may help to strengthen patients’ beliefs in the necessity of their medications, which have been shown to predict non-adherence.55 While information is necessary to improve patients’ knowledge, it is not sufficient as a standalone strategy to change behaviour. Information-only strategies have been found to be generally ineffective at changing complex behaviours such as adherence.56
Clinical and research implications
Medication taking is a complex behaviour that can be difficult to change. Targeting patients identified with an adherence issue rather than all medication-takers may be one strategy to improve the effectiveness of adherence interventions. Cutrona et al 57 reported that ‘broad’ interventions (target all medication-takers) were less effective than ‘focused’ (target non-adherers only). None of the studies identified in this review targeted non-adherers; therefore, it is not yet known whether ‘focused’ interventions would be more appropriate for patients with ACS.
There were a variety of adherence measures used among included interventions, most of which were non-validated self-report tools. While an approach that combines self-reporting with an objective measure (eg, prescription refill records) is considered best practice, just two interventions followed this guidance. No studies used electronic monitors (eg, Medication Event Monitoring System) that provide real-time data on medication-taking behaviour58 and have been used to good effect in studies with patients with hypertension,59 heart failure60 and CAD.61 There is potential for objective measures to be used in conjunction with self-report tools to provide a more reliable and accurate representation of medication-taking behaviour of patients with ACS.
Strengths and limitations
The strengths of this study include the adoption of a comprehensive search strategy that comprised eight online databases and a supplementary grey literature search. Additionally, we applied an existing behaviour change framework to identify specific techniques used among HCP-led adherence interventions, which we believe is a novel approach for trials with patients with ACS. Our study does also include certain limitations. First, while we were successful in BCT identification, there were insufficient data to determine the effectiveness of particular BCTs. A larger data set would be required to undertake the type of meta-regression analyses that have recently been reported within the adherence literature.62 Second, we found relatively high levels of statistical heterogeneity in our random-effects models, which is inherent when comparing methodologically diverse behavioural interventions. We accounted for this variability by removing outliers, which resulted in our final model having moderate statistical heterogeneity. Third, only one researcher was involved in all aspects of the identification, screening, data extraction and risk of bias assessments, although dual-raters coded interventions independently using the BCT taxonomy. Best practice would be to include multiple independent raters in all stages of the review to ensure methodological rigour. Fourth, we decided not to exclude studies based on how medication adherence was measured, which was often done using unreliable self-report methods. A previous review by Santo et al 63 circumvented this issue somewhat by including stricter adherence measurement eligibility criteria. Ultimately, all methods of adherence measurement are limited in terms of practicality, reliability and cost, which represents a wider issue across the adherence literature.
Conclusion
This study suggests that HCP-led interventions have a small positive effect on medication adherence following ACS. An existing BCT taxonomy was used successfully to identify common techniques within adherence interventions. However, data were insufficient to draw firm conclusions regarding the impact of BCTs on intervention effectiveness. Information provision remains the basis of most adherence interventions. Further work is required to understand how intervention design and delivery determines the effectiveness of adherence interventions following ACS.
Footnotes
Contributors: Concept design was undertaken by JC, VA and JW. JC undertook the literature search with VA and JW involved in eligibility screening. LA and SN contributed to behaviour change technique coding and statistical analyses, respectively. JC wrote the first draft, with all authors contributing to the critical revision of the manuscript.
Funding: The authors report that this study was supported by a King’s College London-University of California, San Francisco PhD Studentship (for JC).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 2012;125:882–7. 10.1016/j.amjmed.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 2. Ho PM, Magid DJ, Shetterly SM, et al. . Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008;155:772–9. 10.1016/j.ahj.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 3. Crawshaw J, Auyeung V, Norton S, et al. . Identifying psychosocial predictors of medication non-adherence following acute coronary syndrome: A systematic review and meta-analysis. J Psychosom Res 2016;90:10–32. 10.1016/j.jpsychores.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 4. Nieuwlaat R, Wilczynski N, Navarro T, et al. . Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014:CD000011 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chase JA, Bogener JL, Ruppar TM, et al. . The effectiveness of medication adherence interventions among patients with coronary artery disease: a meta-analysis. J Cardiovasc Nurs 2016;31:357–66. 10.1097/JCN.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laba TL, Bleasel J, Brien JA, et al. . Strategies to improve adherence to medications for cardiovascular diseases in socioeconomically disadvantaged populations: a systematic review. Int J Cardiol 2013;167:2430–40. 10.1016/j.ijcard.2013.01.049 [DOI] [PubMed] [Google Scholar]
- 7. Jörntén-Karlsson M, Pintat S, Molloy-Bland M, et al. . Patient-centered interventions to improve adherence to statins: a narrative synthesis of systematically identified studies. Drugs 2016;76:1447–65. 10.1007/s40265-016-0640-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takiya LN, Peterson AM, Finley RS. Meta-analysis of interventions for medication adherence to antihypertensives. Ann Pharmacother 2004;38:1617–24. 10.1345/aph.1D268 [DOI] [PubMed] [Google Scholar]
- 9. Kubica A, Obońska K, Fabiszak T, et al. . Adherence to antiplatelet treatment with P2Y12 receptor inhibitors. is there anything we can do to improve it? A systematic review of randomized trials. Curr Med Res Opin 2016;32:1441–51. 10.1080/03007995.2016.1182901 [DOI] [PubMed] [Google Scholar]
- 10. Mansoor SM, Krass I, Aslani P. Multiprofessional interventions to improve patient adherence to cardiovascular medications. J Cardiovasc Pharmacol Ther 2013;18:19–30. 10.1177/1074248412442001 [DOI] [PubMed] [Google Scholar]
- 11. Jalal ZS, Smith F, Taylor D, et al. . Pharmacy care and adherence to primary and secondary prevention cardiovascular medication: a systematic review of studies. Eur J Hosp Pharm Sci Pract 2014;21:238–44. 10.1136/ejhpharm-2014-000455 [DOI] [Google Scholar]
- 12. Michie S, Johnston M, Abraham C, et al. . Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005;14:26–33. 10.1136/qshc.2004.011155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Michie S, Richardson M, Johnston M, et al. . The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 14. Johnston N, Weinman J, Ashworth L, et al. . Systematic reviews: causes of non-adherence to P2Y12 inhibitors in acute coronary syndromes and response to intervention. Open Heart 2016;3:e000479 10.1136/openhrt-2016-000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vrijens B, De Geest S, Hughes DA, et al. . A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012;73:691–705. 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conn VS, Ruppar TM, Chase JA, et al. . Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep 2015;17:94 10.1007/s11906-015-0606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JP, Altman DG, Gøtzsche PC, et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodwin L, Ostuzzi G, Khan N, et al. . Can we identify the active ingredients of behaviour change interventions for coronary heart disease patients? A systematic review and meta-analysis. PLoS One 2016;11:e0153271 10.1371/journal.pone.0153271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage publications, 2001. [Google Scholar]
- 21. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons, 2011. [Google Scholar]
- 22. Calvert SB, Kramer JM, Anstrom KJ, et al. . Patient-focused intervention to improve long-term adherence to evidence-based medications: a randomized trial. Am Heart J 2012;163:657–65. 10.1016/j.ahj.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 23. Cossette S, Frasure-Smith N, Dupuis J, et al. . Randomized controlled trial of tailored nursing interventions to improve cardiac rehabilitation enrollment. Nurs Res 2012;61:111–20. 10.1097/NNR.0b013e318240dc6b [DOI] [PubMed] [Google Scholar]
- 24. Costa e Silva R, Pellanda L, Portal V, et al. . Transdisciplinary approach to the follow-up of patients after myocardial infarction. Clinics 2008;63:489-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du L, Dong P, Jia J, et al. . Impacts of intensive follow-up on the long-term prognosis of percutaneous coronary intervention in acute coronary syndrome patients - a single center prospective randomized controlled study in a Chinese population. Eur J Prev Cardiol 2016;23:1077–85. 10.1177/2047487315607041 [DOI] [PubMed] [Google Scholar]
- 26. Giallauria F, Lucci R, D’Agostino M, et al. . Two-year multicomprehensive secondary prevention program: favorable effects on cardiovascular functional capacity and coronary risk profile after acute myocardial infarction. J Cardiovasc Med 2009;10:772–80. 10.2459/JCM.0b013e32832d55fe [DOI] [PubMed] [Google Scholar]
- 27. Giannuzzi P, Temporelli PL, Marchioli R, et al. . Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian cardiac rehabilitation network. Arch Intern Med 2008;168:2194–204. 10.1001/archinte.168.20.2194 [DOI] [PubMed] [Google Scholar]
- 28. Gould KA. A randomized controlled trial of a discharge nursing intervention to promote self-regulation of care for early discharge interventional cardiology patients. Dimens Crit Care Nurs 2011;30:117–25. 10.1097/DCC.0b013e3182052324 [DOI] [PubMed] [Google Scholar]
- 29. Gujral G, Winckel K, Nissen LM, et al. . Impact of community pharmacist intervention discussing patients’ beliefs to improve medication adherence. Int J Clin Pharm 2014;36:1048–58. 10.1007/s11096-014-9993-y [DOI] [PubMed] [Google Scholar]
- 30. Ho PM, Lambert-Kerzner A, Carey EP, et al. . Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med 2014;174:186–93. 10.1001/jamainternmed.2013.12944 [DOI] [PubMed] [Google Scholar]
- 31. Jorstad HT, von Birgelen C, Alings AM, et al. . Effect of a nurse-coordinated prevention programme on cardiovascular risk after an acute coronary syndrome: main results of the RESPONSE randomised trial. Heart 2013;99:1421–30. 10.1136/heartjnl-2013-303989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. M A Jalal ZS, Smith F, Taylor D, et al. . Impact of pharmacy care upon adherence to cardiovascular medicines: a feasibility pilot controlled trial. Eur J Hosp Pharm Sci Pract 2016;23:250–6. 10.1136/ejhpharm-2015-000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotowycz MA, Cosman TL, Tartaglia C, et al. . Safety and feasibility of early hospital discharge in ST-segment elevation myocardial infarction-A prospective and randomized trial in low-risk primary percutaneous coronary intervention patients (the safe-depart trial). Am Heart J 2010;159:117 10.1016/j.ahj.2009.10.024 [DOI] [PubMed] [Google Scholar]
- 34. Kronish IM, Rieckmann N, Burg MM, et al. . The effect of enhanced depression care on adherence to risk-reducing behaviors after acute coronary syndromes: findings from the COPES trial. Am Heart J 2012;164:524–9. 10.1016/j.ahj.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lapointe F, Lepage S, Larrivée L, et al. . Surveillance and treatment of dyslipidemia in the post-infarct patient: can a nurse-led management approach make a difference? Can J Cardiol 2006;22:761–7. 10.1016/S0828-282X(06)70292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller P, Wikoff R, McMahon M, et al. . Influence of a nursing intervention on regimen adherence and societal adjustments postmyocardial infarction. Nurs Res 1988;37:297–302. 10.1097/00006199-198809000-00007 [DOI] [PubMed] [Google Scholar]
- 37. Miller P, Wikoff R, McMahon M, et al. . Personal adjustments and regimen compliance 1 year after myocardial infarction. Heart Lung 1989;18:339–46. [PubMed] [Google Scholar]
- 38. Miller P, Wikoff R, Garrett MJ, et al. . Regimen compliance two years after myocardial infarction. Nurs Res 1990;39:333–6. 10.1097/00006199-199011000-00003 [DOI] [PubMed] [Google Scholar]
- 39. Muñiz J, Gómez-Doblas JJ, Santiago-Pérez MI, et al. . The effect of post-discharge educational intervention on patients in achieving objectives in modifiable risk factors six months after discharge following an episode of acute coronary syndrome, (CAM-2 Project): a randomized controlled trial. Health Qual Life Outcomes 2010;8:137 10.1186/1477-7525-8-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Najafi SS, Shaabani M, Momennassab M, et al. . The nurse-led telephone follow-up on medication and dietary adherence among patients after myocardial infarction: a randomized controlled clinical trial. Int J Community Based Nurs Midwifery 2016;4:199. [PMC free article] [PubMed] [Google Scholar]
- 41. Polack J, Jorgenson D, Robertson P. Evaluation of different methods of providing medication-related education to patients following myocardial infarction. Canadian Pharmacists Journal 2008;141:241–7.doi:10.3821/1913-701X(2008)141[241:EODMOP]2.0.CO;2 [Google Scholar]
- 42. Polsook R, Aungsuroch Y, Thongvichean T. The effect of self-efficacy enhancement program on medication adherence among post-acute myocardial infarction. Appl Nurs Res 2016;32:67–72. 10.1016/j.apnr.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 43. Redfern J, Briffa T, Ellis E, et al. . Choice of secondary prevention improves risk factors after acute coronary syndrome: 1-year follow-up of the CHOICE (Choice of Health Options In prevention of Cardiovascular Events) randomised controlled trial. Heart 2009;95:468–75. 10.1136/hrt.2008.150870 [DOI] [PubMed] [Google Scholar]
- 44. Redfern J, Briffa T, Ellis E, et al. . Patient-centered modular secondary prevention following acute coronary syndrome: a randomized controlled trial. J Cardiopulm Rehabil Prev 2008;28:107–15. 10.1097/01.HCR.0000314204.86805.13 [DOI] [PubMed] [Google Scholar]
- 45. Uysal H, Ozcan Ş. The effect of individual education on patients’ physical activity capacity after myocardial infarction. Int J Nurs Pract 2015;21:18–28. 10.1111/ijn.12193 [DOI] [PubMed] [Google Scholar]
- 46. Xavier D, Gupta R, Kamath D, et al. . Community health worker-based intervention for adherence to drugs and lifestyle change after acute coronary syndrome: a multicentre, open, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:244–53. 10.1016/S2213-8587(15)00480-5 [DOI] [PubMed] [Google Scholar]
- 47. Sharma KK, Gupta R, Mathur M, et al. . Non-physician health workers for improving adherence to medications and healthy lifestyle following acute coronary syndrome: 24-month follow-up study. Indian Heart J 2016;68:832–40. 10.1016/j.ihj.2016.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yorio J, Viswanathan S, See R, et al. . The effect of a disease management algorithm and dedicated postacute coronary syndrome clinic on achievement of guideline compliance. J Investig Med 2008;56:15–25. 10.2310/jim.0b013e3181620295 [DOI] [PubMed] [Google Scholar]
- 49. Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med 2017;99:269–76. 10.1016/j.ypmed.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carter P. Operational productivity and performance in English NHS acute hospitals: unwarranted variations. an independent report for the department of health by lord carter of coles. London: Department of Health, 2016. [Google Scholar]
- 51. Cutrona SL, Choudhry NK, Fischer MA, et al. . Modes of delivery for interventions to improve cardiovascular medication adherence. Am J Manag Care 2010;16:929. [PMC free article] [PubMed] [Google Scholar]
- 52. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555–67. 10.1016/S0022-3999(99)00057-4 [DOI] [PubMed] [Google Scholar]
- 53. Conn VS, Enriquez M, Ruppar TM, et al. . Meta-analyses of theory use in medication adherence intervention research. Am J Health Behav 2016;40:155–71. 10.5993/AJHB.40.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoffmann TC, Glasziou PP, Boutron I, et al. . Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 55. Horne R, Chapman SC, Parham R, et al. . Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS One 2013;8:e80633 10.1371/journal.pone.0080633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Dulmen S, Sluijs E, van Dijk L, et al. . Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res 2007;7:55 10.1186/1472-6963-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cutrona SL, Choudhry NK, Fischer MA, et al. . Targeting cardiovascular medication adherence interventions. J Am Pharm Assoc 2012;52:381–97. 10.1331/JAPhA.2012.10211 [DOI] [PubMed] [Google Scholar]
- 58. El Alili M, Vrijens B, Demonceau J, et al. . A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 2016;82:268–79. 10.1111/bcp.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Márquez-Contreras E, Martell-Claros N, Gil-Guillén V, et al. . Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens 2006;24:169–75. 10.1097/01.hjh.0000198023.53859.a2 [DOI] [PubMed] [Google Scholar]
- 60. Murray MD, Young J, Hoke S, et al. . Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med 2007;146:714–25. 10.7326/0003-4819-146-10-200705150-00005 [DOI] [PubMed] [Google Scholar]
- 61. Park LG, Howie-Esquivel J, Chung ML, et al. . A text messaging intervention to promote medication adherence for patients with coronary heart disease: a randomized controlled trial. Patient Educ Couns 2014;94:261–8. 10.1016/j.pec.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 62. Kassavou A, Sutton S. Automated telecommunication interventions to promote adherence to cardio-metabolic medications: meta-analysis of effectiveness and meta-regression of behaviour change techniques. Health Psychol Rev 2017:1–18. 10.1080/17437199.2017.1365617 [DOI] [PubMed] [Google Scholar]
- 63. Santo K, Kirkendall S, Laba TL, et al. . Interventions to improve medication adherence in coronary disease patients: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 2016;23:1065–76. 10.1177/2047487316638501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2017-000685supp001.pdf (516.4KB, pdf)