Abstract
The regeneration of oligodendrocytes is a crucial step in recovery from demyelination, as surviving oligodendrocytes exhibit limited structural plasticity and rarely form additional myelin sheaths. New oligodendrocytes arise through the differentiation of platelet-derived growth factor receptor α (PDGFRα) expressing oligodendrocyte progenitor cells (OPCs) that are widely distributed throughout the CNS. Although there has been detailed investigation of the behavior of these progenitors in white matter, recent studies suggest that disease burden in multiple sclerosis (MS) is more strongly correlated with gray matter atrophy. The timing and efficiency of remyelination in gray matter is distinct from white matter, but the dynamics of OPCs that contribute to these differences have not been defined. Here we used in vivo genetic fate tracing to determine the behavior of OPCs in gray and white matter regions in response to cuprizone-induced demyelination. Our studies indicate that the temporal dynamics of OPC differentiation varies significantly between white and gray matter. While OPCs rapidly repopulate the corpus callosum and mature into CC1 expressing mature oligodendrocytes, OPC differentiation in the cingulate cortex and hippocampus occurs much more slowly, resulting in a delay in remyelination relative to the corpus callosum. The protracted maturation of OPCs in gray matter may contribute to greater axonal pathology and disease burden in MS.
Keywords: oligodendrocyte progenitor cells, demyelination, gray matter, remyelination, multiple sclerosis
Introduction
In the central nervous system (CNS), oligodendrocytes serve two principal functions; they generate myelin sheaths that enable fast and efficient impulse conduction and, in a more recently appreciated role, they provide both metabolic and trophic support to axons (Funfschilling et al. 2012; Nave 2010). Destruction or loss of myelin results in impaired saltatory conduction and perturbed neural circuitry in the short-term, but if sustained can lead to neurodegeneration. The functional consequences of CNS demyelination are apparent in patients suffering from multiple sclerosis (MS), an immune-mediated disease that destroys myelin, oligodendrocytes and ultimately axons (Ferguson et al. 1997; Tallantyre et al. 2010).
In a reparative process known as remyelination, platelet-derived growth factor receptor α (PDGFRα) expressing oligodendrocyte progenitor cells (OPCs) can proliferate, migrate to demyelinated lesion sites and subsequently differentiate into myelinating oligodendrocytes. Remyelination restores axonal function and health and thus limits neurodegeneration in the long-term (Duncan et al. 2009; Franklin and Ffrench-Constant 2008; Schultz V et al. 2017). Unfortunately, this process is often inefficient or can fail completely for reasons that are not well understood. Genetic fate-mapping studies in mouse models of MS have greatly added to our knowledge of the cellular dynamics of OPC-mediated remyelination (Tripathi et al. 2010; Xing et al. 2014; Zawadzka et al. 2010; Furusho M et al. 2014). While these studies indicate that OPCs are primarily responsible for mediating CNS remyelination, there has been some controversy regarding their ultimate commitment to the oligodendrocyte fate and whether OPCs resident in the parenchyma or those derived from the subventricular zone are primarily responsible for repair (Xing et al. 2014; Brousse et al. 2015; Franco et al. 2008; Guglielmetti et al. 2014; Franklin and Gallo 2014). The cellular composition, extent of inflammation, and extracellular matrix are distinct in gray versus white matter following demyelination (Bo et al. 2003; Brink et al. 2005; Chang et al. 2012; Peterson et al. 2001), but we do not yet understand if the dynamics of de- and remyelination are affected by these differing environments. Thus, further studies using these models are paramount to understanding the cues, both cell intrinsic and environmental, that determine the potential of OPCs to differentiate and remyelinate.
MS was long considered a disease of the white matter, but recent magnetic resonance imaging (MRI) and pathology studies have revealed extensive gray matter demyelination in MS patients, which is thought to make a significant contribution to disease burden (Klaver et al. 2015; Rudick and Trapp 2009). Indeed, gray matter atrophy has been found to correlate more strongly with cognitive and physical disability than white matter atrophy (Fisniku et al. 2008). Notably, observations from cortical lesions spanning both white and gray matter indicate that these differing environments may influence the timing, and potentially the efficiency, of remyelination (Albert et al. 2007; Chang et al. 2012).
To investigate how the cellular dynamics of OPC differentiation and remyelination vary between white and gray matter, we performed in vivo genetic fate tracing in mice following cuprizone-mediated demyelination. Our studies indicate that the maturation of OPCs is significantly slower and remyelination less complete in gray matter than white matter regions. This reduced response of OPCs in gray matter lesions may enhance axonal stress, leading to neurodegeneration and cognitive decline in MS.
Materials and Methods
Animals
The care and treatment of animals in all procedures strictly followed the NIH Guide for the Care and Use of Laboratory Animals and the Johns Hopkins University Institutional Animal Care and Use Committee. Marker Assisted Accelerated Backcrossing (MAX-BAX®, Charles River) technology was used to expedite the breeding of C57BL/6 congenic PDGFαR-CreER mice (Kang et al. 2010). Rosa26-eYFP mice (Jackson Lab Stock # 006148), also on a C57BL/6 background, were purchased from the Jackson Laboratory and crossed to the congenic PDGFRα-CreER mice. Age-, sex-, and genotype-matched mice were used in all experiments as controls. Mice were housed at standard temperature (21°C) and in a light controlled environment with ad libitum access to the food and water.
Cuprizone exposure
Starting at 8 weeks of age, equal numbers of male and female PDGFRα-CreER;Rosa-eYFP mice were fed a diet of milled, irradiated 18% protein rodent diet (Teklad Global) alone (Control) or containing 0.2% w/w bis(cyclohexanone) oxaldihydrazone (Cuprizone, Sigma-Aldrich) for a total of 6 weeks to induce demyelination. Mice were returned to a regular diet after this time to allow remyelination (Fig. 1B).
Figure 1.
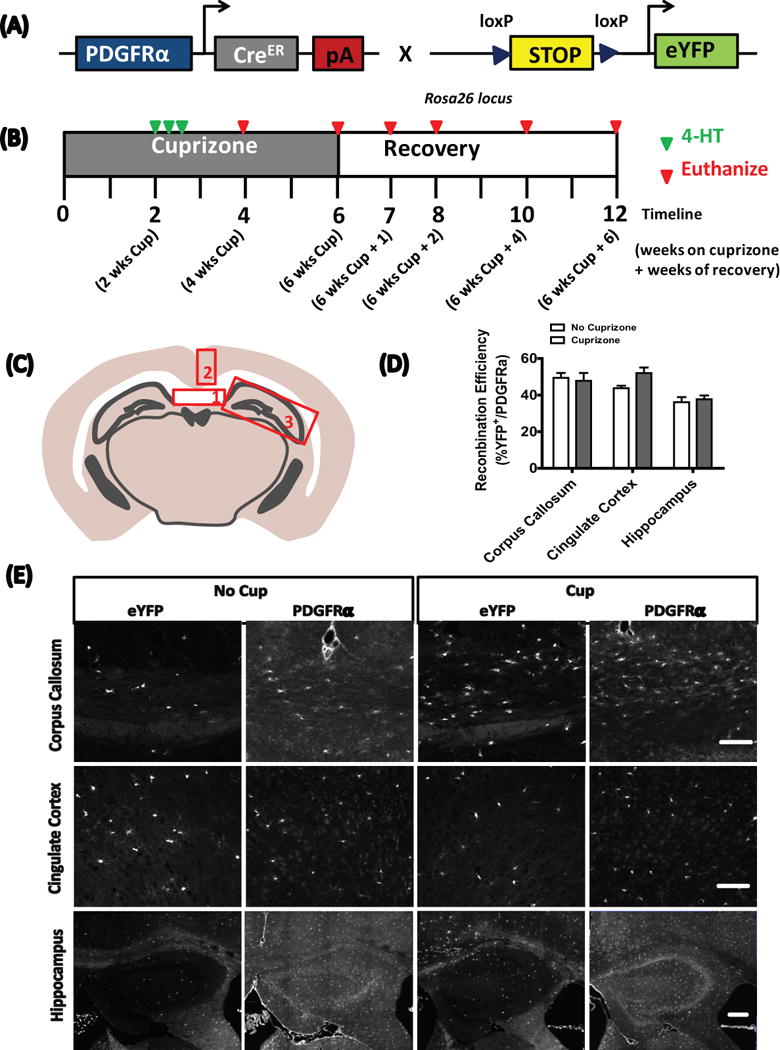
4-Hydroxytamoxifen administration
4-hydroxytamoxifen (4-HT, Sigma-Aldrich) was prepared as previously described (Badea et al. 2003). Two weeks after the start of cuprizone feeding, PDGFRα-CreER;Rosa26-eYFP mice were injected with a total of 3 mg of 4-HT over three days to induce recombination (Fig. 1). Recombination was induced in control mice (no cuprizone exposure) at 10 weeks of age.
Immunohistochemistry and Black Gold II staining
Control or cuprizone-treated mice were euthanized at 4, 6, 7, 8, 10 and 12 weeks following initiation of the experiment (Fig. 1B). Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg BW) and perfused transcardially with chilled PBS followed by 4% paraformaldehyde. Brains were collected, post-fixed overnight, and cryoprotected in 30% sucrose for ∼48 h before freezing. Tissue was sectioned on a cryostat (20 μm) and mounted onto glass slides (Superfrost Plus; Fisher). For immunolabeling of adenomatous polyposis coli (APC), sections were initially treated with boiling 10 mM citric acid buffer (pH 6) for 20 minutes. Sections were permeabilized and blocked in PBS containing 5% normal goat serum and 0.4% Triton X-100 for 1 h at room temperature and then incubated overnight at 4°C in PBS containing 3% normal goat serum, 0.1% Triton X-100, and primary antibody as follows: mouse anti-APC (clone CC1, 1:50, Calbiochem), rabbit anti-PDGFRα (1:600, courtesy of Dr. Bill Stallcup), mouse anti-NeuN (1:1000, Millipore), rabbit ant-GFAP (1:1000, Dako) and chicken anti-GFP (1:1,000, AbCam). Species-specific secondary antibodies directly conjugated to Alexa fluorophores (1:1000, Invitrogen) were used to visualize immunostaining. Sections were incubated in secondary antibodies for 1 h at room temperature before mounting in anti-fade reagent with DAPI (ThermoFisher). No primary controls were included in all experiments to help assess non-specific, background staining.
Black Gold II staining (Black Gold II myelin staining kit, Millipore) was performed according to the manufacturer’s instructions. In brief, slides were incubated in 0.3% Black Gold II at 60°C for 12–20 min until the thinnest fibers were stained. The slides were then fixed in 1% sodium thiosulfate for 3 min, dehydrated using a series of gradated alcohols, cleared in xylene, and cover-slipped with VectaMount Permanent Mounting Media (Vector Laboratories).
Image acquisition and analysis
Black Gold II stained sections were imaged on a BX41 Olympus microscope using ImageProPlus5.1 software (Media Cybernetics). For lineage tracing, slides were imaged using a Zeiss Axio Observer Z1 epifluorescence microscope and Axiovision software with the appropriate excitation and emission filters. A total of 4 sections were examined per mouse, and 8 mice (4 male, 4 female) were analyzed per group. Areas were chosen randomly within the regions of interest. Post-acquisition image analysis and cell counts were performed using Zeiss Zen software.
Statistical analysis
Statistical analyses were conducted using the GraphPad Prism software (GraphPad). Two-tailed Student’s t test was used to analyze normally distributed data. Two way ANOVAs were used to analyze differences between cuprizone and control groups at the same experimental time points. Results were considered significant if the p value was <0.05. Error bars indicate standard error of the mean (SEM) in all the figures.
Results
Recombination efficiency in PDGFRα-CreER;Rosa26-eYFP mice is not affected by cuprizone administration
To follow the fate of OPCs after cuprizone-induced demyelination, we crossed hemizygous PDGFRα-CreER transgenic mice (Kang et al. 2010) with the Rosa26-eYFP reporter strain (Fig 1A). Equal numbers of male and female double transgenic mice were then assigned to either a control or cuprizone diet. As previous studies indicated that OPCs first begin to accumulate in the corpus callosum 2 weeks after initiating the cuprizone challenge, and peak between 2 to three weeks thereafter (Gudi et al. 2009; Xing et al. 2014), we administered 4-HT at 2 weeks of cuprizone-supplementation (or control) in order to yield the largest pool of recombined OPCs (Fig. 1B). To investigate the possibility that cuprizone might influence recombination efficiency, we compared this parameter across groups in three regions of interest; the corpus callosum, the cingulate cortex and the hippocampus. Recombination efficiency was similar in both cuprizone- and control-treated mice after 2 weeks of exposure (Fig. 1C, D, E), thus allowing direct comparisons in OPC fates between experimental groups.
Rapid repopulation of the demyelinated corpus callosum by OPC-derived oligodendrocytes
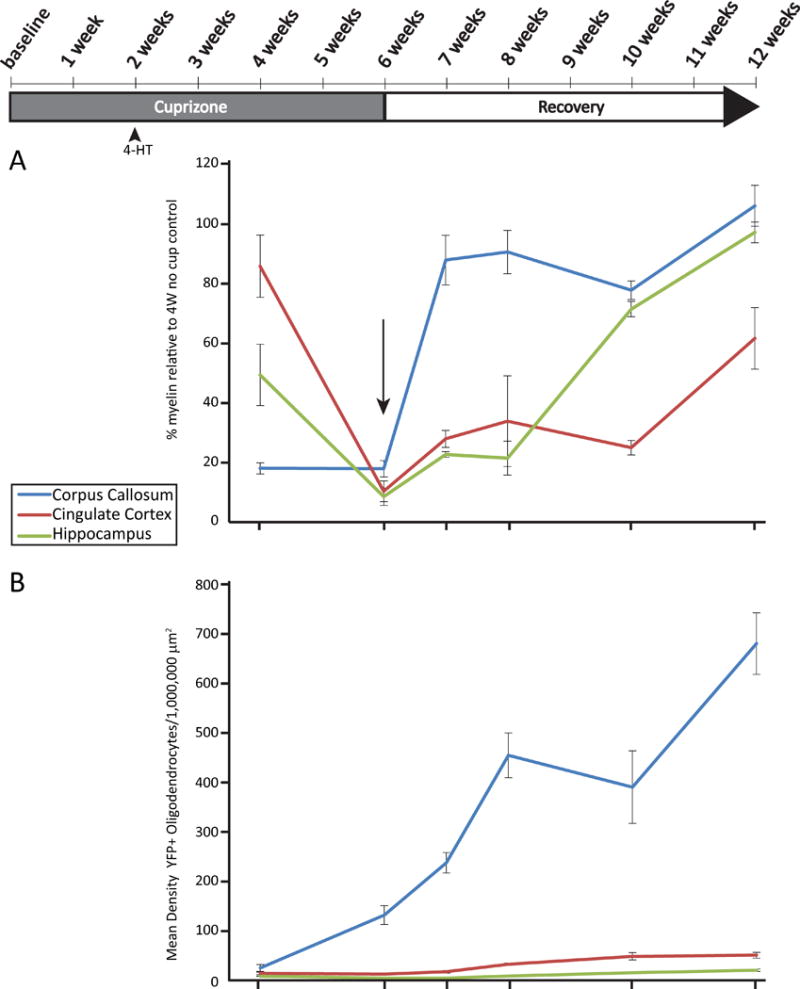
We selected the body of the corpus callosum (above the fornix) to study the dynamics of white matter de- and re-myelination, as this provides fiducial landmarks for comparisons between animals. Black Gold II staining revealed that extensive demyelination was apparent by 4 weeks, with the lowest levels of myelin by 6 weeks of cuprizone treatment (Fig. 2A and C). Remyelination followed, such that by 6 weeks of recovery, the intensity of area stained with Black Gold II was not significantly different from control mice. A significant decrease in the number of CC1-immunoreactive (CC1+) oligodendrocytes was also found after 4 weeks of cuprizone treatment (Fig. 2A, D), but this rapidly rebounded by 6 weeks of cuprizone (despite continued treatment), and persisted over the 6 subsequent recovery weeks. Interestingly, the density of CC1+ oligodendrocytes at 6 weeks of recovery was greater than that of control mice treated for 10 weeks, suggesting that cuprizone-induced injury leads to differentiation of more oligodendrocytes than would be expected without injury. In support of this, the density of PDGFRα+ OPCs at 4 weeks of cuprizone-treatment was ~ 2.5× greater than age-matched controls (Fig. 2A, E) and their density remained elevated until week seven, indicating that there is a massive mobilization of progenitors after demyelination to differentiate and remyelinate the corpus callosum.
Figure 2.
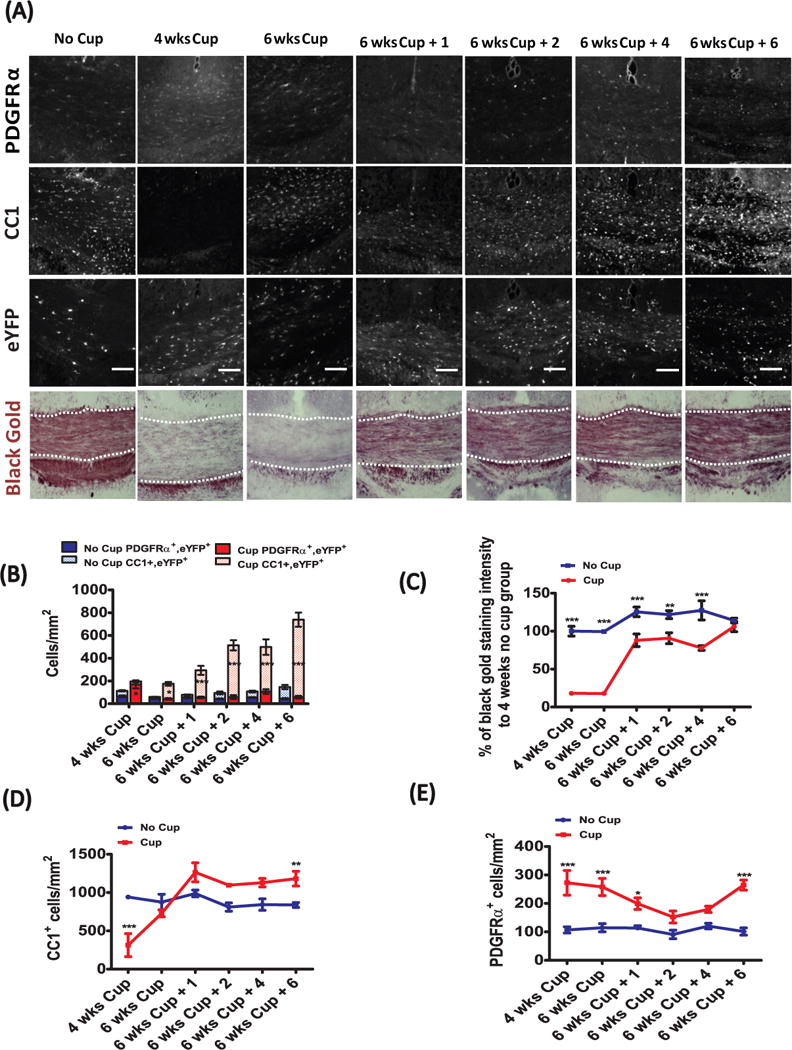
To determine whether new oligodendrocytes were derived from OPCs born after cuprizone exposure, fate-mapping analysis was performed on transgenic mice that were induced to express eYFP in a subset of OPCs during the second week of cuprizone exposure (PDGFRα-CreER;Rosa26-eYFP), and then were analyzed 2 weeks later for eYFP expression in mature oligodendrocytes. The number of PDGFRα+/eYFP+ OPCs was significantly greater post-cuprizone than control-treated animals at this time point. However, unlike the PDGFRα+ OPC counts (Fig. 2E), the number of PDGFRα+/eYFP+ cells (Fig. 2B) did not remain significantly elevated relative to control-treated mice past this initial time-point. Rather, there was a steady and significant rise in the number of CC1+/eYFP+ oligodendrocytes from 6 weeks of cuprizone treatment and across 6 weeks of recovery, indicating that the majority of the OPCs labeled at 2 weeks of cuprizone treatment differentiated into mature oligodendrocytes. This demonstrates that cuprizone-treatment mobilizes a pool of OPCs in the corpus callosum that proliferate and then differentiate into mature oligodendrocytes, at a rate greater than control-fed, age-matched animals.
Gray matter remyelination is mediated through slow repopulation by parenchymal OPCs
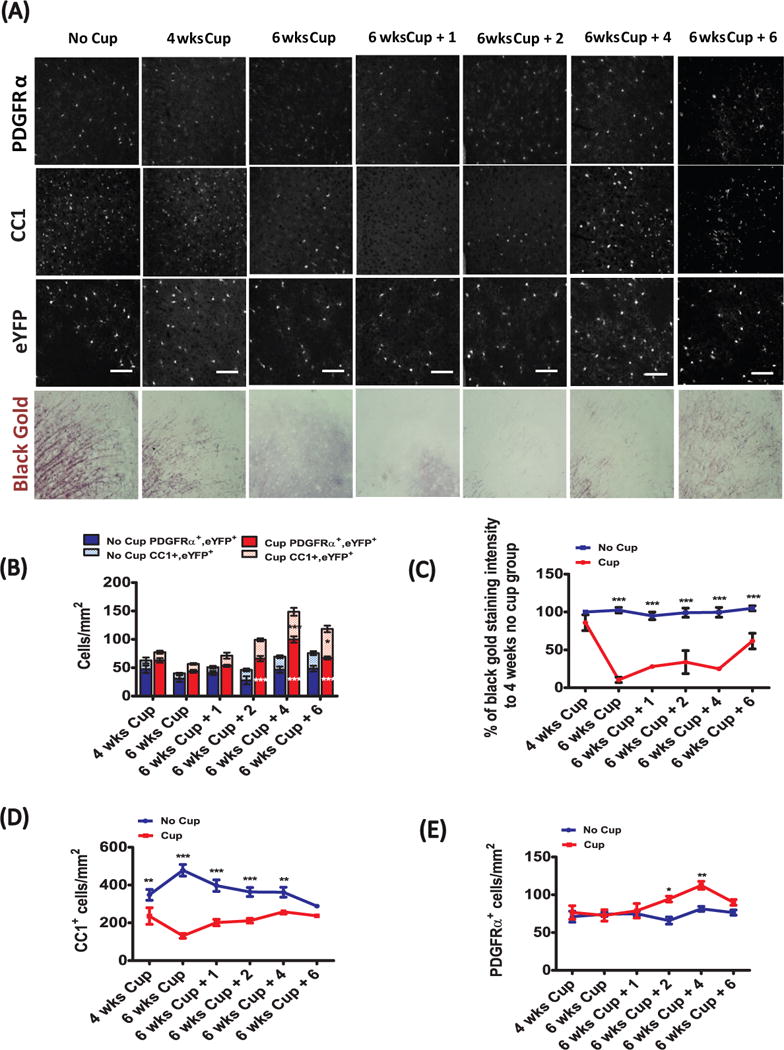
In contrast to the relatively rapid rate in the corpus callosum, the reappearance of myelin occurred more slowly in the cingulate cortex. Initially, similar to the corpus callosum, maximal demyelination occurred at 6 weeks of cuprizone treatment (Fig. 3A, C). However, remyelination progressed more slowly than in the white matter, and only reached about two third of the control value over the 6 weeks of recovery. In agreement with these findings, the density of PDGFRα+ OPCs only became significantly elevated relative to control at 8 and 10 week time-points (or 2 and 4 weeks of recovery; Fig 3A, E). While OPC density increased at 8 and 10 weeks, the density of CC1+ oligodendrocytes remained decreased until 12 weeks (Fig. 3A, D), consistent with delayed recruitment and differentiation of OPCs. Analysis of PDGFRα-CreER;Rosa26-eYFP mice revealed that the number of PDGFRα+/eYFP+ OPCs increased relative to age-matched controls only at 2 and 4 weeks after stopping cuprizone. There was a trend for increased numbers of CC1+/eYFP+ cells by 2 weeks of recovery, that by 4 weeks of recovery was significantly elevated relative to age-matched, indicating that the production of new cortical oligodendrocytes is delayed relative to the corpus callosum and corresponds to a delay in relative proliferation of OPCs after cuprizone-exposure.
Figure 3.
To assess whether this delay in OPC mobilization is specific to this region of the cortex or is a general feature of gray matter demyelination, we examined the dynamics of demyelination and remyelination in the stratum lacunosum moleculare (SLM) region of the hippocampus, a region that contains myelinated fibers from the temporoammonic pathway. The behavior of OPCs, as assessed by counting PDGFRα+ OPCs and PDGFRα+/eYFP+ OPCs in PDGFRα-CreER;Rosa26-eYFP mice, revealed a similarly delayed response as in the cingulate cortex. Black Gold II staining intensity was lowest after 6 weeks of cuprizone-treatment (Fig 4A, C). Unlike recovery in the cingulate cortex, by 6 weeks black gold II staining was comparable to control mice. Moreover, the density of CC1+ oligodendrocytes in the hippocampus was also lowest after 6 weeks of cuprizone treatment, and slowly increased to the level of control mice over 6 weeks of recovery. (Fig. 4A, D). The density of PDGFRα+ cells rose above controls slightly but significantly after 1 week of recovery and through 4 weeks of recovery (Fig. 4E). A significant increase in the number of PDGFRα+/eYFP+ cells was observed at the 4 week recovery time-point, coincident with an increase in the number of CC1+/eYFP+ cells, and persisted to 6 weeks of recovery (Fig. 4A, B). It appears that proliferation of PDGFRα+/eYFP+ cells occurs during cuprizone treatment in white matter, but only after stopping cuprizone treatment in gray matter regions. Together, these results indicate that myelin repair through OPC mobilization, oligodendrogenesis and remyelination are significantly slower in gray matter regions than in the white matter of the corpus callosum (summarized in Figure 6).
Figure 4.
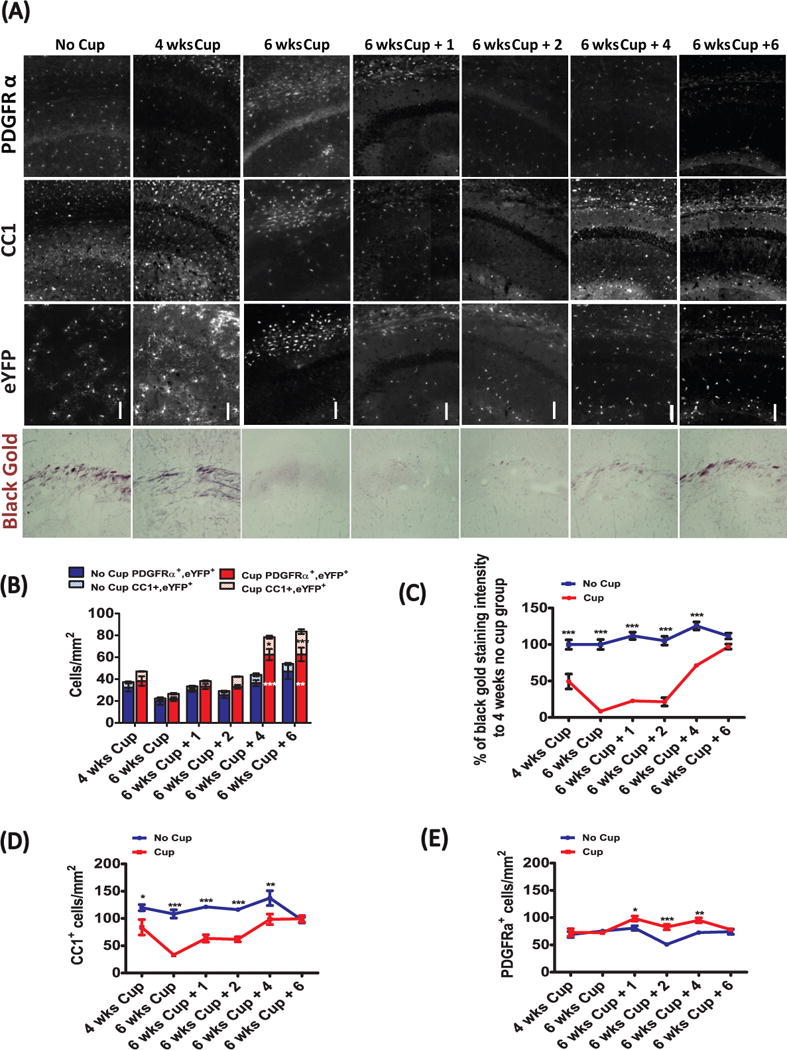
Figure 6.
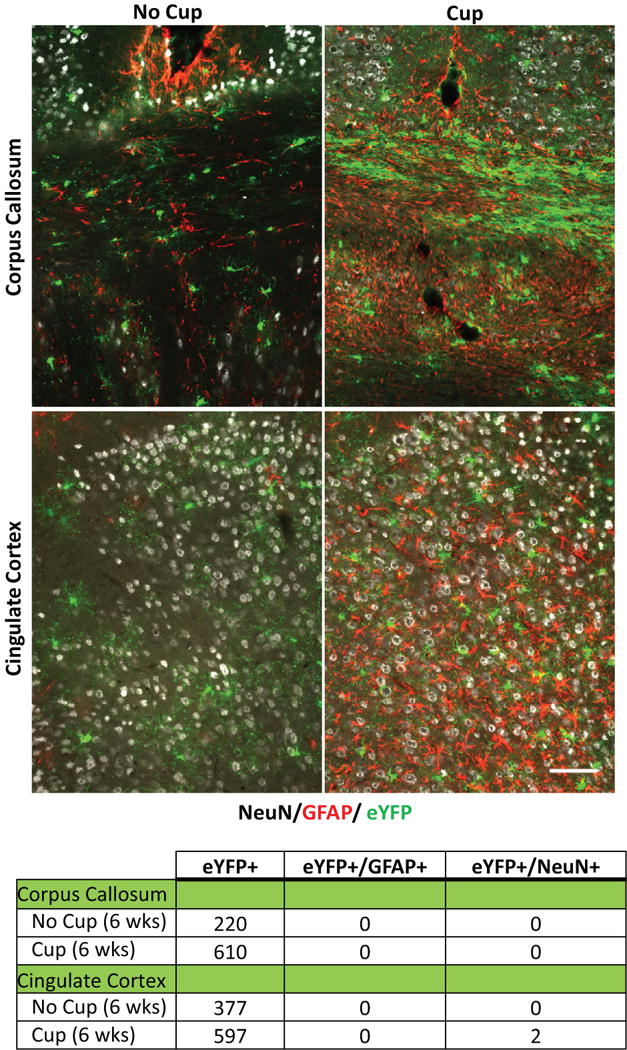
OPCs remain committed to the oligodendrocyte lineage following cuprizone-induced demyelination
OPCs (PDGFRα+, NG2+) have been shown to exhibit lineage plasticity in vitro depending on the combination of growth factors in the extracellular media (Kondo and Raff 2000) and in vivo in response to traumatic injury (Vigano et al. 2016), raising the possibility that these cells may adopt different fates depending as a result of environmental influences. To investigate the potential of OPCs for lineage deviation following cuprizone-induced demyelination, we examined the corpus callosum and cingulate cortex for evidence of eYFP+ cells that were immunoreactive for either GFAP, indicating that these cells have trans-differentiated into astrocytes, or NeuN, indicating that they have developed into neurons. However, no eYFP+ cells that exhibited immunoreactivity for GFAP or NeuN were observed in the corpus callosum of either control or cuprizone fed (at the 6 week time-point) mice (Fig. 5). Similarly, no eYFP+/GFAP+ cells were found in the cingulate cortex in control or cuprizone treated mice, and only a small number of eYFP+/NeuN+ cells (2/597) were detected, consistent with previous studies indicating that a small number of neurons express CreER in PDGFRα-CreER;Rosa26-eYFP mice (Kang et al., 2007). These results indicate that OPCs remain committed to the oligodendrocyte lineage in both gray and white matter after cuprizone mediated demyelination.
Figure 5.
Discussion
OPCs are widely distributed throughout gray and white matter and constitute between 5-8% of the total cell population in the adult CNS (Dawson et al. 2003; Gallo et al. 2008). These cells retain the ability to proliferate and differentiate long after the early postnatal period of intense myelination has ended, and continue to add new oligodendrocytes to brain circuits, albeit at slower rates, establishing these cells as the most abundant progenitors in the adult brain. Although long suspected to be the source of new oligodendrocytes following demyelination (Di Bello et al. 1999; Levine et al. 1993; Reynolds et al. 2002), this hypothesis was confirmed when in vivo genetic labeling of OPCs revealed that they are mobilized after demyelination and differentiate into myelinating oligodendrocytes (Rivers et al. 2008; Tripathi et al. 2010; Xing et al. 2014; Zawadzka et al. 2010). The development and fate of OPCs in both health and disease contexts has been studied extensively in white matter, but much less is known about the dynamics of these progenitors in gray matter regions. In this study, we used double transgenic PDGFRα-CreER;Rosa26-eYFP mice to trace the lineage of OPCs in both white and gray matter regions following cuprizone-induced demyelination. Our results indicate that the dynamics of OPC accumulation and differentiation into mature oligodendrocytes are distinct between gray and white matter. Despite the comparable density of OPCs in gray and white matter, the regeneration of oligodendrocytes occurred substantially slower in gray matter. Since observations from cortical MS lesions also indicate that remyelination efficiency may be affected by the differing environments of gray and white matter (Albert et al. 2007; Chang et al. 2012), these results may have important implications for understanding the dynamics of remyelination in MS.
In the corpus callosum, a 6 week exposure to cuprizone, followed by return to a regular diet, produced a pattern of de- and remyelination, as revealed by Black Gold II staining, consistent with previous observations (Matsushima and Morell 2001; Skripuletz et al. 2011). To define the dynamics of the oligodendrocyte cell lineage in response to this demyelinating insult, we examined both the density of CC1+ oligodendrocytes, PDGFRα+ OPCs and traced the fate of a pool of OPCs that were genetically labeled 2 weeks post-cuprizone treatment, just prior to their maximal accumulation in the corpus callosum (Gudi et al. 2009; Xing et al. 2014). After 4-weeks of cuprizone treatment,, the number of PDGFRα+/eYFP+ cells rose significantly above that observed in control mice, coincident with a significant drop in the density of CC1+ oligodendrocytes. At later time-points, the relative number of PDGFRα+/eYFP+ cells appear to decrease, corresponding to the same time course of remyelination and increase in the CC1+/eYFP+ population increases (depicted in Figure 6, blue lines) over 6 weeks of recovery. In contrast to this relatively rapid repair process within the corpus callosum, the response in both gray matter regions (cingulate cortex and the hippocampus) was slower (Figure 6, red and green lines, respectively). Here, loss of CC1+ oligodendrocytes and maximal demyelination occurred by 6 weeks, in accordance with previous findings (Norkute et al. 2009; Skripuletz et al. 2011; Skripuletz et al. 2008). The production of CC1+/eYFP+ cells (and myelin) in gray matter regions was delayed and remained significantly below control until 4 to 6 weeks of recovery, with the relative increase of CC1+/eYFP+ cells being remarkably slower and delayed as compared to the corpus callosum (Fig. 6B).
Although local OPC proliferation and migration certainly contribute to recovery following a demyelinating insult, a recent study by Xing et. al. (Xing et al. 2014), revealed a significant role for neural precursor cells (NPCs) derived from the subventricular zone (SVZ) to remyelination of the corpus callosum. The authors of this study found that OPCs derived from Nestin positive NPCs, accumulated in the corpus callosum as early as 2 weeks post-cuprizone challenge. Since this time-frame would allow for genetic recombination and eYFP labeling in our experimental paradigm (since NPC-derived OPCs would by this point express PDGFRα+), it is possible that the expanded pool of PDGFRα+/eYFP cells we observe in the corpus callosum at 4 weeks post-cuprizone includes some NPC-derived OPCs. The late (week 12) increase in PDGFRα+ absolute counts but not PDGFRα+/YFP+ cells (seen in figure 2b and e) appears to represent newly formed OPCs even after the time of 4HT induced recombination at week 2, which could reflect late mobilization of NPC from the SVZ. It has been suggested that these newly generated OPCs may have an enhanced ability to undergo differentiation and thus may outcompete resident OPCs in the repair process. Although, it is not clear to what extent NPC-derived OPCs contribute to remyelination in gray matter regions or indeed any region further from the SVZ than the corpus callosum, if the dispersion of these SVZ-derived OPCs is limited, it could contribute to the slower rates of oligodendrocyte regeneration and remyelination in gray matter regions distant from the SVZ. Future studies to examine whether SVZ derived OPCs migrate equally to the gray matter as the white matter should be done.
Beyond proximity to the SVZ, there may be environmental factors inherent to the white or gray matter that influence the rate of OPC accumulation and/or differentiation. For example, while cuprizone-induced demyelination of the corpus callosum is accompanied by a rapid and robust microgliosis, the response microglia in the cortex to cuprizone is less pronounced (Buschmann et al. 2012; Gudi et al. 2009). Since activated microglia and macrophages can play a key role in OPC recruitment (Kotter et al. 2005), this muted microglial response could be another potential explanation for delayed OPC mobilization in the cortex. Gray matter regions such as the cortex have also been noted to exhibit reduced astrogliosis in response to cuprizone, when compared to the corpus callosum (Buschmann et al. 2012; Hibbits et al. 2012). While the role of astrocytes in remyelination is still poorly understood, there is evidence that reactive astrocytes may regulate myelin clearance and subsequent remyelination by promoting microglial recruitment (Skripuletz et al. 2013).
Since deviation from the oligodendrocyte lineage has been reported (Guo et al. 2009; Tripathi et al. 2010; Zhu et al. 2008) using in vivo fate tracing approaches similar to those used here, we investigated whether eYFP+ cells might co-immunolabel with either GFAP or NeuN 6 weeks after cuprizone exposure. In agreement with previous work published using this PDGFRα-CreER transgenic mouse line, we did not see any eYFP+ cells that were co-immunolabeled with GFAP, indicating that even under conditions of demyelination and reactive astrogliosis, OPCs do not deviate to an astrocytic fate. We observed rare eYFP+ neurons in these animals, an observation consistent with the small number of neurons that exhibit direct recombination in this line (Kang et al. 2010). Thus, in the context of demyelination, OPCs either remain in the progenitor state or differentiate into oligodendrocytes to restore myelin sheaths.
One limitation of our ex vivo approach is that we cannot definitively exclude whether there is regionally distinct recombination efficiency or cuprizone-induced YFP+ cell death over the time course of cuprizone-induced loss and recovery. Ideally, imaging methods will be developed to longitudinally image the fate of individual cells in both white and gray matter regions simultaneously over the course of demyelination and recovery following cuprizone.
Both white and gray mater demyelination significantly contribute to disease burden in MS. It is therefore of vital importance to understand the factors that may hinder or aide successful remyelination in these regions. Using genetic fate tracing, this study reveals a significant difference in the dynamic response of OPCs to cuprizone-induced demyelination in the corpus callosum when compared to the cingulate cortex and hippocampus. Identifying environmental cues that aide OPC recruitment and/differentiation in gray matter will help to generate strategies to successfully promote remyelination in brain circuits and prevent cognitive decline.
Main Points.
The timing and efficiency of remyelination in gray matter is distinct from white matter.
Our studies indicate that the temporal dynamics of OPC differentiation varies significantly between white and gray matter.
Acknowledgments
This work was supported by the National Institutes of Health Grant R37 NS041435 to P.A.C. The authors wish to thank Shin Kang for providing generously providig the PDGFRα-CreER transgenic mice.
References
- Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–38. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–22. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–31. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- Brink BP, Veerhuis R, Breij EC, van der Valk P, Dijkstra CD, Bo L. The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol. 2005;64:147–55. doi: 10.1093/jnen/64.2.147. [DOI] [PubMed] [Google Scholar]
- Brousse B, Magalon K, Durbec P, Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol Open. 2015;4:980–92. doi: 10.1242/bio.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann JP, Berger K, Awad H, Clarner T, Beyer C, Kipp M. Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. J Mol Neurosci. 2012;48:66–76. doi: 10.1007/s12031-012-9773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Staugaitis SM, Dutta R, Batt CE, Easley KE, Chomyk AM, Yong VW, Fox RJ, Kidd GJ, Trapp BD. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72:918–26. doi: 10.1002/ana.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–88. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Di Bello IC, Dawson MR, Levine JM, Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–81. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- Duncan ID, Brower A, Kondo Y, Curlee JF, Jr, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A. 2009;106:6832–6. doi: 10.1073/pnas.0812500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA, Thompson AJ, Miller DH. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–54. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- Franco PG, Silvestroff L, Soto EF, Pasquini JM. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp Neurol. 2008;212:458–67. doi: 10.1016/j.expneurol.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62:1905–15. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–21. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Roulois AJ, Franklin RJM, Bansal R. Fibroblast Growth Factor Signaling in Oligodendrocyte-Lineage Cells Facilitates Recovery of Chronically Demyelinated Lesions But Is Redundant in Acute Lesions. Glia. 2015;63:1714–28. doi: 10.1002/glia.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Mangin JM, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586:3767–81. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi V, Moharregh-Khiabani D, Skripuletz T, Koutsoudaki PN, Kotsiari A, Skuljec J, Trebst C, Stangel M. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009;1283:127–38. doi: 10.1016/j.brainres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Guglielmetti C, Praet J, Rangarajan JR, Vreys R, De Vocht N, Maes F, Verhoye M, Ponsaerts P, Van der Linden A. Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum. Neuroimage. 2014;86:99–110. doi: 10.1016/j.neuroimage.2013.07.080. [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–70. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbits N, Yoshino J, Le TQ, Armstrong RC. Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN Neuro. 2012;4:393–408. doi: 10.1042/AN20120062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver R, Popescu V, Voorn P, Galis-de Graaf Y, van der Valk P, de Vries HE, Schenk GJ, Geurts JJ. Neuronal and axonal loss in normal-appearing gray matter and subpial lesions in multiple sclerosis. J Neuropathol Exp Neurol. 2015;74:453–8. doi: 10.1097/NEN.0000000000000189. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–7. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol Dis. 2005;18:166–75. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–21. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–16. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–83. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Norkute A, Hieble A, Braun A, Johann S, Clarner T, Baumgartner W, Beyer C, Kipp M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J Neurosci Res. 2009;87:1343–55. doi: 10.1002/jnr.21946. [DOI] [PubMed] [Google Scholar]
- Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–36. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Trapp BD. Gray-matter injury in multiple sclerosis. N Engl J Med. 2009;361:1505–6. doi: 10.1056/NEJMcibr0905482. [DOI] [PubMed] [Google Scholar]
- Schultz V, van der Meer F, Wrzos C, Scheidt U, Bahn E, Stadelmann C, Brück W, Junker A. Acutely damaged axons are remyelinated in multiple sclerosis and experimental models of demyelination. Glia. 2017;65:1350–60. doi: 10.1002/glia.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Gudi V, Hackstette D, Stangel M. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol. 2011;26:1585–97. doi: 10.14670/HH-26.1585. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgartner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain. 2013;136:147–67. doi: 10.1093/brain/aws262. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol. 2008;172:1053–61. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre EC, Bo L, Al-Rawashdeh O, Owens T, Polman CH, Lowe JS, Evangelou N. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult Scler. 2010;16:406–11. doi: 10.1177/1352458510364992. [DOI] [PubMed] [Google Scholar]
- Tripathi RB, Rivers LE, Young KM, Jamen F, Richardson WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–90. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano F, Schneider S, Cimino M, Bonfanti E, Gelosa P, Sironi L, Abbracchio MP, Dimou L. GPR17 expressing NG2-Glia: Oligodendrocyte progenitors serving as a reserve pool after injury. Glia. 2016;64:287–99. doi: 10.1002/glia.22929. [DOI] [PubMed] [Google Scholar]
- Xing YL, Roth PT, Stratton JA, Chuang BH, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J Neurosci. 2014;34:14128–46. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–90. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]


