Abstract
The Pax gene family encodes DNA binding transcription factors that control critical steps in embryonic development and differentiation of specific cell lineages. Often, Pax proteins are re-expressed or ectopically expressed in cancer and other diseases of abnormal proliferation, making them attractive targets for tissue specific inhibition by small molecules. In this report, we used a homology model of the Pax2 paired domain and a virtual screen to identify small molecules that can inhibit binding of the paired domain to DNA and Pax2 mediated transcription activation. Candidates from the virtual screen were then confirmed in a cell based Pax2 transactivation assay. Subsequently, we tested analogs of these hits to identify a single compound that effectively blocked Pax2 activity and DNA binding with a Kd of 1.35–1.5 µM. The compound, termed EG1, was used to inhibit embryonic kidney development, a process directly dependent on Pax2 activity. Furthermore, we show that EG1 can inhibit proliferation of Pax2 positive renal and ovarian cancer cell lines but has little effect on Pax2 negative cancer cells. These data confirm that small molecules targeting the DNA binding paired domain can be identified and may be good lead compounds for developing tissue and cell-type specific anticancer therapies.
Graphical abstract

The mammalian Pax genes are a family of nine developmental control genes that were first identified due to sequence homology with the Drosophila segmentation genes paired and gooseberry, based on a highly conserved DNA-binding domain known as the paired box.1 While all encode an amino terminal paired box domain, the Pax gene family can be divided into four subgroups based on downstream structural domains including an octapeptide sequence and a homeodomain, which are present in various combinations among the family members.2,3 Pax genes encode nuclear transcriptional regulators that are expressed in a variety of developing structures during embryogenesis. They play a critical role in lineage determination and ultimately regulate tissue specification. For example, Pax5 is expressed in B-cell precursors4 and Pax6 in the optic cup,5,6 where they are required for B-cell maturation and eye development, respectively. While Pax loss of function is typically associated with severe developmental defects, gain of function mutations have been reported in an assortment of cancers including genomic rearrangements involving Pax3 or Pax7 in cases of pediatric rhabdomyosarcoma.7,8
Pax proteins are defined by an evolutionarily conserved 128 amino acid element, the paried domain, which confers DNA-binding ability to these proteins. The structures of the Drosophila Prd,9 human Pax5,10 and human Pax611 paired domains cocrystallized with their respective DNA sequences have identified two globular domains connected by an extended and disordered linker. These structures indicate a tripartite binding pattern in which the N-terminal and C-terminal subdomains adopt a helix–turn–helix structural motif and make contact with nucleotide bases in the major groove while the linker makes extensive contact within the minor groove. While the two helix–turn–helix subdomains are capable of independently binding DNA,12 both are required for proper target gene regulation.13 The sheer number of mutations within the paired domain that have been described to disrupt DNA-binding and are associated with human disease states highlight the biological significance of this domain.
The kidney and reproductive tract are derived from the intermediate mesoderm,14,15 which express Pax2 and its homologue Pax8 among the earliest markers for this region.16 As development proceeds, Pax2 is strongly expressed in the epithelium of the developing collecting ducts that undergo branching morphogenesis and in the progenitor cells of the nephron that aggregate at the tips of the branching ducts. In vertebrates, Pax2 is essential for proper development of the kidney and the reproductive system.17–19 However, expression is down-regulated in mature nephron epithelial cells in adults.20,21 Ectopic or reactivated Pax2 expression is observed in proliferative diseases of the kidney such as renal cell carcinoma,22 Wilms’ tumor,20,23 and polysystic kidney disease.24 Interestingly, in mouse models of polycystic kidney disease, a reduction in Pax2 levels led to decreased cyst formation and slowed disease progression.24,25 Furthermore, a decrease in Pax2 in renal carcinoma cells reduces proliferation, increases apoptosis, and sensitizes cancer cells to chemotherapeutic agents.26,27 Together these data suggest that Pax2 is an excellent target for therapeutic intervention in renal diseases characterized by abnormally proliferating epithelial cells.
Despite being implicated in a multitude of urogenital disorders, Pax2 has not been investigated as a therapeutic target. In fact, DNA-binding transcription factors, as a whole, represent an under-investigated class of potential drug targets due in part to their nuclear localization and the charged nature of the DNA binding domain. Regardless, several DNA binding proteins have been successfully targeted by small molecules including C/EBPα,28 c-Myc,29,30 and Stat3.31,32 Although no Pax proteins have been targeted for inhibition by small molecules to date, we hypothesize that inhibiting Pax2 activity could provide a therapeutic window with a high degree of specificity for renal disease. To discover small molecules capable of targeting the Pax2 paired domain, we used structure based virtual screening to identify compounds that bind to the Pax2 DNA binding domain followed by cell based and in vitro validation and characterization. Targeting tissue-specific developmental control genes represents a novel therapeutic approach, which has the potential to reduce deleterious off-target effects and improve current treatment regimens.
RESULTS AND DISCUSSION
In Silico Screening for Paired Domain Interacting Molecules
One of the most efficient ways to inhibit Pax2 transactivation would be to block binding of the protein to its cognate DNA sequence. In order to identify an inhibitor that can disrupt the Pax2 paired domain from binding to DNA, we applied a virtual screening approach using a three-dimensional structure of Pax2 built by homology modeling (Figure 1). Homology modeling, coupled with further structural refinement using molecular mechanics and molecular dynamics simulations, provides an alternative to obtaining a three-dimensional structure of the target protein with a sufficiently high accuracy for drug design. Fortunately, there are two closely related paired domain structures, the Pax5/Ets-1 in complex with DNA (1K78)10 and Pax6 in complex with DNA (6PAX).11 Amino acid identity throughout the paired DNA binding domain is 97% between Pax2 and Pax5 and 76% between Pax2 and Pax6. On the basis of the high degree of identity and the 2.25 Å resolution of the Pax5 bound to DNA, in complex with the ETS1 protein, the Pax5 paired domain was used as the template for the structural homology model of Pax2.
Figure 1.
A homology model of the Pax2 paired domain and virtual screen. (A) A three-dimensional homology model of the Pax2 paired domain bound to DNA as based on the structure of the Pax5/Ets-1 complex with DNA. (B) The DNA binding pocket used for virtual screening is marked by the red square. Critical amino acid residues within the DNA binding pocket are indicated. For cell based screening of candidate hits, a Cys63 to tyrosine mutation was used as a control because it inhibits DNA binding and Pax2 mediated transactivation activity.33 (C) Flowchart of the number of candidate compounds identified at each step resulting in 227 candidate compounds after virtual screening.
A DNA binding pocket was defined based on the homology model and several point mutations (C63Y and Q47P) that were known to be disease associated and also prevented the binding of Pax2 to DNA33,34 (Figure 1A). The C63 residue sits in a well-defined DNA binding pocket and is surrounded by hydrophobic residues, including L32, V59, and I67 (Figure 1B). The homology model underwent 10 ns of molecular dynamics using Amber 11, and the produced trajectories were clustered using the MMTSB toolset. In each cluster, a structure with the minimum energy was chosen for virtual screening. Using the Glide docking program, we performed computational structure based database searching of the subset drug-like compound library (97 378 compounds) available at the Center for Chemical Genomics (CCG) at the University of Michigan, which were filtered in order to avoid problematic compounds known as PAINS (Pan-Assay Interference Structures).35 These were screened for their potential to dock with the Pax2 paired domain DNA binding pocket, as centered around the C63 active site. A cubic box, or active site, with a length of side of 10 Å represented the volume of the protein for which grids were calculated using Glide 5.5 and used for docking studies. Glide 5.5 standard-precision (SP) mode and extra-precision (XP) of Schrödinger were used for the virtual screening. The top 2000 candidate small-molecules with the best scores obtained by Glide SP were rescreened using the XP mode. After the reranking, the top 227 scored compounds with reasonable docking poses were considered as potential small molecule inhibitors of Pax2.
Cell-based Activity Assays Confirm in Silico Discovery of a Novel Pax2 Inhibitor
Of the 227 potential Pax2 inhibitors identified from the virtual screen, 225 were tested in a cell based in vivo assay developed for Pax2 mediated transcriptional activation (Figure 2). A HEK293 cell line was engineered to carry an integrated Pax2 reporter gene (PRS4-Luc) that had been previously characterized.36 Transiently transfecting these cells with a Pax2 expression construct promotes Pax2 binding to the PRS4 (Pax Response Sequence) DNA sequence, the recruitment of PTIP and the MLL3/4 complex, an increase in H3K4 trimethylation, and induction of luciferase gene expression.36,37 By transfecting cells in large plates and subsequent transfer to 384-well plates containing compounds, a Z′ factor of 0.65–0.71 was reached. Initial dose response experiments were done with compounds prepared and stored at the University of Michigan Center for Chemical Genomics, and 31 hits were identified of 225 candidate molecules from the virtual screen. The IC50 values for the 31 hits ranged from approximately 3 µM to greater than 500 µM. Fresh powder stocks of these 31 hits were ordered and retested in our primary activity assay in order to confirm that the activity was due to the compound and not mislabeling, contamination, or a degradation product. To ensure that compounds were specifically inhibiting Pax2 and not interfering with basic cellular processes (transcription, translation, proliferation, etc.) or with the luciferase enzyme itself, we set up a counter-screen in which HEK293 cells used in the primary screen were transfected with a constitutive luciferase expression vector (CMV-Luc) and assayed for any loss of luciferase activity.
Figure 2.
In vitro validation of computational screening method and discovery of a small molecule inhibitor of Pax2. (A) Diagram of in vitro validation workflow. (B) Chemical structure of lead compound EG1. (C) Dose response curves for PRS4-Luc reporter cells transfected with CMVPax2 expression vector (gray) or CMV-Luc expression vector (black) and treated with increasing concentrations of EG1 shows that EG1 dose dependently inhibits luminescence in a Pax2 dependent manner. (D) Similar experiment as in C but with the closely related proteins Pax5 and Pax8 activating the PRS4-Luc reporter. Note EG1 inhibits transactivation by all 3 Pax proteins. (E) Dose response of EG1 on the ability of BMP4 and Smad1 to activate a BMP response element. EG1 has little effect on the activity of the BRE-luc reporter cell line in the presence of BMP4. (F) Western blot of PRS4-EGFP cells transfected with CMV-Pax2b or CMV-Pax2bC63Y (Mut) and treated with increasing concentrations of EG1 shows that EG1 dose dependently inhibits Pax2 transactivation activity in PRS-EGFP cells.
After the retesting and counter-screening, five compounds were confirmed as Pax2 specific inhibitors of luciferase activity (Table 1). The five confirmed hits could be further divided into two pairs of structurally similar compounds and a single unrelated compound. Since these compounds had relatively mild inhibitory effects, with IC50 values from 50 to 240 µM, we purchased commercially available analogs of the hits and tested them in the cell-based assays. Of the 64 analogs tested, IC50 values ranged from approximately 10 µM to greater than 150 µM. From these analogs, EG1 (Figure 2B, PubChem CID 2193203) was selected for further analysis because of its increased potency in the Pax2 dependent assay and complete lack of luciferase inhibition in the CMV-Luc control assay. EG1 inhibits Pax2 mediated expression of the luciferase reporter genes in a dose dependent manner, with an IC50 of approximately 10 µM, but had no inhibitory effect on CMV-Luc in the counter-screen (Figure 2C). The paired domains of Pax5 and Pax8 are 97% and 92% identical to Pax2, respectively, with the sequences around the DNA binding pocket being identical. EG1 was able to inhibit PRS4-Luc activation by Pax5 and Pax8 with similar kinetics as with Pax2 mediated activation (Figure 2D). However, a BMP dependent luciferase reporter (BRE-Luc), which depends on P-Smad mediated transactivation, was unaffected by increasing amounts of EG1 in HEK293 cells (Figure 2E). As a second independent Pax2 reporter, we also tested EG1 activity in a cell line that contained a PRS4-EGFP reporter (Figure 2F). Similarly, EG1 was capable of inhibiting Pax2 mediated expression of EGFP without significant effects on Pax2 protein levels (Figure 2F). These data suggest that EG1 is a specific and efficacious Pax2 inhibitor.
Table 1.
Pax2 Specific Inhibitors Identified by Virtual Screening
| PRS4-LUC | CMV-LUC | ||
|---|---|---|---|
| Structure | CCG ID | IC50 (µM) | IC50 (µM) |

|
3036 | 106.7 ± 11.3 | >500 |

|
3647 | 126.5 ± 26.5 | >500 |

|
21766 | 126.4 ± 19.8 | >500 |
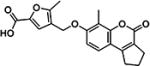
|
21767 | 51.9 ± 4.9 | >500 |
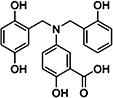
|
100613 | 48.6 ± 6.5 | 291.3 ± 75.6 |
EG1 Directly Binds the Paired Domain of Pax2 and Inhibits Pax2–DNA Interactions
To confirm that EG1, as predicted by our in silico model, binds to the paired domain of Pax2 and to examine its binding kinetics, we utilized biolayer interferometry, a label free technique for determining binding affinities (Kd) between two molecules. For this experiment, we immobilized the biotinylated recombinant Pax2 paired domain, containing amino acids 1–81, onto streptavidin biosensors and measured the binding affinity of EG1 to the immobilized Pax2 paired domain (Figure 3A). Calculated Kon and Koff rates gave a binding affinity (Kd) of 1.35 µM, whereas steady state analysis of the binding curves calculated a Kd of 1.5 µM for this interaction (Figure 3B).
Figure 3.
EG1 directly interacts with Pax2 and inhibits Pax2-DNA binding. (A) Binding affinity and kinetic data of EG1 interacting with the Pax2 paired domain by biolayer interferometry. Pax2 protein was immobilized on streptavidin sensors, and EG1 was tested at concentrations of 0.5, 1.0, 2.0, 4.0, and 8.0 µM. The binding curves were fitted with global fitting models using instrument software. (B) Steady state analysis calculates a Kd of 1.5 µM, consistent with Kon and Koff rates determined in A. (C) EMSA using isotope labeled PRS oligos incubated with recombinant Pax2 paired domain and DMSO or increasing concentrations of EG1 shows that EG1 disrupts the Pax2–DNA interaction.
To see if the EG1-Pax2 interaction had an effect on the ability of Pax2 to bind to DNA, we set up an electrophoretic mobility shift assay (EMSA, Figure 3C). For this assay, a double stranded PRS oligo was end labeled with [32P]-dATP and mixed with the full length recombinant Pax2 paired domain, containing amino acids 1–170, that had been incubated with vehicle or increasing concentrations of EG1. After incubating at RT, samples were loaded and run on native polyacrylamide gels to visualize the Pax2–DNA complex. Significant inhibition of Pax2-DNA binding was observed at 10 µM with near complete inhibition of binding at 100 µM of EG1. These data demonstrate specific binding of EG1 to the DNA binding pocket of the Pax2 paired domain that can prevent Pax2-DNA interactions in vitro.
EG1 Inhibits Kidney Development and Pax2 Target Gene Expression
Pax2 activity is essential for kidney development, which encompasses morphogenetic processes such as epithelial branching and conversion of metanephric mesenchyme cells to epithelia. Loss of Pax2 blocks branching morphogenesis of the ureteric bud, prevents the aggregation of mesenchyme cells at the bud tips, and completely inhibits the conversion of mesenchyme to epithelia. If EG1 is a true Pax2 inhibitor, then we would expect EG1 treatment to recapitulate these findings. To determine if this was the case, we used a well-described organ culture model system. Kidney rudiments were microdissected at embryonic day 11.5 (E11.5) and grown on transwell filters in the presence of vehicle or increasing concentrations of EG1 for 48 h (Figure 4). As a control, we also cultured lung rudiments from E11.5 embryos. Lung buds also undergo branching morphogenesis but do not express Pax2 or related Pax proteins and should be resistant to the effects of EG1. After 48 h in culture, kidney rudiments were stained with antibodies against Pax2 to visualize mesenchyme and epithelial cells and with anticytokeratin, which stains only the branching ureteric tree (Figure 4A). Branch points were counted in both kidney and lung cultures treated with increasing amounts of EG1 (Figure 4B). Branching morphogenesis was significantly inhibited by EG1 in the kidney rudiments but not in the lung buds, with up to 75% inhibition at the highest dose. Furthermore, Pax2 positive cells failed to aggregate completely at the ureteric bud tips and were found dispersed throughout the kidney rudiment. Thus, EG1 did not affect the amounts of Pax2 protein expression but did prevent aspects of renal development consistent with a loss of Pax2 function.
Figure 4.
EG1 treatment disrupts nephrogenesis but not lung development. (A) E11.5 kidney rudiments treated with increasing concentrations of EG1 show a reduction in branching morphogenesis and metanephric mesenchyme condensation around ureteric bud tips consistent with reduction in Pax2 activity. Examples of the ureteric bud tips are indicated by the arrows. Note the Pax2 positive cells (green) condense around the ureteric bud tips (arrowheads) in control cultures but fail to condense and remain diffuse at the highest concentrations of EG1 (arrowheads). E11.5 lung rudiments, which branch independently of Pax2 activity, treated with increasing concentrations of EG1, show no change in branching morphogenesis. (B) Quantification of branch tips in embryonic kidney or lung rudiments cultured in the presence of EG1 show significant changes in kidney (n = 37, 24, 25, and 38, respectively) but not lung (n = 10, 7, 7, and 12, respectively). *P < 0.001, **P < 0.0001.
Recently, we described genes regulated by Pax2 in renal progenitor cells isolated from embryonic kidneys.38 Numerous kidney developmental regulators were found to be down-regulated following a loss of Pax2 while a number of genes associated with the interstitial stroma and paraxial mesoderm were found to be upregulated. After 48 h, Pax2 target gene expression was analyzed from kidney organ cultures using either whole mount in situ hybridization or qRT-PCR (Figure 5). Whole mount in situ hybridization for Cited1, a direct target of Pax2, reveals a dramatic reduction in expression levels in the Pax2 positive renal progenitor cells (Figure 5A). Although expression levels are severely reduced, residual expression still marks the appropriate cell types aggregating at the tips of the buds, suggesting that we are not just killing the progenitor cells. By qRT-PCR, we also see a clear reduction of Cited1 and HNF4a expression, both of which are in renal progenitor cells, but less so for Bmp7, which is expressed in both stroma and epithelial progenitor cells (Figure 5B). In Pax2 mutant cells isolated directly from embryos, we had described an increase in the expression of stromal markers. Thus, we examined several stromal genes in the EG1 treated culture and found increased levels of FoxD1, Msx1, and Twist2, again consistent with a loss of Pax2 activity (Figure 5C). These data indicate a specific effect of EG1 on kidney organ cultures that are entirely consistent with a loss of Pax2 activity.
Figure 5.
EG1 treatment leads to gene expression changes consistent with reduction in Pax2 activity in an ex vivo organ culture model. (A) Whole mount in situ hybridization for Cited1 shows that EG1 inhibits expression of a Pax2 target gene expressed in renal epithelial progenitor cells (arrows). Note a loss of strong Cited1 expression in EG1 treated cultures at the ureteric bud tips. (B) Quantitative RT-PCR for mRNAs of critical regulators of nephrogenesis shows reduced expression of these genes following EG1 treatment. (C) Quantitative RT-PCR for interstitial stroma and paraxial mesodermal mRNAs show increased expression following EG1 treatment.
EG1 Decreases Viability in Pax2 Positive Renal Cancer Cell Lines
Suppression of Pax2 expression in human renal cancer cell lines can suppress proliferation and sensitize cells to the effects of cisplatin.22,26,27 Thus, we examined whether EG1 treatment can impact the growth and survival of Pax2 positive cancer cell lines (Figure 6). We used three cell lines that were Pax2 positive, a renal cell carcinoma line (RCC111) and two ovarian carcinoma cell lines (TOV112D and ES-2). To test for specificity, we also examined three cancer cell lines that were Pax2 negative by Western blotting, including two prostate cancer cell lines (22Rv1 and PC-3) and one ovarian cancer cell line (SK-OV-3, Figure 6A). Cell viability was measured after 48 h of culture with EG1 at 12.5 and 25 µM (Figure 6B). All Pax2 positive cells exhibited a significant decrease in cell viability, whereas none of the Pax2 negative cells were affected. We also measured the levels of phospho–histone H3 (P–H3) in response to EG1, as a measure of cell proliferation and/or cell cycle arrest (Figure 6C). As with the viability assay, all Pax2 positive cells showed decreased levels of P–H3 with increasing amounts of EG1, whereas the Pax2 negative cells were unchanged. The renal carcinoma cell line RCC111, which had the highest level of Pax2 protein expression, was the least sensitive to EG1, whereas the TOV-112D and ES-2 cells showed a more dramatic decease in viability and P–H3 levels. These data are consistent with a role for Pax2 in regulating cell viability and proliferation in Pax2 positive cancer cells of renal and ovarian origin and suggest that Pax2 is a viable target for anticancer therapy.
Figure 6.
Effects of EG1 on Pax2 positive cancer cell lines. (A) A Western blot shows the characteristic Pax2 doublet at 46–48 kDa in the cancer cell lines RCC111, TOV112D, and ES-2 but not in the prostate cell lines 22Rv1 and PC3 or the ovarian cancer cell line SK-OV-3. Nonspecific band at 30 kDa and equivalent levels of total histone H3 are loading controls. (B) Cell viability assays with increasing amounts of EG1. Statistically significant differences in cell viability are indicated (*p < 0.05). Pax2 positive cells are in the left panel and Pax2 negative cells in the right panel. (C) Western blots of P–H3 from cells cultured with increasing amounts of EG1. Quantified P–H3 levels, normalized to total H3, are shown below the individual. Pax2 positive cells are on the left, and Pax2 negative cells are in the right panel. Note no significant effects of EG1 on P–H3 in Pax2 negative cell lines.
In this study, we used the crystal structure of Pax510 to build a homology model of the Pax2 protein and used it for applying structure based virtual screening to identify small molecules with the potential to interact with the amino-terminal subdomain of the Pax2 paired domain. A total of 227 top scoring molecules with reasonable docking poses were identified, of which, 225 were available in the CCG library and tested for their ability to specifically inhibit Pax2 mediated transactivation of a reporter gene. After this primary screen, 31 compounds were confirmed hits and retested with fresh purchased stocks. Following in vitro activity, dose response, and counter screening assays, five specific inhibitors remained. Structural analyses of the five specific hits identified two clusters and one singleton compound. To further characterize structure–activity relationships, we ordered analogs and tested these in the primary and counter screen assays. This led to the discovery of a more potent inhibitor EG1, which was selected for further characterization. As predicted by our homology modeling approach, EG1 was found to directly bind to the N-terminal subdomain of the Pax2 paired domain with a Kd of 1.35–1.5 µM. Moreover, this interaction abolishes the DNA binding ability of Pax2. Of note, EG1 was initially labeled active in 8 out of 541 other bioassays listed on PubChem, although none of the eight screens considered EG1 a potential lead compound due to low activity or poor dose response.
Loss of Pax2 activity during kidney development inhibits branching morphogenesis of the ureteric bud epithelium and prevents conversion of the metanephric mesenchyme to renal tubules.18,39,40 EG1 reduces both of these Pax2 dependent biological processes in a kidney organ culture model yet has no effect on the branching of lung epithelia under similar conditions. Of note, lung buds do not express any Pax proteins in epithelia or in mesenchyme. Upon outgrowth of the ureteric bud and its invasion into the metanephric mesenchyme, Pax2 positive mesenchymal cells aggregate around the tips of the bud and receive WNT signals that initiate their conversion to epithelial cells.41 As in Pax2 genetic mutants, EG1 prevents the aggregation of Pax2 positive cells at these bud tips. This is the strongest evidence that EG1 is a specific Pax2 inhibitor that phenocopies a genetic loss of function. Furthermore, we observed EG1 dependent changes in gene expression patterns in the kidney organ culture system that were similar to genetic loss of Pax2 function, including the loss of Cited1,42 a marker of renal progenitor cells, and an increase in stromal cell marker genes FoxD143 and Msx1.
High levels of Pax2 are observed in aberrantly proliferating renal epithelial cells, such as in Wilms’ tumor,20,23 juvenile cystic and dysplastic kidneys,44 renal cell carcinoma,22,45,46 and polycystic kidney disease.24,25 In such abnormal epithelia, Pax2 has been implicated as a pro-survival factor that triggers continued proliferation and grants tumor cells resistance to apoptosis. Consistent with this idea, a reduction in Pax2 gene dosage was found to decrease cyst formation and slow the progression of disease in mouse models of PKD.24,25 Furthermore, targeting Pax2 with short interfering RNA in renal carcinoma cells reduces proliferation, increases apoptosis, and sensitizes cancer cells to chemotherapeutic agents.26,27 Similarly, we observe a reduction in cell proliferation and cell viability in Pax2 positive cancer cell lines when cultured with EG1, but not in Pax2 negative lines.
Currently, effective therapeutic options for renal cell carcinoma (RCC) are few. Globally, kidney cancer, of which 90% is RCC, accounts for more than 270 000 new cases and over 116 000 deaths annually.47 Until the year 2000, the recommended treatment was immunotherapy using interferon alpha and interleukin-2 (IL-2), with high-dose IL-2 being the only approved method of treatment for patients with metastatic disease.48 The discovery that Von Hippel–Lindau tumor suppressor inactivation is observed in the majority of clear cell renal carcinomas,49 which make up 70–75% of all RCCs,50 leading to up-regulation of vascular endothelial growth factor (VEGF) and other angiogenic factors revolutionized the field. Now, VEGF inhibitors including sunitinib, pazopanib, or bevacizumab are first line therapy.51 Even though the benefits of targeting VEGF are significant, response rates are low, resistance is frequently developed, and long-term survival rates have not increased dramatically. Clearly there is a need for better drugs to combat renal diseases characterized by proliferation of epithelial cells. Because of its tissue and cell type specific expression, molecular targeting of Pax2 may provide an avenue of therapy in which systemic side effects typically seen with conventional chemotherapeutic agents that target all dividing cells are minimized.
The high-degree of homology within the paired domain could allow EG1 to target other members of the Pax family and expand the range of affected tissues. In particular, the other members of group II, Pax5 and Pax8, have the highest degree of homology and are also inhibited by EG1. Pax5 is expressed in B-cell precursors,4 whereas Pax8 is expressed in the thyroid and partially overlaps with Pax2 expression in the urogenital tract.52 Although inhibiting these proteins in the process of treating renal disease could prove deleterious, there are instances when targeting them may be beneficial. For instance, Pax5 overexpression is seen in a variety of hematological malignancies including follicular lymphoma and a subset of non-Hodgkin’s lymphomas.53 Additionally, Pax8 mis-expression is observed in T-cell lymphomas54 and numerous urogenital tumors.55 Of note, a common feature among Pax gene family members is their sensitivity to haploinsufficiency. Thus, it may not be necessary to completely inhibit Pax activity in order to show a therapeutic effect.
Because a computational screen requires a good molecular model of protein structure, we focused on the DNA binding domain of Pax2. However, Pax2 mediated transcriptional activation requires interactions with the adapter protein PTIP, the recruitment of epigenetic complexes, and subsequent chromatin remodeling.37,56 Phosphorylation of Pax2 transactivation domain by c-Jun N-terminal kinase enhances the activation,33,57 which results in recruitment of the mixed lineage leukemia (MLL) histone methyl transferase complex and an increase in histone H3K4 di- and trimethylation. However, PTIP can be displaced by the corepressor Grg4/Tle4, which recruits a complex of proteins, including PPM1B, PRMT5, and proteins of the Polycomb repressor 2 complex. This results in dephosphorylation of Pax2 and histone H4R3 and H3K27 methylation to repress gene expression.36,58 Thus, the complexity involved in the biochemical regulation of Pax2 provides many additional opportunities for targeting its activity. The major benefits to the computational approach presented here are (1) time and monetary savings due to the limited number of small molecules ordered and tested, (2) a clearly defined target making identifying the mechanism of action much simpler, and (3) increased tissue specificity by going directly after Pax2 rather than ubiquitously expressed proteins such as PTIP. Nevertheless, additional screening methods for unbiased Pax inhibitors could provide a diversity of compounds that act at the level of protein–protein interactions and could, in combination, enhance the activity of EG1 further.
In summary, this report demonstrates a viable strategy for developing new compounds that can inhibit DNA binding transcription factors, which control cellular fate and are mis-expressed in disease. The EG1 lead compound can serve as a backbone for developing new and better inhibitors of Pax proteins with similar DNA binding domains. Such compounds could prove effective in combating kidney disease for which specific therapies are still limited.
MATERIALS AND METHODS
Virtual Screening
The three-dimensional (3D) structure of the Pax2 has not been determined yet; thus we used homology modeling to model the 3D structure of the target molecule. There are two related structures reported, the Pax5/Ets-1 in complex with DNA (PDB ID: 1K78) and Pax6 in complex with DNA (PDB ID: 6PAX). Amino acid identity throughout the paired DNA binding domain is 97% between Pax2 and Pax5 and 76% between Pax2 and Pax6. On the basis of the high degree of identity and the higher resolution of the Pax5 structure bound to DNA in complex with the ETS1 protein (2.25 Å), Pax5 (PDB ID 1K78, chain A) was used as the template for the structural homology model of Pax2. The initial models were generated employing Prime 3.0 of Schrödinger. The homology modeled 3D structure of the Pax2 paired domain underwent 10 ns of molecular dynamics using Amber 11. The produced trajectories were clustered using the MMTSB toolset. In each cluster, a structure with the minimum energy was chosen for virtual screening.
Virtual screening was performed against the compound library (151 634 compounds) available at the Center for Chemical Genomics (CCG) at the University of Michigan. The library was filtered in order to avoid possible problematic compounds (known as PAINS) and use drug-like compounds for the virtual screening by applying multiple criteria including molecular weight in the range of 300 to 670 Da, calculated logP in the range 2 to 7, aromatic rings in the range 0 to 5, number of hydrogen bond donors from 0 to 6, number of hydrogen bond acceptors from 2 to 12, and others. A total of 97 378 molecules were selected and taken for structure-based virtual screening (molecular docking approach) using the Glide docking program. Cys63, which is involved in the binding of DNA, was defined as the center of the active site. A cubic box (the active site) with a length of side of 10 Å represented the volume of the protein for which grids were calculated using Glide 5.5. The produced grids were used for docking studies. Glide 5.5 standard-precision (SP) mode and extra-precision (XP) of Schrödinger were used for the virtual screening. The top 1000 candidate small molecules with the best scores obtained by Glide SP were rescreened using the XP mode. After the reranking, the top 227 scored compounds with reasonable docking poses were considered as potential small molecule inhibitors of Pax2. Of these, 225 were available for screening in a cell-based assay for the inhibition of Pax2 mediated transactivation.
Cell Culture
HEK293 cells containing PRS-EGFP or PRS-Luc reporter, described previously,36 were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) supplemented with 10% FCS (Sigma), 100 U/mL penicillin, 100 mg mL−1 streptomycin (Gibco), and 400 ng/mL genetecin (Gibco) and were maintained under humidified 5% CO2/95% air at 37 °C.
Transfection
HEK293 cells were transiently transfected with CMV-Pax2b, CMV-Pax2bC63Y, CMV-Pax5, CMV-Pax8, or CMV-Luciferase expression vector. Briefly, 3 × 106 cells were plated in a 100 mm tissue culture dish (Corning) and incubated overnight. The next morning, the media were replaced with 5 mL of antibiotic free DMEM, and transfection reactions were prepared. In a 2 mL Eppendorf tube containing 896 µL of Opti-MEM I (Gibco) was added 4 µL of 1 µg/µL plasmid. In a second 2 mL Eppendorf tube containing 886 µL of Opti-MEM I was aded 14 µL of Lipofectamine2000 (Life Technologies) and was allowed to incubate at RT for 5 min. The contents of the two tubes were mixed by pipetting and allowed to incubate at RT for 20 min. The transfection mix was added dropwise to a 100 mm dish of cells. The plate was shaken gently and placed in the incubator for 6 h. The plate of transfected cells was trypsinized with 0.05% trypsin (Gibco) for 3 min. The cells were harvested, counted, plated in six-well culture dishes (500 000 cells/well for Western blot analysis) or 96-well dishes (30 000 cells/well for luminescence assays) containing DMSO or increasing concentrations of compound, and placed in the incubator overnight.
Luciferase Reporter Assays
For luciferase reporter assays, test plates were removed from the incubator and equilibrated to RT for 10 min before the media were reduced and an equal volume of Steady-Glo (Promega) was added. The plates were incubated at RT for another 10 min and were read on a PHERAstar multimode plate reader (BMG).
Western Blot Analysis
For Western blot analysis, the media were removed from test plates, and cells were lysed in 2× SDS buffer (20% glycerol, 4% sodium dodecyl sulfate, 0.2 M dithiothreitol, 125 mM Tris, pH 6.8), as described.59 Total protein was separated on 4–12% Bis-Tris gels (Life Technologies), transferred to PVDF membranes, and immunoblotted with anti-Pax2 antibody,20 anti-EGFP antibody (Santa Cruz), antiactin (Cell Signaling Tech.), anti-P-Histone H3 (Cell Signaling Tech.), anti-Histone H3 (Cell Signaling Tech.), or anti-Actin (Sigma).
Electrophoretic Mobility Shift Assays
PRS4 probe was obtained by generating a double stranded oligo, end labeling the double stranded oligo with [32P]dATP using the polynucleotide kinase reaction, and purifying the product with a mini Quick Spin DNA column (Roche). The following oligos were used to generate the PRS4 probe: TCGAGATATCTAGAGCGGAAGGTGAGCCCAGTGA and TCACTGGGCTCACCGTTCCGCTCTAGATATCTCGA. Binding reactions were performed in 0.5 × Z-Buffer (10% glycerol, 12.5 mM HEPES at pH 7.8, 6.25 mM MgCl2, 0.5 mM DTT, 0.05 M KCl, 0.05% NP4) and contained 3 pg of recombinant Pax2 Paired domain, 100 ng of poly(dI-dC), 32P-labeled probe (10 000 c.p.m.), and DMSO or increasing concentrations of EG1. Binding reactions were carried out at RT for 20 min. Samples were resolved at RT on 6% native polyacrylamide gels in 0.5 × TBE at 120 V.
Affinity Binding Kinetics
The Pax2 paired domain protein containing the first DNA binding pocket defined in our homology model (aa 1–81) was expressed as a His tagged protein in E. coli and purified by Ni affinity. After biotinylation, the protein was bound to saturation to the biosensor of an Octet RED Multichannel platform (ForteBio). All binding and equilibration was done in PBS with 0.1% DMSO. For binding to the compound, sensors were incubated for 300 s in EG1 at the following concentrations: 0.5, 1, 2, 4, 8, 16, 31, 62, 125, and 250 µM. Disassociation was then measured for 350 s in PBS to determine affinity constants.
Organ Cultures
Mice were kept according to National Institutes of Health guidelines. All procedures were approved by the University Committee on Use and Care of Animals at the University of Michigan. Wild type FVB mice aged 8–12 weeks were used in this study (Jackson Lab). Kidney and lung rudiments were microdissected at embryonic day 11.5 (E11.5) and were cultured on 0.4 µm Transwell filter inserts (Costar) in Dulbecco’s Modified Eagle Medium (Gibco) supplemented with 10% FCS (Sigma), 100 U/mL penicillin, 100 mg mL−1 streptomycin (Gibco), and DMSO (Sigma) or increasing concentrations of EG1 under humidified 5% CO2/95% air at 37 °C for 2 days.
In Situ Hybridization
In situ hybridization was performed on 2 days ex vivo cultured embryonic kidney rudiments as described previously.38 Briefly, the samples were fixed in 4% PFA in PBS for 1 h at 4 °C, then washed three times in PBS+0.1% Tween-20 (PBT) for 10 min at RT and dehydrated through a PBT/methanol series. Templates for digoxigenin (DIG)-labeled riboprobes were generated by PCR amplification of E11.5–E15.5 mouse embryo cDNAs and sequenced. The following primer pair was used for riboprobe template as described:38
Cited1-ATGCCAACCAGGAGATGAAC, CGATGTTAATACGACTCACTATAGGGCAACAGAATCGGTGGCTTTT
RNA Reverse Transcription and Real-Time PCR
Total RNA was extracted from 2 day ex vivo cultured kidney rudiments using TRIzol reagent (Life Technologies) and an RNeasy Mini Kit (Qiagen). Following extraction, 200 ng of total RNA was reverse transcribed into cDNA using SuperScript Vilo Reverse Transcriptase (Life Technologies). cDNA templates were amplified with iTaq Universal SYBR Green Supermix (Bio-Rad) in a Mx3005P Real-Time PCR System (Stratagene). The following primer pairs which were described previously38 were used in this study:
Bmp7: CAGCCAGAATCGCTCCAAGA, GCAATGATCCAGTCCTGCCA
Cited1: CTCTGGGAAGGAGGATGCC, CCAGAGGAGCTAGTGGGAAC
Foxd1: TTCGGATTCTTGGACCAGAC, CAAGTCAGGGTTGCAGCATA
Hnf4a: TACTCCTGCAGGTTTAGCCG, CAGCCCGGAAGCACTTCTTA
Hprt: GTTGGGCTTACCTCACTGCT, TCATCGCTAATCACGACGCT
Msx1: GCCCCGAGAAACTAGATCGG, GGACTCAGCCGTCTGGC
Twist2: GTCTCAGCTACGCCTTCTCC, CAGGTGGGTCCTGGCTTG
Immunofluorescence
Immunofluorescence was performed on 2 days ex vivo cultured embryonic kidney and lung rudiments as described previously.60 Briefly, the samples were fixed in ice cold methanol for 20 min, then washed in PBS+0.1% Tween-20 (PBT), immunolabeled with anti-Pax2 (1:400), and anti-Cytokeratin (1:400) (Sigma) antibodies overnight at 4 °C in PBT containing 5% goat serum (Sigma); washed with PBT; incubated with Alexa Fluor 488 and Alexa Fluor 594 secondary antibodies (1:500) (Life Technologies) overnight at 4 °C; washed with PBT; and mounted on microscope slides. Images were taken at 10× and 20× magnification.
Viability and Apoptosis Assays
Cancer cell lines were cultured in T75 flasks (Greiner) containing RPMI-1640 (Gibco) supplemented with 10% FCS (Sigma), 100 U/mL penicillin, and 100 mg mL−1 streptomycin (Gibco). Cancer cell lines were maintained under humidified 5% CO2/95% air at 37 °C. Cancer cell lines were trypsinized with 0.25% trypsin, plated in 12 well culture dishes (Corning) or 96 well culture dishes (Greiner) at 50 000 cells/well or 500 cells/well, respectively, and were incubated overnight. DMSO or increasing concentrations of EG1 and vehicle were added to the wells, and the plates were once again incubated overnight. Cells from the 12 well plates were lysed in 2 × SDS lysis buffer and used for Western blotting. Ninety-six well plates were removed from the incubator and allowed to equilibrate to RT for 10 min before CellTiter-Glo (Promega) was added, and the luminescence was read on the PHERAstar multimode plate reader.
Acknowledgments
We are especially thankful to M. Larsen and the University of Michigan Center for Chemical Genomics for help with the automated screening, S. Larsen for help in selecting analogs, K. Cho for the ovarian cancer cells, A. Chinnaiyan for the prostate cancer cell lines, and V. Groppi and the Center for the Discovery of New Medicines for advice and funding. This work was funded in part by the Proteome Informatics of Cancer Training Program CA140044 to E.G. and National Institutes of Health grant DK054740 to G.R.D.
Footnotes
The authors declare no competing financial interest.
References
- 1.Bopp D, Burri M, Baumgartner S, Frigerio G, Noll M. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes in Drosophila. Cell. 1986;47:1033–1040. doi: 10.1016/0092-8674(86)90818-4. [DOI] [PubMed] [Google Scholar]
- 2.Noll M. Evolution and role of Pax genes. Curr. Opin. Genet. Dev. 1993;3:595–605. doi: 10.1016/0959-437x(93)90095-7. [DOI] [PubMed] [Google Scholar]
- 3.Walther C, Guenet JL, Simon D, Deutsch U, Jostes B, Goulding MD, Plachov D, Balling R, Gruss P. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- 4.Urbanek P, Wang Z-Q, Fetka I, Wagner EF, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994;79:901–912. doi: 10.1016/0092-8674(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 5.Ton CCT, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, Royer-Pokora B, Collins F, Swaroop A, Strong LC, Saunders GF. Positional cloning and characterization of a paired box and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 6.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 7.Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001;20:5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- 8.Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the Pax3 paired box gene in the paediatric solid tumour alvelar rhabdomyosarcoma. Nat. Genet. 1995;3:113–117. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- 9.Xu W, Rould MA, Jun S, Desplan C, Pabo CO. Crystal structure of a paired domain-DNA complex at 2.5 A resolution reveals structural basis for Pax developmental mutations. Cell. 1995;80:639–650. doi: 10.1016/0092-8674(95)90518-9. [DOI] [PubMed] [Google Scholar]
- 10.Garvie CW, Hagman J, Wolberger C. Structural studies of Ets-1/Pax5 complex formation on DNA. Mol. Cell. 2001;8:1267–1276. doi: 10.1016/s1097-2765(01)00410-5. [DOI] [PubMed] [Google Scholar]
- 11.Xu HE, Rould MA, Xu W, Epstein JA, Maas RL, Pabo CO. Crystal structure of the human Pax6 paired domain-DNA complex reveals specific roles for the linker region and carboxy-terminal subdomain in DNA binding. Genes Dev. 1999;13:1263–1275. doi: 10.1101/gad.13.10.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein JA, Glaser T, Cai J, Jepeal L, Walton DS, Maas RL. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes Dev. 1994;8:2022–2034. doi: 10.1101/gad.8.17.2022. [DOI] [PubMed] [Google Scholar]
- 13.Pellizzari L, Tell G, Damante G. Co-operation between the PAI and RED subdomains of Pax-8 in the interaction with the thyroglobulin promoter. Biochem. J. 1999;337(Pt 2):253–262. [PMC free article] [PubMed] [Google Scholar]
- 14.Dressler GR. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 15.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–2970. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, Schughart K. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- 20.Dressler GR, Douglass EC. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc. Natl. Acad. Sci. U. S. A. 1992;89:1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan G, Steele-Perkins V, Morris J, Rauscher FJ, III, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development. 1995;121:867–875. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- 22.Gnarra JR, Dressler GR. Expression of Pax-2 in human renal cell carcinoma and growth inhibition by antisense oligonucleotides. Cancer Res. 1995;55:4092–4098. [PubMed] [Google Scholar]
- 23.Eccles MR, Wallis LJ, Fidler AE, Spur NK, Goodfellow PJ, Reeve AE. Expression of the Pax2 gene in human fetal kidney and Wilms’ tumor. Cell Growth and Differ. 1992;3:279–289. [PubMed] [Google Scholar]
- 24.Ostrom L, Tang MJ, Gruss P, Dressler GR. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev. Biol. 2000;219:250–258. doi: 10.1006/dbio.2000.9618. [DOI] [PubMed] [Google Scholar]
- 25.Stayner C, Iglesias DM, Goodyer PR, Ellis L, Germino G, Zhou J, Eccles MR. Pax2 gene dosage influences cystogenesis in autosomal dominant polycystic kidney disease. Hum. Mol. Genet. 2006;15:3520–3528. doi: 10.1093/hmg/ddl428. [DOI] [PubMed] [Google Scholar]
- 26.Hueber PA, Iglesias D, Chu LL, Eccles M, Goodyer P. In vivo validation of PAX2 as a target for renal cancer therapy. Cancer Lett. 2008;265:148–155. doi: 10.1016/j.canlet.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Hueber PA, Waters P, Clarke P, Eccles M, Goodyer P. PAX2 inactivation enhances cisplatin-induced apoptosis in renal carcinoma cells. Kidney Int. 2006;69:1139–1145. doi: 10.1038/sj.ki.5000136. [DOI] [PubMed] [Google Scholar]
- 28.Rishi V, Potter T, Laudeman J, Reinhart R, Silvers T, Selby M, Stevenson T, Krosky P, Stephen AG, Acharya A, Moll J, Oh WJ, Scudiero D, Shoemaker RH, Vinson C. A high-throughput fluorescence-anisotropy screen that identifies small molecule inhibitors of the DNA binding of B-ZIP transcription factors. Anal. Biochem. 2005;340:259–271. doi: 10.1016/j.ab.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Berg T, Cohen SB, Desharnais J, Sonderegger C, Maslyar DJ, Goldberg J, Boger DL, Vogt PK. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3830–3835. doi: 10.1073/pnas.062036999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo H, Henriksson M. Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6344–6349. doi: 10.1073/pnas.0601418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Dong Z, Chen Y, Wang F, Wang CJ, Peng H, He Y, Hangoc G, Pollok K, Sandusky G, Fu XY, Broxmeyer HE, Zhang ZY, Liu JY, Zhang JT. Small-molecule inhibitors targeting the DNA-binding domain of STAT3 suppress tumor growth, metastasis and STAT3 target gene expression in vivo. Oncogene. 2016;35:783–792. doi: 10.1038/onc.2015.215. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Dong Z, Wang F, Peng H, Zhang JT, Liu J-Y. A small molecule compound targeting STAT3 DNA binding domain inhibits cancer cell proliferation, migration, and invasion. ACS Chem. Biol. 2014;9:1188–1196. doi: 10.1021/cb500071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Y, Brophy PD, Levitan I, Stifani S, Dressler GR. Groucho suppresses Pax2 transactivation by inhibition of JNK-mediated phosphorylation. EMBO J. 2003;22:5522–5529. doi: 10.1093/emboj/cdg536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Moriniere V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L. Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum. Mutat. 2012;33:457–466. doi: 10.1002/humu.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 36.Patel SR, Bhumbra SS, Paknikar RS, Dressler GR. Epigenetic mechanisms of Groucho/Grg/TLE mediated transcriptional repression. Mol. Cell. 2012;45:185–195. doi: 10.1016/j.molcel.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SR, Kim D, Levitan I, Dressler GR. The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev. Cell. 2007;13:580–592. doi: 10.1016/j.devcel.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranghini EJ, Dressler GR. Evidence for intermediate mesoderm and kidney progenitor cell specification by Pax2 and PTIP dependent mechanisms. Dev. Biol. 2015;399:296–305. doi: 10.1016/j.ydbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- 40.Soofi A, Levitan I, Dressler GR. Two novel EGFP insertion alleles reveal unique aspects of Pax2 function in embryonic and adult kidneys. Dev. Biol. 2012;365:241–250. doi: 10.1016/j.ydbio.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev. Biol. 2008;313:234–245. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- 44.Winyard PJD, Risdon RA, Sams VR, Dressler GR, Woolf AS. The PAX2 transcription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J. Clin. Invest. 1996;98:451–459. doi: 10.1172/JCI118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong GX, Melamed J, Mansukhani M, Memeo L, Hernandez O, Deng FM, Chiriboga L, Waisman J. PAX2: a reliable marker for nephrogenic adenoma. Mod. Pathol. 2006;19:356–363. doi: 10.1038/modpathol.3800535. [DOI] [PubMed] [Google Scholar]
- 46.Tong GX, Memeo L, Colarossi C, Hamele-Bena D, Magi-Galluzzi C, Zhou M, Lagana SM, Harik L, Oliver-Krasinski JM, Mansukhani M, Falcone L, Hibshoosh H, O’Toole K. PAX8 and PAX2 immunostaining facilitates the diagnosis of primary epithelial neoplasms of the male genital tract. Am. J. Surg. Pathol. 2011;35:1473–1483. doi: 10.1097/PAS.0b013e318227e2ee. [DOI] [PubMed] [Google Scholar]
- 47.Ljungberg B, Campbell SC, Cho HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur. Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 48.Molina AM, Nanus DM. Recent advances in the management of renal cell carcinoma. F1000Research. 2016;5:391. doi: 10.12688/f1000research.7935.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh F-M, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 50.Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, Martignoni G, Rini BI, Kutikov A. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur. Urol. 2015;67:85–97. doi: 10.1016/j.eururo.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 51.Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015;67:913–924. doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- 53.Krenacs L, Himmelmann AW, Quintanilla-Martinez L, Fest T, Riva A, Wellmann A, Bagdi E, Kehrl JH, Jaffe ES, Raffeld M. Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B cells and in subsets of B-cell lymphomas. Blood. 1998;92:1308–1316. [PubMed] [Google Scholar]
- 54.Morgan EA, Pozdnyakova O, Nascimento AF, Hirsch MS. PAX8 and PAX5 are differentially expressed in B-cell and T-cell lymphomas. Histopathology. 2013;62:406–413. doi: 10.1111/his.12020. [DOI] [PubMed] [Google Scholar]
- 55.Ozcan A, de la Roza G, Ro JY, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch. Pathol. Lab. Med. 2012;136:1541–1551. doi: 10.5858/arpa.2012-0072-OA. [DOI] [PubMed] [Google Scholar]
- 56.Patel SR, Ranghini E, Dressler GR. Mechanisms of gene activation and repression by Pax proteins in the developing kidney. Pediatr. Nephrol. 2014;29:589–595. doi: 10.1007/s00467-013-2603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Y, Lechner MS, Nihalani D, Prindle MJ, Holzman LB, Dressler GR. Phosphorylation of Pax2 by the c-Jun N-terminal kinase and enhanced Pax2-dependent transcription activation. J. Biol. Chem. 2002;277:1217–1222. doi: 10.1074/jbc.M109663200. [DOI] [PubMed] [Google Scholar]
- 58.Abraham S, Paknikar R, Bhumbra S, Luan D, Garg R, Dressler GR, Patel SR. The Groucho-associated phosphatase PPM1B displaces Pax transactivation domain interacting protein (PTIP) to switch the transcription factor Pax2 from a transcriptional activator to a repressor. J. Biol. Chem. 2015;290:7185–7194. doi: 10.1074/jbc.M114.607424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang P, Cai Y, Soofi A, Dressler GR. Activation of Wnt11 by Transforming Growth Factor-beta Drives Mesenchymal Gene Expression through Non-canonical Wnt Protein Signaling in Renal Epithelial Cells. J. Biol. Chem. 2012;287:21290–21302. doi: 10.1074/jbc.M112.357202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125:4806–4815. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]









