Abstract
Background
Monoclonal antibodies against Programmed Death 1 (PD-1) and Programmed Death Ligand 1 (PD-L1) are effective therapies in NSCLC. We performed a systematic review investigating differences in the toxicities of PD-1 and PD-L1 inhibitors.
Methods
An electronic literature search was performed of public databases (MEDLINE, EMBASE) and conference proceedings for trials utilizing PD-1 inhibitors (nivolumab, pembrolizumab) and PD-L1 inhibitors (atezolizumab, durvalumab, avelumab) in NSCLC patients. A formal systematic analysis was conducted with Comprehensive Meta-Analysis software (Version 2.2). Clinical and demographic characteristics, response, and toxicity data were compared between both groups.
Results
Twenty-three studies reported between 2013 and 2016 were eligible for analysis. The total number of patients evaluated for toxicities were 3,284 patients in the PD-1 group and 2,460 patients in the PD-L1 group. The baseline patient characteristics of the two groups were similar, although there was a trend towards increased squamous histology in the PD-L1 group (32% versus 25%, p=0.6). There was no difference in response rate between PD-1 (19%) and PD-L1 (18.6%) inhibitors, p=0.17. The incidence of overall adverse events (AEs) was comparable between PD-1 and PD-L1 inhibitors (64% (CI 63-66%) versus 66% (CI 65-69%), p=0.8). Fatigue is the most frequently reported AE with both classes. Patients treated with PD-1 inhibitors have a slightly increased rate of immune related AEs (IRAEs) (16 (CI 14-17%) versus 11% (CI 10-13%), p=0.07) and pneumonitis (4% (CI 3-5) versus 2% (CI 1-3), p=0.01) compared to patients who received PD-1 inhibitors.
Conclusions
In this systematic review involving 5,744 patients, the toxicity and efficacy profiles of PD-1 and PD-L1 inhibitors in NSCLC patients are similar.
Keywords: Immune checkpoint inhibitors, NSCLC, adverse events, immune related adverse events
Introduction
Immune checkpoint inhibition is an effective treatment strategy in multiple tumor types, including non-small cell lung cancer (NSCLC). The immune checkpoint Programmed Death 1 (PD-1) receptor is expressed on activated T cells and binds to its ligands PD-L1 and PD-L2 to limit T cell activation and prevent autoimmunity in peripheral tissues1. Activation of the PD-1 pathway induces T cell exhaustion, which is necessary to prevent continued immune activation following successful removal of cells carrying the antigen of interest2. Several tumors overexpress PD-L1 and evade detection by T cells, which leads to immune tolerance of the tumor. Overexpression of PD-L1 is also associated with more aggressive phenotypes and poor outcomes in NSCLC3-7.
Monoclonal antibodies against PD-1 and PD-L1 have emerged as effective therapies in patients with advanced NSCLC. These agents have been shown to be tolerated well and exert anti-cancer effects even in patients who have failed multiple prior lines of therapy. The PD-1 inhibitors nivolumab and pembrolizumab and the PD-L1 inhibitor atezolizumab are superior to docetaxel in the salvage setting with improved survival outcomes and toxicity8-11. More recently, pembrolizumab was shown to be superior to platinum doublet chemotherapy in treatment naïve metastatic NSCLC patients with high tumoral PD-L1 expression, resulting in a major shift in treatment paradigm12.
The immune checkpoint inhibitors have unique mechanism-based toxicities compared to cytotoxic chemotherapy. Inhibition of the PD-1 pathway can lead to autoimmune toxicities, some of which can be severe and even fatal reactions13, 14_ENREF_14. Lung cancer patients are particularly more vulnerable for toxicities given the older age of the patient population and presence of co-morbid conditions. Of particular interest in NSCLC patients is the development of autoimmune pneumonitis, which led to a few treatment-related deaths in early phase studies of these agents15-17. As clinicians have increasing number of checkpoint inhibitors to choose from in the treatment of advanced stage NSCLC patients, it will be important to understand potential differences in efficacy and toxicity profiles of these agents. There has been speculation that since monoclonal antibodies against PD-L1 still allow for the interaction of PD-1 with its other ligand PD-L2, this could lead to less blockade of the negative inhibitory signal and result in reduced autoimmunity. Whether this can also have implications on the efficacy of the individual agents is not known. Given that it is highly unlikely for comparative clinical trials to be conducted to compare the check point inhibitors against one another, there is an urgent need to understand key differences in efficacy and toxicity between the various immune checkpoint inhibitors. In particular, comparison of PD-1 versus PD-L1 inhibitors is of immense clinical interest. Therefore, we conducted a systematic review of clinical trials to evaluate differences in toxicity profiles between PD-1 and PD-L1 inhibitors used as monotherapy in NSCLC.
Methods
Search Strategy
A comprehensive and methodical search of the literature of electronic databases (Medline, Embase, Cochrane) for studies published between 2000 and 2016 was conducted. Applicable terms, such as “PD-1, PD-L1 and NSCLC,” were used with the filters “clinical trial,” “humans,” and “all adult: 19+ years” for the MEDLINE searches. Relevant abstracts were manually searched and retrieved from the conference proceedings of annual meetings of the American Society of Clinical Oncology (ASCO) (2011-2016), the World Conference on Lung Cancer (2011-2016), and from the European Society of Medical Oncology (2011-2016).
Study Eligibility
All studies potentially meeting the eligibility criteria defined by the search strategy were independently reviewed by three of the authors (RP, MB and SSR), which was followed by a consensus meeting to determine the final list of eligible studies. All trials utilizing PD-1 inhibitors (nivolumab, pembrolizumab) and PD-L1 inhibitors (atezolizumab, durvalumab, avelumab) as monotherapy in NSCLC patients were included in this analysis. Phase 1 trials, trials that enrolled less than 10 patients, and trials that utilized PD-1 or PD-L1 inhibitors in combination with chemotherapy, other immunotherapies, or radiation were excluded from the analysis. Studies that did not report toxicities were excluded from the analysis. For data that were both presented at a meeting and subsequently published in full form, only the data from the full publication was included. If data had been presented multiple times, then the data from the most current presentation was used, and the older data excluded. All prospective randomized, non-randomized, and single arm studies that met the inclusion criteria were included in the analysis. Retrospective studies were excluded from this analysis.
Data extraction and statistical analysis
The studies were reviewed for any available data. Standard data extraction templates were used to collect any analyzable data that were reported. The extracted data included demographics, treatment, and clinical outcomes including; overall response rate (ORR), progression free survival (PFS), overall survival (OS), and toxicities. Not all of the trials reported all outcome measures. Toxicities were extracted as the primary outcome for this analysis.
The outcome data extracted for each arm were analyzed using fixed-effect model which controls for time invariant variables and reported as weighted measures. Each study in the fixed effect analysis is weighted based on its sample size. Toxicities were extracted and reported as overall adverse events, Grade 3-5 adverse events, most common adverse events and immune related adverse events (IRAEs). IRAEs were defined as toxicities mediated by an autoimmune mechanism, defined by the authors to include pneumonitis, colitis, thyroid disorders, and inflammatory conditions reported in any organ system, such as hepatitis or nephritis. All analyses were performed using Comprehensive Meta-Analysis software (CMA Version 2.2) and SPSS statistical package (version 22.0, IBM Corp.). The comparisons between the two arms PD-1 and PD-L1 inhibitors were conducted based on weighted estimates. Two-tailed T-test with a significance level of 0.05 was used for all comparisons. Heterogeneity among studies was assessed used the I2 test, which provides the percentage of variation across studies that is due to the heterogeneity. The overall AE rate was reported as pooled proportions of the percentage of patients treated with each drug that experienced AEs; there was no test of significance between the drugs.
Results
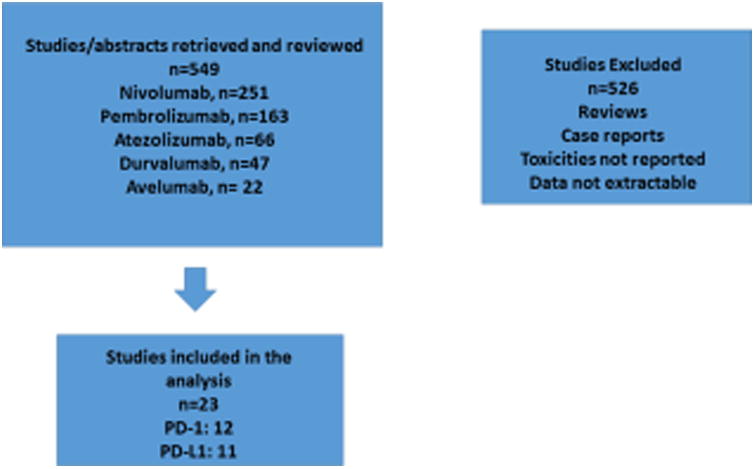
A total of 549 studies were retrieved and reviewed from the searches, out of which twenty-three studies met our inclusion criteria. Of these, twelve trials utilized PD-1 inhibitors and eleven trials utilized PD-L1 inhibitors. The study selection scheme is shown in Figure 1. The trials with PD-1 inhibitors and PD-L1 inhibitors that met criteria studies included in the analysis are outlined in Table 1.
Figure 1. CONSORT Diagram.
Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature
Table 1. Studies with PD-1 and PD-L1 Inhibitors in NSCLC.
| Study | Drug | Line of Therapy | Number of Patients Evaluated for Safety |
|---|---|---|---|
| Gettinger 201517 | Nivolumab 1, 3, or 10 mg/kg | Salvage | 129 |
| Rizvi 201519 | Nivolumab 3 mg/kg | Salvage | 117 |
| Brahmer 20158 | Nivolumab 3 mg/kg | Salvage | 135 |
| Borghaei 20159 | Nivolumab 3 mg/kg | Salvage | 292 |
| Garon 201516 | Pembrolizumab 2 or 10 mg/kg | Salvage | 495 |
| Sakai 201520 | Nivolumab 3 mg/kg | Salvage | 111 |
| Bauer 201521 | Nivolumab 3 mg/kg | Salvage | 824 |
| Herbst 201610 | Pembrolizumab 2 mg/kg | Salvage | 344 |
| Herbst 201610 | Pembrolizumab 10 mg/kg | Salvage | 346 |
| Goldberg 201622 | Pembrolizumab 10 mg/kg | Salvage | 18 |
| Gettinger 201623 | Nivolumab 3 mg/kg | First-line | 52 |
| Socinski 201624 | Nivolumab 3 mg/kg | First-line | 267 |
| Reck 201612 | Nivolumab 3 mg/kg | First-line | 154 |
| Brahmer 201425 | Durvalumab | Salvage | 155 |
| Rizvi 201526 | Durvalumab | Salvage | 228 |
| Spigel 201327 | Atezolizumab | Salvage | 52 |
| Horn 201528 | Atezolizumab | Salvage | 88 |
| Spigel 201529 | Atezolizumab | Salvage | 137 |
| Besse 201530 | Atezolizumab | Salvage | 659 |
| Fehrenbacher 201611 | Atezolizumab | Salvage | 144 |
| Verschraegen 201631 | Avelumab | Salvage | 145 |
| Gulley 201532 | Avelumab | Salvage | 184 |
| Antonia 201633 | Durvalumab | First-line | 59 |
| Barlesi 201634 | Atezolizumab | Salvage | 609 |
A total of 5,744 patients were evaluated for toxicity, including 3,284 patients treated with PD-1 and 2,460 treated with PD-L1 inhibitors. The patient characteristics are described in Table 2. The median age of the PD-1 inhibitor treated population was 64 years and that of the PD-L1 inhibitor was 64.5 years (p=0.5). The PD-1 inhibitor treated population had a slightly higher proportion of males compared to PD-L1 (59% vs. 56%, p=0.4). The two cohorts had a similar proportion of patients with smoking history (83%, p=0.5). Although the two cohorts were comparable in baseline characteristics, trials utilizing PD-L1 enrolled a higher proportion of patients with squamous histology compared to the PD-1 trials (32% vs. 25%, p=0.6).
Table 2. Baseline Characteristics of Patients.
| PD-1 Inhibitor (n=3284) | PD-L1 Inhibitor (n=2460) | P-value | |
|---|---|---|---|
| Median age (yrs) | 64 | 64.5 | 0.5 |
| Males (%) | 59 | 56 | 0.4 |
| Smokers (%) | 83 | 83 | 0.5 |
| Squamous histology | 25 | 32 | 0.6 |
| ECOG PS 0/1(%) | 31.5/68 | 32/68 | 0.2/0.4 |
The objective response rate in the PD-1 inhibitor treated population (n= 3,284) was identical to the PD-L1 inhibitor group (n=2,460) (19% vs. 18.6%, p=0.17; Table 3). The progression free survival and overall survival data were only reported in few studies and were not sufficient to make a comparable analysis.
Table 3. Adverse Events (AEs), Immune Related Adverse Events (IRAEs), and Overall Response Rates with PD-1 versus PD-L1 Inhibitors.
| PD-1 Inhibitors (n=3284) | PD-L1 Inhibitors (n=2460) | p-value | |
|---|---|---|---|
| Overall AEs (%) | 64 | 66 | 0.8 |
| Grade 3-5 AEs (%) | 13 | 21 | 0.15 |
| Fatigue, any grade (%) | 19 | 21 | 0.4 |
| Diarrhea, any grade (%) | 9 | 12 | 0.4 |
| Rash, any grade (%) | 9 | 7 | 0.8 |
| IRAEs (%) | 16 | 11 | 0.07 |
| Grade 3-5 IRAEs (%) | 3 | 5 | 0.4 |
| Hypothyroidism, any grade (%) | 6.7 | 4.2 | 0.07 |
| Pneumonitis, any grade (%) | 4 | 2 | 0.01 |
| Colitis, any grade (%) | 1.7 | 1 | 0.4 |
| Overall Response Rate (ORR) (%) | 19 | 18.6 | 0.17 |
Toxicities
The overall incidence of adverse events were found to be similar between the PD-L1 and PD-1 inhibitor treated cohorts (Table 3), with patients treated with PD-1 inhibitors experiencing a comparable incidence of adverse events compared to PD-L1 inhibitors (64% in PD-1 (CI 63-66%) vs. 66% (65-69%) in PD-L1, p= 0.8). Grade 3-5 toxicities were observed in 13% (CI 12-14%) in the PD-1 cohort vs. 21% (CI 19-23%) in PD-L1 cohort (p= 0.15). Fatigue was the most common toxicity in both cohorts of patients (19% in PD-1 (CI 17-20%) vs. 21% (CI 19-23%) in PD-L1 cohorts, p=0.4). The overall immune related adverse events (IRAEs) had a trend towards higher incidence with PD-1 inhibitors compared to the PD-L1 (16% (CI 14-17%) vs. 11% (CI 10-13%); p=0.07; Table 3), although the rate of grade 3-5 IRAEs was equivalent in the two groups (3%, p=0.4). The most common IRAE was hypothyroidism in both groups. Although the incidence of hypothyroidism was numerically higher with PD-1 inhibitors compared to PD-L1 inhibitors, this was not significant (6.7% in PD-1 (CI 6-8%) vs. 4.2% (3-5%) in PD-L1, p=0.07). The incidence of pneumonitis was higher in PD-1 cohort vs. PD-L1 (4% (CI 3-5%) vs. 2% (1-3%), p-value= 0.01). In analyzing the incidence of toxicities by type of drug, durvalumab was associated with the highest proportion of toxicities (Table 4).
Table 4. Overall Adverse Event Rate by Drug.
| Drug | AE Rate (%) |
|---|---|
| Nivolumab | 62 |
| Pembrolizumab | 67.5 |
| Atezolizumab | 65 |
| Durvalumab | 75 |
| Avelumab | 67 |
Discussion
Immune checkpoint inhibition with PD-1 and PD-L1 inhibitors has become a standard of care for NSCLC patients with metastatic disease. While each drug has activity in NSCLC, it is unclear how these agents compare in terms of efficacy and toxicity. Our systematic review of 5,744 NSCLC patients treated with PD-1 and PD-L1 inhibitors sheds light on this important clinical issue and provides guidance to treating physicians and researchers. The objective response rate appears similar for PD-1 and PD-L1 inhibitors in an unselected population with advanced stage NSCLC. The toxicity profiles are also comparable between the two classes of checkpoint inhibitors with relatively minor differences. First, the overall incidence of clinically significant adverse events was low with immune checkpoint inhibition, which represents a significant improvement in therapeutic index relative to systemic chemotherapy. Fatigue was the most common AE with both PD-1 and PD-L1 inhibitors. Even among IRAEs, the differences between the two classes of agents were relatively minor. In our view, the most notable difference was observed for grades 3/4 immune-mediated pneumonitis, which was slightly higher with PD-1 inhibitors compared to PD-L1 inhibitors.
This is a large dataset and the first systematic review of toxicity profiles of immune checkpoint inhibitors in NSCLC to our knowledge. This analysis adds to the understanding of differences between PD-1 and PD-L1 inhibitors in NSCLC. A major strength of the analysis is that several of the trials included were conducted in the multi-national setting, thus representative of a heterogeneous and representative patient population from across the world. This included a phase IIIB/IV safety study, the Checkmate 153 study, which collected data on advanced NSCLC patients treated with nivolumab in community oncology practices. This study demonstrates that checkpoint inhibitor therapy with nivolumab is well tolerated even in patients with a performance status of 2 with a 53% rate of treatment related AEs and reflects a more real world practice setting. The majority of these studies enrolled patients primarily from North America and Europe; we did identify phase II data from Sakai et al that reported on the use of nivolumab in 111 patients in Japan. From this report, the efficacy and toxicity in Japanese patients appears similar to the overall population of patients analyzed. Asia is the only region that was not represented proportionally, and hence it will be important to study this issue as more trials are specifically conducted in Asia.
There are limitations to this analysis despite the comprehensive nature of our review. We selected the toxicity data by using reported treatment related adverse events (TRAEs) whenever possible. However, not all the studies had toxicity reported as TRAEs. In addition, the attribution of an AE as treatment-related could be somewhat subjective and vary between clinical trials and drugs.
Another caveat of this analysis is the availability of toxicity data. When possible we used full publications to extract toxicity data for each clinical trial. Some of the newer data were only available as presentations from conferences. In these cases, there was limited toxicity data available for extraction. This could lead to a selection bias of more toxicity reporting in the studies with more complete AE data in the full publication. It is of note that since the PD-1 inhibitors have been in clinical development longer, more of the studies for these agents were in full publication format. A higher proportion of studies for PD-L1 inhibitors were only reported in conferences and could explain some of the differences in toxicities seen between PD-1 and PD-L1 inhibitors.
The most significant finding of our analysis is the doubling in pneumonitis seen in patients treated with PD-1 inhibitors. Again, this may have been partly due to less complete AE reporting with the PD-L1 inhibitors; these drugs are in earlier phases of clinical development and also have fewer complete publications available for review. The rate of pneumonitis could have been influenced by different inclusion criteria used in each study in regards to allowing patients with prior interstitial lung diseases. We did not have access to the inclusion criteria for all of the clinical trials included in our analysis. Another potential confounding factor contributing to the difference in pneumonitis incidence could be the underreporting of pneumonitis as other toxicities, such as pneumonia, dyspnea, or interstitial lung disease.
We also acknowledge that the meta-analysis was not based on individual patient data, which would have been an ideal way to study this issue. An individual patient data meta-analysis would have been more comprehensive and would have allowed for better time-to-toxicity assessment. However, given that the trials were conducted primarily by the respective pharmaceutical companies, gaining access to primary datasets is challenging.
Our comparison of efficacy in terms of response rate is a secondary and exploratory endpoint and also has limitations. The patients included in this analysis were from clinical trials in different phases of drug development. There was also variability in regards to number of treatment lines received. Furthermore, some of the patients enrolled in these studies were selected by PD-L1 expression. There is an association between higher likelihood of response and tumor expression of PD-L1,18 and the heterogeneity of PD-L1 expression in these patients could have also impacted the reported ORR and toxicities.
Despite these limitations, this systematic review adds to our knowledge of the toxicity profiles and efficacy of PD-1 and PD-L1 inhibitors in NSCLC. It will be interesting to see how information changes with more frontline data and combination strategies being reported. Since these studies are among the first to evaluate this entirely new class of anti-cancer agents, it is likely that the steep learning curve for early recognition and management of toxicities with more clinical experience will result in an even more favorable toxicity profile for checkpoint inhibitors in the next wave of clinical trials. Another important way to improve the risk-benefit ratio is to choose treatment based on biomarkers, which is an area of active investigation. PD-L1 expression in the tumor tissue has emerged as a valuable selection method, though it is subject to certain limitations. Presently the cost of PD-1 and PD-L1 inhibitors are also similar in the United States, and hence treatment choice will primarily be based on proven efficacy in the chosen setting and the schedule of administration.
In summary, PD-1 and PD-L1 inhibitors are both associated with comparable efficacy and toxicity in patients with advanced stage NSCLC.
Acknowledgments
Funding: This work was supported by P30CA138292 and U10CA180864 grants to Winship Cancer Institute and U10CA180950 grant to ECOG-ACRIN.
Footnotes
Previous presentations: This work has been presented in part at the American Society of Clinical Oncology Annual Meeting 2016 and the World Conference on Lung Cancer 2016.
Disclosures: None of the authors have any conflicts of interest to report.
Author Contributions: Conceptualization: R. Pillai, M. Behera, S. Ramalingam
Methodology: M. Behera, S. Ramalingam
Software: M. Behera
Validation: R. Pillai, M. Behera
Formal analysis: M. Behera
Investigation: R. Pillai, M. Behera, S. Ramalingam
Resources: M. Behera
Data curation: R. Pillai, M. Behera
Writing – original draft: R. Pillai, M. Behera
Writing – review and editing: T. Owonikoko, A. Kamphorst, S. Pakkala, C. Belani, F. Khuri, R. Ahmed, S. Ramalingam
Visualization: R. Pillai
Supervision: S. Ramalingam
Project administration: R. Pillai, M. Behera, S. Ramalingam
Funding acquisition: S. Ramalingam
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 3.Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi: 10.1016/j.ejca.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1) Lung Cancer. 2016;98:69–75. doi: 10.1016/j.lungcan.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Sun JM, Zhou W, Choi YL, et al. Prognostic Significance of PD-L1 in Patients with Non-Small Cell Lung Cancer: A Large Cohort Study of Surgically Resected Cases. J Thorac Oncol. 2016;11:1003–1011. doi: 10.1016/j.jtho.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) J Thorac Oncol. 2013;8:803–805. doi: 10.1097/JTO.0b013e318292be18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;4:203–208. doi: 10.3978/j.issn.2218-6751.2015.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015 doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 13.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–574. doi: 10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 17.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai H, Nishio M, Hida T, et al. Phase II Studies of Nivolumab in Patients with Advanced Squamous (SQ) or Non-Squamous (NSQ) Non-Small Cell Lung Cancer (NSCLC) Eur J Cancer. 2015;51:S110–111. [Google Scholar]
- 21.Bauer TM, McCleod M, Chandler JC, et al. An ongoing phase IIIb/IV safety trial of nivolumab (NIVO) in patients (pts) with advanced or metastatic non-small-cell lung cancer (NSCLC) who progressed after receiving 1 or more prior systemic regimens. J Clin Oncol. 2015;33 abstr 3013. [Google Scholar]
- 22.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34:2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Socinski M, Creelan B, Horn L, et al. CheckMate 026: A Phase 3 Trial of Nivolumab vs Investigator's choice (IC) of Platinum-Based Doublet Chemotherapy (PT-DC) as First-Line Therapy for Stage IV/Recurrent Programmed Death Ligand 1 (PD-L1) Positive NSCLC. Ann Oncol. 2016;27 [Google Scholar]
- 25.Brahmer J, Rizvi N, Lutsky J, et al. Clinical activity and biomarkers of MEDI4736, an anti-PDL1 antibody, in patients with NSCLC. J Clin Oncol. 2014;32 abstr 8021. [Google Scholar]
- 26.Rizvi N, Brahmer J, Ou SH, et al. Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33 abstr 8032. [Google Scholar]
- 27.Spigel DR, Gettinger S, Horn L, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) J Clin Oncol. 2013;31 abstr 8008. [Google Scholar]
- 28.Horn L, Spigel DR, Gettinger S, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase 1a study. J Clin Oncol. 2015;33 abstr 8029. [Google Scholar]
- 29.Spigel DR, Chaft JE, Gettinger S, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1-selected patients with non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33 abstr 8028. [Google Scholar]
- 30.Besse B, Johnson M, Janne PA, et al. Phase II, single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell lung cancer (NSCLC) Eur J Cancer. 2015;51:S717–718. [Google Scholar]
- 31.Verschraegen CF, Chen F, Spigel DR, et al. Avelumab (MSB0010718C; anti-PD-L1) as a first-line treatment for patients with advanced NSCLC from the JAVELIN Solid Tumor phase 1b trial: safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34 abstr 9036. [Google Scholar]
- 32.Gulley JL, Spigel DR, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: a phase Ib, open-label expansion trial in patients progressing after platinum-based chemotherapy. J Clin Oncol. 2015;33 abstr 8034. [Google Scholar]
- 33.Antonia S, Kim S, Spira AI, et al. Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naive patients with advanced non-small-cell lung cancer. J Clin Oncol. 2016;34 abstr 9029. [Google Scholar]
- 34.Barlesi F, Park K, Ciardello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol. 2016;27 abstract LBA44_PR. [Google Scholar]


