Abstract
Objective
The aim of this article is to review why the first 1000 days of life are a vulnerable period of human development, and the long-term effects of a nutrition experiment carried out in Guatemala (1969–1977).
Methods
In 1969–77, a supplement called Atole, containing high quality protein, energy and micronutrients, was provided to women during pregnancy and lactation and to children <7 years of age in two villages while in two control villages a low-energy drink called Fresco was provided. The villages were assigned at random to the treatment groups.
Results
Several reasons explain the vulnerability of the first 1000 days: rapid growth and development, high nutritional requirements, greater susceptibility to infections, high sensitivity to programming effects and full dependence on others for care, nutrition, and social interaction. Compared to Fresco, Atole improved total nutrient intakes (protein, energy and micronutrients) and reduced stunting, but only in children < 3 years of age. A study in 2002–2004 showed that schooling, reading, and intelligence were improved in Atole villages, but only in those who received Atole before the age of 3 years. Wages of men were increased by 46% in those provided Atole through the age of 2 years. Findings for cardiovascular disease risk factors were inconclusive, perhaps because of the young age of the sample. A new study focusing on chronic diseases is ongoing (ages 38–54 years).
Conclusions
The Guatemalan studies indicate that substantial improvement in adult human capital and economic productivity resulted from the nutrition intervention. This provides a powerful argument for promoting improvements in nutrition in pregnant women and young children in low income countries.
Keywords: nutrition interventions, first 1000 days, growth and development, stunting, short and long term consequences of child nutrition, human capital, adult health
INTRODUCTION
The first 1000 days of life, which includes gestation and the first two years of life, are a vulnerable period in human development when poor nutrition can have short and long lasting consequences on human health and function. The importance of this period was recognized in the 2008 Lancet series on Maternal and Child Undernutrition but it was referred to as “pregnancy and the first two years of life,” using eight words (Victora et al, 2008). Hillary Clinton, in a speech given in 2010, was probably the first person to use the words “1000 days” in a public event at the launching of a foundation with the same name (http://thousanddays.org/). The catchy, easy to remember name has helped with advocacy for the importance of this period.
The first 1000 days are a window of opportunity because this is when improvements in nutrition can have the greatest impact in populations with poor nutrition. Support for this claim comes from follow-up studies of a nutrition experiment carried out in Guatemala (1969–1977) by the Institute of Nutrition of Central America and Panama (INCAP) in women and young children that have shown long term effects on adult health and human capital. Human capital refers to “the collective skills, knowledge, or other intangible assets of individuals that can be used to create economic value for the individuals, their employers, or their community” (http://www.dictionary.com/browse/human-capital). Human capital includes aspects that can be measured easily such as schooling, reading, and intelligence, as well as biological attributes such as adult height, muscle mass, and work capacity. The origin of the concept of human capital is attributed to Adam Smith who held that the wealth of nations depends in part on the health, nutrition, skills and knowledge of their peoples (Smith, 1776). He believed that poor health and nutrition and lack of education lower economic productivity. The INCAP studies show that this is true and draw attention to the urgent need to address maternal and child undernutrition in poor countries.
The first 1000 days
Rapid growth and development
The fundamental reason why the first 1000 days are a vulnerable period is that this is a time of very rapid growth and development. This can be illustrated by the developmental changes that occur in the brain in this period [Figure 1 here]. There is proliferation and migration of cells, mostly during fetal development, followed by explosive experience-dependent synaptogenesis that creates more neural connections that will ever be needed; for this reason, there is pruning throughout childhood of little used connections in an effort to increase neural efficiency. The three curves shown, from left to right, refer to sensory pathways (vision, hearing), language, and higher cognitive function, respectively. During early life more so than later, lack of key nutrients such as essential amino acids, essential fatty acids, iron, and iodine as well as poor stimulation and social neglect will have long-lasting consequences on learning capacity, behavior, and the ability to regulate emotions.
Figure 1.
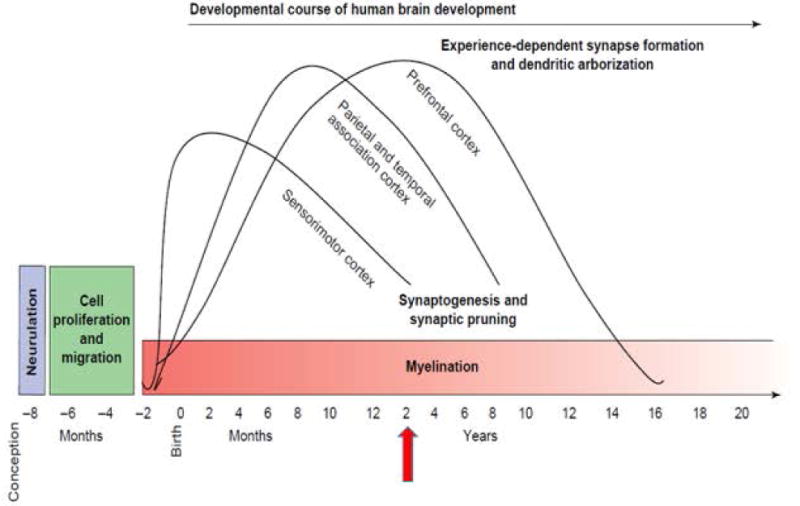
Human brain development. [Source: Casey BJ, Tottenham N, Liston C, Durston S. 2005. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences 9:104–110.]
Other organ systems also undergo rapid growth and development in the first 1000 days. Linear growth reflects growth in bone, muscle, and fat. In fetal life, crown-heel length grows rapidly until about the 20th week of gestation, when it achieves maximum velocity of about 10 cm per 4 weeks, and then decelerates [Figure 2 here] (Tanner, 1978). Linear growth continues to decelerate after birth as reflected in growth velocities for length/height [Figure 3 here]. However, growth velocities continue to be higher during the first two years of age compared to middle childhood; in the neonatal period it is about 24 cm per year but this falls to less than 10 cm by two years of age. Growth velocities accelerate again during puberty and in boys peak height velocity is about 10 cm per year.
Figure 2.
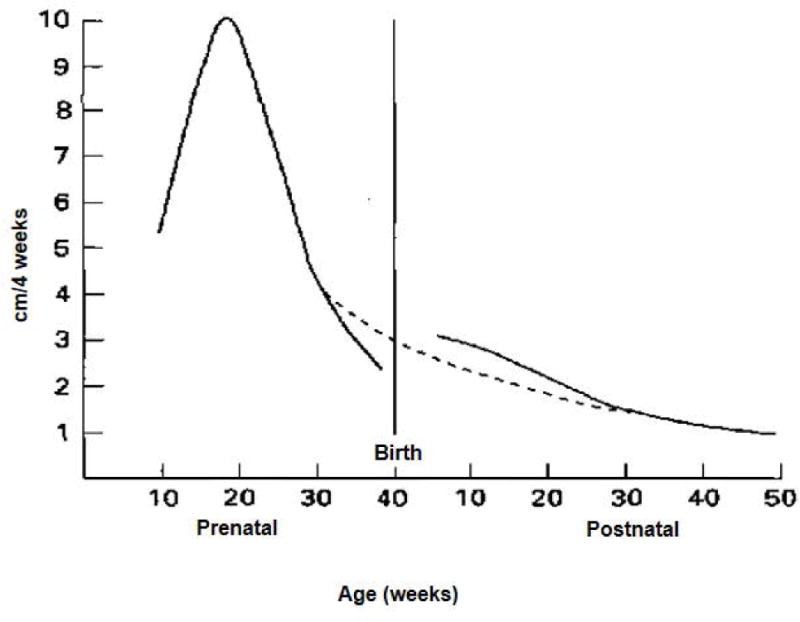
Velocity curve (cm/4 weeks) for growth in crown-heel length in prenatal and early postnatal periods in boys. The interrupted line represents the theoretical curve if no uterine restriction had taken place. [Source: Tanner JM. Foetus into Man. 1978. Cambridge, MS: Harvard University Press, p 250.]
Figure 3.
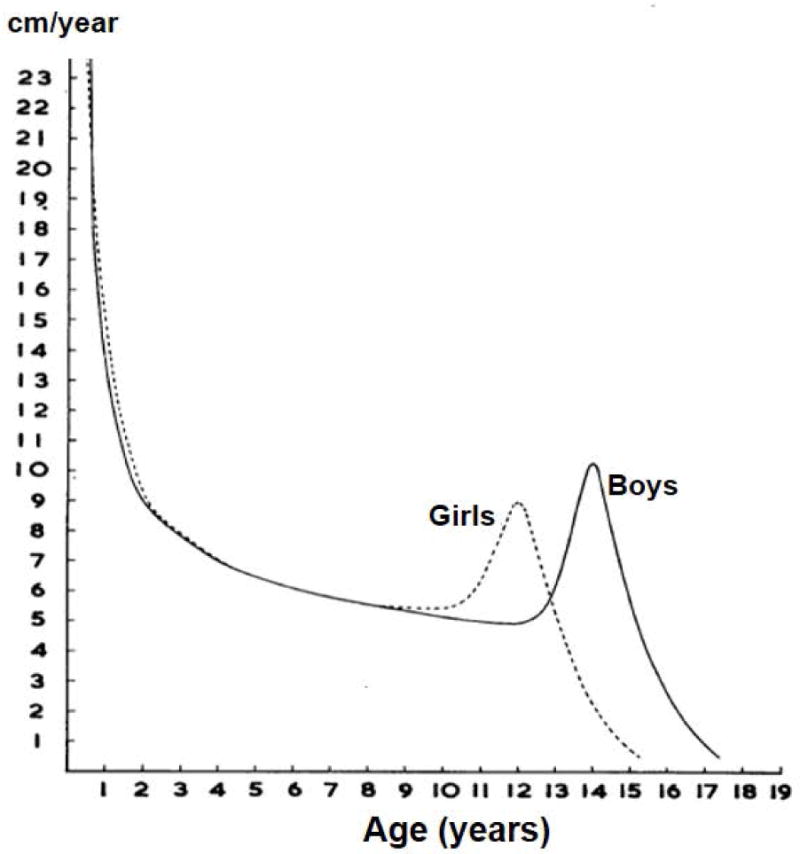
Typical velocity curves for length/height in English girls and boys (Source: Tanner JM, Whitehouse RH and Takaishi M. 1965. Standards from birth to maturity for height, weight, height velocity and weight velocity; British children. Arch Dis In Child 41:454–71; 613–35.]
Growth potential across the world
There is now conclusive evidence that growth potential in the first 1000 days is similar across populations of different ethnicities under optimal heath, nutritional, and environmental conditions. The Multicentre Growth Reference Study (MGRS) of the World Health Organization (WHO) studied healthy populations of children from birth to 5 years of age from the USA, Brazil, Norway, Ghana, Oman and India (de Onis et al, 2006). Rather than include a representative sample from these countries, the study included women and households with adequate environmental sanitation, optimal health care and food security; in addition, the women did not smoke, the children were breastfed, and there was timely complementary feeding. The study followed a “prescriptive” approach in an effort to create a “standard” of growth that describes how children should grow. Differences among the sites were inconsistent, small, and biologically unimportant, and the data were pooled to generate a single standard. More recently, the INTERGROWTH-21st study has shown similar findings for fetal growth (Papageorghiou et al, 2014). The same conceptual approach as the MGRS study was followed in selecting healthy populations from Brazil, China, India, Italy, Kenya, Oman, the UK, and the USA. Several dimensions of fetal growth were measured by ultrasound. The analyses showed that there were no differences among the sites and single standards for fetal growth were generated. Also, there were no differences in term, newborn size between the MGRS and INTERGROWTH-21st studies. We do not have similar, incontrovertible evidence that later growth, particularly during adolescence, is similar across healthy populations from around the world.
Within populations, height varies greatly among individuals, and in healthy populations, an important source of variation is genetic. Is the finding that growth potential in linear growth in the first 1000 days is similar across healthy populations surprising? Viewed from the perspective of the candelabra model of human evolution that I was exposed to as a graduate student at the University of Washington, this is indeed surprising. One of my courses used “The Origin of Races” by Carlton S. Coon as a textbook (Coon, 1962). Coon held that there were five races and that these had great antiquity, going back all the way to Homo erectus. Each of these races evolved into Homo sapiens at different times in their respective ancestral regions. Modern Chinese, for example, were viewed as descendants of Homo erectus pekinensis populations, the so called “Peking man” fossils from as long ago as 750,000 years. The consensus is now vastly different. Our species, Homo sapiens, only left Africa some 50 to 60 thousand years ago and quickly spread throughout the world; the crossing to the Americas occurred about 15,000 years ago (Henn et al, 2012). Our departure coincided with the disappearance of all other hominids and possible reasons include greater facility with language, better hunting weapons and techniques, diseases brought from Africa, and direct aggression, among others. A bit of mixing took place as well. A small portion of DNA is of Neanderthal origin in people with ancestry from outside Africa, whose ancestors encountered Neanderthals in Eurasia. From the perspective of the current consensus about human evolution, the similarity in growth potential in the first 1000 days around the world does not seem as surprising.
Growth of children living in poverty
Knowing how children ought to grow in the first 1000 days of life, permits us to assess confidently patterns of growth failure in children growing in the context of poverty. Victora and colleagues (2010) analyzed anthropometric data from national nutrition surveys and plotted height Z-score values by age by WHO region [Figure 4 here]. Z-scores were computed with reference to the WHO standards. While there is variation in the degree of growth retardation across regions, the pattern with age is qualitatively similar. Values are negative in the early months, suggesting some intrauterine growth failure, and then become progressively negative with age until about 24 months of age. This is more clearly seen when showing one region (Panel B) or one country (Panel C).
Figure 4.
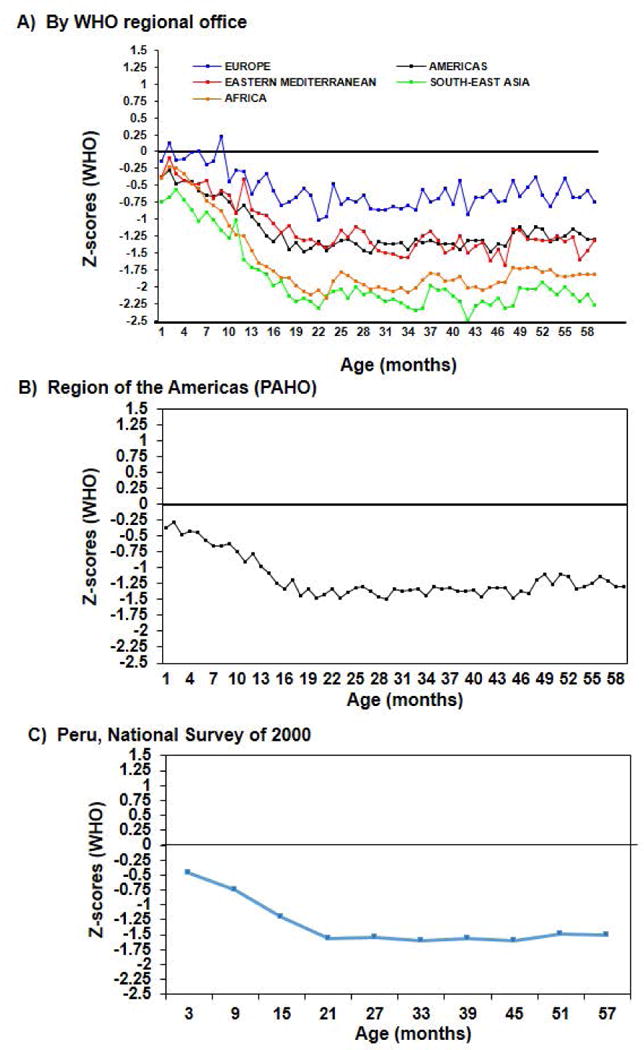
Stature for age z-scores; analyses of national surveys. [Source of Panels A and B : Víctora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. 2010. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics 125(3):e473–80.]
Stunting is defined as a value more than 2 standard deviations (< −2 Z) below the age-sex specific mean, preferably using the WHO standard as the reference population. Stunting is the best summary indicator of the extent of growth failure in the first 1000 days and hence of child undernutrition. Countries track the prevalence of stunting over time, often in children under 5 years of age, to evaluate the effectiveness of policies and programs. Stunting is also used in research as a key indicator of impact of interventions to address undernutrition. The synonym “chronic malnutrition” is used instead of stunting in juxtaposition to “acute malnutrition” (low weight for length, also defined as < −2 Z below the age-sex specific mean of the distribution). One is cumulative and therefore chronic and the other is more reflective of current nutritional status. I find that the use of the term “chronic” confuses some program managers because they do not take into account that stunting is an outcome of growth failure in the first 1000 days and not thereafter. Surveys of students in first grade are common; these often show that a large percentage of children are “chronically malnourished,” and these results have been used by some to call for interventions in school children to reduce stunting.
Results from the 2014–15 National Survey of Maternal and Child Health in Guatemala illustrate well that stunting is a product of growth failure prior to age 2 years [Figure 5 here]. About 30% of children 0–5 months of age are stunted, in part reflecting intrauterine growth failure. The prevalence of stunting then increases until about 2 years of age, when it reaches 55%. The overall prevalence in children less than 5 years, 46.5 %, is one of the highest in the world, with little change since the last survey in 2008–09. In 2014–15, the average Z-score in Guatemalan children was −1.9, nearly 2 standard deviations from the mean of 0 in the WHO standard, the result of a marked shift of the entire distribution in Guatemala to the left (http://www.osarguatemala.org/osartemporal/Archivos/PDF/201603/259_4.pdf).
Figure 5.
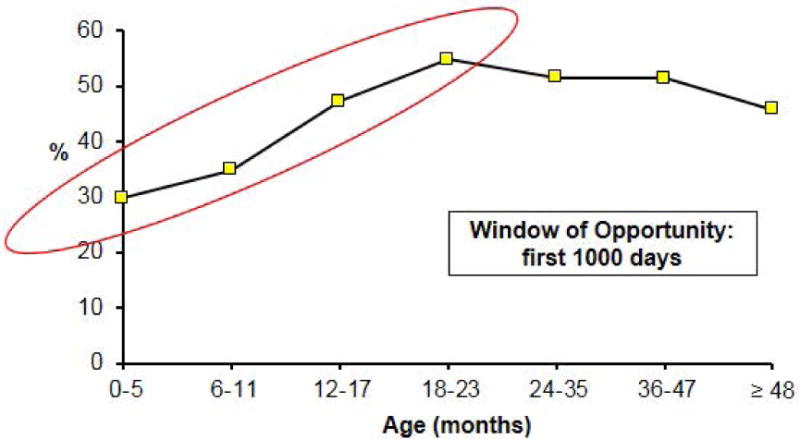
Prevalence of stunting (< −2 SD) in Guatemala in the National Survey of Maternal and Child Health (ENSMI) of 2014/2015. [Drawn using data in http://www.osarguatemala.org/osartemporal/Archivos/PDF/201603/259_4.pdf. Accessed June 20, 2016]
Causes of growth failure
What causes such catastrophic growth failure? The UNICEF conceptual framework of child malnutrition divides the causes into those that operate at societal, household/family, and individual levels. The immediate causes operating at the individual level are: inadequate dietary intakes and disease [Figure 6 here]. Relative nutritional needs, expressed as nutrient amounts per kg of body weight, are very high in infants compared to older children and adults on account of rapid growth and development. Inadequate feeding practices as well as insufficient access to high quality foods lead to poor dietary intakes. Diarrheal diseases and other infections are the second immediate cause of malnutrition. Water, sanitation, and hygiene are deficient, as are access to quality preventive and curative health services, which increase infections. Young children are particularly vulnerable to infections because they have naïve immune systems; diarrheal diseases decline rapidly after 2–3 years of age as children who survive the infections of early life build up immunity. At the household/family level, UNICEF also recognizes the importance of caring practices in addition to food and health. We are born totally dependent on caretakers for our nutritional and social stimulation needs, which requires time and attention. In developing countries, mothers face severe time constraints due to work in and outside the home and the need to take care of several children. This is compounded by inadequate and/or inappropriate knowledge of best caring practices. At the societal level, poverty and related factors constrain the resources households are able to access and control.
Figure 6.
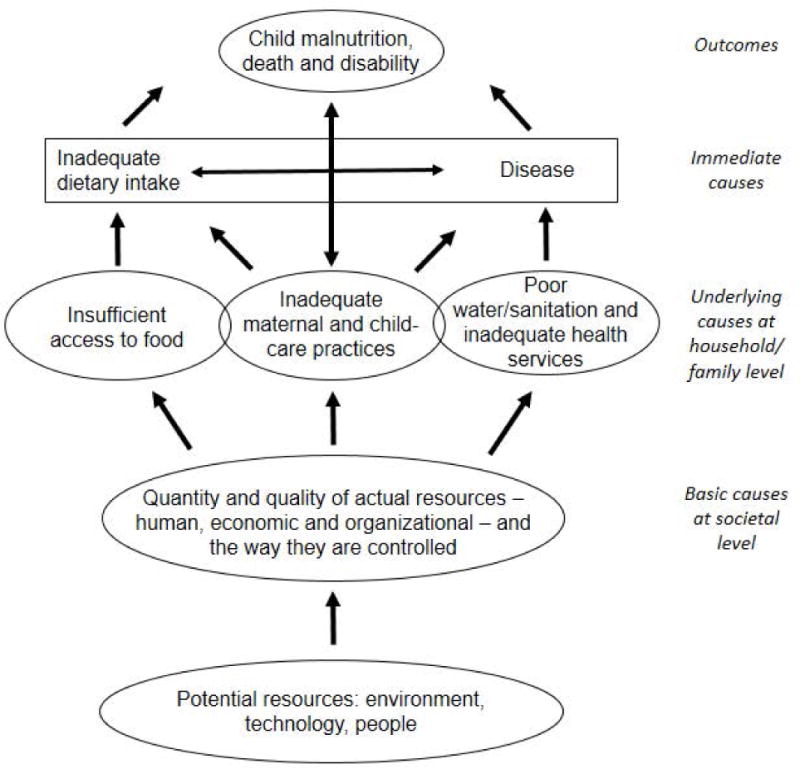
Causes of child malnutrition [Modified from: United Nations Children’s Fund (UNICEF). 1998. The State of the World’s Children: A UNICEF Report. Malnutrition: Causes, Consequences, and Solutions. New York: Oxford University Press, p 131.]
Susceptibility to programming
The first 1000 days are also a period of high sensitivity to programing effects from external factors such as the maternal physiological environment in utero, breast milk, and diet (Godfrey et al, 2010). It is during this time that that there is high plasticity in development, a process which results in phenotypes that may or may not be suited to the adult environment. The geneticists Neel suggested in the 1960s that exposure during many generations to difficult nutritional conditions, including famines, might have led to a “thrifty genotype” that would confer metabolic advantages and enhance survival (Godfrey et al, 2010). David Barker later advanced the “fetal origins and infant origins of adult disease,” proposing that coronary heart disease, type 2 diabetes, stroke, hypertension and other chronic diseases develop partly due to poor nutrition during early life leading to “thrifty phenotypes” (Godfrey et al, 2010). Epigenetic mechanisms responding to external or environmental factors provide the molecular basis for the processes of developmental plasticity. Epigenetic mechanisms such as DNA methylation, changes in histone structure, and small non-coding RNA activity, cause genes to be switched off and affect how cells read genes rather than change the DNA sequence (the genotype) itself. The enhanced risk of being metabolically thrifty comes from a mismatch between scarcity in early life and abundance in adulthood. The world, particularly developing countries, is experiencing changes in diet and lifestyle that are no doubt fueling the epidemic of obesity and related chronic diseases. The epidemic may also be accelerated because poor nutrition enhances risk of chronic diseases. For example, low birth weight has been consistently found to be associated with the development of type 2 diabetes because fetal undernutrition predisposes individuals to insulin resistance and reduced β-cell mass and function (Chen et al, 2012).
Today, scientists speak more broadly about the “developmental origins of metabolic disease” in recognition that both scarcity as well as excess in early life can also enhance risk of chronic diseases (Godfrey et al, 2010). Increasingly, children are born to poor women in developing countries who are short due to stunting in early life, deficient in many micronutrients (e.g., iron deficiency anemia) but also obese and even diabetic (Black et al, 2013); undoubtedly, this evolving intrauterine environment is programming development in ways in which we are only now beginning to understand. Finally, it has been suggested that nutrition interventions in early life aimed at essential short term gains, such as improved birthweight and infant survival, could have longer term effects on individuals throughout their life-course, and that such outcomes might not always be beneficial (Gluckman et al, 2009).
The 2008 Lancet series on Maternal and Child Undernutrition emphasized the first 1000 days as a critical window for health and nutrition interventions (Victora et al, 2008). The 2013 series updated the scientific evidence supporting this claim, but also drew attention to the importance of interventions prior to conception for success in the first 1000 days (Bhutta et al, 2013). Short stature in women, largely the result of stunting in early life, increases the risk of delivery complications, such as obstructed labor, but this needs to be addressed in the first 1000 days of the mother herself (Black et al, 2013). Reproductive health and family planning programs can reduce unwanted pregnancies, improve birth spacing and optimize age at first birth, most importantly preventing adolescent pregnancies that are associated with adverse maternal and birth outcomes (Bhutta et al, 2013). Other interventions can improve micronutrient stores prior to conception (Bhutta et al, 2013). Periconceptional folic acid is effective in prevent most neural tube defects. Iron deficiency anemia leads to adverse maternal, fetal, and child outcomes, and this is best addressed prior to conception. Finally, achieving a healthy body composition at conception, neither underweight nor obese, is desirable as noted above.
In summary, several reasons explain the vulnerability of the first 1000 days: rapid growth and development, high nutritional requirements, greater susceptibility to infections, high sensitivity to programming effects, and full dependence on others for care, nutrition and social interaction. Achieving a healthy preconceptional maternal status will optimize nutrition interventions during the first 1000 days.
Long-term studies of nutrition in the first 1000 days and human capital: studies from Guatemala
In this section I review the history and design of a nutrition experiment carried out in Guatemala by the Institute of Nutrition of Central America and Panama (INCAP) from 1969 to 19777, as well as the history and design of key follow-up studies and their results. These studies support the claim that the first 1000 days are a window of opportunity.
The INCAP Longitudinal Study (1969–1977)
The National Institute of Child Health and Human Development (NIHCHD) provided funding to INCAP to carry out a community randomized trial to test the hypothesis that improved protein intakes lead to better child development test scores (Martorell et al, 1995). In the 1960s, protein deficiency was seen as the major dietary limitation in the diets of the poor in developing countries. To facilitate data collection, Spanish rather than Mayan speaking villages were selected for the study. After comparing characteristics of a large number of villages, the investigators settled on two large (~900 people) and two small (~500 people) communities that were similar to each other in health, nutrition, and socio-economic aspects. Villages were randomized within pairs to receive a high-protein drink called Atole or a control drink called Fresco. The Atole was made from INCAPARINA (a vegetable protein mixture developed by INCAP), dry skim milk, sugar, and a flavoring agent. The Atole was available mid-morning and mid-afternoon every day at a supplementation center. The Fresco was originally intended to be a placebo and was to contain cyclamates for sweetening, but fears about carcinogenicity led to a formula that used only sugar and a flavoring agent. The Fresco was designed to control for the social interaction created by attending the supplementation centers and it was also available twice a day, every day. To sharpen the contrast in protein, vitamins and minerals were added to the Fresco in equal concentrations as were present in the Atole; the added nutrients were iron, fluoride, thiamin, riboflavin, niacin, vitamin C and vitamin A. Per cup (180 ml), the Atole provided 163 kcal and 11.5 g of protein while the Fresco provided 59 kcal per serving. All four villages received high quality preventive and curative medical services staffed by INCAP nurses and physicians.
Attendance at the supplementation centers by pregnant and lactating women and children 6 months to 7 years in age was encouraged. Children and mothers were followed longitudinally and information on child development, growth, diet, supplement consumption, morbidity, and social and demographic aspects were collected. All children <7 years of age in 1969 were followed until they turned 7 years or to the study end in 1977, all newborns during the study period were studied until they turned 7 years or to the study end, and all mothers during pregnancy and lactation were included as well. The design proved to be very useful in the analyses because children were exposed during different ages to the supplements; for example, some began to receive the Atole only after three years of age whereas other were exposed earlier during the first 1000 days window.
Supplement consumption was fastidiously measured. Any amount left from the initial serving of 180 ml was poured into a beaker and measured. Additional servings and possible leftovers were measured similarly. Dietary intakes in children 15 months and older were measured every three months through 24-hour recall surveys. Using these data, it was estimated that total dietary intakes (home diets plus supplement) in children in Atole villages 15 to 36 months of age were increased by 9 g of protein and 100 kcal/day compared to children in Fresco villages. Because greater amounts of the Atole were consumed than the Fresco in children, there were also differences in micronutrients across experimental groups. The design, therefore, was unable to isolate a “protein” effect since diets in Atole villages improved in energy, protein, and micronutrients. Any effect found, therefore, is best attributed to a general improvement in dietary intakes.
The main motivation for the study was to assess effects on child development and the many psychologists who worked on the project supervised the collection of a wide range of tests (Pollitt et al, 1995). The findings were suggestive of an Atole effect but were not definitive, and the conclusion at the end of the study was that improved nutrition did not substantially and consistently improve child development. It was thought that perhaps other inputs, such as social stimulation, were needed as well.
Anthropometric measures were included in the study in part to establish whether or not the Atole was efficacious in improving growth compared to Fresco. This was important to interpret the child development findings; for example, lack of effects on child development and lack of efficacy in improving growth would call into question the integrity of the experiment. As it turns out, the Atole improved linear growth (length, arm length) and head circumference but not weight for length (Martorell et al, 1982). Length for age in children was similar in Atole and Fresco villages in 1968, prior to the initiation of the study. A village-level analysis found that children in Atole villages at three years of age who had been exposed to the supplement during their entire life, were about 3 cm taller than three year old children at baseline. In Fresco villages, there was only a slight increase, perhaps due to medical care and the energy and micronutrients in the Fresco villages. The difference-in-difference contrast was highly significant in favor of Atole villages [Figure 7 here]. There was also a clear dose relationship between categories of average lifetime Atole intake and length at three years of age; relative to children in 1968, boys and girls in the highest category of intake were 4.5 and 5.8 cm taller, respectively, than the average child in 1968 [Figure 8 here]. An important finding was that the Atole improved length only in the first three years of life (Schroeder et al, 1995).
Figure 7.
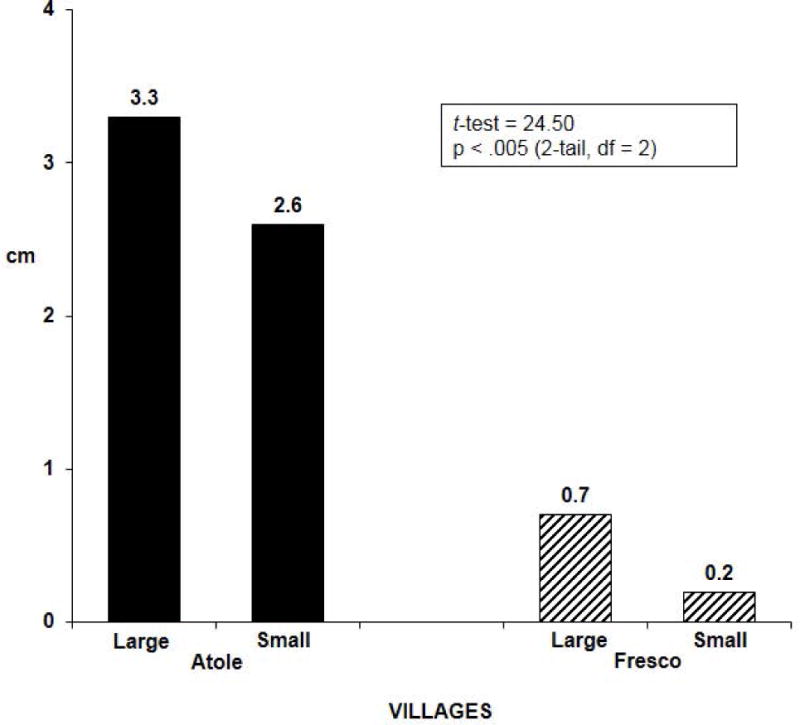
Difference in length at 3 years of age between children (n = 453) exposed to supplement during their entire lives and those measured at baseline in 1968: village level analysis. [Drawn from data in Habicht J-P, Martorell R, Rivera JA. 1995. Nutritional Impact of Supplementation in the INCAP Longitudinal Study: Analytic Strategies and Inferences. J Nutr 125(S4):S1042-S50.]
Figure 8.
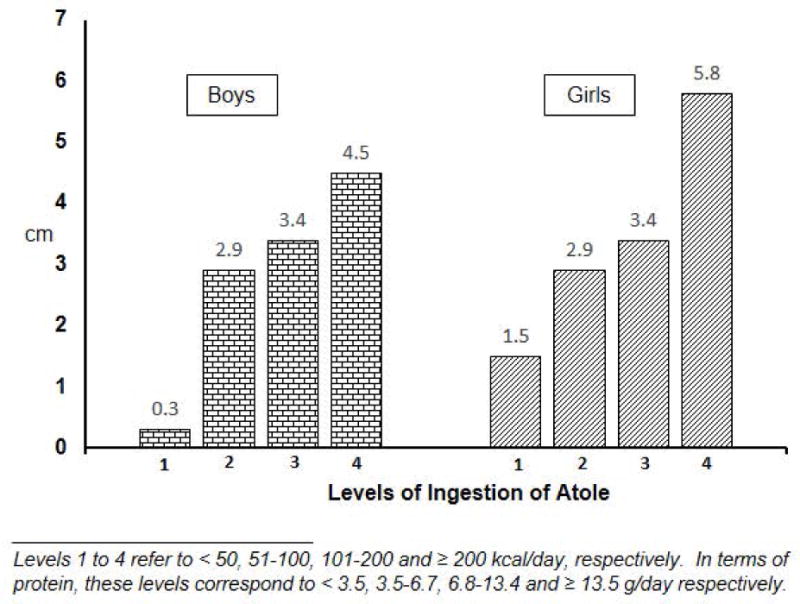
Dose response relationship: Differences in length at 3 years of age between children consuming different amounts of Atole and those measured at baseline. [Drawn from data in Martorell R, Habicht J-P and Klein RE. 1982. “Anthropometric indicators of changes in nutritional status in malnourished populations.” Joint U.S.-Japan Malnutrition Panels, U. S.-Japan Cooperative Medical Science Program, Bethesda, Maryland. In: B. A. Underwood (ed.), Methodologies for Human Population Studies in Nutrition Related to Health. NIH Publication No. 82–2462. U.S. Government Printing Office: Washington, D.C., p 99–110.]
Follow-up studies
There have been 7 follow-up studies of the original cohort, and the most relevant for this article are the ones carried out in 1988–89, 2002–2004, and 2014–2019. The follow-up study of 1988–89 was carried out when the participants were 11–26 years old and the one in 2002–04 when they were 26–41 years old. Residents of the study and nearby villages as well as migrants to the capital city of Guatemala were included in both studies; in the 2002–2004 study, migrants to the rest country were also included. Some 1574 and 1571 participants were measured, respectively, in these two follow-up studies, which in 2002–2004 represented 85% to those known to be living in the country.
When putting together the team for the 1988–89 study, I approached the late Ernesto Pollitt, a leading authority on nutrition and child development who was on the faculty at the University of California at Davis. He was reluctant to join because he expected null findings, but I convinced him that, either way, we were answering important questions about the long-term effects of a nutrition intervention, a topic about which very little was known. To our surprise, we found better performance on tests of knowledge, numeracy, reading comprehension, and vocabulary in Atole compared to Fresco villages (Pollitt et al, 1995). We don’t have a good explanation for the contrasting results in the original study in preschool children and later in the follow-up study. Possible reasons are that we were testing different aspects in children and youth; in the follow-up study, the focus was on skills. Also, it is possible that small differences in trajectories between children in Atole and Fresco villages became larger over time. Also on the team were Jean-Pierre Habicht and Jere Haas from the Division of Nutritional Sciences at Cornell; Juan Rivera, now at the National Institute of Public Health of Mexico, coordinated the study at INCAP, and Paul Melgar was the field director. Other findings from the 1988–1989 study were that the youth in Atole villages were taller, had greater fat free mass and in the case of males, better work capacity (Martorell et al, 2010). Although the findings of the 1988–89 study were novel and important, there were important limitations for studying effects on human capital due to the young age of the participants: 11 to 26 years old. Some had not yet finished growing, not yet finished their schooling, not yet chosen an occupation, and not yet married or formed unions.
The 2002–2004 follow-up study sought to address these limitations by focusing on measures of human capital and was dubbed the “Human Capital Study” (Martorell et al, 2005). This required collaboration with economists and Jere Behrman of the University of Pennsylvania, and John Maluccio and John Hoddinott, both then at International Food Policy Research Institute (IFPRI), came on board, joining Aryeh Stein at Emory University and Ruben Grajeda, Manuel Ramirez, and Paul Melgar (again the field director) at INCAP. The findings convincingly demonstrated long-term impact on human capital. Significant effects were found of exposure to Atole compared to Fresco but only up to 3 years of age on schooling, reading, and cognition, the latter measured by the Raven Progressive Matrices (Maluccio et al, 2009). The most quoted findings are those concerning wages, which were published in the Lancet along with the first Lancet Series on Maternal and Child Nutrition (Hoddinott et al, 2008). The analyses took advantage of the fact that children were exposed to the Atole at different ages or windows and showed that the impact was greatest and significant for the 0–24 month window and least and not significant for the 37–72 month window [Table 1 here]. For the 0–24 month window, Atole increased wages by US 67 cents, which represented an increase of 46% in wages and US $870 of additional annual income (the mean annual income in men was about US $3200). The impact on wages was seen in men only, most likely because of the low participation of women in the labor market. We know that women had the same potential as men to be more productive because women also saw improvements in schooling, reading, and cognition, the variables mediating the relationship between improved nutrition and wages, rather than through physical size and work capacity (Behrman et al, 2010).
Table 1.
Impact of exposure to Atole during various age windows on hourly wages (US$); n=602 men; age ~32 years.+
| Window (months) | Coefficient (95% C.I.) | P |
|---|---|---|
| 0 – 24 | 0.665 (0.15 to 1.17) | 0.009 |
| 0 – 36 | 0.622 (0.17 to 1.07) | 0.007 |
| 37 – 72 | 0.215 (−0.29 to 0.72) | 0.406 |
Source of data: Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. 2008. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet 371(9610):411–6.
We also examined effects on chronic disease indicators in the 2002–2004 Human Capital Study and concluded that the nutrition intervention did not increase risk factors of cardiovascular disease, as some feared (Stein et al, 2006). Rather, there was evidence of a small protective effect. However, the population was relatively young to study these health outcomes, around 32 years on average.
A new follow-up study began in 2014 with the objective of investigating whether improving early life nutrition can attenuate the development of cardiovascular disease and diabetic risk by influencing the metabolomics and cardiometabolic profiles. The range in age of the participants during field work is 39–53 years and more appropriate to the study of chronic diseases, which are increasing alarmingly in Guatemala. The study incorporates basic scientists from Emory University and uses established and novel biomarkers and approaches to characterize risk and presence of metabolic disease. The study also updates the cohort demographic and socioeconomic histories and retains the participation of our economist colleagues. Aryeh Stein is the principal investigator of this ambitious study.
Consequences of Stunting
The Human Capital Study of 2002–2004 study provides data to assess the consequences of stunting (Hoddinott et al, 2013). These analyses complement the analyses that use the experimental design but require careful control for confounding. On the other hand, stunting summarizes all influences on growth and not just supplementation. We used instrumental variable regression to correct for estimation bias, an approach that included a variable that represents the nutrition intervention. In addition we adjusted for many confounding factors at the individual (sex, birth year), family (schooling of father and mother, household wealth at baseline in 1967, etc.) and village levels (village dummy variables, community development variables, etc.). The results are compelling; stunting has profound negative consequences for human capital and sharply increases the risk of living in poverty as an adult [Table 2 here]. Moreover, stunted individuals do not do well in what economists call the marriage market; affected individuals marry at younger ages and have partners with less schooling and who are shorter (Hoddinott et al, 2013). Women who were stunted are younger at their first birth, have more pregnancies and more children, aspects with important maternal and child health and socio-economic implications (Hoddinott et al, 2013).
Table 2.
Stunting at 2 years of life and adult human capital in Guatemala: Instrumental variable analysis (n = 1338).++++
| Adults who were stunted at 2 y: | Coefficient | 95% C.I. | P |
|---|---|---|---|
| Left school earlier | −3.1 years | (−5.9, −0.4) | P = 0.026 |
| Have less schooling | −4.6 grades | (−7.8, −1.5) | P = 0.004 |
| Have lower reading scores+ | −1.3 SD | (−2.3, −0.3) | P = 0.003 |
| Are less intelligent++ | −1.1 SD | (−2.0, −0.3) | P = 0.006 |
| Have lower per capita household expenditure | −53% | (−73, −18) | P = 0.006 |
| Gave a greater probability of living in poverty+++ | 42 pp | (2, 82) | P = 0.040 |
Inter-American Series test score of reading and vocabulary, standardized with mean ± SD of 0 ± 1 in the sample.
Raven’s Standard Progressive Matrices test score, standardized with a mean ± SD of 0 ± 1 in the sample.
Per capita household expenditure below the poverty line for Guatemala; pp, percentage points.
Source of data: Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisimbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. 2013. Adult consequences of growth failure in early childhood. Am J Clin Nutr 98(5):1170–8.
Consequences of Famines
Famines are natural experiments that can provide information about the long term effects of poor nutrition in early life. The most studied has been the Dutch famine of World War II (“Hungry Winter”), which was of short duration, severe, and which occurred in a previously well-nourished population. Effects of early prenatal exposure on mental health have been found, notably on schizophrenia (Susser and St Clair, 2013). Effects on risk factors on chronic disease have been reported with some consistency for diabetes and BMI (Lumey et al, 2011). No effects on human capital (intelligence, IQ, schooling, etc.) had been reported until a recent report linking prenatal exposure during the first trimester with a lower probability of employment at around 55 years of age (Scholte et al, 2015).
The Chinese famine of 1969–61 was precipitated by Mao’s disastrous polices of the “Great Leap Forward” strategy of economic development that involved, among many changes, the concentration of peasants in communes, thereby severely disrupting agricultural production and leading to the death of some 30 to 60 million people, perhaps the largest death toll ever from a famine. The Chinese famine lasted several years, was very severe and was superimposed on an already malnourished and extremely poor population. Studies of the long-term effects of the Chinese famine began only recently but there are now some results, including from our own studies. These document negative effects of exposure to the famine during the first 1000 days on adult height, mental health, obesity, hypertension, and income (Chen and Zhou, 2007; Huang et al, 2010; Huang et al, 2013).
Policy and Program Implications
The first 1000 days are both a window of vulnerability as well as opportunity. Rapid growth and development, high nutritional requirements, greater susceptibility to infections, high sensitivity to programming effects, and full dependence on others for care, nutrition, and social interaction are among the reasons why malnutrition is more common and severe in this period. The corollary is that efforts to improve nutrition in the first 1000 days will have the greatest impact on survival, child health and nutrition, and adult outcomes, making this period a window of opportunity. The Guatemalan studies indicate that substantial improvement in adult human capital and economic productivity resulted from a nutrition intervention, but only if received in the first 1000 days. This evidence provides a powerful argument for promoting improvements in nutrition in pregnant women and young children in low income countries. Advocacy for the importance of the first 1000 days has been successful, spawning world efforts and funding to reduce stunting, including the Scaling Up Nutrition, or SUN movement. SUN unites people—from governments, civil society, the United Nations, donors, businesses and researchers—in a collective effort to improve nutrition during the critical 1,000 day window of opportunity (https://www.google.com/?gws_rd=ssl#q=scale+up+nutrition+initiative).
The World Health Assembly (WHA) is the decision-making body of WHO. Annually, the Ministers of Health of the member states meet to set policies and, in 2012, they endorsed the target of reducing stunting in children less than 5 years of age by 40% by the year 2025, relative to the baseline value of 2010. This means reducing the number of stunted children from 167 million to 100 million. Despite significant progress, the reduction in the prevalence of stunting is too slow to reach the target, and current projections are that there will be 127 million children stunted by the year 2025 (Black et al, 2013). In Africa, the rate of decline is so slow and population growth so fast that the absolute number of stunted children is actually increasing. The other bad news is that childhood overweight and obesity are increasing alarmingly and notably in Africa (Black et al, 2013). We are thus faced with a double burden, the unresolved problems of malnutrition as well as the emerging epidemic of obesity and related chronic diseases.
The ecology of poor nutrition in low and middle income countries is evolving. The maternal environment to which the fetus is exposed increasingly combines 1) a history of undernutrition and poor development in the first 1000 days which leads to short maternal stature and possible complications during delivery, 2) overweight/obesity and gestational diabetes leading to a glucose rich milieu, and 3) micronutrient deficiencies like iron deficiency and anemia due to the poor quality of the maternal diets. After birth, deficient infant feeding practices, including absent or deficient breastfeeding practices and inappropriate and untimely complementary feeding, together with high rates of diarrheal diseases caused by unhygienic environments, lead to undernutrition and stunting, with dire consequences for human capital. As children leave the 1000 day period and face the obesogenic environment of today’s world, many become overweight or obese. Fast weight gain after age 2 years is predictive of risk of chronic disease, and this relationship strengthens with age (Adair et al, 2013).
The challenge we face is that of promoting healthy growth without falling into the obesity trap. The Lancet series on maternal and child nutrition of 2008 and 2013 provide a review of effective nutrition-specific (direct nutrition) interventions and nutrition-sensitive interventions (that are not nutritional in nature but that have nutrition impact) (Victora et al, 2008; Black et al, 2013). A clear message from these publications is that we know a great deal about what to do, and that what we need most are political will, funding, evidence-based policies and programs, sound implementation, and careful monitoring and evaluation. There are also other Lancet series on several topics such as child development, breastfeeding, obesity and physical activity that provide reviews of effective interventions.
Raymond Pearl (1878–1940) was a towering figure in human biology in the first half of the 20th century who made wide-ranging contributions in zoology and human studies. Although a supporter earlier in his life, in 1927, he was the first American biologist to denounce the fallacy of eugenics, the belief in the supremacy of heredity (Hendricks, 2006). To improve human capital, the eugenics movement proposed that defective people should be prevented from breeding. In this talk, I emphasized the powerful, detrimental effects of environments of poverty on human development and the need to address these problems with urgency, a proposal that offers a radically different and humane policy for improving human capital. The proposal is not new; Adam Smith advocated for it 240 years ago (Smith, 1776).
Acknowledgments
I am honored that the Human Biology Association selected me as the 2016 Raymond Pearl Memorial Lecturer. I am grateful to the former participants of the 1969–77 INCAP Longitudinal Study for their consent and participation in many follow-up studies and to my many colleagues with whom I have collaborated in analyzing and publishing the results from these rich databases.
Grant sponsorship: Supported by the National Institutes of Health (5R01HD075784)
Footnotes
AUTHOR CONTRIBUTIONS
The author wrote the article and had sole responsibility for its final content.
LITERATURE CITED
- Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris S, Micklesfield L, Hallal P, Victora C, for the COHORTS Group Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birthcohort studies. The Lancet. 2013;382(9891):525–34. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman J, Hoddinott, Maluccio JA, Martorell R. Brains versus Brawn: Labor Market Returns to Intellectual and Physical Health Human Capital in a Developing Country. Mimeo, International Food Policy Research Institute; Washington, DC: 2010. p. 38. [ http://dx.doi.org/10.2139/ssrn.1471316] [Google Scholar]
- Bhutta ZA, Das JK, Arjumand R, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE, The Lancet Nutrition Interventions Review Group, and The Maternal and Child Nutrition Study Group Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? The Lancet. 2013;382(9890):452–77. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- Black RE, Victoria CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R, Maternal and Child Nutrition Study Group Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Sciences. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Review Nat Rev Endocrinol. 2012;(8):228–36. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhou L-A. The long-term health and economic consequences of the 1959–1961 famine in China. J Health Econ. 2007;26:659–681. doi: 10.1016/j.jhealeco.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Coon CS. The Origin of Races. New York: Knopf Publishing; 1962. p. 844. [Google Scholar]
- de Onis M, Garza C, Onyango AW, Martorell R, editors. WHO child growth standards. Acta Paediatrica. 2006;95(450S):1–101. [Google Scholar]
- Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, Anokhin K, Bougnères, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. The Lancet. 2009;373(9675):1654–1657. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrin Met. 2010;21(4):1999–2014. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Habicht J-P, Martorell R, Rivera JA. Nutritional Impact of Supplementation in the INCAP Longitudinal Study: Analytic Strategies and Inferences. J Nutr. 1995;125(S4):S1042–S50. doi: 10.1093/jn/125.suppl_4.1042S. [DOI] [PubMed] [Google Scholar]
- Hendricks M. Raymond Pearl’s “Mingled Mess”. John Hopkins Magazine; 2006. http://pages.jh.edu/jhumag/0406web/pearl.html [Accessed July 5, 2016] [Google Scholar]
- Henn BM, Cavalli-Sforza LL, Feldman MW. The great human expansion. Proc Natl Acad Sci USA. 2012;109:17758–17764. doi: 10.1073/pnas.1212380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98(5):1170–8. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddinott J, Maluccio JA, Behrman JR, Flores R, Martorell R. Effect of a nutrition intervention during early childhood on economic productivity in Guatemalan adults. Lancet. 2008;371(9610):411–6. doi: 10.1016/S0140-6736(08)60205-6. [DOI] [PubMed] [Google Scholar]
- http://www.dictionary.com/browse/human-capital [Accessed June 20, 2016]
- https://www.google.com/?gws_rd=ssl#q=scale+up+nutrition+initiative [Accessed July 5, 2016]
- Huang C, Li Z, Wang M, Martorell R. Early life exposure to the 1959–1961 Chinese famine has long-term health consequences. J Nutr. 2010;140(10):1874–8. doi: 10.3945/jn.110.121293. [DOI] [PubMed] [Google Scholar]
- Huang C, Phillips MR, Zhang Y, Zhang J, Shi Q, Song Z, Ding Z, Pang S, Martorell R. Malnutrition in early life and adult mental health: evidence from a natural experiment. Soc Sci Med. 2013;97:259–66. doi: 10.1016/j.socscimed.2012.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu Rev Public Health. 2011;32:1–26. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluccio JA, Hoddinott J, Behrman JR, Martorell R, Quisumbing AR, Stein AD. The impact of improving nutrition during early childhood on education among Guatemala adults. Economic Journal. 2009;119:734–63. [Google Scholar]
- Martorell R, Behrman JR, Grajeda R, Hoddinott J, editors. The Human Capital 2002–04 Study in Guatemala: A Follow-up to the INCAP Longitudinal Study 1969–77. Food Nutr Bull. 2005;26(2 Suppl 1):S1–S124. [Google Scholar]
- Martorell R, Habicht J-P, Klein RE. Anthropometric indicators of changes in nutritional status in malnourished populations. Joint U.S.-Japan Malnutrition Panels, U. S.-Japan Cooperative Medical Science Program, Bethesda, Maryland. In: Underwood BA, editor. Methodologies for Human Population Studies in Nutrition Related to Health. U.S. Government Printing Office; Washington, D.C.: 1982. pp. 99–110. (NIH Publication No. 82-2462). [Google Scholar]
- Martorell R, Habicht J-P, Rivera JA. History and design of the INCAP Longitudinal Study (1969–77) and its Follow-Up (1988–89) J Nutr. 1995;125:1027S–1041S. doi: 10.1093/jn/125.suppl_4.1027S. [DOI] [PubMed] [Google Scholar]
- Martorell R, Melgar P, Maluccio JA, Stein AD, Rivera JA. The development and legacy of the INCAP Oriente studies 1969–2009: The nutrition intervention improved adult human capital and economic productivity. J Nutr. 2010;140(2):411–4. doi: 10.3945/jn.109.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorghiou AT, Ohuma EO, Altman DG, Todros T, Ismail LC, Lambert A, Jaffer YA, Bertino E, Gravett MG, Purwar M, Noble JA, Pang R, Victora CG, Barros FC, Carvalho M, Salomon LJ, Bhutta ZA, Kennedy SH, Villar J, for the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) International standards for fetal growth based on serial ultrasound measurements: The Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;284:869–79. doi: 10.1016/S0140-6736(14)61490-2. [DOI] [PubMed] [Google Scholar]
- Pollitt E, Gorman KS, Engle P, Martorell R, Rivera JA. Early Supplementary Feeding and Cognition: Effects Over Two Decades. Monographs of the Society for Research in Child Development. 1993;58(7):1–123. Serial No. 235. [PubMed] [Google Scholar]
- Scholte RS, van den Berg GJ, Lindeboom M. Long-run effects of gestation during the Dutch Hunger Winter famine on labor market and hospitalization outcomes. J Health Econ. 2015;39:17–30. doi: 10.1016/j.jhealeco.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Schroder DG, Martorell R, Rivera JA, Ruel MT, Habicht J-P. Age differences in the impact of nutritional supplementation on growth. J Nutr. 1995;125:1051S–1059S. doi: 10.1093/jn/125.suppl_4.1051S. [DOI] [PubMed] [Google Scholar]
- Smith A. An Inquiry into the Nature and Causes of the Wealth of Nations. Chicago, IL: University of Chicago Press; 1776. p. 568. [Google Scholar]
- Susser E, Clair D. Prenatal famine and adult mental illness: Interpreting concordant and discordant results from the Dutch and Chinese famines. Soc Sci Med. 2013;97:325–330. doi: 10.1016/j.socscimed.2013.02.049. [DOI] [PubMed] [Google Scholar]
- Stein AD, Wang M, Ramirez-Zea M, Flores R, Grajeda R, Melgar P, Ramakrishnan U, Martorell R. Exposure to a nutrition supplementation intervention in early childhood and risk factors for cardiovascular disease in adulthood: Evidence from Guatemala. Am J Epidemiol. 2006;164(12):1160–70. doi: 10.1093/aje/kwj328. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Foetus into Man. Cambridge, MS: Harvard University Press; 1978. p. 250. [Google Scholar]
- Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity and weight velocity; British children. Arch Dis In Child. 1965;41:454–71. 613–35. doi: 10.1136/adc.41.219.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Children’s Fund (UNICEF) The State of the World’s Children: A UNICEF Report Malnutrition: Causes, Consequences, and Solutions. New York: Oxford University Press; 1998. p. 131. [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS, for the Maternal and Child Undernutrition Study Group Maternal and child undernutrition 2: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide Timing of Growth Faltering: Revisiting Implications for Interventions. Pediatrics. 2010;125(3):473–480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- VI Encuesta Nacional de Salud Materno Infantil. ENSMI 2014–2015. Informe de Indicadores Básicos. Guatemala: Noviembre. 2015. http://www.osarguatemala.org/osartemporal/Archivos/PDF/201603/259_4.pdf [Accessed June 20, 2016] [Google Scholar]


