Abstract
Importance
Despite extensive knowledge of hypertension treatment, the prevalence of uncontrolled hypertension is high and increasing in low- and middle-income countries.
Objective
To test whether a community health worker (CHW)-led multicomponent intervention would improve blood pressure (BP) control among low-income patients with hypertension.
Design, Setting, and Participants
A cluster randomized trial was conducted in 18 centers for primary healthcare within a national public system providing free medications and healthcare to uninsured patients in Argentina. A total of 1,432 low-income adult patients with uncontrolled hypertension were recruited between June 2013 and April 2015 and followed to October 2016.
Intervention
Nine centers (743 patients) were randomized to the multicomponent intervention, which included a CHW-led home intervention (health coaching, home BP monitoring, and BP audit and feedback), a physician intervention, and a text-messaging intervention over 18 months. Nine centers (689 patients) were randomized to usual care without study intervention.
Main Outcomes and Measures
The co-primary outcomes were the differences between the intervention and control groups in systolic and diastolic BP changes from baseline to end of follow-up in patients with hypertension. Secondary outcomes included the proportion of patients with controlled hypertension (BP<140/90 mmHg). Three BP measurements were obtained at each of two baseline and two termination visits using a standard protocol, and the means were used for analyses.
Results
Among 1,432 participants (mean age, 55.8 years; 772 [53.0%] women), 1,357 (94.8%) completed the trial. Baseline mean BP was 151.7 and 149.8 mmHg for systolic, and 92.2 and 90.1 mmHg for diastolic in the intervention and control groups, respectively. Systolic BP reduction from baseline to month 18 was 19.3 mmHg (95% confidence interval [CI]: 17.9, 20.8) in the intervention group and 12.7 mmHg (95% CI: 11.3, 14.2) in the control group; difference in the reduction was 6.6 mmHg (95% CI: 4.6, 8.6; p<0.001). Diastolic BP decreased by 12.2 mmHg (95% CI: 11.2, 13.2) in the intervention group and 6.9 mmHg (95% CI: 5.9, 7.8) in the control group; difference in the reduction was 5.4 mmHg (95% CI: 4.0, 6.8; p<0.001). The proportion of controlled hypertension increased from 17.0% at baseline to 72.9% at 18 months in the intervention group and from 17.6% to 52.2% in the control group; difference in the increase was 20.6% (95% CI: 15.4, 25.9%; p<0.001). No adverse events were reported.
Conclusion and Relevance
Among low-income patients with uncontrolled hypertension in Argentina, a CHW-led multicomponent intervention compared with usual care resulted in a greater decrease in systolic and diastolic BP over 18 months. Further research is needed to assess generalizability and cost-effectiveness of this intervention, and to understand which components may have contributed most to the outcome.
Trial Registration
clinicaltrials.gov Identifier: NCT01834131
Hypertension is a leading global modifiable risk factor for cardiovascular disease and premature death.1,2 Despite extensive knowledge of hypertension prevention and treatment, the global prevalence of hypertension is high and increasing, while the proportion of controlled hypertension is low, especially in low- and middle-income countries (LMICs).3 It was estimated that 31.1% of the world’s adults had hypertension in 2010, and 75% of those with hypertension lived in LMICs. Furthermore, only 7.7% of patients with hypertension had their blood pressure (BP) controlled to <140/90 mmHg in LMICs.3 Therefore, developing and implementing effective, affordable, and sustainable programs for hypertension control is a public health priority in LMICs.
Barriers at the healthcare system, physician, patient, and community levels hinder BP control.4,5 Strategies for overcoming these barriers, including pharmacist-led and nurse-led interventions, were shown to improve BP control in patients with hypertension.6,7 For example, pharmacist-led interventions were associated with a 7.6 mmHg reduction in systolic BP (95% confidence interval [CI] 6.3, 9.0) and nurse-led interventions were associated with a 3.5 mmHg reduction in systolic BP (95% CI 1.1, 5.9) in previously published meta-analyses.6,7 However, there are limited data on effective intervention strategies for hypertension control in LMICs.8,9 Furthermore, the effect of community health worker (CHW)-led interventions, a more affordable and sustainable approach for low-income settings, has not been well tested in randomized trials.
The Hypertension Control Program in Argentina (HCPIA) was a cluster randomized trial aiming to test whether implementation of a CHW-led multicomponent intervention over 18 months lowered systolic and diastolic BP and improved hypertension control among low-income patients with uncontrolled hypertension in Argentina.
Methods
Study Design and Oversight
The HCPIA trial was a cluster randomized trial conducted among 18 centers for primary healthcare within a national public system (Remediar+Redes Program) in Argentina (trial protocol in Supplement 1). Details of the trial’s design and analysis plan have been published previously.9 The Institutional Review Boards of Tulane University and Hospital Italiano de Buenos Aires in Argentina approved the study. Informed consent was signed by all participants during screening.
Study Participants
After the 1998–2002 economic depression, a large proportion of the Argentine population did not have health insurance, and health care for the uninsured was provided by an overloaded national network of public clinics and hospitals.10,11 In response, the Remediar+Redes Program was funded by the Argentina Ministry of Health to provide free medications and healthcare to low-income, uninsured patients.12 Over the past decade, the program has evolved to become the main public primary healthcare network in Argentina, covering almost all provinces and municipalities with almost 7,000 centers for primary healthcare across the country (>90% of all public clinics).
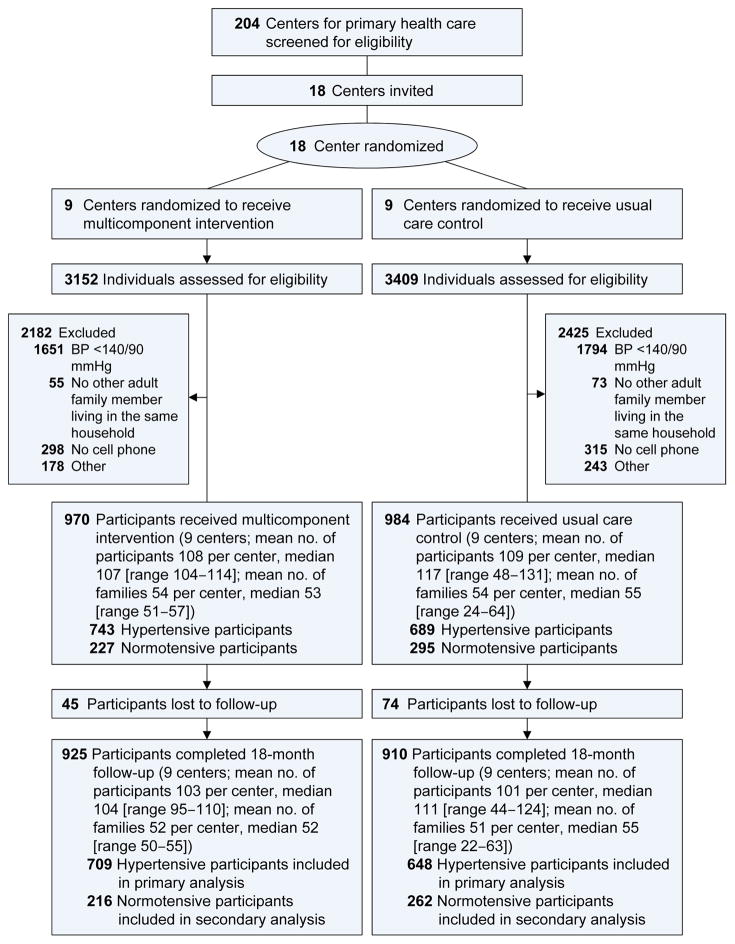
The main eligibility criteria for centers for primary healthcare were an affiliation with the Remediar+Redes Program, location in a poor urban area, and employment of CHWs in addition to general practitioners and nurses. A total of 204 centers from Buenos Aires, Misiones, Tucuman, Corrientes, and Entre Ríos were screened for potential participation. Among centers that met the eligibility criteria, 18 were selected based on recommendations from the Remediar+Redes Program (Figure 1). Cluster randomization was stratified by geographic region and conducted at the data coordinating center at Tulane University. The randomization schedules were generated using PROC PLAN in SAS software.
Figure 1. Flow diagram of trial participants.
Normotensive participants were spouses of hypertensive participants who had systolic blood pressure <140 mmHg, diastolic blood pressure <90 mmHg, and no use of antihypertensive medications. The Remediar+Redes Program national coordinating center screened 204 centers for primary health care from five provinces. Many centers met the eligibility criteria. The Remediar+Redes Program recommended 18 centers to the study based on their geographic distribution, their willingness to participate, and their previous experience collaborating with the coordinating center. The centers were not randomly selected.
The main eligibility criteria for index participants were uncontrolled BP (systolic ≥140 mmHg and/or diastolic ≥90 mmHg on at least 2 separate screening visits), aged ≥21 years, uninsured and receiving primary care from the participating centers, and spouses or adult hypertensive family members aged ≥21 years living in the same household who were willing to participate in the study. The study nurses reviewed the clinic appointment schedules daily and identified all patients with hypertension. Two screening visits at least one day apart were conducted to assess patients’ eligibility. Eligible index participants, as well as their spouses and adult hypertensive family members, were recruited for the study between June 2013 and April 2015. To avoid selection bias, participants remained eligible for the study as hypertensive participants if they received antihypertensive treatment after the screening visits and their BP was <140/90 mmHg at the baseline examination.
Interventions
An 18-month multicomponent intervention program, including a CHW-led home-based intervention (health coaching and home BP monitoring and audit), physician education and BP feedback, and weekly text-messaging was implemented in the intervention clinics.
CHWs were trained to coach patients and their family members on lifestyle modification, home BP-monitoring, and medication adherence during a 2-day interactive training session followed by onsite field testing and certification. They were also trained to function as case managers for the patients and their families by coordinating intervention activities and facilitating patient care. CHWs visited participants’ homes monthly for the first six months and every other month thereafter. The family-based intervention started with an initial 90-minute home visit at a time when all family members in the household were available to discuss general knowledge about hypertension prevention and treatment. During subsequent 60-minute monthly or bimonthly follow-up visits, CHWs provided tailored counseling to participants and their families on lifestyle modification, home BP monitoring, and medication adherence skills. They reviewed specific strategies for lifestyle modification, such as weight loss, dietary sodium reduction, physical activity, alcohol moderation, and the DASH (the Dietary Approaches to Stop Hypertension) diet, with patients and their families. Patients were encouraged to adopt lifestyle modification strategies that were the most suitable for their individual needs. All patients with hypertension in the intervention group were given an automatic home BP monitor and log and were trained to record their BP weekly. Additionally, they were provided 7-day pill organizers and counseled on techniques for improving medication adherence. Home visits also focused on goal setting, problem solving, social support, and maintaining motivation during challenging situations. CHWs helped patients schedule appointments with primary care physicians and delivered antihypertensive medications to patients’ homes if they did not have access to transportation.
Primary care physicians took part in an online education course on hypertension management followed by an onsite in-person half-day intensive training and certification. The physician training program focused on standard treatment algorithms for stepped-care BP management based on clinical guidelines.13,14 In addition, annual online hypertension management courses were provided for re-certification. Feedback was given to primary care physicians, based on home BP monitoring data collected by CHWs, to encourage medication adjustment when needed.
Individualized text-messages to promote lifestyle changes and reinforce medication adherence were sent out weekly to participants’ mobile phones by an eHealth platform at the Institute for Clinical Effectiveness and Health Policy in Buenos Aires, Argentina. Messages were based on hypertension status and perceived barriers to behavioral change identified during CHW home visits and consisted of motivational statements and behavior-change techniques to reinforce in-person education interventions. CHWs also collected information on participants’ receipt of text messages.
Usual Care Control
In the centers randomized to the control group, neither physicians nor CHWs were trained to conduct study interventions. Additionally, participants did not receive CHW home visits, home BP monitors, or text-messages. Participants were encouraged to follow regular the clinical visit schedule of the Remediar + Redes Program: monthly among patients after pharmacological treatment initiation and every 3–6 months among patients who had controlled BP.
Blinding
Study physicians, CHWs, and participants were not blinded to intervention assignment. However, study outcomes were collected by nurses who were not involved in the intervention.
Outcomes
The co-primary outcomes were the differences between the intervention and control groups in mean systolic and diastolic BP changes from baseline to the end of follow-up in patients with hypertension. Secondary outcomes included the proportion of patients with controlled hypertension (BP<140/90 mmHg), self-reported antihypertensive medication adherence, intensification (titration and/or addition) of antihypertensive medications, cost per additional percentage of hypertension controlled, and weight change over the 18-month intervention. In addition, difference in the changes of systolic and diastolic BP among normotensive participants were secondary outcomes.
Trained and certified research nurses who did not engage in the intervention collected all study data at baseline and 6, 12, and 18 months of follow-up in participants’ homes using standard questionnaires and measurement methods. Two visits between 1 and 14 days apart at baseline and at 18 months were conducted to obtain repeated BP measurements. Three BP measurements were obtained at each data collection visit, and the mean of all measurements at each time-point was used for analysis. BP was measured according to a standard protocol recommended by the American Heart Association.15 BP was measured with participants in a seated position after 5 minutes of quiet rest. In addition, participants were required to avoid alcohol, cigarettes, coffee/tea, and exercise for at least 30 minutes before their BP measurement. An auto-BP cuff (Intellisense Digital BP Monitor; model OMRON HEM-907 XL) was used, and one of four cuff sizes (pediatric, regular adult, large, or thigh) was chosen based on each participant’s arm circumference. Patient adherence to antihypertensive medication was quantified using the eight-item Morisky Medication Adherence Scale.16 Intensification of antihypertensive medications, including titration or addition of a new medication, was assessed by questionnaire and medical records. Intensification was used as an indicator of primary care physician adherence to the intervention program and related clinical guidelines. Adverse events, such as hypotension, syncope, and injurious falls, were queried at nurse visits.
Costs related to the intervention (i.e., program coordination, CHW salaries, physician training, home visits, BP monitors, and eHealth platform programming) were recorded at each clinic or study coordinating center. Costs related to healthcare (i.e., drug expenditures, laboratory tests, physician office visits, and hospitalizations) were collected from patients, clinics, and hospitals using standard questionnaires.
Statistical Analysis
The trial was designed to provide 80% statistical power to detect a ≥4.0 mmHg reduction in systolic BP at a significance level of 0.05 using a 2-tailed test.6,7 Eighteen centers (9 in each group) with an average cluster size of 62 patients with hypertension, an 85% follow-up rate, an inter-cluster correlation of 0.06, and a standard deviation of 10.0 mmHg were assumed, and the cluster design was taken into consideration in the power calculation.17,18
The intention-to-treat principle was used for all analyses. Only patients with hypertension were included in the primary analysis, according to the study protocol, because we aimed to test the effect of the CHW-led multicomponent intervention on BP control among patients with hypertension.9 A mixed-effects regression analysis, in which participants were nested in families, which were nested in centers, which were further nested in randomization groups, was used to estimate difference in the changes of BP from baseline to 6, 12, and 18 months, separately. In addition, the mean difference in the changes of BP during the intervention period were estimated. In these models, participants, families, and centers were assumed to be random effects, and the intervention was assumed to be a fixed effect. An autoregressive correlation structure was selected for these repeated measures. In addition, generalized estimating equations were used to compare baseline variables and the proportions of binary outcomes at 6, 12, and 18 months. Cluster effects were accounted for by assuming a compound-symmetry covariance structure, and standard errors were estimated using a robust variance estimator. In secondary analyses, important co-variables were adjusted, and predefined subgroup analyses were conducted. In these analyses, pairwise deletion of missing data was used to preserve all information observed. Additionally, multiple imputation for missing data in the multivariable analyses was conducted using the Markov chain Monte Carlo method. PROC GLIMMIX and PROC GENMOD of SAS version 9.4 (SAS Institute Inc., Cary, NC) were used to obtain point estimates and standard errors and to test for differences between randomization groups. A 2-sided p-value <0.01 was considered statistically significant because five main study outcomes were compared.
The incremental cost per additional percentage of patients achieving hypertension control at 18 months was calculated using patient-level data.19 Costs related to intervention and healthcare, but not study data collection, were included. Costs were documented in Argentine pesos and converted to US dollars as of May 2017 (one dollar =15.8 pesos). The 95% CI of incremental cost effectiveness ratio was estimated by bootstrapping 1000 replications of the main analysis.20
Results
From June 2013 to April 2015, a total of 6561 patients with hypertension and their family members were screened, and 1,954 who met eligibility criteria were enrolled (Figure 1). Of them, 970 participants (743 hypertensive and 227 normotensive participants) were recruited from the 9 intervention centers, with a median 107 participants per center (range 104–114), and 984 participants (689 hypertensive and 295 normotensive participants) from the 9 control centers, with a median 117 participants per center (range 48–131). Among 1,432 participants with hypertension, 1,357 (94.8%) completed the 18-month follow-up.
The mean age of patients with hypertension was 55.8 years and 53.0% were women. In general, baseline characteristics of patients with hypertension were balanced between intervention and control groups (Table 1). However, the intervention group had a slightly higher proportion of individuals with self-reported major cardiovascular disease and hypercholesterolemia, as well as higher levels of mean systolic (151.7 vs. 149.8 mmHg) and diastolic (92.2 vs. 90.1 mmHg) BP, compared with the control group. Likewise, baseline characteristics of normotensive participants were balanced between intervention and control groups except for physical activity and diastolic BP, which were slightly lower in the intervention compared to control group (eTable 1 in Supplement 2).
Table 1.
Baseline Characteristics of Hypertensive Participants
| Characteristicsa | Intervention (n=743) | Control (n=689) | P-value |
|---|---|---|---|
| Age, mean (SD), year | 56.1 (13.6) | 55.5 (13.0) | .45 |
| Female sex, n (%) | 394 (52.6) | 378 (53.4) | .53 |
| Currently smoking, n (%) | 144 (19.2) | 134 (19.2) | .99 |
| Weekly alcohol drinking, n (%) | 247 (33.4) | 208 (30.1) | .19 |
| Physical activity, median (IQR), MET/wk | 8 (0, 24) | 9 (0, 28) | .30 |
| History of major CVDb, n (%) | 93 (12.7) | 62 (9.0) | .03 |
| History of hypercholesterolemia, n (%) | 313 (42.4) | 254 (36.8) | .04 |
| History of diabetes, n (%) | 175 (23.7) | 146 (21.1) | .26 |
| Body-mass index, mean (SD), kg/m2 | 31.8 (6.6) | 31.5 (6.5) | .36 |
| Systolic BPc, mean (SD), mm Hg | 151.7 (16.8) | 149.8 (15.5) | .03 |
| Diastolic BPc, mean (SD), mm Hg | 92.2 (12.2) | 90.1 (12.9) | .002 |
| Use of antihypertensive medications, n (%) | 639 (86.0) | 575 (83.5) | .18 |
| Morisky scored, mean (SD) | 6.3 (1.9) | 6.3 (2.0) | .69 |
Abbreviations: CVD, cardiovascular disease; IQR, inter-quartile range; SD, standard deviation.
Generalized estimating equations were used to compare baseline variables accounting for cluster effects from families and clinics.
Major cardiovascular disease includes myocardial infarction and stroke.
Mean blood pressure from screening and baseline visits.
Eight-item Morisky Medication Adherence Scale scores range from 0 to 8 with low adherence defined as a score <6, medium adherence as scores of 6 or 7, and high adherence with a score of 8.
Implementation indicators
During the 18-month intervention, CHWs completed 92.8% (8272/8916) of planned home-based interventions, and patients completed 84.2% (26342/31287) of anticipated home BP measurements. In addition, the eHealth platform sent out 91.2% of scheduled text-messages and 76.3% of participants reported receiving messages weekly. The proportion of high adherence to antihypertensive medication (Morisky score=8) increased from 31.3% at baseline to 66.1% at 18 months in the intervention group and from 38.0% to 53.0% in the control group (Table 2). The difference in the proportion of high adherence to antihypertensive medication was 13.1% (95% CI: 7.0, 19.2%; p<0.001) at 18 months. Proportions of medication intensification from baseline to 18 months were 57.6% in the intervention group and 42.8% in the control group (p<0.001).
Table 2.
Effects of the Multicomponent Intervention on Primary and Secondary Outcomes in Hypertensive Patients
| Intervention | Control | Net difference (95% CI) | P Value | Adjusted net Differencea (95% CI) | P Value | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. | Mean or No. and Proportion (95% CI) | No. | Mean or No. and Proportion (95% CI) | |||||
| Co-primary outcome: change in systolic BP from baseline, mmHg | ||||||||
| At month 6 | 722 | −11.9 (−13.3,−10.5) | 682 | −7.4 (−8.9,−5.9) | −4.5 (−6.6,−2.4) | <.001 | −3.7 (−5.7,−1.8) | <.001 |
| At month 12 | 719 | −15.6 (−16.8,−14.4) | 654 | −10.1 (−11.3,−8.8) | −5.5 (−7.3,−3.8) | <.001 | −4.8 (−6.3,−3.3) | <.001 |
| At month 18 | 709 | −19.3 (−20.8,−17.9) | 648 | −12.7 (−14.2,−11.3) | −6.6 (−8.6,−4.6) | <.001 | −5.8 (−7.5,−4.1) | <.001 |
| Overall | 722 | −15.6 (−16.8,−14.3) | 682 | −10.0 (−11.3,−8.8) | −5.5 (−7.3,−3.8) | <.001 | −4.8 (−6.3,−3.2) | <.001 |
| Co-primary outcome: change in diastolic BP from baseline, mmHg | ||||||||
| At month 6 | 722 | −6.5 (−7.4,−5.5) | 682 | −3.5 (−4.4,−2.6) | −2.9 (−4.3,−1.6) | <.001 | −2.1 (−3.4,−0.8) | .001 |
| At month 12 | 719 | −9.4 (−10.2,−8.5) | 654 | −5.2 (−6.0,−4.4) | −4.2 (−5.3,−3.0) | <.001 | −3.3 (−4.3,−2.4) | <.001 |
| At month 18 | 709 | −12.2 (−13.2,−11.2) | 648 | −6.9 (−7.8,−5.9) | −5.4 (−6.8,−4.0) | <.001 | −4.6 (−5.7,−3.4) | <.001 |
| Overall | 722 | −9.3 (−10.2,−8.5) | 682 | −5.2 (−6.0,−4.4) | −4.1 (−5.3,−3.0) | <.001 | −3.3 (−4.3,−2.3) | <.001 |
| Proportion of controlled hypertensionb, No, % | ||||||||
| At baseline | 743 | 127, 17.0 (14.4, 20.0) | 689 | 122, 17.6 (15.0, 20.7) | −0.6 (−4.6, 3.4) | .77 | 1.9 (−1.1, 5.0) | .22 |
| At month 6 | 722 | 333, 46.1 (42.5, 50.0) | 682 | 277, 40.4 (36.8, 44.4) | 5.7 (0.4, 11.0) | .04 | 8.0 (2.9, 13.1) | .002 |
| At month 12 | 719 | 439, 61.0 (57.3, 64.8) | 654 | 288, 43.9 (40.2, 47.9) | 17.1 (11.7, 22.5) | <.001 | 18.4 (13.2, 23.6) | <.001 |
| At month 18 | 709 | 517, 72.9 (69.6, 76.3) | 648 | 340, 52.2 (48.4, 56.4) | 20.6 (15.4, 25.9) | <.001 | 22.1 (17.1, 27.2) | <.001 |
| Proportion of high adherence in patients taking antihypertensive medications,c,d No, % | ||||||||
| At baseline | 620 | 197, 31.3 (27.6, 35.6) | 570 | 223, 38.0 (34.0, 42.5) | −6.7 (−12.6,−0.9) | .03 | −6.3 (−12.1,−0.5) | .03 |
| At month 6 | 627 | 309, 48.3 (44.2, 52.8) | 575 | 237, 41.2 (37.0, 45.7) | 7.1 (1.0, 13.2) | .02 | 8.2 (2.4, 14.0) | .005 |
| At month 12 | 633 | 353, 54.5 (50.4, 58.9) | 550 | 280, 49.6 (45.3, 54.4) | 4.9 (−1.4, 11.1) | .13 | 6.0 (−0.1, 12.1) | .05 |
| At month 18 | 629 | 422, 66.1 (62.2, 70.4) | 542 | 292, 53.0 (48.7, 57.7) | 13.1 (7.0, 19.2) | <.001 | 14.9 (8.8, 20.9) | <.001 |
| Proportion of intensification of antihypertensive treatment from baseline,e No, % | ||||||||
| At month 6 | 722 | 248, 34.5 (31.1, 38.2) | 683 | 177, 26.0 (22.9, 29.5) | 8.5 (3.7, 13.4) | <.001 | 7.8 (3.0, 12.7) | .001 |
| At month 12 | 719 | 339, 47.2 (43.8, 51.0) | 655 | 244, 37.3 (33.7, 41.3) | 9.9 (4.7, 15.2) | <.001 | 8.9 (3.7, 14.2) | <.001 |
| At month 18 | 709 | 407, 57.6 (54.1, 61.3) | 648 | 276, 42.8 (39.0, 47.0) | 14.8 (9.4, 20.2) | <.001 | 13.7 (8.3, 19.1) | <.001 |
Adjusted for age, sex, history of major cardiovascular disease, history of hypercholesterolemia, alcohol drinking, physical activity, baseline body-mass index, and systolic (or diastolic) blood pressure.
Controlled hypertension was defined as systolic BP <140 mmHg and diastolic DBP <90 mmHg among patients with hypertension.
High adherence was defined as eight-item Morisky Medication Adherence Scale score equal to eight. Patients who were not taking antihypertensive medication did not collect information on medical adherence.
Adjusted for age, sex, history of major cardiovascular disease, history of hypercholesterolemia, alcohol drinking, physical activity, and baseline body-mass index, systolic blood pressure, and eight-item Morisky Medication Adherence Scale score.
Titration or addition of new antihypertensive medications since baseline.
Co-primary outcomes
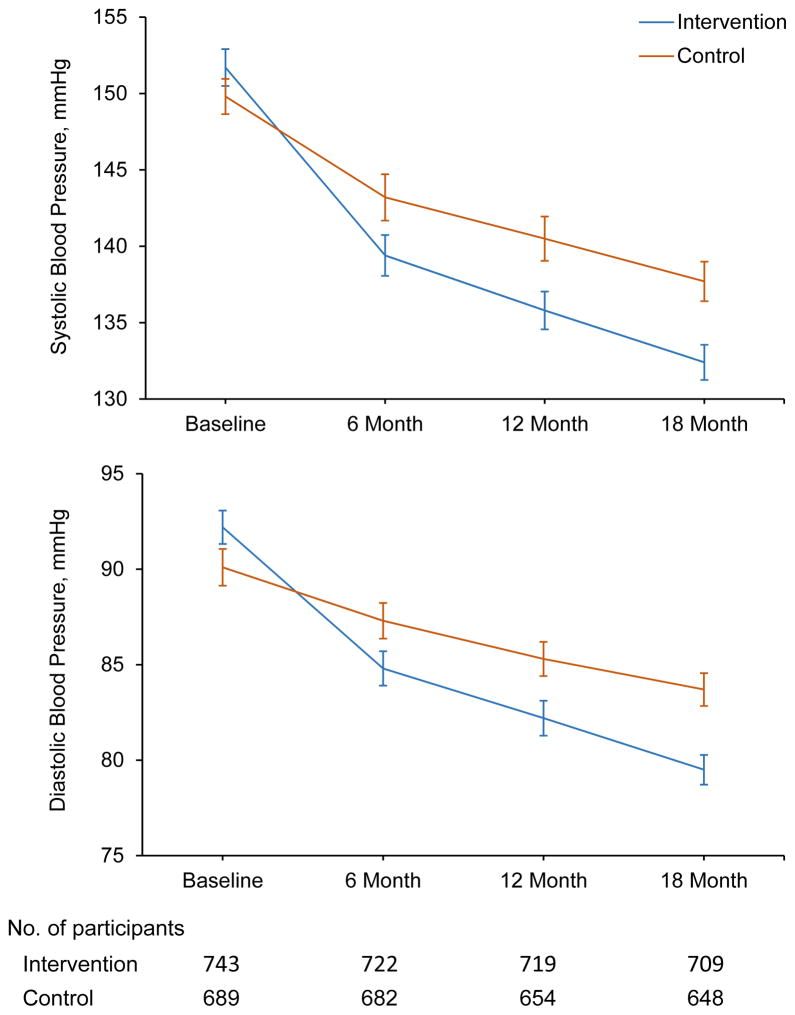
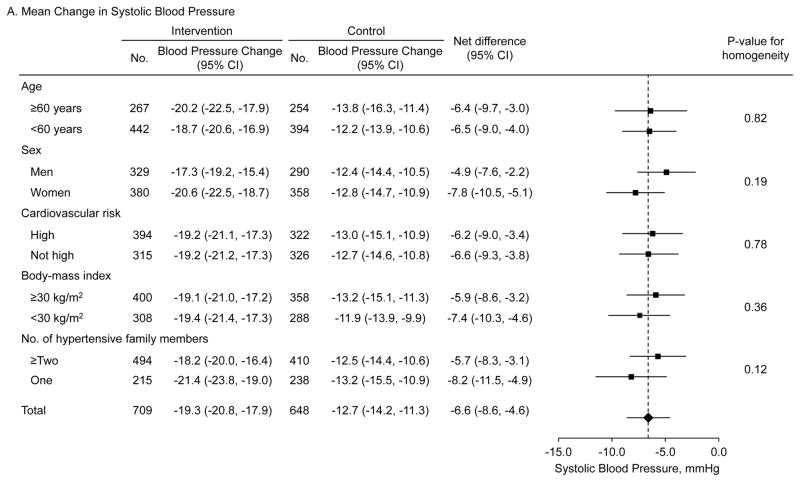
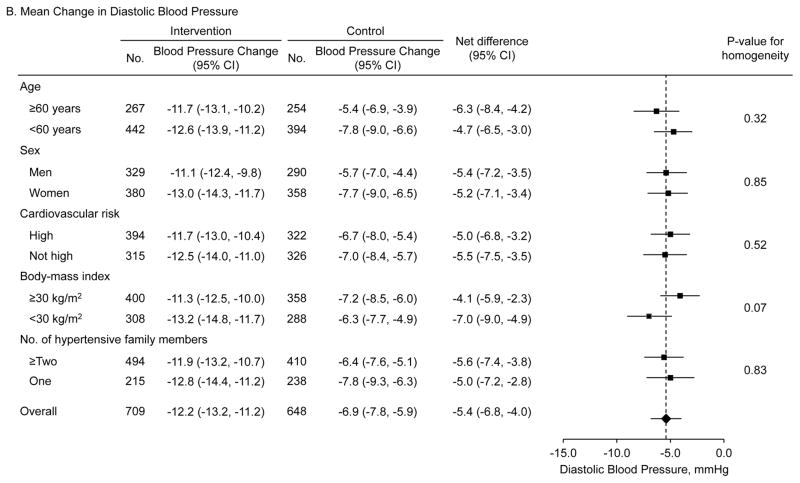
Systolic BP (95% CI) was significantly reduced from 151.7 (150.5, 152.9) at baseline to 132.4 (131.2, 133.5) mmHg at 18 months in the intervention group and from 149.8 (148.7, 151.0) to 137.7 (136.4, 139.0) mmHg in the control group (Figure 2, eTable 2 in Supplement 2). Diastolic BP was reduced from 92.2 (91.3, 93.0) to 79.5 (78.7, 80.2) mmHg in the intervention group and from 90.1 (89.1, 91.1) to 83.7 (82.9, 84.6) mmHg in the control group. Difference in the reduction in systolic BP was 6.6 mmHg (95% CI: 4.6 to 8.6; p<0.001) and in diastolic BP was 5.4 mmHg (95% CI: 4.0 to 6.8; p<0.001) (Table 2). The intra-class correlation coefficients were 0.0768 and 0.0713 for changes in systolic and diastolic BP, respectively. Net reductions in systolic and diastolic BP were consistent by age, sex, cardiovascular risk (history of major cardiovascular disease, hypercholesterolemia, and diabetes), body-mass index, and number of hypertensive family members in predefined subgroup analyses (Figure 3).
Figure 2. Mean blood pressure during trial follow-up in intervention and control groups among patients with hypertension.
Systolic blood pressure (upper panel) and diastolic blood pressure (lower panel). Six blood pressure measurements at baseline and 18 months from two visits as well as three blood pressure measurements at 6 months and 12 months from one visit were obtained. The point estimates are mean blood pressure and error bars indicate 95% confidence intervals.
Figure 3. Mean difference in the changes of systolic and diastolic blood pressure among patients with hypertension by subgroups.
Mean differences in systolic (upper panel) and diastolic (lower panel) blood pressure changes from baseline to 18-month follow-up between the intervention and control groups. Data markers indicate mean difference in the changes and error bars indicate 95% confidence intervals. High cardiovascular risk subgroup includes participants with a history of coronary heart disease, heart failure, stroke, hypercholesterolemia, or diabetes.
Secondary outcomes
The proportion of controlled hypertension increased from 17.0% at baseline to 72.9% at 18 months in the intervention group, and from 17.6% at baseline to 52.2% at 18 months in the control group. The difference in the increase in proportion of controlled hypertension was 20.6% (95% CI: 15.4, 25.9%; p<0.001). The intra-class correlation coefficient was 0.0415 for hypertension control. There were no significant differences in body weight or waist circumference changes between intervention and control groups (eTable 3 in Supplement 2).
There were no significant changes in BP in normotensive participants during the 18-month intervention (eTable 4 in Supplement 2). For example, differences in BP changes over 18 months were 0.6 mmHg (95% CI: −1.6, 2.7; p=0.60) for systolic and 1.8 mmHg (95% CI: 0.1, 3.5; p=0.04) for diastolic.
No adverse events were reported.
Cost-effectiveness of intervention
Mean intervention cost per patient was $114.6 (95% CI: 113.7, 115.6) or $6.36 per patient per month. There were no significant differences in mean healthcare costs per patient between the two groups: $62.2 (95% CI: 44.6, 79.7) in intervention and $67.6 (95% CI: 41.9, 93.3) in control. The total cost per patient over the 18-month follow-up was $178.6 (95% CI: 161.0, 196.1) in the intervention group and $67.6 (95% CI: 41.9, 93.3) in the control group. The mean adjusted total cost difference was $102.7 (95% CI: 61.0, 144.4), and the incremental cost effectiveness ratio was $464.7 per additional percentage of patients achieving hypertension control at 18 months (95% CI: 335.2, 771.7).
Sensitivity analysis
After multiple imputation for missing data, the difference in the reduction in BP over 18 months was 6.7 mmHg (95% CI: 4.7, 8.7; p<0.001) for systolic and 5.1 mmHg (95% CI: 3.8, 6.5, p<0.001) for diastolic (eTable 5 in Supplement 2). The difference in the increase in the proportion of controlled hypertension was 19.2% (95% CI: 14.1, 24.3%; p<0.001).
Discussion
This cluster randomized trial indicated that a CHW-led multicomponent intervention was effective in reducing systolic and diastolic BP and improving hypertension control among low-income, uninsured patients with hypertension in Argentina. The multicomponent intervention significantly increased patients’ adherence to antihypertensive medication and physicians’ adherence to treatment guidelines.
These findings may have public health significance. About 80% of all cardiovascular mortality occurs in LMICs, where the greatest burden of hypertension is observed.3,21 Although clinical trials have documented that BP lowering reduces the risk of cardiovascular disease and premature death, and affordable antihypertensive medications and lifestyle interventions are widely available, hypertension control rates continue to be low in LMICs.3,22–24 Lack of effective and sustainable strategies to overcome barriers is a major obstacle for hypertension control in under-served populations.25 Therefore, widespread scaling-up of this proven effective intervention in LMICs should result in a substantial reduction in uncontrolled hypertension and related cardiovascular disease.
Several strategies have been documented to improve BP control in patients with hypertension.6,7,26 In addition, multicomponent interventions targeting healthcare systems, physicians, and patients have been shown to be more effective.27,28 However, the effects of these intervention strategies have not been well studied in low-income settings. In a cluster randomized trial, Ogedegbe and colleagues reported that a multicomponent intervention, including patient education, home BP monitoring, lifestyle counseling, physician education, and BP audit and feedback, did not improve BP control compared to usual care in African-American hypertensives receiving care in low-resource primary care practices.29 The HCPIA trial was also conducted in low-income patients who received healthcare from a resource-limited public primary care system in Argentina. The major differences between the two trials are that the intervention was led by CHWs and conducted at patients’ homes in HCPIA. In another cluster randomized trial, Jafar and colleagues reported that CHW-led home health education or general practitioner training alone did not reduce BP.30 However, the combination of home health education and practitioner training led to a significant 5.0 mmHg reduction in systolic BP among patients with hypertension in Pakistan.30 These results support CHW-led multicomponent interventions for BP control in low-income settings. In many LMICs, CHWs are already employed within the public primary care system for infectious disease control and maternal and child healthcare. Training and engaging them in hypertension management may provide an effective, affordable, and sustainable approach for BP control in LMICs.
This study showed that CHWs can play an important role in hypertension control among low-income communities. They provided health coaching to patients and families about lifestyle modification and medication adherence; trained and tracked patients’ home BP monitoring; served as mediators between patients and the healthcare system and physicians; arranged physicians’ appointments and delivered medications when needed; and listened to patients and their family members, motivated them, and provided social support.31
A significant BP reduction in patients from control centers was also observed. In the Remediar+Redes Program, only 11.6% of patients with hypertension had their BP controlled.32 In this study, 52.3% of patients achieved hypertension control at 18 months in the usual care group. Patients received repeated BP measurements every six months and were interviewed about behaviors related to antihypertensive medication adherence, which might have contributed to improvement in medication adherence and treatment intensification, and eventually, BP reduction. In addition, intervention contamination could have occurred and contributed to the findings observed in the control group. Furthermore, BP reduction in patients might be partially due to regression to the mean because participants were selected to have elevated BP.
This study has several limitations. It used a cluster randomized trial design because the multilevel and multicomponent interventions were implemented at the primary care center level. It was not practical to recruit all participants prior to randomization. Therefore, selection bias could have occurred. However, patients with hypertension and their family members were systematically recruited to avoid selection bias.33 Important co-variables were also adjusted to limit potential confounding effects. Another limitation is that intervention contamination, if any occurred, might have diluted the observed effect. In addition, the incremental cost effectiveness ratio for quality adjusted life years saved was not calculated because extensive assumptions were necessary for modeling which was outside the scope of this report. Therefore, the cost-effectiveness of this CHW-led multicomponent intervention for BP control could not be directly compared with other interventions for various conditions.34
Conclusion
Among low-income patients with uncontrolled hypertension in Argentina, a CHW-led multicomponent intervention compared with usual care resulted in a greater decrease in systolic and diastolic BP over 18 months. Further research is needed to assess generalizability and cost-effectiveness of this intervention, and to understand which components may have contributed most to the outcome.
Supplementary Material
Key Points.
Question
Can a community health worker-led multicomponent intervention improve blood pressure control among low-income patients with hypertension?
Findings
In this cluster randomized trial among 1,432 low-income, uninsured patients with hypertension in Argentina, the community health worker-led multicomponent intervention significantly reduced systolic blood pressure by 6.6 mmHg and diastolic blood pressure by 5.4 mmHg compared to usual care over 18 months.
Meaning
A community health worker-led multicomponent intervention may improve hypertension control in low-income populations.
Acknowledgments
Funding/Support: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number U01HL114197 and partially by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM109036.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Role of the Funder/Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Meeting Presentation: Parts of these data were presented at the American Heart Association Scientific Sessions 2016 Late-Breaking Clinical Trial Session, November, 14, 2016, New Orleans, Louisiana.
Author Contributions: Dr. He had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: He, Irazola, Mills, Poggio, Beratarrechea, Krousel-Wood, Bazzano, Jing Chen, and Rubinstein.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: He
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Chung-Shiuan Chen
Administrative, technical, or material support: He, Irazola, Mills, Dolan, and Rubinstein
Supervision: He, Irazola, Mills, and Rubinstein
Additional Contributions: We acknowledge the contributions of all investigators, coordinators, and staff of the Hypertension Control Program in Argentina (HCPIA) Study. We thank Dr. Lawrence J. Fine, MD, Dr. PH. of National Heart, Lung, and Blood Institute for his support of this study and for his comments on this manuscript; he did not receive compensation. We also acknowledge Miss Katherine Obst for her editorial assistance; she was compensated as an employee of Tulane University. A complete list of the investigators, coordinators, and staff from all participating sites is provided in Supplement 2, available at JAMA.com. Use of the Morisky Medication Adherence Scale is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E Morisky ScD ScM MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E Young Drive South, Los Angeles, CA 90095.
References
- 1.Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 2.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–34. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He J, Muntner P, Chen J, Roccella EJ, Streiffer RH, Whelton PK. Factors associated with hypertension control in the general population of the United States. Arch Intern Med. 2002;162(9):1051–8. doi: 10.1001/archinte.162.9.1051. [DOI] [PubMed] [Google Scholar]
- 5.Odedosu T, Schoenthaler A, Vieira DL, Agyemang C, Ogedegbe G. Overcoming barriers to hypertension control in African Americans. Cleve Clin J Med. 2012;79(1):46–56. doi: 10.3949/ccjm.79a.11068. [DOI] [PubMed] [Google Scholar]
- 6.Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3(2):e000718. doi: 10.1161/JAHA.113.000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. doi: 10.1136/bmj.c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogedegbe G, Gyamfi J, Plange-Rhule J, et al. Task shifting interventions for cardiovascular risk reduction in low-income and middle-income countries: a systematic review of randomised controlled trials. BMJ Open. 2014;4(10):e005983. doi: 10.1136/bmjopen-2014-005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills KT, Rubinstein A, Irazola V, et al. Comprehensive approach for hypertension control in low-income populations: rationale and study design for the hypertension control program in Argentina. Am J Med Sci. 2014;348(2):139–45. doi: 10.1097/MAJ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Bank. [Accessed February 7, 2017];The health sector in Argentina: current situations and options for improvement. http://documents.worldbank.org/curated/en/227331468768555246/pdf/261440AR.pdf.
- 11.Belló M1, Becerril-Montekio VM. The health system of Argentina. Salud Publica Mex. 2011;53(Suppl 2):s96–s108. [PubMed] [Google Scholar]
- 12.Homedes N, Ugalde A. Improving access to pharmaceuticals in Brazil and Argentina. Health Policy Plan. 2006;21(2):123–31. doi: 10.1093/heapol/czj011. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez RA, Ayala M, Baglivo H, et al. Latin American guidelines on hypertension. Latin American Expert Group. J Hypertens. 2009;27(5):905–22. doi: 10.1097/HJH.0b013e32832aa6d2. [DOI] [PubMed] [Google Scholar]
- 15.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005;7(2):102–9. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57(8):785–94. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Donner A, Klar N. Statistical considerations in the design and analysis of community intervention trials. J Clin Epidemiol. 1996;49(4):435–439. doi: 10.1016/0895-4356(95)00511-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–72. doi: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: A non-parametric approach to confidence interval estimation. Health Economics. 1997;6(4):327–40. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 21.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 23.Gu D, He J, Coxson PG, et al. The cost-effectiveness of low-cost essential antihypertensive medicines for hypertension control in China: a modelling study. PLoS Med. 2015;12(8):e1001860. doi: 10.1371/journal.pmed.1001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinberg D, Bennett GG, Svetkey L. The DASH Diet, 20 Years Later. JAMA. 2017;317(15):1529–1530. doi: 10.1001/jama.2017.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on Public Health Priorities to Reduce and Control Hypertension in the U.S. Population, Institute of Medicine. A population-based policy and systems change approach to prevent and control hypertension. Washington DC: National Academy Press; 2010. [PubMed] [Google Scholar]
- 26.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;3:CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 27.Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med. 2006;145(3):165–75. doi: 10.7326/0003-4819-145-3-200608010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Pladevall M, Brotons C, Gabriel R, et al. Multicenter cluster-randomized trial of a multifactorial intervention to improve antihypertensive medication adherence and blood pressure control among patients at high cardiovascular risk (the COM99 study) Circulation. 2010;122(12):1183–91. doi: 10.1161/CIRCULATIONAHA.109.892778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension: cluster-randomized clinical trial main effects. Circulation. 2014;129(20):2044–51. doi: 10.1161/CIRCULATIONAHA.113.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med. 2009;151(9):593–601. doi: 10.7326/0003-4819-151-9-200911030-00004. [DOI] [PubMed] [Google Scholar]
- 31.Brownstein JN, Chowdhury FM, Norris SL, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32(5):435–447. doi: 10.1016/j.amepre.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Soriano ER, Dawidowski AR, Pereiro N, et al. Gaps between prescription of anti-hypertensive and hypertension control in older adults of Buenos Aires suburbs. Rev Fac Cien Med Univ Nac Cordoba. 2011;68(4):141–8. [PubMed] [Google Scholar]
- 33.Brierley G, Brabyn S, Torgerson D, Watson J. Bias in recruitment to cluster randomized trials: a review of recent publications. J Eval Clin Pract. 2012;18(4):878–86. doi: 10.1111/j.1365-2753.2011.01700.x. [DOI] [PubMed] [Google Scholar]
- 34.Gu D, He J, Coxson PG, Rasmussen PW, Huang C, Thanataveerat A, et al. The cost-effectiveness of low-cost essential antihypertensive medicines for hypertension control in China: a modelling study. PLoS Med. 2015;12(8):e1001860. doi: 10.1371/journal.pmed.1001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.