Abstract
Background
The secondary T790M mutation accounts for more than 50% of acquired TKI resistance in EGFR-mutated NSCLC patients. Recent reports suggest this resistance mutation may be more common among patients with longer PFS on first-line TKI therapy, but much is still unknown.
Materials and Methods
Our group collected medical records from patients who underwent a biopsy for T790M mutation testing while screening for clinical trials involving the drug rociletinib (CO-1686), a T790M mutation specific TKI. Medical records were retrospectively analyzed for demographic data, PFS, and best response to previous therapies.
Results
Our patient cohort included 69 T790M+ patients and 28 T790M− patients. Patients who later developed a T790M mutation had a longer PFS on first-line TKI therapy (12.0 vs. 9.0 months, p = 0.021), but ORR was the same (75.0% vs 81.0%, p = 0.76). There was no difference in PFS on TKI rechallenge (4.0 vs. 3.0 months, p = 0.94), though there was a trend towards higher ORR in T790M+ patients (22.2% vs. 0%, p = 0.12). T790M+ patients had a longer PFS on initial chemotherapy treatment (5.0 vs. 4.0 months, p = 0.025) and a trend towards higher ORR (40.0% vs 21.4%, p = 0.31).
Conclusion
Our study confirms that tumors expressing T790M have a more indolent progression of disease compared to their T790M− counterparts when treated with both first-line TKI and cytotoxic chemotherapy.
Keywords: acquired resistance, EGFR tyrosine kinase inhibitor, chemotherapy, progression-free survival, targeted therapy
Introduction
Mutations in the epidermal growth factor receptor (EGFR) tyrosine kinase have emerged as an important treatment target for non-small cell lung cancer (NSCLC), a disease for which the current five-year survival rate for stage IV patients is only 1%.1 This molecular subtype of EGFR mutations is present in up to half of NSCLC patients in Asian populations2 and approximately 10% in Western populations.3–5 It is also known to be more common in females, never-smokers, and those with adenocarcinoma histology.2,6
First-generation tyrosine kinase inhibitors (TKI’s), including erlotinib and gefitinib, against EGFR have been shown to be superior to chemotherapy as first-line treatment for patients with EGFR-mutated NSCLC, leading to longer progression-free survival (PFS).7–14 Overall survival (OS), however, has not been shown to be statistically different between first-generation TKI’s and chemotherapy, possibly due to crossover at disease progression.7,8,10,11,13,14 Of the two most common types of EGFR mutations, patients with the exon 19 deletion have recently been suggested to have a longer progression-free survival (PFS) on TKI’s and also a longer OS than those with the L858R point mutation,6,15,16 although data has been somewhat conflicting.17,18
Despite the initial beneficial response to TKI’s, most NSCLC patients with EGFR-activating mutations develop resistance, generally 9–14 months after treatment initiation.6–9,12,19,20 While multiple mechanisms have been described, a secondary EGFR mutation Thr790Met (T790M) in exon 20 accounts for more than 50% of acquired TKI resistance.21–23 Second generation TKI’s including afatinib and dacomitinib were developed in order to combat such acquired resistance, but limited efficacy has been observed.24–27 More recently, third-generation drugs, including rociletinib and osimertinib, have been designed to irreversibly inhibit the tyrosine kinase activity of mutant EGFR, including both activating and the T790M resistance mutations, without affecting wild-type EGFR.28,29
Investigation into acquired resistance due to the T790M mutation is ongoing, but much is still unknown. Some studies have shown that T790M positive patients have a longer PFS on erlotinib treatment,23 a longer post-progression survival (PPS) following progression on TKI treatment,30,31 and a longer OS compared to T790M negative patients,23,30,32,33 suggesting that those patients who develop a T790M mutation may have a more favorable prognosis and a more indolent progression of disease than those who develop another resistance mechanism to TKI’s. Our study aimed to examine patients harboring a T790M mutation in terms of their response to treatment, including not only EGFR-TKI’s but also chemotherapy, to help further characterize this important subset of patients.
Materials and Methods
Patients and study design
The patient cohort for this study was acquired from enrolled and screen-failed patients for the clinical trials NCT02147990 (TIGER-2) and NCT01526928 (TIGER-X)34,35 involving the third-generation TKI rociletinib (CO-1686), an irreversible inhibitor of the T790M resistance mutation, as well as the exon 19 and 21 mutations commonly present in EGFR-mutated NSCLC.29,36 Inclusion criteria for these studies included histologically or cytologically confirmed metastatic or unresectable locally advanced NSCLC, life expectancy of at least 3 months, Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, adequate hematological and biological function as confirmed by laboratory values, and documented evidence of an activating mutation in EGFR, in addition to undergoing a biopsy of either primary or metastatic tumor tissue within 28 days (TIGER-2) or 60 days (TIGER-X) of starting the study drug. Patients were also required to have disease progression on treatment with a prior EGFR-directed therapy (eg erlotinib, gefitinib, neratinib, afatinib, or dacomitinib) and have documented evidence of a T790M mutation in EGFR following disease progression on most recent prior EGFR-directed therapy, with the exception of phase 1 of TIGER-X for which T790M mutation testing was not required. T790M mutation testing was performed by PCR-based testing of tumor tissue using the UCLA central laboratory.
Medical records were retrospectively analyzed for demographic data, PFS, best response (BR) to previous therapies, and T790M mutation status. No analysis was done on clinical trial data. Analysis was performed only on therapies given prior to study drug, with the exception of chemotherapy, for which 5 patients received initial chemotherapy after study drug. As most of the therapies were not on a clinical trial and often administered at outside institutions, the date of progression was defined based on either (1) radiographic progression based on RECIST 1.1 (Response Evaluation Criteria in Solid Tumours)37 or (2) sufficient growth of a tumor in a known site of disease to make a clinician note progression or discontinue therapy as documented in the patient’s medical chart, if data to assess by RECIST was unavailable. PFS was then recorded in months as the interval between date of start of treatment and date of progression. The characterization of best treatment response as CR (complete response), PR (partial response), SD (stable disease), or PD (progressive disease) was assessed either using (1) RECIST 1.1 criteria37 directly from radiographic scans or (2) clinician documentation of patient response in the medical chart, if data to assess by RECIST was unavailable.
Statistical analysis
The associations between T790M and patient demographics/clinical characteristics were analyzed using two-sample t-test and chi-squared tests. Progression-free survival was estimated using the Kaplan-Meier method and compared across two groups using the log-ranked test. Univariable and multivariable analysis were performed using Cox proportional hazard regression analysis. In multivariable analysis, all variables with p values <0.2 by univariable analysis were added into the basic model which included the study variable of T790M mutation status. A final model was obtained first by backward elimination and then verified by the stepwise model selection method, retaining the study variable of T790M mutation status. Results were considered statistically significant if p value < 0.05.
Results
Patient population
From April 2012 to October 2016, 125 patients were screened at our institution for clinical trials involving the third-generation TKI rociletinib (Figure 1). Nineteen patients were excluded from our study because of unknown T790M status due to (1) a nondiagnostic biopsy, (2) biopsy not being obtained at our institution as a result of poor performance status or transfer of care, or (3) biopsy not being required for clinical trial screening given patient was being screened for phase 1 of TIGER-X. Out of the 106 patients who received a diagnostic biopsy identifying the presence or absence of a T790M mutation, 8 patients (6 T790M positive and 2 T790M negative) were excluded because they were treated with erlotinib and/or chemotherapy in the adjuvant setting after definitive surgery or radiation with no evidence of disease, and the PFS and response rate endpoints in this adjuvant setting were not considered comparable to that in the metastatic setting. One T790M negative patient was also excluded because they possessed an EGFR mutation known to be non-sensitizing to approved EGFR TKI’s (exon 20 insertion). Ninety-seven patients had advanced disease (stage IV) at the time of TKI or chemotherapy treatment that was either advanced at diagnosis or recurrent from stage I, II, or III disease at diagnosis. Out of those 97 patients, 69 had a biopsy positive for the T790M mutation and 28 were found to be T790M negative.
Figure 1.
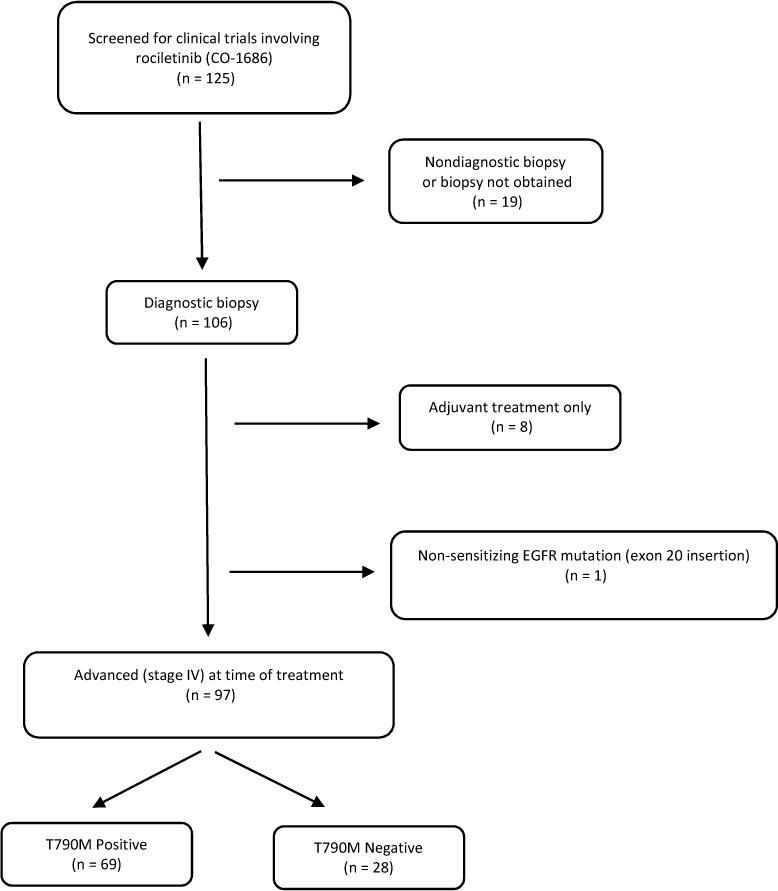
Flowchart of Study Population
Characteristics of the 97 patients are outlined in Table 1. In both T790M positive and negative patients, most were female and never smokers, and the exon 19 deletion was the most common EGFR activating mutation. Seven patients (4 T790M positive, 3 T790M negative) had unknown EGFR mutational status for primary EGFR mutation given incomplete documentation. Though the distinction between exon 19 deletion and L858R was not made, it was documented for these seven patients that primary EGFR mutations were sensitizing and/or appropriate for erlotinib therapy. Almost all patients had advanced stage IV disease at diagnosis, but a small number had recurrent disease after definitive therapy, such as surgery, chemotherapy, radiation, or TKI treatment. There was no difference in clinical characteristics of patients based on T790M mutation status. The type of activating EGFR mutation (exon 19 deletion or L858R) did not correlate with T790M mutation status (p = 0.57), nor did ethnicity (p = 0.14). Overall, 76 patients (51 T790M positive, 25 T790M negative) had data available to assess progression-free survival and best response by RECIST criteria. Twenty-one patients (18 T790M positive, 3 T790M negative) did not have radiographic scans available.
Table 1.
Patient Demographics and Clinical Characteristics
| T790M Positive | T790M Negative | p value | ||
|---|---|---|---|---|
| Total number of patients | 69 | 28 | ||
| Age (mean ± standard deviation) | 65.2 ± 11.3 | 70.3 ± 11.5 | p = 0.052 | |
| Gender | ||||
| Male | 22 | 6 | p = 0.30 | |
| Female | 47 | 22 | ||
| Ethnicity | ||||
| Caucasian | 13 | 11 | p = 0.14 | |
| Black | 1 | 0 | ||
| Asian | 22 | 4 | ||
| Hispanic | 5 | 2 | ||
| Other | 5 | 0 | ||
| Unknown | 23 | 11 | ||
| Smoking Status | ||||
| Current or Former Smoker | 18 | 4 | p = 0.21 | |
| Never Smoker | 51 | 24 | ||
| EGFR Mutation | ||||
| Exon 19 Deletion | 43 | 15 | p = 0.57 | |
| L858R | 20 | 10 | ||
| Exon 19 Deletion and L858R | 2 | 0 | ||
| Unknown | 4 | 3 | ||
| Stage | ||||
| IV at Diagnosis | 58 | 25 | p = 0.51 | |
| Recurrent | 11 | 3 | ||
| Tumor Histology | ||||
| Adenocarcinoma | 67 | 26 | p = 0.28 | |
| Squamous Cell Carcinoma | 0 | 1 | ||
| Adenosquamous Carcinoma | 2 | 1 | ||
| Data Analysis | ||||
| By RECIST | 51 | 25 | ||
| By Medical chart | 18 | 3 | ||
| Treated with first-line TKI | 66 | 28 | ||
| Treated with TKI rechallenge | 29 | 14 | ||
| Treated with chemotherapy | 42 | 18 | ||
Progression-Free Survival and Tumor Response to First TKI Treatment
Analysis of first-line TKI treatment was performed for 66 T790M positive patients and 28 T790M negative patients. Three T790M positive patients were excluded from analysis because they received TKI therapy before progression to stage IV disease (but received chemotherapy once disease was metastatic so were not excluded from the overall study). The first-line TKI for all patients was erlotinib with the exception of 1 T790M positive patient who was treated first with gefitinib and 1 T790M negative patient who was treated first with afatinib. Seven T790M positive patients and 2 T790M negative patients were given their first erlotinib treatment in combination with other therapies, including as part of clinical trials. These combination agents included celecoxib (2 patients), tivantinib (1 patient), bevacizumab (3 patients), fulvestrant (2 patients), and cytotoxic chemotherapy (1 patient).
The median PFS for each patient’s first TKI treatment was 12.0 months in 66 T790M positive patients versus 9.0 months in 28 T790M negative patients (Figure 2, p = 0.086 by log-rank test). Univariable and multivariable analysis were performed to analyze T790M mutation status, age, sex, ethnicity, smoking history, type of primary EGFR activating mutation (exon 19 deletion or L858R), stage (recurrent from stage I, II, or III at diagnosis versus advanced stage IV at diagnosis), TKI treatment alone or in combination with other agents, and timing of TKI therapy in relation to chemotherapy (before versus after) as independent predictors of progression-free survival (Table 2). As association was observed for shorter progression-free survival with the absence of a T790M mutation (p = 0.021, HR 1.75, 95% CI 1.09–2.82), male sex (p = 0.010, HR 1.88, 95% CI 1.16–3.03), and positive smoking history (p = 0.011, HR 1.93, 95% CI 1.16–3.20). There was also a trend towards longer PFS with TKI given after chemotherapy (p = 0.0070 by log-rank test), though not significant in the multivariable model that contained T790M mutation status. When the 9 patients treated with first-line TKI in combination with other therapies were removed from the analysis, the median PFS was 12.0 months in 59 T790M positive patients and 10.5 months in 26 T790M negative patients (p = 0.036, HR 1.72, 95% CI 1.04–2.84). When the 1 patient that received first-line TKI in combination with chemotherapy was removed from the analysis, the median PFS was 12.0 months in 65 T790M positive patients and 9.0 months in 28 T790M negative patients (p = 0.019, HR 1.77, 95% CI 1.10–2.85). Overall response rate (ORR) to initial TKI treatment was 75.0% in 48 T790M positive patients and 81.0% in 21 T790M negative patients (Table 3, p = 0.76).
Figure 2.
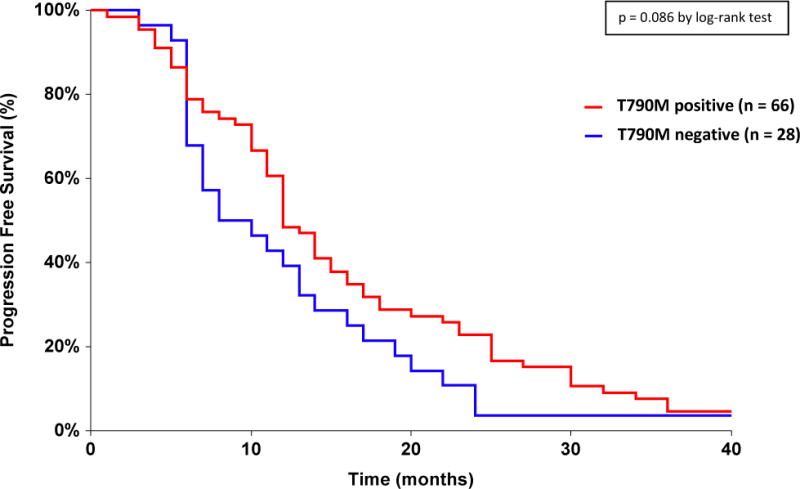
Progression-Free Survival on First-line TKI Treatment Depending on T790M Mutation Status
Table 2.
Univariable and Multivariable Cox Proportional Hazard Regression Analysis for Progression-Free Survival on First-line TKI
| Parameter | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| p value | Hazard Ratio (95% Confidence Interval) | p value | Hazard Ratio (95% Confidence Interval) | |
| T790M mutation: negative vs positive | 0.10 | 1.46 (0.93–2.29) | 0.021 | 1.75 (1.09–2.82) |
| TKI: in combination vs alone | 0.57 | 1.22 (0.61–2.44) | N/A | |
| TKI: before vs after chemotherapy | 0.015 | 2.02 (1.14–3.56) | N/A | |
| Sex: male vs female | 0.021 | 0.59 (0.37–0.92) | 0.010 | 1.88 (1.16–3.03) |
| Race: Caucasian vs Asian | 0.58 | 1.19 (0.65–2.16) | N/A | |
| Race: Other vs Asian | 0.64 | 1.18 (0.59–2.36) | N/A | |
| Age as continuous variable (as 1 year increase) | 0.90 | 1.00 (0.98–1.02) | N/A | |
| Smoking status: yes vs no | 0.007 | 1.99 (1.21–3.29) | 0.011 | 1.93 (1.16–3.20) |
| Exon 19 deletion: absent vs present | 0.25 | 1.31 (0.83–2.06) | N/A | |
| L858R: present vs absent | 0.13 | 1.41 (0.90–2.22) | N/A | |
| Stage: recurrent vs advanced at presentation | 0.45 | 1.28 (0.68–2.41) | N/A | |
Table 3.
Best Response to First-line TKI, TKI Rechallenge, and Chemotherapy Based on T790M Mutation Status
| T790M+ | T790M− | |||||
|---|---|---|---|---|---|---|
| Treatment | Response | No. of Patients (%) | No. of Patients (%) | |||
| First-line TKI | CR | 2 (4.2%) | 0 (0%) | |||
| PR | 34 (70.8%) | 17 (81.0%) | ||||
| SD | 12 (25.0%) | 3 (14.3%) | ||||
| PD | 0 (0%) | 1 (4.8%) | ||||
| ORR (CR + PR) | 36 (75.0%) | 17 (81.0%) | p = 0.76 | |||
| TKI Rechallenge | CR | 0 (0%) | 0 (0%) | |||
| PR | 4 (22.2%) | 0 (0%) | ||||
| SD | 7 (38.9%) | 5 (38.5%) | ||||
| PD | 7 (38.9%) | 8 (61.5%) | ||||
| ORR (CR + PR) | 4 (22.2%) | 0 (0%) | p = 0.12 | |||
| Chemotherapy | CR | 2 (6.67%) | 2 before TKI 0 after TKI |
0 (0%) | 0 before TKI 0 after TKI |
|
| PR | 10 (33.3%) | 4 before TKI 6 after TKI |
3 (21.4%) | 1 before TKI 2 after TKI |
||
| SD | 14 (46.7%) | 5 before TKI 9 after TKI |
5 (35.7%) | 2 before TKI 3 after TKI |
||
| PD | 4 (13.3%) | 1 before TKI 3 after TKI |
6 (42.9%) | 1 before TKI 5 after TKI |
||
| ORR (CR + PR) | 12 (40.0%) | 3 (21.4%) | p = 0.31 | |||
Abbreviations: CR, complete response; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease
Progression-Free Survival and Tumor Response to TKI Rechallenge
Twenty-nine T790M positive and 14 T790M negative patients received TKI rechallenge, defined as TKI therapy given after progression on first TKI treatment (and prior to enrollment in clinical trial for rociletinib). Out of the T790M positive patients, 22 patients received erlotinib and 7 patients received afatinib. Seventeen T790M positive patients received combination therapy, which included chemotherapy (with erlotinib, 6 patients), cabozantinib (with erlotinib, 5 patients), MK-2206 (with erlotinib, 1 patient), REGN1400 (with erlotinib, 1 patient), bevacizumab (with erlotinib, 1 patient), phosphoinositide 3-kinase (PI3K) inhibitor (with erlotinib, 1 patient), ramucirumab (with erlotinib, 1 patient), and cetuximab (with afatinib, 1 patient). In the T790M negative patients, 9 patients received erlotinib, 4 patients received afatinib, and 1 patient received gefitinib. Seven T790M negative patients received combination therapy, which included chemotherapy (with erlotinib, 1 patient), MK-2206 (with erlotinib, 1 patient), LY2875359 (with erlotinib, 2 patients), PI3K inhibitor (with erlotinib, 1 patient), bevacizumab (with afatinib, 1 patient), and cetuximab (with afatinib, 1 patient).
The median PFS for each patient’s TKI rechallenge was 4.0 months in 29 T790M positive patients versus 3.0 months in 14 T790M negative patients (p = 0.94, HR 0.97, 95% CI 0.50–1.88). Univariable and multivariable analysis were performed to include the same variables as above for first-line TKI with the addition of type of TKI treatment (afatinib or erlotinib) and combination of TKI with chemotherapy versus without chemotherapy. None of these variables were found to be significant. When the 7 patients treated with TKI and chemotherapy together were excluded, the median PFS was unchanged in the two groups (p = 0.80, HR 0.91, 95% CI 0.45–1.84). Response to TKI rechallenge was 22.2% in 18 T790M positive patients versus 0% in 13 T790M negative patients (Table 3, p = 0.12).
Progression-Free Survival and Tumor Response to Chemotherapy
Forty-two T790M positive patients and 18 T790M negative patients were treated with chemotherapy at some point during their disease course. The median PFS on chemotherapy for all 60 patients was 4.75 months. The median PFS for patients who received chemotherapy before TKI (16 T790M positive patients, 4 T790M negative patients) was 5.5 months versus 4.0 months for patients who received chemotherapy after TKI (26 T790M positive patients of which 5 received chemotherapy after trial drug rociletinib, 14 T790M negative patients) (p = 0.019 by log-rank test). The median PFS for 42 T790M positive patients, whether it was given before or after TKI therapy, was 5.0 months versus 4.0 months for 18 T790M negative patients (Figure 3, p = 0.014 by log-rank test). Univariable analysis showed an association with shorter PFS on chemotherapy with the absence of a T790M mutation (Table 4, p = 0.025, HR 1.95, 95% CI 1.09–3.49). Response to chemotherapy was 40.0% in 30 T790M positive patients compared to 21.4% in 14 T790M negative patients (Table 3, p = 0.31).
Figure 3.
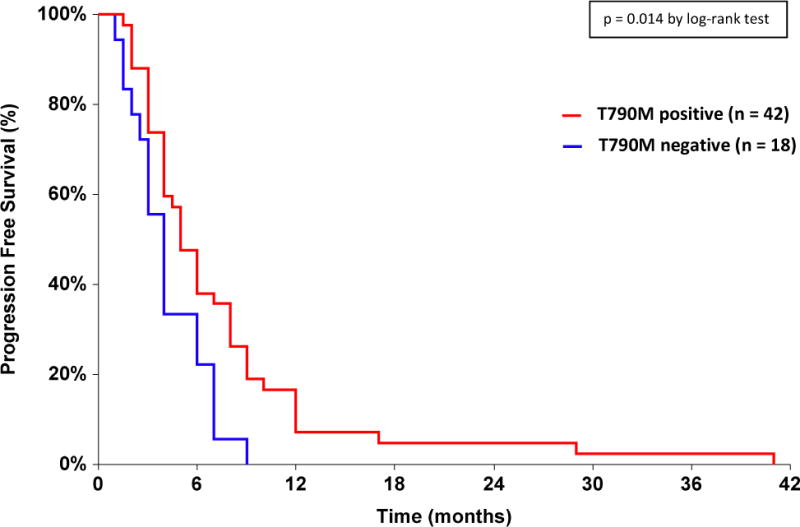
Progression-Free Survival on Chemotherapy Treatment Depending on T790M Mutation Status
Table 4.
Univariable and Multivariable Cox Proportional Hazard Regression Analysis for Progression-Free Survival on Chemotherapy
| Parameter | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| p value | Hazard Ratio (95% Confidence Interval) | p value | Hazard Ratio (95% Confidence Interval) | |
| T790M mutation: negative vs positive | 0.025 | 1.95 (1.09–3.49) | 0.025 | 1.95 (1.09–3.49) |
| Chemotherapy: after vs before TKI | 0.036 | 1.87 (1.04–3.34) | N/A | N/A |
| Sex: female vs male | 0.14 | 1.50 (0.88–2.55) | N/A | N/A |
| Race: Caucasian vs Asian | 0.99 | 1.00 (0.50–1.98) | N/A | N/A |
| Race: Other vs Asian | 0.25 | 0.63 (0.28–1.40) | N/A | N/A |
| Age as continuous variable (as 1 year increase) | 0.40 | 1.01 (0.99–1.03) | N/A | N/A |
| Smoking status: yes vs no | 0.91 | 1.04 (0.55–1.94) | N/A | N/A |
| Exon 19 deletion: absent vs present | 0.94 | 1.02 (0.59–1.77) | N/A | N/A |
| L858R: present vs absent | 0.63 | 1.15 (0.65–2.03) | N/A | N/A |
| Stage: recurrent vs advanced at presentation | 0.40 | 0.69 (0.30–1.63) | N/A | N/A |
Discussion
Our results indicate that T790M positive patients have a significantly longer progression-free survival on first-line TKI treatment compared to T790M negative patients (12.0 months versus 9.0 months, p = 0.021). This is consistent with Kuiper et al. who also showed a statistically longer PFS for first TKI treatment in T790M positive patients23 and other studies which demonstrated a trend towards longer PFS on TKI treatment for tumors with T790M positivity.30,32,38,39 Response rate to first-line TKI treatment, however, demonstrated no difference based on T790M mutation status (ORR 75.0% in T790M positive versus 81.0% in T790M negative, p = 0.76), consistent with other reports.31,39–41 In addition to T790M mutation status, our results showed an association for a shorter PFS on first-line TKI treatment with male sex (p = 0.010) and a positive smoking history (p = 0.011) as has been shown in other studies.6,41,42 We did not find the same positive association with exon 19 deletion compared to L858R that had been documented in some reports.6,15 Results were also unchanged if patients that received TKI in combination with other agents were removed from the analysis, which is in line with current data that there has not yet been a proven benefit for combination use of TKI with other agents.43–45 There has been an improved PFS noted for first-line erlotinib plus bevacizumab,46 but our patient sample was too small (3 patients) to analyze this subgroup alone.
For progression-free survival on TKI rechallenge (TKI treatment given after progression on first-line TKI and prior to enrollment in clinical trial for rociletinib), there was no difference in PFS based on T790M mutation status (4.0 months versus 3.0 months, p = 0.94). Sun et al. also showed no difference in progression-free survival on TKI rechallenge with afatinib.38 However, there have been other studies to suggest that PFS of patients who received a TKI beyond progression to be longer in T790M positive patients.31,32 Response rate did show a numerically higher ORR in T790M positive patients (22.2% in T790M positive versus 0% in T790M negative, p = 0.12).
T790M positive patients also had a significantly longer PFS on chemotherapy (p = 0.025), but no difference in ORR compared to T790M negative patients (40.0% versus 21.4%, p =0.31). To our knowledge, this is the first study to report on differences in PFS and response to chemotherapy based on T790M mutation status. While overall survival was not assessed in this analysis given selective follow-up of patients enrolled in the clinical trials with T790M positivity (and lack of data on screen-failed T790M negative patients), other reports have indicated a longer post-progression survival30,31 and a longer overall survival for T790M positive patients.23,30,32,33,38
Our study, as well as the others documented above, therefore demonstrate that patients with and without the T790M mutation after progression on TKI have different clinical features, with the T790M positive tumors showing a more indolent progression of disease compared to their T790M negative counterparts. Preclinical data is supportive of this theory in that cells harboring the T790M mutation have slower growth47 and mice expressing the T790M mutation develop tumors with a longer latency than other EGFR mutations.48
If the presence or absence of the T790M mutation confers different disease characteristics, different interventions may be considered for tumors with a T790M mutation present and those without. T790M positive patients may benefit from treatment post-progression with TKI’s to preserve indolent T790M positive cells and prevent repopulation of T790M negative accelerated growth cells. Several studies have suggested that continuing TKI treatment beyond progression is a feasible option and may be of benefit in terms of overall survival.49–51 It has also been shown that stopping TKI therapy in patients who develop acquired resistance can result in progression52 and even rapid worsening of symptoms or “disease flare” due to regrowth of TKI-sensitive clones,53 though no association was noted between such disease flare and T790M positivity at time of progression.53 However, data is not yet conclusive at this point as evidenced by the Soria et al IMPRESS study which demonstrated that continuing TKI therapy beyond progression is not of benefit.45 Furthermore, T790M subgroup analysis from this study actually showed that continued TKI maintenance during chemotherapy was more beneficial for T790M negative than T790M positive patients.54 Further studies should be performed to further evaluate TKI continuation beyond progression, specifically in relation to T790M mutation status.
Third-generation TKI’s have been developed to specifically inhibit the T790M mutation. On the basis of clinical efficacy demonstrated in the AURA and AURA2 trials,55,56 osimertinib was granted FDA approval in November 2015 for treatment of patients whose tumors harbor EGFR T790M and whose disease worsened after treatment with other EGFR-inhibiting therapies.57 The most recently published AURA3 study showed increased efficacy for osimertinib compared to chemotherapy in T790M positive EGFR-mutant patients who had progressed on first-line EGFR TKI therapy.58 Although data was also initially promising for rociletinib based on the original publication from the phase 1/2 studies,34 the updated confirmed response rates35 led to termination of drug development.
We found that patients who received chemotherapy before TKI had a trend toward a longer PFS compared to those who received chemotherapy after TKI (p = 0.019). This is consistent with Zeng et al. who demonstrated that frontline EGFR TKI treatment significantly reduced the PFS and response rate of subsequent chemotherapy in comparison to TKI-naïve patients who received frontline chemotherapy.59 Importantly, the response rate and PFS of TKI treatment were similar in chemotherapy-naïve and -refractory patients, suggesting that TKI treatment after chemotherapy would not reduce its efficacy.6,59 Our study actually demonstrated a numerically improved PFS on TKI if TKI was given after chemotherapy (p = 0.0070). Overall survival has likewise been shown to be longer for patients receiving frontline versus post-TKI chemotherapy.59,60 Several preclinical studies have also suggested that EGFR TKI and chemotherapy may influence the efficacy of each other.61,62 And, while there is generous evidence that TKI’s are superior to chemotherapy for PFS when both are given as first-line treatment, an overall survival benefit for first-line TKI over first-line chemotherapy has never been demonstrated, though this has been attributed to crossover at disease progression.7–14 In T790M positive patients in particular, the third-line TKI osimertinib has been shown to be more efficacious than chemotherapy following progression on first-like TKI therapy.58 As all of these studies including ours are retrospective, a prospective trial is needed to evaluate the extent of interaction between TKI and chemotherapy, as there may be a benefit to first-line chemotherapy in EGFR-mutated NSCLC patients.
Our study includes several limitations. First, our data set is of limited size and the data is retrospective. There was also a predominance of T790M positive patients compared to T790M negative patients, likely due to referral bias (some patients were sent for clinical trial screening after already obtaining a biopsy positive for T790M at an outside institution). Second, our sample only included patients who were able to undergo a diagnostic biopsy. While the excluded patients were mostly those whose biopsy did not obtain an adequate tissue sample or those who did not need a biopsy to enroll in the clinical trial, there were also patients who never underwent biopsy due to poor performance status and were presumably sicker individuals with a poorer overall prognosis that could have affected our analysis. Third, there are limitations to our measurements of progression-free survival and response rate, as radiological scans were not available for all patients, given they were treated as standard of care and not a clinical trial protocol and also at outside institutions. Lastly, our study does not differentiate based on other possible mechanisms of resistance to EGFR TKI treatment, such as MET amplification, HER2 amplification, BRAF mutations, PIK3CA mutations, epithelial-to-mesenchymal transition, or small cell transformation,43,63 and the non-T790M patients most likely are not a homogenous group.
Conclusion
In conclusion, our study validates that tumors expressing the T790M resistance mutation have a more indolent progression of disease than their T790M negative counterparts as evidenced by a longer PFS on first-line TKI and chemotherapy, though no difference in PFS was noted for TKI rechallenge. Improved knowledge of the molecular mechanisms of T790M positive and negative forms of resistance is necessary to develop and further evaluate new therapeutic strategies.
Clinical Practice Points.
The clinical efficacy of EGFR-TKI’s in EGFR-mutated NSCLC is limited by the development of drug resistance mutations, most commonly the T790M resistance mutation.
The present study aimed to further characterize patients harboring a T790M mutation in terms of their response to various treatments including EGFR-TKI’s (first-line and rechallenge) and chemotherapy.
In our cohort, T790M positive patients were found to have a longer PFS on first-line TKI (p = 0.021), but there was no difference in response rate based on T790M mutation status (p = 0.76).
There was no difference in PFS on TKI rechallenge based on T790M mutation status (p = 0.94), though there was a trend towards higher response rate in T790M positive patients (p = 0.12).
T790M positive patients also had a longer PFS on chemotherapy (p = 0.025) and a trend towards higher response rate compared to their T790M negative counterparts (p = 0.31).
Our study confirms that T790M positive patients have a more indolent progression of disease compared to T790M negative patients.
Acknowledgments
Research reported in this publication was supported by National Cancer Institute of the National Institutes of Health under award number RO1CA208403. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. JW Goldman reports grant support, personal fees, and non-financial support from Clovis Oncology and has served on the advisory board for them.
Abbreviations
- BR
best response
- ECOG
Eastern Cooperative Oncology Group
- EGFR
epidermal growth factor receptor
- NSCLC
non-small cell lung cancer
- ORR
overall response rate
- OS
overall survival
- PI3K
phosphoinositide 3-kinase
- PFS
progression-free survival
- PPS
post-progression survival
- RECIST
Response Evaluation Criteria in Solid Tumours
- TKI
tyrosine kinase inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest: JW Goldman reports grant support, personal fees, and non-financial support from Clovis Oncology and has served on the advisory board for them.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). She is responsible for communication with the other authors about progress, submissions of revisions, and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from dgaut@mednet.ucla.edu.
Signed by all authors as follows:
Daria Gaut 5/30/17
Myung Shin Sim 5/26/17
Yuguang Yue 5/25/17
Brian R. Wolf 5/24/17
Phillip A. Abarca 5/26/17
James M. Carroll 5/26/17
Jonathan W. Goldman 5/30/17
Edward B. Garon 5/30/17
References
- 1.Non-small cell lung cancer survival rates, by stage. American Cancer Society. http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-survival-rates. Updated May 16, 2016. Accessed November 6, 2016.
- 2.Shi Y, Au JS-K, Thongprasert S, et al. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non–Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gahr S, Stoehr R, Geissinger E, et al. EGFR mutational status in a large series of Caucasian European NSCLC patients: data from daily practice. Br J Cancer. 2013;109(7):1821–1828. doi: 10.1038/bjc.2013.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinersman JM, Johnson ML, Riely GJ, et al. Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. J Thorac Oncol. 2011;6(1):28–31. doi: 10.1097/JTO.0b013e3181fb4fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlesi F, Blons H, Beau-Faller M, et al. Biomarkers (BM) France: Results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts) J Clin Oncol. 2013;31(suppl) abstr 8000. [Google Scholar]
- 6.Rosell R, Moran T, Queralt C, et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 7.Gao G, Ren S, Li A, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer. 2012;131(5):E822–E829. doi: 10.1002/ijc.27396. [DOI] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 9.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 11.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 13.Han J-Y, Park K, Kim S-W, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y-L, Zhou C, Liam C-K, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Sheng J, Kang S, et al. Patients with Exon 19 Deletion Were Associated with Longer Progression-Free Survival Compared to Those with L858R Mutation after First-Line EGFR-TKIs for Advanced Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS One. 2014;9(9):e107161. doi: 10.1371/journal.pone.0107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackman DM, Yeap BY, Sequist LV, et al. Exon 19 Deletion Mutations of Epidermal Growth Factor Receptor Are Associated with Prolonged Survival in Non–Small Cell Lung Cancer Patients Treated with Gefitinib or Erlotinib. Clin Cancer Res. 2006;12(13):3908–3914. doi: 10.1158/1078-0432.CCR-06-0462. [DOI] [PubMed] [Google Scholar]
- 17.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer. 2006;95(8):998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic Implication of EGFR, KRAS, and TP53 Gene Mutations in a Large Cohort of Japanese Patients with Surgically Treated Lung Adenocarcinoma. J Thorac Oncol. 2009;4(1):22–29. doi: 10.1097/JTO.0b013e3181914111. [DOI] [PubMed] [Google Scholar]
- 19.Jänne PA, Wang X, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol. 2012;30(17):2063–2069. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ke E-E, Wu Y-L. EGFR as a Pharmacological Target in EGFR-Mutant Non-Small-Cell Lung Cancer: Where Do We Stand Now? Trends Pharmacol Sci. 2016;37(11):887–903. doi: 10.1016/j.tips.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuiper JL, Heideman DAM, Thunnissen E, et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patients. Lung Cancer. 2014;85(1):19–24. doi: 10.1016/j.lungcan.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 25.Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 26.Mok T, Kazuhiko N, Rosell R, et al. Phase III randomized, open label study (ARCHER 1050) of first-line dacomitinib (D) versus gefitinib (G) for advanced (adv) non-small cell lung cancer (NSCLC) in patients (pts) with epidermal growth factor receptor (EGFR) activating mutation(s) J Clin Oncol. 2013;31(suppl) abstr TPS8123. [Google Scholar]
- 27.Ellis PM, Shepherd FA, Millward M, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014;15(12):1379–1388. doi: 10.1016/S1470-2045(14)70472-3. [DOI] [PubMed] [Google Scholar]
- 28.Yap TA, Popat S. Toward precision medicine with next-generation EGFR inhibitors in non-small-cell lung cancer. Pharmgenomics Pers Med. 2014;7:285–295. doi: 10.2147/PGPM.S55339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462(7276):1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hata A, Katakami N, Yoshioka H, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor. Cancer. 2013;119(24):4325–4332. doi: 10.1002/cncr.28364. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer. 2014;84(3):295–300. doi: 10.1016/j.lungcan.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Uramoto H, Yano S, Tanaka F. T790M is associated with a favorable prognosis in Japanese patients treated with an EGFR-TKI. Lung Cancer. 2012;76(1):129–130. doi: 10.1016/j.lungcan.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Sequist LV, Soria J-C, Goldman JW, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372(18):1700–1709. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 35.Sequist LV, Soria J-C, Camidge DR. Update to Rociletinib Data with the RECIST Confirmed Response Rate. N Engl J Med. 2016;374(23):2296–2297. doi: 10.1056/NEJMc1602688. [DOI] [PubMed] [Google Scholar]
- 36.Forde PM, Ettinger DS. Managing acquired resistance in EGFR-mutated non-small cell lung cancer. Clin Adv Hematol Oncol. 2015;13(8):528–532. http://www.ncbi.nlm.nih.gov/pubmed/26351816. [PubMed] [Google Scholar]
- 37.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Sun J-M, Ahn M-J, Choi Y-L, Ahn JS, Park K. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer. 2013;82(2):294–298. doi: 10.1016/j.lungcan.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Sakai K, Horiike A, Irwin DL, et al. Detection of epidermal growth factor receptor T790M mutation in plasma DNA from patients refractory to epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Sci. 2013;104(9):1198–1204. doi: 10.1111/cas.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M Mutation and BRCA1 mRNA Expression in Erlotinib-Treated Advanced Non–Small-Cell Lung Cancer Patients with EGFR Mutations. Clin Cancer Res. 2011;17(5):1160–1168. doi: 10.1158/1078-0432.CCR-10-2158. [DOI] [PubMed] [Google Scholar]
- 41.Su K-Y, Chen H-Y, Li K-C, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30(4):433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 42.Faehling M, Eckert R, Kuom S, Kamp T, Stoiber KM, Schumann C. Benefit of Erlotinib in Patients with Non-Small-Cell Lung Cancer Is Related to Smoking Status, Gender, Skin Rash and Radiological Response but Not to Histology and Treatment Line. Oncology. 2010;78(3–4):249–258. doi: 10.1159/000315731. [DOI] [PubMed] [Google Scholar]
- 43.Zhou C, Yao LDi. Strategies to Improve Outcomes of Patients with EGFR-Mutant Non– Small Cell Lung Cancer: Review of the Literature. J Thorac Oncol. 2016;11(2):174–186. doi: 10.1016/j.jtho.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(32):4105–4114. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soria J-C, Wu Y-L, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 46.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 47.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regales L, Balak MN, Gong Y, et al. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS One. 2007;2(8):e810. doi: 10.1371/journal.pone.0000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oxnard GR, Lo P, Jackman DM, et al. Delay of chemotherapy through use of post-progression erlotinib in patients with EGFR-mutant lung cancer. J Clin Oncol. 2012;30(suppl) abstr 7547. [Google Scholar]
- 50.Nishie K, Kawaguchi T, Tamiya A, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors Beyond Progressive Disease: A Retrospective Analysis for Japanese Patients with Activating EGFR Mutations. J Thorac Oncol. 2012;7(11):1722–1727. doi: 10.1097/JTO.0b013e31826913f7. [DOI] [PubMed] [Google Scholar]
- 51.Park K, Yu C-J, Kim S-W, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation–Positive Non–Small-Cell Lung Cancer. JAMA Oncol. 2016;2(3):305. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 52.Riely GJ, Kris MG, Zhao B, et al. Prospective Assessment of Discontinuation and Reinitiation of Erlotinib or Gefitinib in Patients with Acquired Resistance to Erlotinib or Gefitinib Followed by the Addition of Everolimus. Clin Cancer Res. 2007;13(17):5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 53.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease Flare after Tyrosine Kinase Inhibitor Discontinuation in Patients with EGFR-Mutant Lung Cancer and Acquired Resistance to Erlotinib or Gefitinib: Implications for Clinical Trial Design. Clin Cancer Res. 2011;17(19):6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soria J, Kim S, Wu Y, et al. Gefitinib/chemotherapy vs chemotherapy in EGFR mutation-positive NSCLC resistant to firstline gefitinib: IMPRESS T790M subgroup analysis. J Thorac Oncol. 2015;10(9):S207–S208. [Google Scholar]
- 55.Jänne PA, Yang JC-H, Kim D-W, et al. AZD9291 in EGFR Inhibitor–Resistant Non– Small-Cell Lung Cancer. N Engl J Med. 2015;372(18):1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Ramalingam SS, Jänne PA, et al. Osimeritinib (AZD9291) in pre-treated pts with T790M-positive advanced NSLCC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol. 2016;11(4S):S152–S155. [Google Scholar]
- 57.FDA approves new pill to treat certain patients with non-small cell lung cancer. U.S. Food & Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm472525.htm. Updated November 24, 2015. Accessed March 7, 2017.
- 58.Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng Z, Yan H, Zhang X, et al. Reduced chemotherapy sensitivity in EGFR-mutant lung cancer patient with frontline EGFR tyrosine kinase inhibitor. Lung Cancer. 2014;86(2):219–224. doi: 10.1016/j.lungcan.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Gridelli C, Ciardiello F, Gallo C, et al. First-Line Erlotinib Followed by Second-Line Cisplatin-Gemcitabine Chemotherapy in Advanced Non-Small-Cell Lung Cancer: The TORCH Randomized Trial. J Clin Oncol. 2012;30(24):3002–3011. doi: 10.1200/JCO.2011.41.2056. [DOI] [PubMed] [Google Scholar]
- 61.Deng Q, Su B, Zhao Y, Zhou C. Sensitivity of two cell lines with acquired resistance to gefitinib to several chemotherapeutic drugs. Zhonghua Zhong Liu Za Zhi. 2008;30(11):813–816. http://www.ncbi.nlm.nih.gov/pubmed/19173824. [PubMed] [Google Scholar]
- 62.Tseng J-S, Yang T-Y, Chen K-C, et al. Prior EGFR tyrosine-kinase inhibitor therapy did not influence the efficacy of subsequent pemetrexed plus platinum in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma. Onco Targets Ther. 2014;7:799. doi: 10.2147/OTT.S62639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New Strategies in Overcoming Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Lung Cancer. Clin Cancer Res. 2011;17(17):5530–5537. doi: 10.1158/1078-0432.CCR-10-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]


