Abstract
Background
The burden of influenza infections in patients with hematological malignancies (HMs) is not well defined.
Objective
To describe the associated outcomes at two comprehensive cancer centers (center 1 in the United States and center 2 in Mexico).
Patients/Methods
Clinical and laboratory data on patients with HMs and influenza infection diagnosed from April 2009 to May 2014 at the two centers were reviewed retrospectively.
Results
In our cohort of 190 patients, the majority were male (63%) with a median age of 49 years (range, 1–88 years), and had active or refractory HMs (76%). Compared to center 1, patients in center 2 were significantly sicker (active cancer, decreased albumin levels, elevated creatinine levels, or hypoxia at influenza diagnosis) and experienced higher lower respiratory tract infection (LRI) rate (42% vs. 7%; P<0.001). In multivariable logistic regression analysis (odds ratio, 95% confidence interval), leukemia, (3.09, 1.23–7.70), decreased albumin level (3.78, 1.55–9.20), hypoxia at diagnosis (14.98, 3.30–67.90), respiratory co-infection (5.87, 1.65–20.86), and corticosteroid use (2.71, 1.03–7.15) were significantly associated with LRI; and elevated creatinine level (3.33, 1.05–10.56), hypoxia at diagnosis (5.87, 1.12–30.77), and respiratory co-infection (6.30, 1.55–25.67) were significantly associated with 60 day mortality in both centers.
Conclusions
HM patients with influenza are at high risk for serious complications such as LRI and death, especially if they are immunosuppressed. Patients with respiratory symptoms should seek prompt medical care during influenza season.
Keywords: Influenza, H1N1, pneumonia, leukemia, lymphoma, myeloma, cancer
Background
Influenza vaccine uptake and circulating virus strain play a major role in determining outcomes of influenza infection in the general population. However, in cancer patients, such as those with hematological malignancies (HMs) and recipients of hematopoietic cell transplants (HCTs), many additional factors determine the severity of influenza infection, such as the underlying malignancy, immunosuppression, delay in presentation, and timing of antiviral therapy.1–4 Influenza infections are more likely to progress from upper respiratory tract infections (URIs) to lower respiratory tract infections (LRIs) with subsequent bacterial co-infections in these patients than in the general population.5–8 Also, immunocompromised patients experience persistent viral shedding, need hospitalization and mechanical ventilation, and have high mortality rates following acquisition of influenza infections.9–12 Researchers have shown that delay from onset of influenza symptoms to seeking medical care and initiation of antiviral therapy is correlated with poor outcomes, mainly in HCT recipients.2–4
Influenza is increasingly recognized as a serious infection with fatal consequences in patients with HMs. However, only few studies focusing on influenza in cancer patients have been published.12–15 Most of these published studies included few patients with influenza during the H1N1 pandemic in the 2009 and 2010 influenza seasons,8,10,12, 16, 17 and few of them described the clinical presentations, risk factors, and outcomes during the pandemic and post-pandemic seasons in this vulnerable patient population.
Objectives
Therefore, we conducted this retrospective study described herein to determine the characteristics, clinical presentation, risk factors, and outcomes of influenza infection during and after the 2009–2010 H1N1 pandemic in patients with HM from two comprehensive cancer centers: one located in Houston, Texas, and the other one located in Mexico City, Mexico.
Study Design
Patients
The medical records of all patients with HMs and laboratory-confirmed diagnoses of influenza who received care at The University of Texas MD Cancer Center in Houston, Texas, (center 1) and Instituto Nacional de Cancerología in Mexico City, Mexico, (center 2) from April 2009 to April 2014 were reviewed. The institutional review boards of both centers approved the study, informed consent was waived, no patient contacts were made, and patient confidentiality was protected.
Collected data included demographics, comorbidities, underlying malignancies, oncological treatment, immunosuppressive therapy, clinical presentations, radiological and laboratory investigation results, concurrent co-infections, treatments, and outcomes. All patients were observed until resolution of all signs and symptoms of infection or death.
Definitions
A case of influenza was defined as a patient with an HM (leukemia, lymphoma, or multiple myeloma) having acute respiratory illness and influenza infection confirmed via viral culture, direct fluorescent antigen testing (DFA), and/or real-time polymerase chain reaction (PCR) assay. The infection was classified as 1) community-acquired if the onset of symptoms occurred before or within the first 2 days after hospital admission or 2) nosocomial if the symptoms developed any time after that during hospitalization. URI was characterized by onset of rhinorrhea, nasal/sinus congestion, and pharyngitis or cough with or without expectoration, and its diagnosis was confirmed using any of the tests listed above and by a normal or unchanged chest radiograph or computed tomography scan at the time of diagnosis. LRI was characterized by new or changing pulmonary infiltrates suggestive of viral etiology on chest imaging and confirmed via examination of respiratory specimens, including nasal washes, endotracheal tube aspirates, sputum specimens, and bronchoalveolar lavage fluid specimens. A concurrent infection was considered when another organism was isolated from a patient within 3 days after diagnosis of influenza. Neutropenia was defined as an absolute neutrophil count less than 500 cells/mL, and lymphopenia was defined as an absolute lymphocyte count less than 200 cells/mL. Obesity was defined as a body mass index (BMI) of at least 30 kg/m2 in adults and a BMI percentile of at least 95% in children up to 18 years old.
Statistical analysis
After checking patient data for accuracy and consistency, the clinical presentations and outcomes of influenza infection in the study patients at the two centers were compared for categorical variables using a chi-square test or the Fisher exact test and for continuous variables using the Student t-test or Wilcoxon rank sum test. Using multivariable logistic regression modeling, differences in the characteristics of the patients with LRIs and of those with URIs were identified. Risk factors associated with mortality 60 days after influenza diagnosis also were identified using multivariable logistic regression analysis. The probability of death in influenza cases stratified by type of malignancy and development of LRI in the two centers was compared using Kaplan-Meier failure curves. A two-sided P value of 0.05 was considered statistically significant for all analyses, which were performed using the Stata software program (version 13; StataCorp, College Station, TX).
Results
We identified a total of 190 patients with laboratory-confirmed influenza in the two cancer centers (135 at center 1 and 55 at center 2) during the study period. Their median age was 49 years (range, 1–88 years), and 120 of them (63%) were male. The majority of the patients had leukemia (54%) and an active or refractory underlying malignancy (76%). Distribution of age, sex, and type of underlying malignancy did not differ between the two centers. However, the patient ethnicities were more diverse in center 1 than in center 2 (P<0.001). When compared with the patients in center 1, those in center 2 were sicker: 89% had an active or refractory malignancy at the time of influenza diagnosis (P=0.008), 27% had an elevated creatinine level (P=0.03), and 62% had an albumin level less than 3.5 mg/dL (P<0.001). In addition, we observed more patients in center 2 with LRIs (40% vs. 27%; P=0.089) or hypoxia at influenza diagnosis (75% vs. 7%; P<0.001) (Table 1).
Table 1.
Clinical Characteristics of and Outcomes of Influenza in Patients with HMs in the Two Cancer Centers
| Number of patients (%) | |||
|---|---|---|---|
|
|
|||
| Characteristic | Center 1 (n=135) |
Center 2 (n=55) |
P |
| Median age, years (range) | 52 (1–88) | 45 (15–75) | 0.181 |
| Sex | 0.081 | ||
| Male | 80 (59) | 40 (73) | |
| Female | 55 (41) | 15 (27) | |
| Race | <0.001 | ||
| Non-Hispanic white | 75 (56) | 1 (2) | |
| Black | 23 (17) | 0 | |
| Hispanic | 29 (21) | 54 (98) | |
| Other | 8 (6) | 0 | |
| Type of malignancy | 0.387 | ||
| Acute leukemia | 52 (39) | 19 (35) | |
| Chronic leukemia | 25 (19) | 6 (11) | |
| Lymphoma | 38 (28) | 20 (36) | |
| Myeloma | 17 (13) | 10 (18) | |
| Other | 3 (2) | 0 | |
| Influenza season | 0.978 | ||
| H1N1 (April 2009–May 2010) | 42 (31) | 17 (31) | |
| Post-H1N1 (November 2010–February 2014) | 93 (69) | 38 (69) | |
| Cancer status | 0.008 | ||
| Remission | 39 (29) | 6 (11) | |
| Active/refractory | 96 (71) | 49 (89) | |
| Systemic chemotherapy use | 101 (75) | 44 (80) | 0.494 |
| Corticosteroid use | 26 (19) | 25 (45) | <0.001 |
| Median BMI, kg/m2 (range) | 27 (13–63) | 25 (17–45) | 0.071 |
| WHO classification of BMI | 0.145 | ||
| Underweight (<18.5 kg/m2) | 15 (11) | 4 (7) | |
| Normal (18.50–24.99 kg/m2) | 35 (26) | 21 (38) | |
| Overweight (25.00–29.99 kg/m2) | 36 (27) | 18 (33) | |
| Obesea (≥30 kg/m2) | 48 (36) | 12 (22) | |
| Infection site at diagnosis | 0.089 | ||
| Upper respiratory tract | 98 (73) | 33 (60) | |
| Lower respiratory tract | 37 (27) | 22 (40) | |
| Laboratory abnormalities at presentation | |||
| Leukocytosis (WBC >11,000 cells/mm3) | 11 (8) | 8 (15) | 0.134 |
| Neutropenia (ANC <500 cells/mL) | 26 (19) | 12 (22) | 0.689 |
| Lymphocytopenia (ALC <200 cells/mL) | 26 (19) | 12 (22) | 0.689 |
| Elevated creatinine level (>1.3 mg/dL) | 19 (14) | 15 (27) | 0.031 |
| Decreased albumin level (<3.5 g/dL) | 41 (30) | 34 (62) | <0.001 |
| Hypoxia at influenza diagnosis | 9 (7) | 40 (73) | <0.001 |
| Outcome | |||
| Admission to hospital | 76 (56) | 47 (85) | <0.001 |
| Median length of hospital stay, days (range) | 7 (2–75) | 11 (1–49) | 0.039 |
| ICU admission | |||
| At onset of infection | 4 (3) | 1 (2) | 1.000 |
| Later | 11 (8) | 13 (24) | 0.001 |
| Mechanical ventilation | 8 (6) | 19 (35) | <0.001 |
| Oxygen supplementation | 33 (24) | 39 (71) | 0.510 |
| Antiviral therapy | 112 (83) | 49 (89) | 0.287 |
| Within 24 hours of symptom onset | 13 (10) | 8 (15) | 0.327 |
| Within 48 hours of symptom onset | 35 (26) | 19 (35) | 0.232 |
| Duration of antiviral therapy | 6 (2–18) | 9 (1–28) | 0.099 |
| Progression from URI to LRI | 7/98 (7) | 14/33 (42) | <0.001 |
| Co-infection | 26 (19) | 10 (18) | 0.864 |
| Respiratory co-pathogen | 23 (17) | 5 (9) | 0.183 |
| All-cause mortality | 10 (7) | 20 (36) | <0.001 |
| Median time from influenza diagnosis to death, days (range) | 16 (7–39) | 7 (1–47) | 0.095 |
Abbreviations: WHO, World Health Organization; WBC, white blood count; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; ICU, intensive care unit.
Obesity was defined as a BMI of at least 30 kg/m2 in adults 18 years of age or older or a BMI percentile of 95–100% in children 1 to 18 years old.
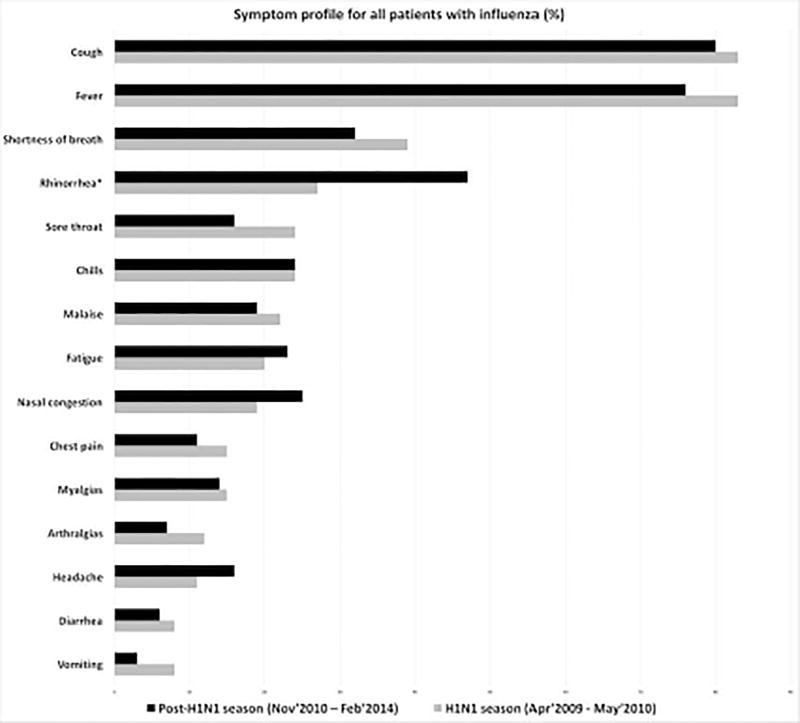
Cough, fever, rhinorrhea, shortness of breath, and chills were the most frequently reported symptoms in this cohort, which did not differ between the two centers or between the H1N1 influenza pandemic (April 2009–May 2010) and post-pandemic (November 2010–May 2014) periods (Figure 1).
Figure 1.
Symptom profile for HM patients with influenza in the 2009–2010 H1N1 pandemic and postpandemic periods in both cancer centers
Clinical characteristics of and influenza outcomes in the patients at both centers are listed in Table 1. The majority of the influenza infections (131 [69%]) were URIs at diagnosis. However, more patients at center 2 experienced progression from URI to LRI (42% vs. 7%; P<0.001), were hospitalized (85% vs. 56%; P<0.001), and needed mechanical ventilation (35% vs. 6%; P<0.001) as per table 1. A total of 80 (42%) patients had LRIs, and 30 (16%) died within 60 days after influenza diagnosis. A majority of the patients (85%) received antiviral therapy. However, only 54 patients (28%) began receiving antiviral therapy within 48 hours after onset of symptom(s). We identified no significant associations between initiation of antiviral therapy and poor outcome in either center.
In bi-variable regression analysis, cared for at center 2 was closely associated with LRI. However, this association was not significant when adjusted for the following factors in multivariable logistic regression analysis: age, sex, influenza season, type of malignancy, status of cancer at the time of infection diagnosis, corticosteroid use within 30 days prior to infection, BMI, neutropenia, lymphocytopenia, decreased albumin level, elevated creatinine level, hypoxia at diagnosis, receipt of antiviral therapy within 24 hours after diagnosis, and respiratory co-infections. In this analysis, leukemia (odds ratio [OR], 3.09 [95% confidence interval (CI), 1.23–7.70]), corticosteroid use (OR, 2.71 [95% CI, 1.03–7.15]), decreased albumin level (OR, 3.78 [95% CI, 1.55–9.20]; P=0.003), hypoxia at diagnosis (OR, 14.98 [95% CI, 3.30–67.90]; P<0.001), and respiratory co-infection (OR, 5.87 [95% CI, 1.65–20.86]; P=0.006) were independently associated with LRI (Table 2). When we restricted the analysis to patients who presented with URIs only, we found that the only independent predictor of progression to LRI was hypoxia at diagnosis (OR, 28.22 [95% CI, 3.00–265.60]).
Table 2.
Factors Associated with URI and LRI in HM Patients with Influenza (n=190)
| Number of patients (%) |
Entire cohort | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristic | URI (n=110) |
LRI (n=80) |
Unadjusted OR (95% CI) |
P | Adjusted OR (95% CI) |
P |
| Age | 49 (1–80)a | 50 (8–88)a | 1.12 (0.97–1.29)b | 0.119 | 1.20 (0.95–1.52)b | 0.120 |
| Sex | 0.654 | 0.450 | ||||
| Female | 42 (38) | 28 (35) | 1.00 | 1.00 | ||
| Male | 68 (62) | 52 (65) | 1.15 (0.63–2.09) | 1.39 (0.59–3.27) | ||
| Influenza season | 0.188 | 0.573 | ||||
| H1N1 (April 2009–May 2010) | 30 (27) | 29 (36) | 1.00 | 1.00 | ||
| Post-H1N1 (November 2010–February 2014) | 80 (73) | 51 (64) | 0.66 (0.35–1.26) | 0.76 (0.29–1.99) | ||
| Type of malignancy | 0.545 | 0.016 | ||||
| Lymphoma/myeloma | 53 (48) | 35 (44) | 1.00 | 1.00 | ||
| Leukemia | 57 (52) | 45 (56) | 1.20 (0.67–2.13) | 3.09 (1.23–7.70) | ||
| Cancer status | 0.090 | 0.305 | ||||
| Remission | 31 (28) | 14 (18) | 1.00 | 1.00 | ||
| Active/refractory | 79 (72) | 66 (82) | 1.85 (0.91–3.77) | 0.61 (0.24–1.56) | ||
| Corticosteroid use | 0.002 | 0.043 | ||||
| No | 90 (82) | 49 (61) | 1.00 | 1.00 | ||
| Yes | 20 (18) | 31 (39) | 2.85 (1.47–5.51) | 2.71 (1.03–7.15) | ||
| BMI | 0.135 | 0.159 | ||||
| Normal/underweight | 39 (35) | 37 (46) | 1.00 | 1.00 | ||
| Overweight/obese | 71 (65) | 43 (54) | 0.64 (0.35–1.15) | 0.53 (0.22–1.28) | ||
| Neutropenia | 0.004 | 0.287 | ||||
| No | 96 (87) | 56 (70) | 1.00 | 1.00 | ||
| Yes | 14 (13) | 24 (30) | 2.94 (1.41–6.14) | 1.87 (0.59–5.89) | ||
| Lymphocytopenia | 0.012 | 0.533 | ||||
| No | 95 (86) | 57 (71) | 1.00 | 1.00 | ||
| Yes | 15 (14) | 23 (29) | 2.56 (1.23–5.29) | 1.44 (0.46–4.53) | ||
| Decreased albumin level | <0.001 | 0.003 | ||||
| No | 89 (81) | 26 (33) | 1.00 | 1.00 | ||
| Yes | 21 (19) | 54 (67) | 8.80 (4.52–17.15) | 3.78 (1.55–9.20) | ||
| Elevated creatinine level | 0.793 | 0.742 | ||||
| No | 91 (83) | 65 (81) | 1.00 | 1.00 | ||
| Yes | 19 (17) | 15 (19) | 1.11 (0.52–2.34) | 1.20 (0.40–3.59) | ||
| Hypoxia at diagnosisc | <0.001 | <0.001 | ||||
| No | 100 (91) | 39 (49) | 1.00 | 1.00 | ||
| Yes | 9 (9) | 40 (50) | 11.40 (5.06–25.68) | 14.98 (3.30–67.90) | ||
| Antiviral therapy within 24 hours of onset of symptoms | 0.588 | 0.591 | ||||
| No | 99 (90) | 70 (88) | 1.00 | 1.00 | ||
| Yes | 11 (10) | 10 (12) | 1.29 (0.52–3.19) | 1.46 (0.36–5.88) | ||
| Respiratory co-infection | <0.001 | 0.006 | ||||
| No | 104 (95) | 58 (73) | 1.00 | 1.00 | ||
| Yes | 6 (5) | 22 (27) | 6.57 (2.52–17.14) | 5.87 (1.65–20.86) | ||
| Center | <0.001 | 0.649 | ||||
| 1 | 91 (83) | 44 (55) | 1.00 | 1.00 | ||
| 2 | 19 (17) | 36 (45) | 3.92 (2.02–7.59) | 0.72 (0.17–3.01) | ||
Median (range),
Age interval of 10 years,
data available for 188 patients only
The mortality rate 60 days after influenza diagnosis was significantly higher in patients in center 2 (36%) than in those in center 1 (7%), but after adjusting for other factors in the multivariable model (Table 3), this association was not statistically significant. Risk factors for mortality 60 days after influenza diagnosis in the entire cohort included elevated creatinine level (OR, 3.33 [95% CI, 1.05–10.56]; P=0.041), hypoxia at diagnosis (OR, 5.87 [95% CI, 1.12–30.77]; P=0.036), and respiratory co-infection (OR, 6.30 [95% CI, 1.55–25.67]; P=0.01). Neutropenia, lymphopenia, type of underlying malignancy, cancer status, and receipt of antiviral therapy within 24 hours after onset of symptoms were not predictors of mortality when adjusted for the differences between the patients in the two cancer centers. The average time from onset of symptoms to diagnosis of influenza was 3 days (range, 0–21 days), and we did not observe any significant differences in this duration between patients who died and those who recovered from their infections in either center.
Table 3.
Risk Factors for Mortality 60 Days After Influenza Diagnosis in HM Patients (n=190)
| Number of patients (%) |
||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristic | Alive (n=160) |
Dead (n=30) |
Unadjusted OR (95% CI) |
P | Adjusted OR (95% CI) |
P |
| Age | 49 (1–88)a | 52 (13–75)a | 1.13 (0.92–1.38)b | 0.246 | 1.10 (0.78–1.55)b | 0.577 |
| Sex | 0.664 | 0.455 | ||||
| Female | 60 (38) | 10 (33) | 1.00 | 1.00 | ||
| Male | 100 (62) | 20 (67) | 1.20 (0.53–2.73) | 0.65 (0.21–2.02) | ||
| Influenza season | 0.470 | 0.605 | ||||
| H1N1 (April 2009–May 2010) | 48 (30) | 11 (37) | 1.00 | 1.00 | ||
| Post-H1N1 (November 2010–February 2014) | 112 (70) | 19 (63) | 0.74 (0.33–1.67) | 0.74 (0.23–2.34) | ||
| Type of malignancy | 0.018 | 0.113 | ||||
| Leukemia | 92 (58) | 10 (33) | 1.00 | 1.00 | ||
| Lymphoma/myeloma | 68 (42) | 20 (67) | 2.71 (1.19–6.15) | 2.37 (0.82–6.88) | ||
| Cancer status | 0.030 | 0.586 | ||||
| Remission | 43 (27) | 2 (7) | 1.00 | 1.00 | ||
| Active/relapse | 117 (73) | 28 (93) | 5.15 (1.18–22.52) | 1.63 (0.28–9.39) | ||
| Corticosteroid use | 0.009 | 0.574 | ||||
| No | 123 (77) | 16 (53) | 1.00 | 1.00 | ||
| Yes | 37 (23) | 14 (47) | 2.91 (1.30–6.51) | 1.37 (0.46–4.14) | ||
| BMI | 0.226 | 0.181 | ||||
| Normal/underweight | 61 (38) | 15 (50) | 1.00 | 1.00 | ||
| Overweight/obese | 99 (62) | 15 (50) | 0.62 (0.28–1.35) | 0.47 (0.16–1.41) | ||
| Neutropenia | 0.140 | 0.477 | ||||
| No | 131 (86) | 21 (14) | 1.00 | 1.00 | ||
| Yes | 29 (76) | 9 (24) | 1.94 (0.80–4.66) | 1.64 (0.42–6.43) | ||
| Lymphocytopenia | 0.140 | 0.995 | ||||
| No | 131 (82) | 21 (70) | 1.00 | 1.00 | ||
| Yes | 29 (18) | 9 (30) | 1.94 (0.80–4.66) | 0.99 (0.26–3.80) | ||
| Decreased albumin level | <0.001 | 0.423 | ||||
| No | 108 (68) | 7 (23) | 1.00 | 1.00 | ||
| Yes | 52 (32) | 23 (77) | 6.82 (2.75–16.93) | 1.65 (0.49–5.57) | ||
| Elevated creatinine level | 0.020 | 0.041 | ||||
| No | 136 (85) | 20 (67) | 1.00 | 1.00 | ||
| Yes | 24 (15) | 10 (33) | 2.83 (1.18–6.79) | 3.33 (1.05–10.56) | ||
| Hypoxia at diagnosis | <0.001 | 0.036 | ||||
| No | 131 (82) | 8 (27) | 1.00 | 1.00 | ||
| Yes | 27 (17) | 22 (73) | 13.34 (5.38–33.12) | 5.87 (1.12–30.77) | ||
| Antiviral therapy within 24 hours of onset of symptoms | 0.665 | 0.951 | ||||
| No | 143 (89) | 26 (87) | 1.00 | 1.00 | ||
| Yes | 17 (11) | 4 (13) | 1.29 (0.40–4.16) | 1.05 (0.20–5.54) | ||
| Respiratory co-infection | 0.003 | 0.010 | ||||
| No | 142 (89) | 20 (67) | 1.00 | 1.00 | ||
| Yes | 18 (11) | 10 (33) | 3.94 (1.60–9.74) | 6.30 (1.55–25.67) | ||
| Center | <0.001 | 0.369 | ||||
| 1 | 125 (78) | 10 (33) | 1.00 | 1.00 | ||
| 2 | 35 (22) | 20 (67) | 7.14 (3.06–16.65) | 2.26 (0.38–13.35) | ||
Median (range),
interval of 10 years,
data available for 188 patients only
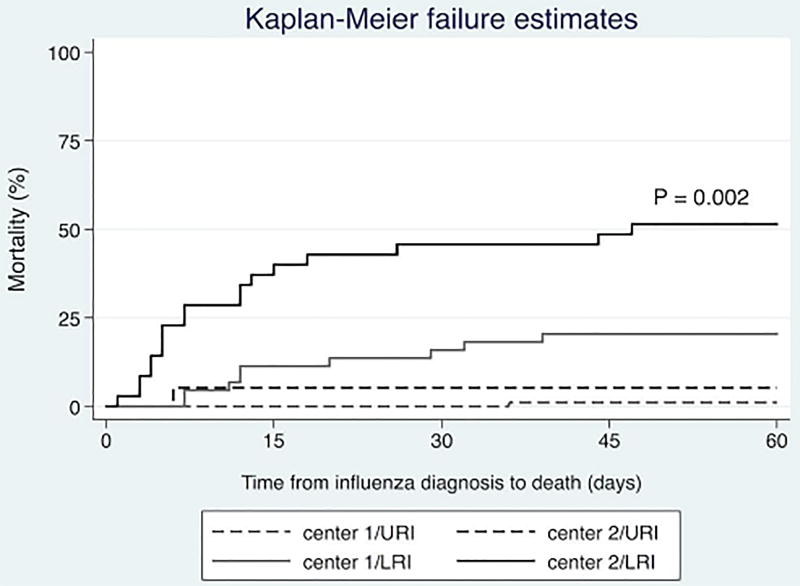
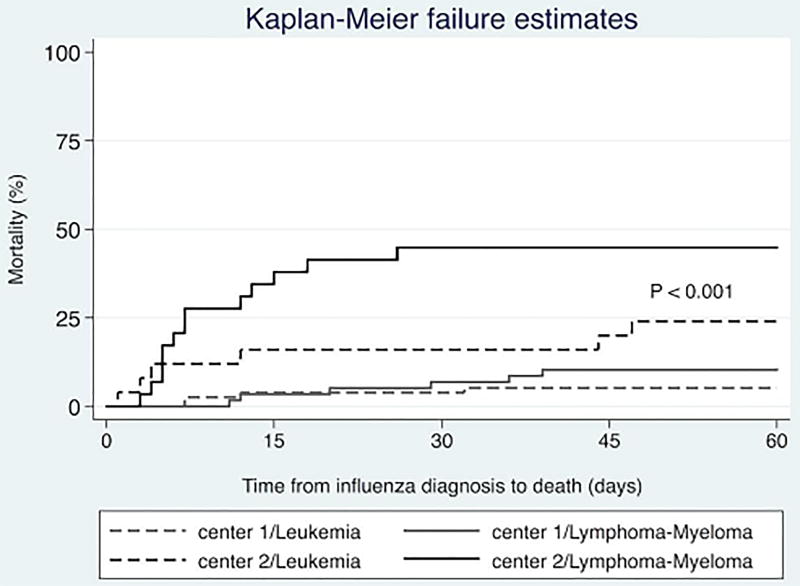
Kaplan-Meier curves showed that the 60-day all-cause mortality rate was significantly higher in patients in center 2 than in those in center 1 (P<0.001) after stratification for the type of malignancy (leukemia or lymphoma/myeloma). However, the mortality rates in center 2 were similar for all types of HM (Figure 2). Similarly, we observed a significantly higher all-cause mortality rate in patients with LRIs in center 2 than in those in center 1 (P=0.002 [log-rank test]). The mortality rates in patients without LRIs did not differ significantly between the two centers, though (Figure 3).
Figure 2.
All-cause mortality rates for the study patients following influenza diagnosis stratified according to cancer center and type of malignancy
Figure 3.
All-cause mortality rates for the study patients following influenza diagnosis stratified by cancer center and LRI
Discussion
Herein, we describe the clinical presentations and outcomes of influenza in a cohort of patients with HMs during the 2009–2010 H1N1 pandemic and the four post-pandemic influenza seasons. Compared with the patients in the American center, those in Mexico were sicker (having active cancer, decreased albumin level, elevated creatinine level, hypoxia at diagnosis) and had markedly higher rates of LRI and mortality. We did not identify any differences in outcome between the two centers with respect to receipt of early antiviral therapy (within 24 hours of influenza diagnosis). To our knowledge, this is one of the largest studies of HM patients with influenza in two cancer centers.
Besides the expected differences in ethnicity of HM patients between the two cancer centers, we observed other significant differences, mainly in their clinical characteristics, such as a higher rate of active or refractory HM and use of corticosteroids, higher creatinine levels, and lower serum albumin levels at the time of influenza diagnosis in center 2. These characteristics are surrogate markers for advanced underlying malignancy and subsequent increased immunosuppression, indicating that patients in center 2 were much sicker than those in center 1. Furthermore, physicians diagnosed influenza at later stages in patients in center 2, and they had worse outcomes than did patients in center 1.
Patients with HMs have a wide range of influenza-associated LRI rates (21–80%).8, 11, 13 In our cohort, the observed rate was on the higher end of the spectrum, with a total of 80 (42%) patients having LRIs. Patients with leukemia, a decreased albumin level, hypoxia at diagnosis, a respiratory co-infection or receiving corticosteroids were at increased risk for LRI. Of these risk factors, lymphocytopenia and corticosteroid use have been related to severe influenza and poor outcomes in other studies of HM patients and HCT recipients.8,12,14–15 Authors also reported that hypoalbuminemia is a risk factor for pneumonia in a single-center cohort of patients with hematological disease having influenza.13 Respiratory co-infections are very common (20–40%) in immunocompromised cancer patients with influenza and are especially troublesome if the co-infection is affecting the lungs.1,4,5 We observed that 28 (15%) of our patients had respiratory co-infections, which was associated with increased rates of poor outcome (LRI and death). We did not observe a correlation between BMI and poor outcome following influenza infection.
After adjusting for factors such as elevated creatinine level, hypoxia at diagnosis, and respiratory co-infection in multivariable analyses, the difference in mortality rate between the two centers was not significant. In a previous study, investigators analyzed these risk factors in HCT recipients with influenza.17 Authors reported that other factors, such as nosocomial infection, profound lymphocytopenia, neutropenia, and delayed antiviral treatment, increased the risk of adverse outcomes in HCT recipients.1,2,11,18 However, we did not identify such associations.
Multiple studies have shown the benefit of prompt antiviral therapy for influenza (within 48 hours after diagnosis) in HM patients and HCT recipients in preventing LRI and death.2,4 Physicians prescribed antiviral therapy for influenza in most our patients. However, only a small proportion of them received it within 48 hours after onset of influenza symptoms. After adjustment for all differences in various risk factors in the patients in the two centers, delayed initiation of antiviral therapy was not associated with LRI or death. The time from onset of symptoms to administration of antiviral therapy was similar in the two centers, although a markedly higher number of patients in center 2 than in center 1 presented with LRIs. This may explain the lack of association between late antiviral therapy and poor outcome of influenza in this population. Nevertheless, all HM patients diagnosed with influenza are recommended to receive adequate antiviral therapy as early as possible, preferably within 48 hours after symptom onset, as it may prevent severe complications of influenza in immunocompromised patients.2,4
Our study has few limitations. First, this was a retrospective study of data collected from laboratory reports (DFA or culture in center 1 and PCR or culture in center 2) and medical records in centers in two different countries, which may have led to some information bias. However, we assumed this bias to be minimal, as both institutions are cancer referral centers with standardized center-specific management protocols (oseltamivir-based regimens) for patients with influenza. Furthermore, trained infectious disease physicians collected the data using a common data collection form, and they performed rigorous data-quality checks, resolving discrepancies in the data via mutual discussion. Not all of the outpatients with influenza-like illness (ILI) at center 2 were screened for influenza, which is a possible source of selection bias. This underscores the importance of recommending thorough screening for ILI in cancer patients. Furthermore, the criteria for hospitalization in the two cancer centers may have differed. At center 2, patients with mild influenza were probably missed because of the identification was mostly targeted to patients at high risk for LRI. Also, most of the patients did not undergo evaluation for prolonged viral shedding and data on vaccination status for patients or staff were not available for comparison. Nevertheless, the strengths of this study included one of the largest cohorts of patients with HMs in two cancer referral centers in North America, a diverse ethnic population with different severities of influenza, and a study period covering five influenza seasons including patients with influenza A, and B.
In conclusion, HM patients with influenza are at high risk for poor outcomes, such as LRI and death, especially if they are immunosuppressed. The mortality rate after influenza diagnosis was higher in center 2 than in center 1, owing in part to a group of sicker patients who were diagnosed with influenza at late stages of their infections (i.e., after onset of LRI) in the former center. Patient education about seeking prompt medical care during influenza season is highly recommended for those with HMs.
Article Summary.
-
-
Influenza infections in patients with hematological malignancies differed significantly between 2 cancer centers.
-
-
Patient education about seeking prompt medical care during influenza season is highly recommended.
Acknowledgments
R.F.C. received research grants from GlaxoSmithKline.
Funding: Supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
We thank Mr. Donald Norwood, Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for her editorial support.
This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Financial Disclosures: The remaining authors declare no competing financial interests.
Author Contributions
D.V.C, D.P.S. and R.F.C. conceptualized and designed the study. D.V.C., J.V., and D.P.S. performed the clinical research and data validation. P.C.J., A.G.H., P.V., and R.F.C. helped with data acquisition. D.P.S. performed the statistical analyses. D.V.C., D.P.S., and R.F.C. wrote the paper. All authors helped critically review the manuscript and checked the final version of it.
References
- 1.Cordero E, de la Torre-Cisneros J, Moreno A, Pérez-Romero P, Riera M. The impact of influenza A(H1N1) pandemic 2009 infection in immunosuppressed patients. Enferm Infecc Microbiol Clin. 2012;30:38–42. doi: 10.1016/S0213-005X(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 2.Choi SM, Boudreault AA, Xie H, Englund JA, Corey L, Boeckh M. Differences in clinical outcomes following 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood. 2011;117(19):5050–6. doi: 10.1182/blood-2010-11-319186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah DP, El Taoum KK, Shah JN, Vigil KJ, Adachi JA, Granwehr BP, et al. Characteristics and outcomes of pandemic 2009/H1N1 versus seasonal influenza in children with cancer. Pediatric Infectious Disease Journal. 2012;31(4):373–8. doi: 10.1097/INF.0b013e3182481ef8. [DOI] [PubMed] [Google Scholar]
- 4.Kmeid J, Vanichanan J, Shah DP, El Chaer F, Azzi J, Ariza-Heredia EJ, et al. Outcomes of Influenza Infections in Hematopoietic Cell Transplant Recipients: Application of an Immunodeficiency Scoring Index. Biol Blood Marrow Transplant. 2016;22(3):542–8. doi: 10.1016/j.bbmt.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikulska M, Del Bono V, Gandolfo N, Dini S, Dominietto A, Di Grazia C, et al. Epidemiology of viral respiratory tract infections in an outpatient hematology facility. Ann Hematol. 2014;93:669–76. doi: 10.1007/s00277-013-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martino R, Porras R, Rabella N, Williamns JV, Rámila E, Margall N, et al. Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem transplants for hematological malignancies. Biol Blood Bone Marrow Transplant. 2005;11:781–6. doi: 10.1016/j.bbmt.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Li Z, Zhang S, Song S, Julong W, Lin Y, et al. Viral etiology of acute respiratory tract infections in hospitalized children and adults in Shandong Province, Chine. Virol J. 2015;12:168. doi: 10.1186/s12985-015-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redelman-Sidi G, Sepkowitz K, Huang CK, Park S, Stiles J, Eagan J, et al. Influenza infection in cancer patients and hematopoietic stem cell transplant recipients. J of Infect. 2009;60:257–63. doi: 10.1016/j.jinf.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Chemaly RF, Ghosh S, Bodey GP, Rohatgi N. Respiratory viral infections in adults with hematological malignancies and human stem cell transplantation recipients: a retrospectivestudy at a major cancer cancer center. Medicine (Baltimore) 2006;85:278–87. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 10.Tai Y, Lee TC, Chang HL, Chen KT. Epidemiology and outcomes of hospitalization of influenza in the cancer population in Taiwan. J Cancer Res Clin Oncol. 2009;135:1061–6. doi: 10.1007/s00432-009-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Bone Marrow Transplant. 2001;(Suppl 7):5–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Schwartz B, Vallabhaneni S, Nixon M, Chin-Hong PV, Miller SA, et al. Pandemic (H1N1) 2009 infection in patients with hematologic malignancy. Emer Infect Dis. 2010;16:1910–7. doi: 10.3201/eid1612.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad M, Hayajneh W, Mubarak S, Yousef I, Awad H, Elbjeirami W, et al. Clinical presentations and outcomes of influenza infection among hematology/oncology patients from a single cancer center: pandemic and post-pandemic seasons. Scand J Infect Dis. 2014;46:770–8. doi: 10.3109/00365548.2014.943282. [DOI] [PubMed] [Google Scholar]
- 14.Minnema BJ, Husain S, Mazzulli T, Hosseini-Mogaddam SS, Patel M, Brandwein J, et al. Clinical characteristics and outcome associated with pandemic (2009) H1N1 influenza infection in patients with hematologic malignancies: a retrospective cohort study Leuk & Lymphoma. 2013;54:1250–5. doi: 10.3109/10428194.2012.740558. [DOI] [PubMed] [Google Scholar]
- 15.Irga N, Osak M, Jaworski R, Bronk M, Kosiak W, Adamkiewicz-Drozynaka E. Pandemic [H1N1] 2009 influenza -real threat or unjustified panic? The experience of one pediatric hematology-oncology center. Cent Eur J Med. 2012;7:295–301. [Google Scholar]
- 16.Tekgündüz E, Yüksel MK, Erbay C, Aribas B, Özdilekcan C, Arsian H, et al. Pandemic 2009 H1N1 influenza in patients with hematopoietic stem cell transplantation and hematologic malignancy: Single center experience. Oncology. 2010;79:409–14. doi: 10.1159/000320789. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa-Aguilar L, Green JS, Forrest GN, Ball ED, Maziarz RT, et al. Novel H1N1 influenza in hematopoietic stem cell transplantation recipients: Two centers' experiences. Biol Blood Bone Marrow Transplant. 2009;17:566–73. doi: 10.1016/j.bbmt.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 18.George B, Ferguson P, Kerridge I, Gilroy N, Gottlieb D, Hertzberg M. The clinical impact of infection with swine flu (H1/N109) strain of influenza virus in hematopoietic stem cell transplant recipients. Biol Blood Bone Marrow Transplant. 2011;17:147–53. doi: 10.1016/j.bbmt.2010.07.004. [DOI] [PubMed] [Google Scholar]