Abstract
SLE is a serious, debilitating autoimmune disease that affects various organs and body systems. Of all the heterogeneous autoimmune diseases, SLE is perhaps the most heterogeneous. Patients with SLE, who are primarily female, have diverse disease manifestations and severity. SLE is characterised by substantial concentrations of autoantibodies against nuclear antigens, which are thought to be caused by immune cell dysregulation. Until recently, several immunosuppressant agents were used to treat this disease. Efforts to develop drugs against targets potentially involved in disease mechanisms have resulted in the identification and use of BAFF (B-cell activating factor)/APRIL (a proliferation-inducing ligand) inhibitors to treat SLE. Drugs in late-stage development that focus on pathways that are dysregulated in SLE include those that target the interferon pathway, T-cell signalling and B-cell signalling. New therapeutic agents are still necessary because of the unmet medical needs associated with this disease, including insufficient disease control, poor health-related quality of life, comorbidities, toxicity of the majority of therapies and diminished survival. Despite the substantial long-term investment of research, clinical activity and resources for identifying new treatments for this disease, only one new therapy, the biological belimumab, has been approved in the past 50 years. Efforts to develop drugs to address these needs are challenged by problems associated with disease heterogeneity, variable disease mechanisms and trial design. This review provides an overview of current and future treatments, discusses challenges in the SLE drug development process and offers recommendations for overcoming these challenges.
Keywords: systemic lupus erythematosus, lupus nephritis, interferon, cytokines, treatment
Background
SLE is a heterogeneous autoimmune disease with clinical manifestations, organ involvement, disease severity and laboratory findings that vary greatly among patients.1 2 Prevalence of SLE worldwide is estimated to be as great as 150 per 100 000 individuals, with an incidence of approximately 1 to 10 per 100 000 person-years.3 Approximately 90% of patients afflicted with SLE are women, with the greatest age-specific incidence rates in North America observed for women in their late teens to early 20s and for those in their 50s.3
SLE can target many organs and body systems, including the skin, kidneys, joints, cardiovascular system and central nervous system.1 2 The disease is characterised by autoantibodies against nuclear antigens (ANA), which are a consequence of immune system dysregulation.1 The pathogenesis of SLE is still under investigation. Along with immune system dysregulation and the presence of ANA, other relevant factors in SLE pathogenesis encompass genetic susceptibility, environmental triggers and innate and adaptive immune system activation. Treatment currently focuses on immune suppression to control lupus disease activity, prevent organ damage, reduce morbidity and improve patient survival and health-related quality of life.1
Patients with SLE have several unmet medical needs. Few options exist for disease control for patients who fail to respond to currently available and approved therapies or who are unable to tolerate the adverse effects related to these therapies. Patients with SLE have significantly worse health-related quality of life than healthy controls or patients with other chronic diseases.4 Although 5-year survival for patients with SLE has improved from 50% in the 1950s to more than 90% currently, there is still a need to improve long-term survival.4 Additional approaches are necessary to prevent organ damage from both the disease and its treatment. Other unmet medical needs include reducing the number and severity of comorbidities and decreasing drug toxicity.
This review focuses on current and future treatment regimens and potential challenges that may affect the success of new treatments based on our evolving knowledge of SLE.
Currently available treatments
Organ involvement and its related disease activity dictate the treatment for SLE. For constitutional symptoms and mild-to-moderate SLE, current guidelines recommend the use of antimalarial drugs, glucocorticosteroids (GCS), non-steroidal anti-inflammatory drugs (eg, for arthritis and serositis) and often immunosuppressive therapy for treating persistent disease activity and decreasing GCS use.5 For patients with severe SLE, often immunosuppressive therapy and GCS are initiated at the same time (eg, for lupus nephritis). For patients with class III and IV lupus nephritis, GCS, azathioprine, cyclophosphamide, mycophenolic acid/sodium and mycophenolate mofetil (MMF) are recommended as induction drugs. For maintenance therapy, GCS, azathioprine, mycophenolic acid/sodium and MMF are recommended.5 Additional agents suggested for patients with SLE include vitamin D, calcium supplements and antiresorptive agents for osteoporosis prevention, antihypertensive agents and statins.6 7
Belimumab (Benlysta, GlaxoSmithKline, Research Triangle Park, North Carolina, USA) is a human monoclonal antibody specific for B-cell activating factor (BAFF) that is approved in the USA, Canada and Europe to treat adult patients with autoantibody-positive SLE whose disease is active despite receiving standard therapy.8 BAFF was originally identified as a target for SLE because of its required role in mature B-lymphocyte survival. Along with another family member named a proliferation-inducing ligand (APRIL), BAFF promotes plasma cell survival, and it also regulates naïve B-lymphocyte repertoire selection.9 Overexpression of BAFF leads to SLE-like symptoms in transgenic mouse models, including anti-DNA autoantibody production.10 BAFF concentrations are elevated for patients with SLE and correlated with lupus disease activity.11
The approval of belimumab for the treatment of SLE was based on two phase III studies, BLISS-52 and BLISS-76, which demonstrated safety and efficacy in treating patients with active SLE without lupus nephritis or central nervous system lupus.12–14 In the two trials, a greater SLE Responder Index (SRI4) response was achieved with belimumab treatment compared with placebo at 52 weeks (BLISS-52: 58% for belimumab 10 mg/kg vs 44% for placebo, P=0.0006; BLISS-76: 43% for belimumab 10 mg/kg vs 34% for placebo, P=0.017).12 13 Observational cohort studies from the USA, Canada and Europe involving patients with SLE receiving belimumab plus standard of care demonstrated improvements in disease activity and laboratory values with a reduction in GCS use and healthcare resource utilisation through 24 months.15–17 More recently, the efficacy of subcutaneous belimumab for moderate-to-severe SLE has been demonstrated.18
Current drug pipeline
Approximately 30 novel agents are currently being evaluated in phase II/III clinical trials for the treatment of SLE, lupus nephritis and cutaneous lupus (tables 1 and 2). In phase III trials, agents include those targeting the interferon (IFN) pathway, BAFF-APRIL pathway, T-cell signalling and B-cell signalling. Furthermore, combinations of novel agents that target different mechanisms of action are being explored in phase II trials, as exemplified by the CALIBRATE and BEAT-LUPUS trials with belimumab and rituximab for lupus nephritis and SLE, respectively.19 20
Table 1.
Pipeline of drugs being evaluated in phase III clinical trials for SLE19
| Drug | Mechanism of action | Overview of current phase III | Overview of phase II/III development in SLE |
| Abatacept | T-cell costimulation modulator (cytotoxic T lymphocyte-associated antigen 4–IgG1 fusion) | Efficacy and safety of abatacept in lupus nephritis on a background of MMF and GCS. Trial is ongoing. | In a 52-week phase II/III trial involving patients with lupus nephritis, there was no difference between treatment groups and placebo in time to confirmed complete response (primary endpoint), although biological activity was observed. Treatment was well tolerated.37 |
| Anifrolumab | Fully human, IgG1 κ monoclonal antibody that binds to and neutralises receptors of all type I IFNs | Two currently ongoing trials are evaluating the efficacy and safety of anifrolumab either at one or two different dosing regimens for patients with moderate-to-severe SLE. A third trial evaluating the long-term safety and tolerability of anifrolumab for patients with moderate-to-severe SLE is recruiting patients who completed one of the above phase III trials. | In a phase IIb trial for patients with moderate-to-severe SLE who did not have active and severe lupus nephritis or neuropsychiatric SLE, a significantly greater percentage of patients receiving anifrolumab 300 mg every 4 weeks achieved an SRI(4) response at week 24 with sustained reduction of GCS compared with placebo (primary endpoint).28 |
| Atacicept | TACI-Fc fusion protein that binds BAFF and APRIL | Trial has been completed and results are published.30 | In a phase II/III trial, atacicept did not improve flare rate (primary endpoint) or time to first flare (main secondary endpoint) during a 52-week trial compared with placebo.30 However, a post hoc analysis indicated that patients with large baseline BAFF and APRIL concentrations may benefit more with this agent.31 More recently, results from a 24-week phase IIb trial showed no significant improvement in SRI(4) with atacicept treatment versus placebo (primary endpoint).32 However, patients with high disease activity did demonstrate significant improvements with atacicept versus placebo in SRI(6) response and incidence of flares.32 |
| Lupuzor (IPP-201101) |
21-mer peptide derived from small nuclear riboprotein U1-70K, which is phosphorylated at Ser140 | Efficacy and safety of Lupuzor plus standard of care for patients with SLE Trial is ongoing. |
In a phase IIb trial, a significantly greater percentage of patients achieved SRI(4) response at week 12 with Lupuzor given once every 4 weeks compared with placebo (primary endpoint). Treatment was well tolerated in general.47 |
| Rituximab | Anti-CD20 monoclonal antibody (B cell) | Evaluation of rituximab plus MMF for flare reduction and steroid-sparing benefit for patients with lupus nephritis (RITUXILUP). Trial is ongoing. | Although previous phase II/III trials did not meet their primary endpoint,43 44 additional clinical studies in combination with different drugs and under various conditions are being explored. |
| Voclosporin | Immunosuppressant, calcineurin inhibitor | Efficacy and safety of voclosporin in patients with active lupus nephritis. Trial is currently recruiting patients. | In a completed phase IIb trial, at 48 weeks of treatment, 49% of patients with lupus nephritis achieved complete remission with the lower dosage voclosporin regimen (23.7 mg two times per week) compared with 24% in the control arm (P<0.001).48 |
APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor; GCS, glucocorticosteroid; IFN, interferon; MMF, mycophenolate mofetil; SRI(4), SLE Responder Index (SRI) with ≥4 point reductions; SRI(5), SRI with ≥5 point reductions; SRI(6), SRI with ≥6 point reductions TACI, transmembrane activator and CAML (calcium-modulating cyclophilin ligand) interactor.
Table 2.
Pipeline of drugs being evaluated in phase II clinical trials for SLE19
| Drug | Mechanism of action | Overview of phase II development in SLE* |
| Aldesleukin (ILT-101) | IL-2 | Evaluate the efficacy, safety and pharmacokinetics of ILT-101 in moderate-to-severe SLE. Trial is currently recruiting patients. |
| Baricitinib (LY3009104) | JAK inhibitor | Evaluate the safety and efficacy of baricitinib for patients with SLE. Trial is ongoing. |
| BIIB059 | Anti-BDCA2 monoclonal antibody | Efficacy of BIIB059 in reducing skin disease activity for patients with SLE and cutaneous lupus erythematosus with or without systemic manifestations. Trial is currently recruiting patients. |
| BI655064 | Anti-CD40 monoclonal antibody | Dosage finding, efficacy and safety of BI655064 for patients with active lupus nephritis. Trial is currently recruiting patients. |
| Bortezomib | Proteasome inhibitor | Change in disease-specific antibody titres with bortezomib. Trial is currently recruiting patients. |
| BT063 | Anti–IL-10 monoclonal antibody | Efficacy and safety of BT063 for patients with SLE. Trial is ongoing. |
| Cenerimod (ACT-334441) | Sphingosine-1-phosphate receptor agonist | Biological activity, safety, tolerability and pharmacokinetics of ACT-334441 for patients with SLE. Trial has been completed; no results posted. |
| Dapirolizumab pegol | Anti-CD40L | Efficacy and safety of dapirolizumab for patients with moderate-to-severe SLE. Trial is currently recruiting patients. |
| Edratide | Peptide based on complementary-determining region I of a human anti-DNA monoclonal antibody | In a 26-week phase II trial, no significant difference was observed between edratide-treated and placebo-treated patients in reduction in SLEDAI-2K and adjusted mean SLEDAI, although positive trends were noted for other endpoints.67 Trial is completed, and results are published. |
| Filgotinib | JAK1 inhibitor | Efficacy of filgotinib for female patients with moderate-to-severe active cutaneous lupus erythematosus. Trial is currently recruiting patients. Efficacy and safety of filgotinib in adults with lupus membranous neuropathy. Trial has not begun recruiting. |
| GS-9876 | SYK inhibitor | Efficacy of GS-9876 for female patients with moderate-to-severe active cutaneous lupus erythematosus. Trial is currently recruiting patients. Efficacy and safety of GS-9876 in adults with lupus membranous neuropathy. Trial has not begun recruiting. |
| Iberdomide (CC-220) | Ubiquitin ligase modulator | Efficacy, safety, tolerability, pharmacodynamics and pharmacokinetics of CC-220 for patients with SLE. A pilot study is ongoing, with a phase II trial currently recruiting patients. |
| IFN-α-kinoid | Anti-IFN-αvaccine | Efficacy, neutralisation of the IFN gene signature and safety of IFN-α-kinoid for patients with SLE. Trial is currently recruiting patients. |
| Iguratimod | Antiinflammatory, NF-κB inhibitor | Efficacy and safety of iguratimod for patients with active diffuse lupus nephritis and refractory lupus nephritis. Studies have not yet started recruiting. |
| Nelfinavir | HIV-1 protease inhibitor | Effect of nelfinavir in reducing anti-dsDNA antibodies. Trial is currently recruiting patients. |
| Obinutuzumab | Anti-CD20 monoclonal antibody | Efficacy and safety of obinutuzumab plus MMF/MPA compared with MMF/MPA-treated placebo for patients with proliferative lupus nephritis. Trial is currently recruiting patients. |
| OMS721 | Anti-MASP-2 monoclonal antibody | Safety and tolerability of OMS721 for patients with lupus nephritis. Trial is currently recruiting patients. |
| Rapamycin (sirolimus) | Immunosuppressant | Two studies have taken place. One was a prospective study evaluating decrease in disease activity and GCS reduction for patients with SLE. The second study evaluated efficacy and safety for patients with idiopathic and lupus-related membranous nephropathy. Trials are completed, and no results are available. |
| RC18 | TACI-antibody fusion protein | Efficacy and safety of RC18 for patients with moderate-to-severe SLE. Trial is currently recruiting patients. |
| RSLV-132 | RNase-Fc fusion protein | Effect of RSLV-132 on cutaneous manifestations for patients with SLE. Trial is currently recruiting patients. |
| SM101 | Soluble Fc-gamma receptor IIb | In a phase IIa trial, the SRI response rate for patients treated with SM101 12 mg/kg once weekly for 4 weeks was approximately twofold higher than placebo at 24 weeks (39% vs 18%).68 |
| Theralizumab (TAB08) | CD28 superagonist (Treg) | Efficacy, safety, pharmacokinetics and pharmacodynamics of TAB08 for patients with SLE not adequately controlled with current concomitant therapy. Trial is currently recruiting patients. |
| Ustekinumab | Anti–IL-12/–23 monoclonal antibody | In a phase II study for patients with active SLE, 60% of patients receiving ustekinumab had an SRI(4) response at week 24 compared with 31% for placebo (P=0.0046).69 |
| Vobarilizumab (ALX-0061) | Anti–IL-6 receptor nanoantibody | Efficacy and safety of vobarilizumab for patients with moderate-to-severe SLE. Trial is ongoing. |
| XmAb5871 | Anti-CD19 monoclonal antibody (B cell) | Efficacy and safety of XmAb5871 for patients with SLE. Trial is currently recruiting patients. |
*Phase I/II trials not reported.
BDCA2, blood dendritic cell antigen-2; dsDNA, double-stranded DNA; GCS; glucocorticosteroid; IFN-α, interferon-α; IL-2, interleukin-2; IL-6, interleukin-6; IL-12, interleukin-12; IL-23, interleukin-23; JAK, Janus kinase; MASP-2, mannan-binding lectin-associated serine protease-2; MMF, mycophenolate mofetil; MPA, mycophenolic acid; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RNase, ribonuclease; SLEDAI, SLE Disease Activity Index; SLEDAI-2K, modified SLEDAI scale introduced in 2002; SRI(4), SLE Responder Index (SRI) with ≥4 point reductions; SYK, spleen tyrosine kinase; TACI, transmembrane activator and CAML (calcium-modulating cyclophilin ligand) interactor; Treg, T-regulatory cell.
IFN pathway inhibitors
Rationale
Type I IFNs are a class of cytokines that play a protective role against viral infections.21 IFN-α, a member of the type I IFN family, promotes the stimulation and differentiation of various immune cells, including the differentiation of autoreactive B lymphocytes to immunoglobulin-secreting plasma cells, maturation of myeloid dendritic cells and inducing their expression of BAFF and APRIL, upregulation of T-cell costimulatory molecules and inactivation of T-regulatory cells (Tregs).21–23
Mouse models indicate that type I IFN receptor deficiency reduces lupus-like symptoms.24 25 Conversely, IFN-α can intensify lupus-like symptoms in a murine model.25
Type I IFN concentrations positively correlate with SLE Disease Activity Index (SLEDAI) score and anti–double-stranded DNA (anti-dsDNA) concentrations in patients with SLE.26 Furthermore, the magnitude of the type I IFN–driven gene signature (IFNGS) is correlated with disease activity.27
Anifrolumab
Anifrolumab (AstraZeneca, Gaithersburg, Maryland, USA) is a fully human, IgG1 κ monoclonal antibody in phase III clinical development for SLE. Anifrolumab binds to and neutralises the IFN-α receptor, in effect blocking type I IFN-dependent cell signalling.28
In a phase IIb trial (the MUSE trial) of patients with moderate-to-severe SLE who did not have active or severe lupus nephritis or neuropsychiatric SLE, a significantly greater percentage of patients receiving anifrolumab 300 mg every 4 weeks achieved an SRI(4) response at week 24 and sustained reduction of GCS compared with placebo (primary endpoint).28 These effects were greater for patients who were IFNGS test–high at baseline.28 In addition, after 1 year of treatment, anifrolumab patients achieved significantly greater rates of improvement in rash, alopecia and joint manifestations than did placebo recipients.29 The incidence of serious adverse events was similar in both treatment groups, although greater percentages of anifrolumab-treated patients than placebo-treated patients developed herpes zoster or influenza.28
Two currently ongoing phase III trials are evaluating efficacy and safety of anifrolumab in either one or two different dosing regimens for patients with moderate-to-severe SLE. A third phase III trial evaluating the long-term safety and tolerability of anifrolumab for patients with moderate-to-severe SLE is currently recruiting patients who completed one of the aforementioned phase III trials.
BAFF-APRIL inhibitors
Rationale
See Currently available treatments section above.
Atacicept
Atacicept (Merck KGaA, Darmstadt, Germany) is a fusion protein composed of the TACI receptor with the modified Fc portion of human immunoglobulin that binds BAFF and APRIL.30 In a phase II/III trial for patients with moderate-to-severe SLE, no difference was observed in the flare rates (primary endpoint) or time to first flare (secondary endpoint) between treatment and placebo groups.30 Adverse events were similar between treatment groups with respect to incidence and severity.30 In a post hoc analysis of this trial, a greater reduction in flare rates was observed for patients with baseline BAFF and APRIL concentrations of ≥1.6 and ≥2.2 ng/mL, respectively, compared with smaller baseline concentrations of both.31 Recently, results reported from a 24-week phase IIb trial demonstrated that the primary endpoint of statistically significant improvement in SRI(4) with atacicept treatment versus placebo was not achieved.32 However, patients with high disease activity did demonstrate significant improvements with atacicept versus placebo in SRI with ≥6 point reductions (SRI6) response and incidence of flares.32
T-cell signalling inhibitors
Rationale
T cells are believed to play an important role in the development and progression of SLE. Autoreactive B lymphocytes are activated in a T-cell-dependent manner that relies on T-follicular helper cells (Tfh).33 Increased concentrations of cells with a Tfh phenotype have been found in the blood of patients with SLE.34 35 Furthermore, dysregulation of Tfh cells has been found to be associated with the development of SLE in mouse models.2 Patients with SLE have autoreactive T cells,1 and concentrations of both CD4+ T-helper cells and CD3+CD4−CD8− T lymphocytes are elevated in patients with SLE, supporting the production of autoantibodies.36 Increased numbers of T-helper cells expressing large concentrations of the proinflammatory cytokine interleukin-17 (Th17 cells) are found as well. Large interleukin-17 concentrations are associated with the development of lupus-like nephritis in several mouse models.33 Finally, defects in regulatory T-cell (Treg) function have been reported in patients with SLE.33 Tregs play an important role in modulating T-cell activity and regulating the immune system.33
Abatacept
Abatacept (Orencia, Bristol-Myers Squibb, Princeton, New Jersey, USA) is a cytotoxic T lymphocyte-associated antigen 4-IgG1 fusion protein that inhibits T-cell activation by modulating T-cell costimulatory events.37 In a phase II/III trial of patients with lupus nephritis, no difference was observed between abatacept-treated patients and placebo-treated patients in the time to confirmed complete response (the primary endpoint) or the percentage of patients who achieved confirmed complete response at the end of treatment (52 weeks). Confirmed complete response was a composite measure that required patients to maintain a defined glomerular filtration rate, minimal proteinuria and inactive urinary sediment maintenance over the 52-week treatment period. Improvements with abatacept treatment over placebo were observed in anti-dsDNA, complement C3 and complement C4 concentrations. Adverse events were similar between treatment arms with respect to incidence and severity.37
B-cell signalling inhibitors
Rationale
Central and peripheral B-cell tolerances to self-antigens are defective in SLE.38 39 This defect results in clonal expansion of autoreactive B lymphocytes that begins at the preclinical stage.40 One reason for such clonal expansion is a decrease in the removal of self-reactive immature B cells through checkpoints in the early stages of B-cell development.38 39 In addition, germinal centre exclusion is defective, resulting in the promotion of autoreactive B-lymphocyte differentiation to pathogenic memory and plasma cells.41
Rituximab
Rituximab (Rituxan, Genentech, South San Francisco, California, USA) is a chimeric anti-CD20 monoclonal antibody that is considered a treatment option in published guidelines for patients with lupus nephritis who are not responsive to first-line therapy.6 42 Rituximab was evaluated in the phase II/III EXPLORER trial (Exploratory Phase II/III SLE Evaluation of Rituximab) for patients with moderate-to-severe extrarenal SLE and a phase III LUNAR trial (Lupus Nephritis Assessment with Rituximab) for patients with class III/IV lupus nephritis.43 44 In the EXPLORER trial, no differences were observed between treatment groups in the primary endpoint (British Isles Lupus Assessment Group (BILAG) response).43 In the LUNAR trial, no significant difference between treatment groups was observed in overall renal response rates (primary endpoint).44 Adverse event rates were similar between treatment groups in both studies.43 44 However, in post hoc analysis of the EXPLORER trial, with rituximab treatment there was a reduction in the risk of subsequent first severe (BILAG A) flare (HR=0.61; P=0.052) and mean annualised flare rate (0.86 vs 1.41; P=0.038) relative to placebo.45
Currently, rituximab is being evaluated in combination with MMF in the phase III RITUXILUP trial as a GCS-sparing agent for patients with lupus nephritis.19 In a pilot study of 50 patients with lupus nephritis, 90% of patients achieved complete or partial response by a median time of 37 weeks (range 4–200) when treated with two doses of rituximab (1 g) and methyl prednisolone (500 mg) on days 1 and 15, followed by maintenance treatment of MMF.46 Two of the 45 responders needed >2 weeks of oral glucocorticoids.46
Additional agents in phase III trials
Lupuzor
Lupuzor (ImmuPharma PLC, London, UK) is a 21-mer peptide derived from small nuclear riboprotein U1-70K and is phosphorylated at the Ser140 position.47 In a phase IIb trial, a significantly greater percentage of patients receiving Lupuzor once every 4 weeks achieved an SRI(4) response at week 12 compared with placebo recipients (primary endpoint).47 Similar incidences of adverse events were reported between treatment groups.47
Voclosporin
Voclosporin (Aurinia Pharmaceuticals, Victoria, Canada) is a calcineurin inhibitor with immunosuppressant activity.48 In a phase IIb trial of patients with lupus nephritis, voclosporin given two times per day for 48 weeks elicited significantly greater complete and partial remission rates than control for both dosing regimens (23.7 and 39.5 mg). At week 48, 49% of patients obtained complete remission and 68% achieved partial remission with the 23.7 mg dosage regimen compared with 24% (P<0.001) and 48% (P=0.07) of the control arm, respectively.48 A phase III trial evaluating the efficacy and safety of voclosporin in patients with active lupus nephritis is currently recruiting patients.
Common reasons for clinical trial failure in SLE and recommendations
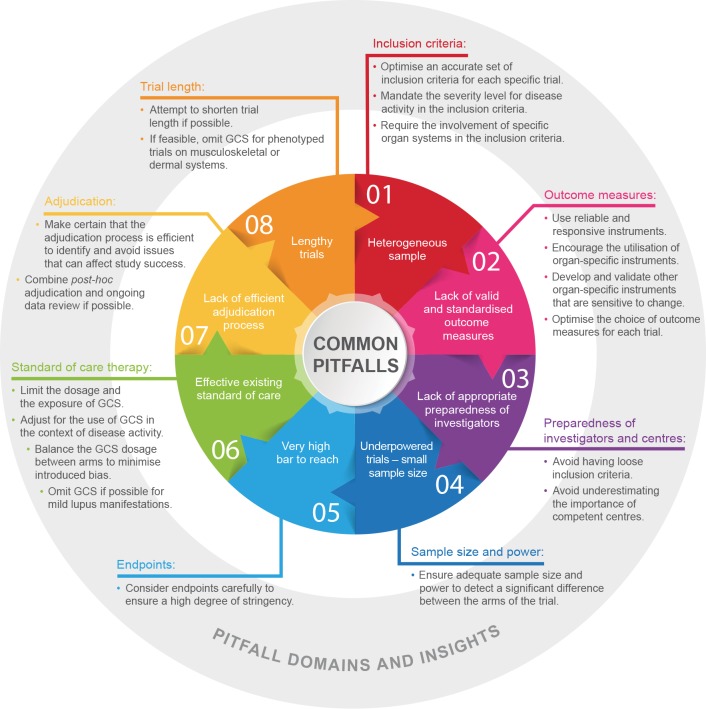
In recent years, various agents in SLE clinical trials have failed to meet their primary endpoints, including epratuzumab, an anti-CD22, B-cell-directed monoclonal antibody (programme terminated)49; rituximab43 44; and tabalumab, an anti-BAFF monoclonal antibody (programme terminated).50 Several potential reasons have been cited for clinical trial failure besides the drugs not being efficacious, including heterogeneity of patients included in a trial, use of outcome measures that were not developed for clinical trials and cannot measure change accurately over time, site investigator inexperience, concomitant medication use during trials and other trial design flaws.2 51 52 Many of these factors continue to challenge trial site staff, investigators and contract research organisations involved in running an SLE trial. An overview of these factors and recommendations for avoiding them is presented in figure 1.53–59
Figure 1.
The most common pitfalls in lupus clinical trials The inner circle lists the most common pitfalls that have hindered the success of lupus clinical trials. The outside statements reflect the domains in which the pitfalls may occur and insights into each of the pitfalls, along with some guidance. GCS, glucocorticosteroid.
In general, homogeneity of enrolled patients in clinical trials is essential, especially for trials with small sample sizes. Stringent and well-chosen criteria ensure patients’ homogeneity. Currently, SLEDAI-2K 4–6 is used to select patients with mild-to-moderate disease activity, but this approach often is not sufficient to ensure homogeneity of the sample. For example, patients who enter a trial with SLEDAI-2K of ≥4–6 can have different manifestations that sum up to the inclusion threshold. Furthermore, the degree of disease activity for the respective manifestation may differ from patient to patient (eg, inflammatory skin rash involving 3% of body surface area (BSA) in one patient vs 10% of BSA in another patient). Inflammatory lupus rash could be related to discoid rash in one patient and subacute rash in another patient, and a particular drug may work better for subacute rash compared with discoid rash. The time to improvement or resolution of these manifestations may vary. All of these points should be considered if the aim is to have a homogenous sample for a specific trial. Moreover, particular inclusion criteria may be overlooked in trial development, such as serological activity thresholds (eg, for ANA), which may have an important role in trial success. When deciding which patients to include in a trial, it may be necessary to classify the disease by immunological mechanisms, such as ANA concentrations (eg, ANA≥1:80). Several lessons learnt from the post hoc analyses in clinical trials may benefit the design of future trials. The post hoc analyses of the phase II/III APRIL study highlighted the importance of baseline biomarkers such as elevated serum concentrations of B-lymphocyte stimulator (BLyS) and APRIL, which may help to identify potential responders to atacicept.31 Another study by Petri et al demonstrated that BLyS concentrations of ≥2.0 ng/mL at screening are an independent prognostic factor for an increased risk of BILAG A or B flares.60 In the MUSE trial, patients with IFNGS-high test results responded better to anifrolumab than patients with IFNGS-low test results.28 In the future, lupus clinical trials will probably include and stratify patients based on their concentrations of cytokines and other biomarkers. Inclusion and exclusion criteria need to be selected carefully so that they will not be too restrictive and thereby fail to identify patients who may potentially benefit in future trials. In addition, excessively restrictive criteria will limit the external validity of the trial results and its generalisability.
We recommend the following actions to address the factors associated with heterogeneous samples. First, an accurate set of inclusion criteria should be optimised for each specific trial to ensure the homogeneity of the sample. For example, the majority of current trials mandate serologically positive patients with SLE (ANA +or dsDNA +antibodies). Second, severity level for disease activity should be mandated in the inclusion criteria. For example, six active joints should be mandated as opposed to ≥2 joints as per SLEDAI-2K. Finally, the inclusion criteria should require involvement of specific organ systems. Using an SLEDAI-2K score of ≥6 or BILAG 1A as inclusion criterion is not sufficient. The inclusion criteria for trials should require the activity in specific organ-systems such as musculoskeletal or dermal systems.
SLE encompasses a spectrum of manifestations, and the commonly used outcome measures in clinical trials lack the required extent of standardisation in the documentation of lupus manifestations. Accurate documentation is crucial for identifying and confirming change over time. Moreover, the different composite indices used, such as SRI and BILAG Composite Lupus Assessment (BICLA), can result in different responder rates, which can complicate between-trial comparisons. For example, SRI response is defined as (1)≥4 point reduction in SLEDAI global score; (2) no new severe disease activity (BILAG A organ score) or >1 new moderate organ score (BILAG B); and (3) no worsening from baseline in Physician’s Global Assessment score (increase <0.3).12 13 BICLA response is defined as (1) baseline BILAG score improvement (eg, all A (severe disease) scores falling to B (moderate), C (mild), or D (no activity), and all B scores falling to C or D); (2) no new BILAG A scores and ≤1 new BILAG B score; (3) no worsening of total SLEDAI-2K score from baseline; (4)≤10% deterioration in Physician’s Global Assessment score; and (5) no initiation of non-protocol treatment.61 One major difficulty for developing uniform outcome measurements is the low number of validated biomarkers available.
We therefore recommend the use of reliable and responsive instruments, for they are very important in clinical trials. Although SLEDAI-2K measures a complete recovery of descriptors, a better approach might be a 50% improvement, which SLEDAI-2K SRI(50) can capture. SLEDAI-2K SRI(50) is superior to SLEDAI-2K for measuring change over time.53–55 Second, the utilisation of organ-specific instruments (eg, Cutaneous Lupus Erythematosus Disease Area and Severity Index, composite renal outcomes, and so on) should be encouraged. Several groups have recently described the development of new indices for assessing lupus activity. Touma et al recently demonstrated that SLE Disease Activity Index Glucocorticosteroid Index (SLEDAI-2KG) identifies more responders at 6 months (92% vs 84%) and at 12 months (89% vs 76%) than SLEDAI-2K for cut-off points of 5, 6 and 7.62 Abrahamowicz et al described the derivation of a new Multivariable Lupus Outcome Score (LuMOS) with data from BLISS-76. LuMOS included a reduction in SLEDAI by ≥4 points, increase in C4, decrease in DNA antibody titre and no new symptoms or worsening in renal BILAG as well as improvements in the mucocutaneous component of BILAG. Early validation of LuMOS with data from BLISS-52 demonstrated superiority in discriminating responders from non-responders compared with SRI-4.63 Furthermore, it is necessary to develop and validate other organ-specific instruments that are sensitive to change (eg, an instrument for central nervous system manifestations such as cognitive impairment; instruments for assessing serositis disease severity). In addition, the choice of outcome measures should be optimised for each trial. For example, recent analyses have shown that urinary red blood cells should not be included as a component of renal composite outcomes.56 Spot urine protein to creatinine ratio should not take the place of 24-hour proteinuria quantification.
In some cases, investigators are not adequately prepared to use the disease activity instruments correctly. Investigators need proper training on the use of outcome measures and the specific instruments selected for the study. Selection of study sites needs to be considered carefully and should have expertise in treating patients with SLE. To address issues associated with a lack of appropriate preparedness of investigators and centres, we recommend avoiding loose criteria. Non-stringent criteria allow the participation of non-competent centres with insufficient skills for assessing and managing lupus. The importance of competent centres should not be underestimated. The inclusion of certified investigators for the use of specific instruments may be insufficient to assure a properly run study if competent centres are not chosen.
It is necessary to ensure adequate sample size and power to detect a significant difference between the arms of the trial.57 58 In view of the heterogeneity of the disease, the sample size in a trial needs to be large enough to obtain a statistically significant result. One case in which low sample size may have been important in determining the significance of a result is the LUNAR trial for rituximab, in which the primary endpoint of a superior renal response rate with rituximab at end of treatment was not achieved.44 In this trial of 144 patients (72 each for rituximab and control), the overall renal response rate was 56.9% for the rituximab cohort compared with 45.8% for the control cohort (P=0.18).44 By comparison, in the BLISS-76 trial for belimumab, a significant improvement (P=0.017) in efficacy (SRI response at end of treatment) was obtained with the 10 mg dosage compared with placebo, although the percentage improvement (43.2% vs 33.5%) was slightly smaller than in the LUNAR trial.13 In BLISS-76, more than twice as many patients completed this trial (n=186 for placebo, n=191 for 10 mg dosage) compared with the LUNAR trial.13 Probably trials with smaller sample sizes can be designed and implemented once disease heterogeneity is controlled and a strict disease phenotype is achieved.
Patient diversity is another consideration in patient recruitment beyond achieving adequate sample size. Centres worldwide should be chosen to promote diversity in the trial participants. Such an approach needs to be taken prudently to avoid certain types of variability associated with different geographical locations, such as high infection rates in certain centres. One method for achieving participant diversity would be to use centres that have substantial population diversity.
Endpoints with high bars can be too restrictive to demonstrate positive results. An example in which this may have occurred is the ILLUMINATE trials for tabalumab. Although the ILLUMINATE-2 trial met its primary endpoint of SRI(5) response at week 52 for the more frequent dosing regimen, it did not meet this endpoint for the less frequent dosing regimen or in ILLUMINATE-1.50 64 In ILLUMINATE-1, similar percentages of patients achieved SRI(5) response at week 52 (31.8% and 35.2% for the two treatment arms compared with 29.3% for placebo.50 Using an SRI(5) response as the primary endpoint, whereby a criteria of a ≥5 point reduction in the Safety of Estrogens in Lupus Erythematosus–National Assessment-SLEDAI score is used as opposed to the ≥4 point reduction in SRI(4) response, may have resulted in trial failure. The successful BLISS-52 and BLISS-76 trials for belimumab used SRI(4) response.12 13 Another example is the phase II/III abatacept trial set. In this case, the bar was set overly high by using spot urine protein to creatinine ratio ≤0.26 g/g (30 mg/mmol), although EULAR (European League of Associations for Rheumatology) guidelines define a complete renal response as <50 mg/mmol.6 37
Standard of care needs to be considered when designing a trial. It is difficult to achieve a significant difference between the placebo and drug treatment arms when patients are receiving standard-of-care treatment. For example, GCS use can increase the response rate in the placebo group and thereby influence trial results. Therefore, the exposure to and dosage of GCS should be limited. In the context of disease activity, use of GCS should be adjusted. This strategy is currently undergoing evaluation in the SLEDAI-2KG trial.65 The GCS dosage should be balanced between arms to minimise introduced bias.57 For mild lupus manifestations, GCS should be omitted if possible. Drug trials focusing on patients with dermal and musculoskeletal SLE manifestations might demonstrate results of experimental therapy more clearly if they omit GCS use as a standard of care. However, this strategy would be unethical to implement for patients with moderate-to-severe lupus.
The adjudication committee review of data is important. It is important to review the data in a timely manner to identify deficiencies and inconsistencies. Two of the more common approaches used are post hoc review and adjudication when all patient visits are finalised and ongoing data review and adjudication. Although the first approach allows for an overall review of the data, the latter approach can identify difficulties with data collection during the trial process. A combination of both approaches is preferable because it would allow the identification of sites that are not adequately trained with respect to inclusion criteria and outcomes.
Drug dosages and regimens should be selected for optimal efficacy and safety. In relation to drug dosing, when safety issues do occur, the safety committee needs to consider carefully if treatment discontinuation is appropriate. For example, in the phase II/III APRIL-SLE trial for atacicept, the 150 mg arm was discontinued prematurely because of two deaths related to infection; no deaths occurred in the placebo arm.30 In this 52-week trial, patients with moderate-to-severe SLE received atacicept two times per week for 4 weeks followed by once weekly for the remaining 48 weeks.30 In the phase IIb ADDRESS II trial of patients with SLE, atacicept 150 mg was given weekly for 24 weeks with no increase in serious adverse events compared with placebo.32 66 Although other factors cannot be excluded, these results indicate that the dosing regimen given in the ADDRESS II trial has a better safety profile than the regimen in the APRIL-SLE trial.
The time to improvement or resolution of disease activity for a particular manifestation often depends on disease phenotype. The length of trials may be too short to observe meaningful effects, and researchers may need more than 1 year for given endpoints (eg, a significant reduction in proteinuria for patients with lupus nephritis, especially because the speed of recovery from proteinuria is slow).59 However, the length of trials for patients with dermal or musculoskeletal manifestations can be shortened, especially if GCS are omitted or tapered and stopped very early. Trials involving patients with mild skin/musculoskeletal manifestations can also be shortened both by omitting the use of GCS and using partial recovery as an endpoint instead of complete recovery.
Conclusions
Recent advancements to our knowledge of the mechanisms involved in SLE development have led to the advancement of novel therapies for this disease. Nevertheless, the heterogeneous and persistent nature of SLE manifestations remains a substantial burden for many patients, and agents need to be developed to address the substantial unmet medical needs for this disease.
Acknowledgments
Editorial support was provided by Alan Saltzman, PhD, of Endpoint Medical Communications, and Michael A. Nissen, ELS, of Astra Zeneca.
Footnotes
Contributors: Both authors conceived of the concept for this review, participated in its design and coordination and drafted and reviewed the content of the manuscript. Both authors read and approved the final manuscript.
Funding: Funding for this manuscript was provided by Astra Zeneca.
Competing interests: The authors have received grants from GSK and have participated in advisory boards for Janssen and Astra Zeneca.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No data sets were generated or analysed in the preparation of this manuscript. The texts of the published references, as cited, were the sources used.
References
- 1.Dema B, Charles N. Advances in mechanisms of systemic lupus erythematosus. Discov Med 2014;17:247–55. [PubMed] [Google Scholar]
- 2.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med 2012;18:871–82. doi:10.1038/nm.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pons-Estel GJ, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 2010;39:257–68. doi:10.1016/j.semarthrit.2008.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res Ther 2012;14(Suppl 4):S4 doi:10.1186/ar3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tunnicliffe DJ, Singh-Grewal D, Kim S, et al. Diagnosis, monitoring, and treatment of systemic lupus erythematosus: a systematic review of clinical practice guidelines. Arthritis Care Res 2015;67:1440–52. doi:10.1002/acr.22591 [DOI] [PubMed] [Google Scholar]
- 6.Bertsias GK, Tektonidou M, Amoura Z, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–82. doi:10.1136/annrheumdis-2012-201940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bultink IE. Osteoporosis and fractures in systemic lupus erythematosus. Arthritis Care Res 2012;64:2–8. doi:10.1002/acr.20568 [DOI] [PubMed] [Google Scholar]
- 8.GlaxoSmithKline LLC. Benlysta (belimumab) prescribing information. 2017. https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG.PDF (accessed 26 Oct 2017).
- 9.Liu Z, Davidson A. BAFF inhibition: a new class of drugs for the treatment of autoimmunity. Exp Cell Res 2011;317:1270–7. doi:10.1016/j.yexcr.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999;190:1697–710. doi:10.1084/jem.190.11.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petri M, Stohl W, Chatham W, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008;58:2453–9. doi:10.1002/art.23678 [DOI] [PubMed] [Google Scholar]
- 12.Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. doi:10.1016/S0140-6736(10)61354-2 [DOI] [PubMed] [Google Scholar]
- 13.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. doi:10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furie RA, Wallace DJ, Aranow C, et al. 7-year safety and efficacy of belimumab in patients with systemic lupus erythematosus. Arthritis Rheumatol 2016;68(Suppl 10):P768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins CE, Dall’Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med 2016;3:e000118 doi:10.1136/lupus-2015-000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touma Z, Sayani A, Pineau CA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada Study. Rheumatol Int 2017;37:865–73. doi:10.1007/s00296-017-3682-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarting A, Schroeder JO, Alexander T, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in germany: results from the OBSErve Germany study. Rheumatol Ther 2016;3:271–90. doi:10.1007/s40744-016-0047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2017;69:1016–27. doi:10.1002/art.40049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov. ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. https://www.clinicaltrials.gov/ (accessed 26 Oct 2017).
- 20.ISRCTN Registry. isrctn.com. Comparison of two methods of lymph node removal in patients suffering from lung cancer. http://www.isrctn.com (accessed 13 Sep 2017).
- 21.Oon S, Wilson NJ, Wicks I. Targeted therapeutics in SLE: emerging strategies to modulate the interferon pathway. Clin Transl Immunology 2016;5:e79 doi:10.1038/cti.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucak H, Yrlid U, Reizis B, et al. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity 2009;31:491–501. doi:10.1016/j.immuni.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 23.Bacher N, Raker V, Hofmann C, et al. Interferon-α suppresses cAMP to disarm human regulatory T cells. Cancer Res 2013;73:5647–56. doi:10.1158/0008-5472.CAN-12-3788 [DOI] [PubMed] [Google Scholar]
- 24.Agrawal H, Jacob N, Carreras E, et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol 2009;183:6021–9. doi:10.4049/jimmunol.0803872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Bethunaickan R, Huang W, et al. Interferon-α accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum 2011;63:219–29. doi:10.1002/art.30087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dall’era MC, Cardarelli PM, Preston BT, et al. Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis 2005;64:1692–7. doi:10.1136/ard.2004.033753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–23. doi:10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furie R, Khamashta M, Merrill JT, et al. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol 2017;69:376–86. doi:10.1002/art.39962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrill J, Furie R, Werth VP, et al. The effect of anifrolumab on cutaneous manifestations and arthritis in moderate to severe systemic lupus erythematosus (SLE) using categorical SLEDAI-2K responses and continuous measures of activity as outcome measures [abstract]. Arthritis Rheumatol 2016;68(Suppl 10):P2009. [Google Scholar]
- 30.Isenberg D, Gordon C, Licu D, et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 2015;74:2006–15. doi:10.1136/annrheumdis-2013-205067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon C, Wofsy D, Wax S, et al. Post hoc analysis of the phase II/III april-sle study: association between response to atacicept and serum biomarkers including BLyS and april. Arthritis Rheumatol 2017;69:122–30. doi:10.1002/art.39809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merck KGaA Press Release. Merck KGaA, Darmstadt, Germany’s Phase IIb ADDRESS II results confirm potential of atacicept as a candidate therapy for SLE. 2016. http://news.emdgroup.com/EMD/CC/EMDNewsRelease.nsf/Open (accessed 7 Apr 2017).
- 33.Suárez-Fueyo A, Bradley SJ, Tsokos GC. T cells in systemic lupus erythematosus. Curr Opin Immunol 2016;43:32–8. doi:10.1016/j.coi.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Liu J, Cui X, et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol 2015;295:46–51. doi:10.1016/j.cellimm.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Lindwall E, Gauthier C, et al. Circulating CXCR5+CD4+helper T cells in systemic lupus erythematosus patients share phenotypic properties with germinal center follicular helper T cells and promote antibody production. Lupus 2015;24:909–17. doi:10.1177/0961203314567750 [DOI] [PubMed] [Google Scholar]
- 36.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol 1989;143:103–12. [PubMed] [Google Scholar]
- 37.Furie R, Nicholls K, Cheng TT, et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol 2014;66:379–89. doi:10.1002/art.38260 [DOI] [PubMed] [Google Scholar]
- 38.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 2005;201:703–11. doi:10.1084/jem.20042251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol 2007;95:83–110. doi:10.1016/S0065-2776(07)95003-8 [DOI] [PubMed] [Google Scholar]
- 40.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. doi:10.1056/NEJMoa021933 [DOI] [PubMed] [Google Scholar]
- 41.Cappione A, Anolik JH, Pugh-Bernard A, et al. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest 2005;115:3205–16. doi:10.1172/JCI24179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahn BH, McMahon MA, Wilkinson A, et al. American college of rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012;64:797–808. doi:10.1002/acr.21664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:222–33. doi:10.1002/art.27233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum 2012;64:1215–26. doi:10.1002/art.34359 [DOI] [PubMed] [Google Scholar]
- 45.Merrill J, Buyon J, Furie R, et al. Assessment of flares in lupus patients enrolled in a phase II/III study of rituximab (EXPLORER). Lupus 2011;20:709–16. doi:10.1177/0961203310395802 [DOI] [PubMed] [Google Scholar]
- 46.Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 2013;72:1280–6. doi:10.1136/annrheumdis-2012-202844 [DOI] [PubMed] [Google Scholar]
- 47.Zimmer R, Scherbarth HR, Rillo OL, et al. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann Rheum Dis 2013;72:1830–5. doi:10.1136/annrheumdis-2012-202460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aurinia Pharmaceuticals Inc. Aurinia announces voclosporin meets 48-week remission endpoints, achieving highest complete remission rate of any global lupus nephritis study. http://ir.auriniapharma.com/press-releases/detail/73 (accessed 13 Sep 2017).
- 49.Clowse ME, Wallace DJ, Furie RA, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase iii randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol 2017;69:362–75. doi:10.1002/art.39856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isenberg DA, Petri M, Kalunian K, et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016;75:323–31. doi:10.1136/annrheumdis-2015-207653 [DOI] [PubMed] [Google Scholar]
- 51.Medina-Rosas J, Al-Rayes H, Moustafa AT, et al. Recent advances in the biologic therapy of lupus: the 10 most important areas to look for common pitfalls in clinical trials. Expert Opin Biol Ther 2016;16:1225–38. doi:10.1080/14712598.2016.1214263 [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Pintó I, Espinosa G, Cervera R. The problems and pitfalls in systemic lupus erythematosus drug discovery. Expert Opin Drug Discov 2016;11:525–7. doi:10.1080/17460441.2016.1181056 [DOI] [PubMed] [Google Scholar]
- 53.Touma Z, Gladman DD, Ibañez D, et al. Development and initial validation of the systemic lupus erythematosus disease activity index 2000 responder index 50. J Rheumatol 2011;38:275–84. doi:10.3899/jrheum.100724 [DOI] [PubMed] [Google Scholar]
- 54.Touma Z, Urowitz MB, Taghavi-Zadeh S, et al. Systemic lupus erythematosus disease activity Index 2000 Responder Index 50: sensitivity to response at 6 and 12 months. Rheumatology 2012;51:1814–9. doi:10.1093/rheumatology/kes146 [DOI] [PubMed] [Google Scholar]
- 55.Touma Z, Gladman DD, Ibañez D, et al. SLEDAI-2K Responder Index 50 captures 50% improvement in disease activity over 10 years. Lupus 2012;21:1305–11. doi:10.1177/0961203312454344 [DOI] [PubMed] [Google Scholar]
- 56.Dall’Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-lupus nephritis cohort. Arthritis Rheumatol 2015;67:1305–13. doi:10.1002/art.39026 [DOI] [PubMed] [Google Scholar]
- 57.Karassa FB, Tatsioni A, Ioannidis JP, Design IJP. Design, quality, and bias in randomized controlled trials of systemic lupus erythematosus. J Rheumatol 2003;30:979–84. [PubMed] [Google Scholar]
- 58.Dall’Era M, Wofsy D. Clinical trial design in systemic lupus erythematosus. Curr Opin Rheumatol 2006;18:476–80. doi:10.1097/01.bor.0000240357.22680.63 [DOI] [PubMed] [Google Scholar]
- 59.Touma Z, Urowitz MB, Ibañez D, et al. Time to recovery from proteinuria in patients with lupus nephritis receiving standard treatment. J Rheumatol 2014;41:688–97. doi:10.3899/jrheum.130005 [DOI] [PubMed] [Google Scholar]
- 60.Petri MA, van Vollenhoven RF, Buyon J, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum 2013;65:2143–53. doi:10.1002/art.37995 [DOI] [PubMed] [Google Scholar]
- 61.Wallace DJ, Kalunian K, Petri MA, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis 2014;73:183–90. doi:10.1136/annrheumdis-2012-202760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Touma Z, Gladman DD, Su J, et al. SLE disease activity index glucocorticoid index (SLEDAI-2KG) identifies more responders than SLEDAI-2K. Arthritis Rheum 2017;69 (Suppl 10):P1624. [Google Scholar]
- 63.Abrahamowowicz M, Esdaile JM, Ramsey-Goldman R, et al. Development and validation of a novel evidence-based lupus multivariable outcome score for clinical trials. [abstract]. Arthritis Rheum 2017;69 (Suppl 10):P1839. [DOI] [PubMed] [Google Scholar]
- 64.Merrill JT, van Vollenhoven RF, Buyon JP, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016;75:332–40. doi:10.1136/annrheumdis-2015-207654 [DOI] [PubMed] [Google Scholar]
- 65.University Health Network. Improving the assessment of SLE disease activity. 2017. https://clinicaltrials.gov/ct2/show/NCT03144063 (accessed 15 Sep 2017).
- 66.Merrill JT, Wallace DJ, Wax S, et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a 24-week randomized, placebo-controlled, phase iib study. Arthritis Rheumatol 2017;68 (Suppl 10):P12L doi:10.1002/art.40360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urowitz MB, Isenberg DA, Wallace DJ. Safety and efficacy of hCDR1 (Edratide) in patients with active systemic lupus erythematosus: results of phase II study. Lupus Sci Med 2015;2:e000104 doi:10.1136/lupus-2015-000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tillmanns S, Kolligs C, D’Cruz DP, et al. SM101, a novel recombinant, soluble, human FcγIIB receptor, in the treatment of systemic lupus erythematosus: results of a double-blind, placebo-controlled multicenter study. Arthritis Rheum 2014;66 (S1238):P2833. [Google Scholar]
- 69.van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an interleukin 12/23 inhibitor, in patients with active systemic lupus erythematosus: results of a phase 2, randomized placebo-controlled study. Arthritis Rheum 2017;69 (Suppl 10):P6L. [Google Scholar]