Abstract
A selective and rapid high-performance liquid chromatography–tandem mass spectrometry method was developed and validated for the quantification of raltegravir using raltegravir-d3 as an internal standard (IS). The analyte and IS were extracted with methylene chloride and n-hexane solvent mixture from 100 µL human plasma. The chromatographic separation was achieved on a Chromolith RP-18e endcapped C18 (100 mm×4.6 mm) column in a run time of 2.0 min. Quantitation was performed in the negative ionization mode using the transitions of m/z 443.1→316.1 for raltegravir and m/z 446.1→319.0 for IS. The linearity of the method was established in the concentration range of 2.0–6000 ng/mL. The mean extraction recovery for raltegravir and IS was 92.6% and 91.8%, respectively, and the IS-normalized matrix factors for raltegravir ranged from 0.992 to 0.999. The application of this method was demonstrated by a bioequivalence study on 18 healthy subjects.
Keywords: Raltegravir, LC–ESI–MS/MS, Negative ionization mode, Human plasma, Bioequivalence study
1. Introduction
Raltegravir (RAL), a hydroxypyrimidinone carboxamide derivative, is an integrase strand-transfer inhibitor (INSTI) used in the treatment and management of human immunodeficiency virus (HIV) infection [1]. It was first approved by USFDA in 2007 for the treatment of HIV treatment-experienced patients [2]. RAL is considered as the first generation INSTI that has demonstrated considerable efficacy in the treatment of naive as well as HIV treatment-experienced adult patients with viral resistance. It inhibits the catalytic activity of HIV-1 integrase enzyme, which is responsible for viral replication by blocking the viral DNA into the cellular genome by binding to the integrase-viral DNA complex [3], [4], [5]. RAL is approximately 83% plasma bound and gets rapidly absorbed from the gastrointestinal tract, with peak plasma concentration achieved within 0.5–1.3 h. It undergoes hepatic metabolism mainly by uridine diphosphate glucuronosyltranferase enzyme to give an inactive glucuronide metabolite, with only 9% of the administered dose excreted unchanged in the urine [6]. Further, the pharmacokinetics of RAL showed important inter- or intra-subject variability [7].
Selective and sensitive determination of anti-HIVs in plasma is essential for studying drug–drug interaction, pharmacokinetics, pharmacodynamics and therapeutic drug monitoring. Several methods are reported for the determination of RAL as a single analyte [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20] or in combination with its glucuronide (Glu) metabolite [21] or other anti-HIV drugs [22], [23], [24], [25], [26] in different biological samples such as human cell extracts [15], [19], cerebrospinal fluid [16], cervicovaginal fluid [17], dried blood spots [15], bile [9], feces [9] and human plasma [8], [10], [11], [12], [13], [14], [18], [20], [21], [22], [23], [24], [25], [26]. Mainly, liquid chromatography with UV [10], [17], [23], photodiode array [18], [22], fluorescence [11] or mass spectrometry [8], [9], [12], [13], [14], [15], [16], [19], [20], [21], [24], [25], [26] detection has been used for the quantification of RAL in these matrices.
All reported methodologies using LC–MS/MS quantified RAL in the positive ionization mode and few discussed in-source conversion of RAL-Glu to RAL [14], [15], [21], [24] and its possible interference in the quantification of RAL. Thus, it is essential to develop an adequately sensitive, rapid and selective method, with chromatographic resolution of interfering compounds like RAL-Glu to avoid overestimation of RAL concentration. In the present work, negative ionization mode was selected as it showed better selectivity without compromising the sensitivity of the method. Further, the chromatographic conditions were suitably optimized on a Chromolith RP-18e endcapped C18 column under isocratic elution for baseline separation of RAL from RAL-Glu. The method described was used to support a bioequivalence study in healthy Indian subjects.
2. Experimental
2.1. Chemicals and materials
Reference standard of raltegravir (98.5%) was procured from Hetero Drugs Limited (Hyderabad, India). Raltegravir-d3 (IS, 98%) and raltegravir glucuronide were procured from Toronto Research Chemicals Inc. (Ontario, Canada). HPLC grade methanol, acetonitrile, ammonium formate, dichloromethane (DCM), n-hexane and formic acid were obtained from Merck Specialties Pvt. Ltd. (Mumbai, India). Water used in the entire analysis was prepared from Milli-Q water purification system procured from Millipore (Bangalore, India). Blank human plasma was obtained from Supratech Micropath (Ahmedabad, India) and was stored at −20 °C until use.
2.2. Liquid chromatographic and mass spectrometric conditions
An LC-VP HPLC system (Shimadzu, Kyoto, Japan) was used for chromatographic separation of RAL and IS on Chromolith RP-18e endcapped C18 (100 mm×4.6 mm) analytical column having monolithic silica rod (Phenomenex, Hyderabad, India) maintained at 40 °C in the column oven. For isocratic elution, the mobile phase consisting of 10 mM ammonium formate in water, pH 3.0, and acetonitrile (30:70, v/v) was delivered at a flow-rate of 1.2 mL/min. The total eluate from the column was split in 85:15 (v/v) ratio; flow directed to the electrospray interface was equivalent to 180 μL/min. The autosampler temperature was maintained at 5 °C and the average pressure of the system was 1200 psi. A triple quadrupole mass spectrometer API-4000 (AB/MDS SCIEX, Toronto, Canada) equipped with electrospray ionization and operating in negative ionization mode was used for detection of RAL and IS. For quantitation, multiple reaction monitoring (MRM) was used to monitor precursor→product ion transitions at m/z 443.1→316.1 for RAL and m/z 446.1→319.0 for IS. The nebulizer gas, heater gas, ion spray voltage, heater temperature, curtain gas nitrogen and collisional activation dissociation were optimized at 50 psig, 60 psig, −4500 V, 300 °C, 35 psig and 7 psig, respectively. The compound-dependent parameters like declustering potential, entrance potential, collision energy and collision cell exit potential were maintained at −40.0 V, −10.0 V, −27.0 eV and −7.0 V, respectively. A dwell time of 100 ms was set for both the compounds. Data collection, peak integration, and calculations were performed using Analyst software version 1.4.2.
2.3. Preparation of standard solutions and quality control samples
The standard stock solution of RAL (1000 µg/mL) was prepared by dissolving its accurately weighed amount in methanol. Its working solution was prepared by appropriate dilution of stock solution in methanol:water (50:50, v/v). Calibration standards (CSs) and quality control (QC) samples were prepared by spiking blank plasma with standard spiking solutions. CSs were prepared at 2.0, 4.0, 12.0, 45.0, 90, 180, 480, 1200, 3000 and 6000 ng/mL concentrations while QC samples were prepared at 5000 ng/mL (HQC, high quality control), 2500 ng/mL (MQC, medium quality control), 6.0 ng/mL (LQC, low quality control) and 2.0 ng/mL (LLOQ QC, lower limit of quantification quality control) concentrations. Stock solution (1000 µg/mL) of the IS was prepared by dissolving 2.0 mg of RAL-d3 in 2.0 mL of methanol. An aliquot of 10 µL of this solution was further diluted to 25.0 mL in the same diluent to obtain a solution of 400 ng/mL. Standard stock and working solutions used for spiking were stored at 5 °C, while CSs and QC samples in plasma were kept at −70 °C until use.
2.4. Sample preparation
Prior to analysis, all frozen subject samples, CSs and QC samples were thawed and allowed to equilibrate at room temperature. To an aliquot of 100 µL of spiked plasma/subject sample, 50 µL of IS was added and vortexed for 20 s. Thereafter, 50 µL of 0.1% formic acid in water was added and vortexed for another 20 s. Extraction of samples was carried out with 2.5 mL of DCM and n-hexane (50:50, v/v) on a rotospin for 10 min at 32g. The samples were then centrifuged at 3204g for 5 min at 10 °C. After centrifugation, 2.0 mL of the supernatant organic layer was transferred to an evaporation tube. The supernatant was evaporated to dryness in a thermostatically controlled water-bath maintained at 40 °C under a stream of nitrogen. The dried residue was reconstituted in 150 µL of mobile phase [10 mM ammonium formate in water, pH 3.0 and acetonitrile (30:70, v/v)] and 10 µL was used for injection in the chromatographic system.
2.5. Validation of assay
The bioanalytical method was validated as per the USFDA guidelines [27] and was similar to the one described in our previous reports [28], [29]. System suitability test was performed by injecting six successive injections using an aqueous standard mixture of RAL (2500 ng/mL) and IS (400 ng/mL) at the start of each batch. System performance was checked by injecting one extracted LLOQ sample with IS at the beginning of each analytical batch. Carryover effect of autosampler was verified by sequentially injecting extracted blank plasma→ULOQ sample→extracted blank plasma→LLOQ sample.
The selectivity of the method for endogenous plasma matrix components was evaluated in ten different batches of blank plasma (7 normal K3EDTA plasma and 1 each of lipemic, haemolysed and heparinised plasma). Additionally, lipemic and hemolytic effects of plasma were assessed at HQC and LQC levels in six replicates. These sets were processed along with freshly prepared CSs and qualifying QC samples in duplicate using normal plasma lots. As per the acceptance criteria, the accuracy of lipemic and hemolytic samples should be within 85–115%. The selectivity of the method towards commonly used medications by human volunteers was checked for acetaminophene, cetirizine, domperidone, ranitidine, diclofenac, ibuprofen, nicotine and caffeine in six different batches of human plasma containing K3EDTA as an anticoagulant. Their stock solutions were prepared by dissolving requisite amount in methanol and water (50:50, v/v). Further, a mixed working solution of acetaminophen (1000 µg/mL), cetirizine (20 µg/mL), domperidone (1 µg/mL), ranitidine (27.5 µg/mL), diclofenac (100 µg/mL), ibuprofen (2250 µg/mL), nicotine (5 µg/mL) and caffeine (1000 µg/mL) was prepared in the same diluents, spiked in plasma and analyzed under the same conditions at LQC and HQC levels in six replicates. These sets were processed along with freshly prepared CSs and qualifying QC samples in duplicate. As per the acceptance criteria, the accuracy should be within 85–115%.
The linearity of the method was determined by analysis of five calibration curves containing 10 non-zero concentrations. The area ratio response for the analyte/IS obtained from MRM was used for regression analysis. The calibration curves were analyzed individually by using least square weighted (1/x2) linear regression. The lowest standard on the calibration curve was accepted as the LLOQ, if the analyte response was at least ten times more than that of drug-free (blank) extracted plasma.
Intra-batch accuracy and precision for RAL were determined by analyzing six replicates of QC samples along with calibration curve standards on the same day. The inter-batch accuracy and precision were assessed by analyzing five precision and accuracy batches on three consecutive days. Sample injection reproducibility was also checked by re-injecting one entire validation batch.
Assessment of ion suppression/enhancement was ascertained through post-column analyte infusion. In this experiment, a standard solution containing RAL (2500 ng/mL) was infused post-column via a ‘T’ connector into the mobile phase at 10.0 µL/min, employing an infusion pump. Thereafter, aliquots of 10 µL of extracted control (blank) plasma were injected into the column and MRM chromatograms were acquired for RAL and IS.
The extraction recovery of RAL and IS was estimated by comparing the mean area response of samples spiked before extraction to that of extracts with post-spiked samples (spiked after extraction) at three QC levels. Matrix effect, expressed as matrix factors (MFs), was assessed by comparing the mean area response of post-extraction fortified samples with mean area of solutions prepared in mobile phase solutions (neat standards). IS-normalized MFs (RAL/IS) were calculated to access the variability of the assay due to matrix effects. Relative matrix effect was assessed from the precision (%CV) values of the slopes of the calibration curves prepared from ten different plasma lots/sources.
The standard stock solutions of RAL and IS were evaluated for short-term and long-term stability at 25 and 5 °C, respectively. The analyte stability in the spiked plasma samples was evaluated by measuring the area ratio response (RAL/IS) of stability samples against freshly prepared standards having identical concentration. Bench-top (at room temperature), processed sample stability at room temperature and at refrigerated temperature (5 °C), dry extract (−20 °C), freeze–thaw (−20 and −70 °C) and long term (−20 and −70 °C) stability of RAL in plasma were studied at LQC and HQC levels.
Method ruggedness study was done with two precision and accuracy batches. The first batch was analyzed by a different analyst while the second batch was studied on two different columns of the same make having different batch numbers. Dilution integrity experiment was evaluated by preparing the spiked standard at 30,000 and 8000 ng/mL concentrations for 1/10 and 1/2 dilutions in the screened plasma. The precision and accuracy for dilution integrity standards at 1/10 (3000 ng/mL) and 1/2 (4000 ng/mL) dilution were determined by analyzing the samples against freshly prepared calibration standards.
2.6. Application of the method and incurred sample reanalysis (ISR)
The validated method was applied to quantify plasma RAL concentration for a bioequivalence study in 18 healthy Indian subjects after oral administration of test (400 mg tablets) and a reference (ISENTRESS®, 400 mg raltegravir tablets from Merck & Co., Inc. Whitehouse Station, NJ 08889, USA) formulation under fasting conditions. Written consent was taken from all the subjects after informing them about the objectives and possible risks involved in the study. The study was conducted as per the International Conference on Harmonization, E6 Good Clinical Practice guidelines [30]. Blood samples were collected at 0.0 (pre-dose), 0.33, 0.67, 1.00, 1.25, 1.50, 1.75, 2.00, 2.33, 2.67, 3.00, 3.33, 3.67, 4.00, 4.50, 5.00, 6.00, 7.0, 8.0, 10.0, 12.0, 16.0, 24.0 and 36.0 h after oral administration of test and reference formulations in labeled K3EDTA-vacutainers. Plasma was separated through centrifugation and kept frozen at −70 °C until analysis.
An incurred sample reanalysis was also done by reanalysis of 87 subject samples (10% of total subject samples analyzed) [31]. The selection criterion was based on samples which were near the Cmax and the elimination phase in the pharmacokinetic profile of the drug.
3. Results and discussion
3.1. LC–MS/MS method development
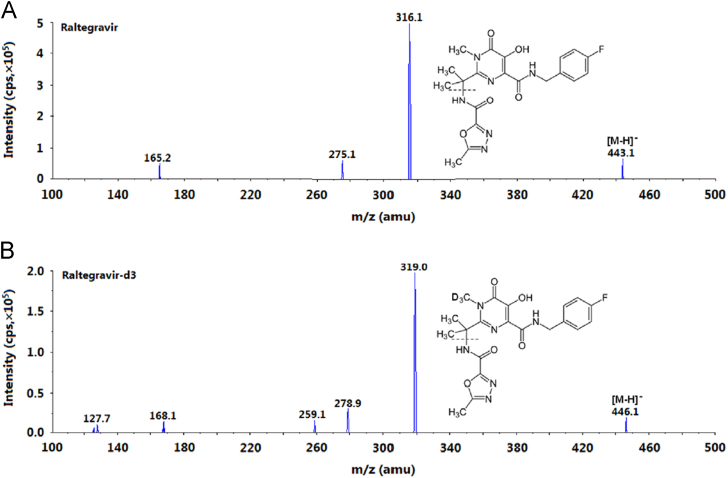
All the reported methods employed positive ionization mode for LC–MS/MS analysis of RAL in different biological matrices [8], [9], [12], [14], [15], [16], [19], [20], [21], [24], [25], [26]. Contrary to this approach, negative ionization mode was selected in the present work as it showed better selectivity without compromising the sensitivity. Furthermore, the response obtained during initial trials with both the polarities was comparable. The intensities of the most stable daughter ions were 4.97e5 cps (443.1→316.1) and 5.01e5 cps (445.1→109.0) in the negative and positive ionization modes, respectively, under the optimized conditions. The same approach was adopted by Djerada et al. [25] for efavirenz, a non-nucleoside reverse transcriptase inhibitor, using UPLC–MS/MS technology. The ESI conditions were optimized so as to have predominant deprotonated precursor [M-H]− ions at m/z 443.1 for RAL and m/z 446.1 for IS in the Q1 MS full scan spectra. In the product ion mass spectrum, the most consistent and intense fragments were observed at m/z 316.1 and 319.0 for RAL and IS, respectively, by applying –27 eV collision energy. These fragments were formed from the deprotonated parent ion by cleavage of oxadiazole moiety containing the amide functionality as shown in Fig. 1. A dwell time of 100 ms for both the compounds was adequate to obtain sufficient data points for quantification.
Fig. 1.
Product ion mass spectra of (A) raltegravir (m/z 443.1→316.1, scan range 100–500 amu) and (B) internal standard, raltegravir-d3 (m/z 446.1→319.0, scan range 100–500 amu), in the negative ionization mode.
Although there are several methods for the quantification of RAL, few studies reported the presence of RAL-Glu metabolite in subject samples [14], [15], [21], [24]. Ter Heine et al. [15] reported a secondary peak corresponding to RAL-Glu in a patient sample which overlapped with the peak of RAL in the positive ionization mode. This was due to the in-source fragmentation of RAL-Glu and its presence was confirmed by lowering the declustering potential. However, as it was difficult to completely eliminate this interference by changing the mass parameters, it was chromatographically separated by changing the elution conditions from isocratic to gradient mode. Similarly, Fayet et al. [24] verified the presence of RAL-Glu in a patient sample (collected at 1.5 h after oral administration of 400 mg RAL) separately by single ion monitoring at m/z 619 corresponding to this metabolite in the negative ionization mode. In another report [14], the presence of this secondary peak was completely eliminated by pre-treatment of patient samples with β-glucuronidase, which effectively broke the bond between RAL and glucuronic acid and released free RAL. Interestingly, all the methodologies employing protein precipitation (PP) for sample preparation reported the presence of RAL-Glu metabolite in subject samples [14], [15], [21], [24]. Thus, gradient elution conditions were implemented for chromatographic separation of the drug and its metabolite. However, in the present work, chromatographic conditions were optimized to have baseline separation of RAL and RAL-Glu under isocratic elution.
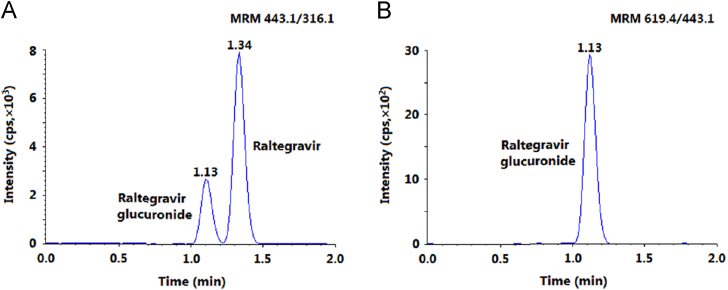
For this experiment, during method development a mixture of RAL (50 ng/mL) and RAL-Glu (15 ng/mL) was chromatographed for baseline separation on different reversed-phase columns like Gemini C18 (50/150 mm×4.6 mm, 5.0 µm), ACE C18 (150 mm×4.6 mm, 5.0 µm), Cosmosil C18 (150 mm×4.6 mm, 5.0 µm), Symmetry C18 (150 mm×4.6 mm, 5.0 µm) and Chromolith RP-18e endcapped C18 (100 mm×4.6 mm) using formic acid/ammonium formate together with methanol/acetonitrile in different compositions. All the columns provided adequate response. However, on Gemini C18, the analytes and IS had limited retention (RT~0.5 min), while the peak shape was unacceptable on ACE C18 and Cosmosil C18 columns at a flow rate ranging from 0.6 to 1.0 mL/min. Symmetry C18 provided adequate response as well as peak shape but the retention time for the analytes was above 4.0 min. Thus, these columns were not considered for further experiment. The best chromatographic conditions were achieved on Chromolith RP-18e column with adequate response, symmetrical peak shape, baseline separation within 2.0 min at a flow rate of 1.2 mL/min (Fig. 2). This column provides high efficiency and an improved performance due to the fast mass transfer kinetics and high binding capacity of monolithic silica compared to particle packed columns. These conditions can serve well for reliable quantification of RAL even in the presence of RAL-Glu, which was not quantified as it is pharmacologically inactive.
Fig. 2.
MRM chromatograms of (A) a mixture of raltegravir and raltegravir glucuronide and (B) raltegravir glucuronide.
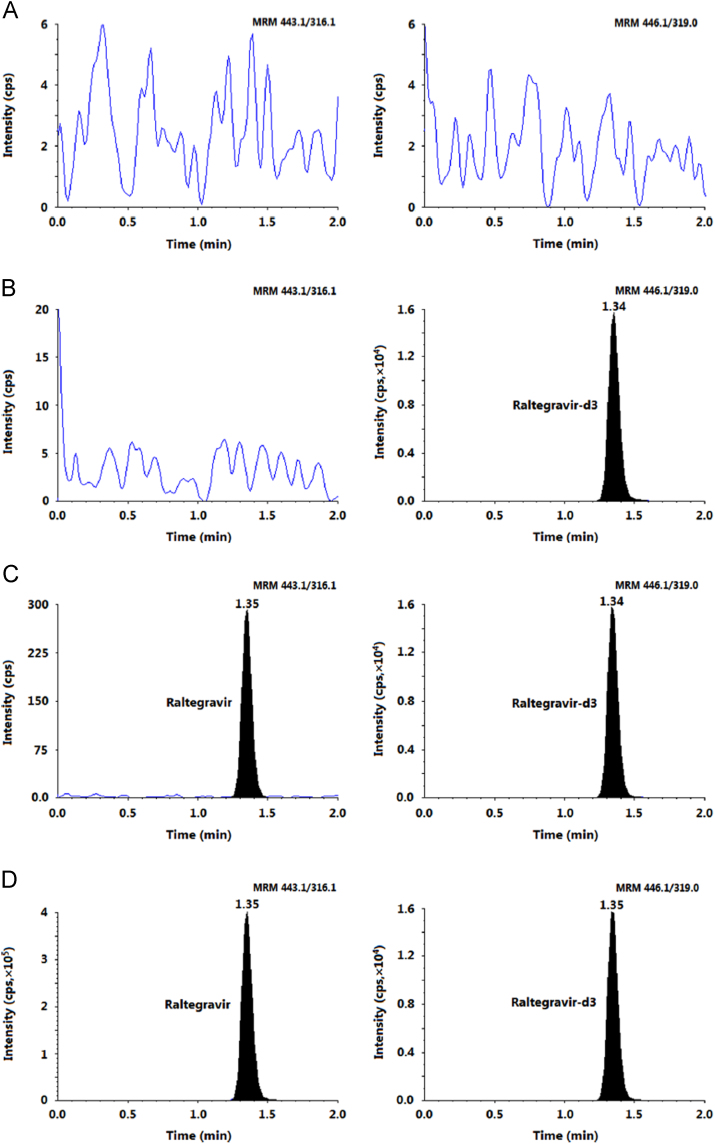
Further, the method selectivity was evident from the MRM chromatograms of double blank plasma, plasma spiked with IS and an LLOQ sample as shown in Fig. 3. There was no interference due to endogenous plasma components at the retention time of RAL and IS. Additionally, all eight commonly used medications studied in this work did not interfere with the quantitation of RAL due to their different MRM transitions. Unlike previous reports [14], [15], [21], [24], there was no peak corresponding to RAL-Glu in healthy Indian subject samples under investigation. Chromatogram in Fig. 3 shows complete absence of this circulating metabolite in plasma samples even up to 4 h after oral administration of the drug.
Fig. 3.
MRM ion-chromatograms of (A) double blank plasma (without analyte and IS), (B) blank plasma with raltegravir-d3 (IS), (C) raltegravir at LLOQ and IS and (D) subject sample at Cmax after administration of 400 mg dose of raltegravir.
Several assays [8], [11], [12], [13], [18] have employed liquid–liquid extraction (LLE) with n-hexane–DCM (50:50, v/v) in the presence of ammonium acetate buffer (pH 4.0) prior to extraction. In the present work, 0.1% formic acid was used to break high drug–protein binding and render the analyte in an unionized state. Further, reconstitution with the mobile phase afforded better peak shape and overall chromatographic performance with quantitative and precise recovery for RAL (91.2–94.2%) across QC levels.
3.2. Assay validation results
The precision values for system suitability ranged from 0.03% to 0.11% for the retention time and 0.48–1.32% for the area ratio response of RAL/IS. For system performance, the S/N ratio at LLOQ was ≥40. The evaluation of autosampler carry-over was performed in each analytical run so as to ensure that it does not affect the accuracy and the precision of the proposed method. There was practically negligible carry-over (≤0.003%) during carry-over experiment in the extracted blank plasma (without IS and analyte) after subsequent injection of highest CS at the retention time of RAL and IS as shown in Supplementary Fig. 1.
The calibration curves showed good linearity over the established concentration range of 2.0–6000 ng/mL (r2≥0.9978) for RAL. The mean linear equation for calibration curves was y=(0.00242±0.00021)x+(0.0061±0.0022), where y is the peak area ratio of the RAL/IS and x is the concentration of RAL. The accuracy and precision (%CV) observed for the CSs ranged from 93.1% to 104.0% and 1.6% to 3.5%, respectively, for RAL. The intra-batch precision (%CV) ranged from 2.77% to 3.64% and the accuracy was within 98.3–103.9% for RAL. Similarly, for inter-batch experiments, the precision varied from 0.87% to 2.53% and the accuracy was within 96.3–102.2% (Table 1).
Table 1.
Intra- and inter-batch precision and accuracy for raltegravir.
| QC samples (ng/mL) | Intra-batch (n=6; single batch) |
Inter-batch (n=30, 6 from each batch) |
||||
|---|---|---|---|---|---|---|
| Mean conc. found (ng/mL) | CV (%) | Accuracy (%) | Mean conc. found (ng/mL) | CV (%) | Accuracy (%) | |
| HQC (5000) | 4915.7 | 2.77 | 98.3 | 4815.7 | 0.87 | 96.3 |
| MQC (2500) | 2483.7 | 2.98 | 99.4 | 2485.4 | 1.43 | 99.4 |
| LQC (6.0) | 6.12 | 2.92 | 102.1 | 6.13 | 2.38 | 102.2 |
| LLOQ QC (2.0) | 2.08 | 3.64 | 103.9 | 2.04 | 2.53 | 102.1 |
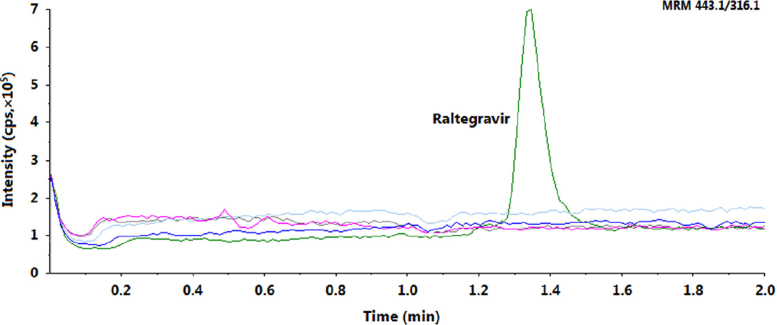
The mean extraction recovery and IS-normalized MFs for RAL are shown in Table 2. The recovery obtained was consistent, ranging from 91.2% to 94.2% across three QC levels. As co-eluting matrix components can directly impact the overall reliability of a validated method, it is suggested to compute MFs to assess the matrix effect. The IS-normalized MFs using stable-isotope labeled IS should be close to unity due to similarities in the chemical properties and elution behavior of the analyte and IS. The IS-normalized MFs ranged from 0.992 to 0.999 for RAL. Additionally, it is required to check the relative matrix effect in lipemic and haemolysed plasma samples together with normal K3EDTA plasma. The coefficient of variation (%CV) of the slopes of calibration lines for relative matrix effect in ten different plasma lots was 2.77 (Table 3). Furthermore, the blank extracts obtained through the LLE procedure were analyzed by the post-column analyte infusion method. The result confirmed the absence of signal suppression or enhancement at the retention time of RAL and IS (Fig. 4).
Table 2.
Extraction recovery and matrix factors for raltegravir and raltegravir-d3.
| QC level (ng/mL) | Area response (n=6) |
Extraction recovery (A/B×100) (Internal standard) | Matrix factor |
||||
|---|---|---|---|---|---|---|---|
| A (CV, %) | B (CV, %) | C (CV, %) | Analyte (B/C) | IS | IS-normalized | ||
| LQC (6.0) | 3974 (2.8) | 4220 (4.7) | 4241 (3.4) | 94.2 (92.6) | 0.995 | 1.003 | 0.992 |
| MQC (2500) | 1,551,632 (5.8) | 1,701,708 (6.6) | 1,723,059 (6.2) | 91.2 (91.8) | 0.987 | 0.998 | 0.988 |
| HQC (5000) | 3,321,302 (4.7) | 3,590,736 (5.6) | 3,596,711 (5.0) | 92.5 (91.1) | 0.998 | 0.999 | 0.999 |
A: mean area response of samples prepared by spiking in extracted blank plasma.
B: mean area response of samples prepared by spiking before extraction.
C: mean area response of samples prepared by spiking in mobile phase (neat samples).
Table 3.
Relative matrix effect in eight different plasma lots for raltegravir.
| Plasma lot | Slope |
|---|---|
| Lot-1 (K3EDTA) | 0.00245 |
| Lot-2 (K3EDTA) | 0.00238 |
| Lot-3 (K3EDTA) | 0.00238 |
| Lot-4 (K3EDTA) | 0.00249 |
| Lot-5 (K3EDTA) | 0.00236 |
| Lot-6 (K3EDTA) | 0.00233 |
| Lot-7 (K3EDTA) | 0.00235 |
| Lot-8 (heparinized) | 0.00254 |
| Lot-9 (haemolysed) | 0.00243 |
| Lot-10 (lipemic) | 0.00249 |
| Mean | 0.00242 |
| Standard deviation | 0.000067 |
| Coefficient of variation (%) | 2.77 |
Fig. 4.
Injection of four extracted blank plasma samples during post-column infusion of raltegravir with a chromatogram of raltegravir at HQC level.
The stock solutions kept for short-term and long-term stability as well as spiked plasma samples showed no evidence of degradation under all the studied conditions. Samples for short-term stability remained stable up to 8 h, while the stock solutions of RAL and IS were stable for a minimum period of 18 days at refrigerated temperature of 5 °C. No significant degradation was observed for the analyte during sample storage and any of the processing steps during extraction. The detailed results for stability studies are presented in Table 4. Sample dilution test was performed to ascertain dilution reliability of samples which were above the ULOQ. The precision (%CV) values for 2-fold and 10-fold dilution were 1.5% and 0.4%, while the accuracy results were 96.0% and 102.4%, respectively. The precision and accuracy for method ruggedness on two different Chromolith RP-18e columns and with different analysts were within 0.9–4.2% and 93.5–103.8%, respectively.
Table 4.
Stability of raltegravir in plasma under various conditions (n=6).
| Storage conditions | Nominal concentration (ng/mL) | Mean stability sample (ng/mL)±SD | Change (%) |
|---|---|---|---|
| Bench-top stability at room temperature (25 °C) for 8 h | 5000 | 4709.67±72.69 | −5.81 |
| 6.0 | 6.43±0.15 | 7.17 | |
| Freeze-thaw stability after 5th cycle at −20 °C | 5000 | 4773.50±106.40 | −4.54 |
| 6.0 | 6.17±0.22 | 2.83 | |
| Freeze-thaw stability after 5th cycle at −70 °C | 5000 | 4804.83±218.23 | −3.90 |
| 6.0 | 6.17±0.24 | 2.83 | |
| Wet extract (autosampler) stability at 4 °C for 65 h | 5000 | 4838.17±209.68 | −3.24 |
| 6.0 | 6.24±0.28 | 4.00 | |
| Wet extract stability at 25 °C for 4 h | 5000 | 4959.33±287.84 | −0.81 |
| 6.0 | 6.23±0.25 | 3.83 | |
| Dry extract stability in deep freezer at −20 °C for 65 h | 5000 | 4874.00±183.04 | −2.52 |
| 6.0 | 6.23±0.25 | 3.83 | |
| Long-term stability at −20 °C for 42 days | 5000 | 4844.00±247.40 | −3.12 |
| 6.0 | 5.94±0.26 | −1.00 | |
| Long-term stability at −70 °C for 42 days | 5000 | 4861.50±315.59 | −2.77 |
| 6.0 | 6.20±0.23 | 3.33 |
3.3. Application to a bioequivalence study and ISR results
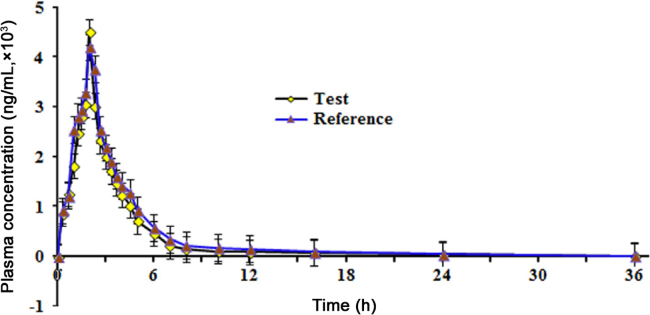
As yet there are no reports on the pharmacokinetics of RAL in Indian subjects. Therefore, the method was applied to monitor RAL concentration in human plasma samples after oral administration of a single 400 mg dose of RAL. Fig. 5 shows the mean plasma concentration vs. time profile for RAL under fasting.
Fig. 5.
Mean plasma concentration-time profile of raltegravir after oral administration of 400 mg (test and reference) tablet formulation to 18 healthy Indian subjects.
Although the area under the plasma-time curve (AUC) increases by about 19% with a high fat meal, RAL can be given without regard to food [1]. The time required to reach peak plasma concentration was ~2 h, which was almost double as observed by Kassahun et al. [9] with 200 mg dose of RAL in healthy subjects. Nevertheless, the Cmax [test: 4642 ng/mL; reference: 4270 ng/mL] and AUC0–36 h values [test: 10,463 h ng/mL; reference: 9708 h ng/mL] were comparable with their work. For studies in healthy subjects, the pharmacokinetics of RAL was reported to be dose-dependent for oral doses ranging from 10 to 1600 mg [9]. Further, the reproducibility of the assay was studied by reanalysis of 87 incurred samples (Supplementary Fig. 2). The % change was within±17%, which was within the acceptance criterion of ±20% [31].
3.4. Comparison with existing methods
The proposed method is more sensitive compared to several methods developed for determination of raltegravir in human plasma [8], [9], [10], [11], [13], [14], [15], [18], [20], [24], [25], [26]. Further, it is more rapid than all other methods except one report [20]. The present method employs small plasma volume (100 µL) for processing, which is much less compared to many reported procedures. A detailed comparison of salient features of different liquid chromatographic methods with mass spectrometric detection developed for RAL is given in Table 5.
Table 5.
Salient features of selected liquid chromatographic methods developed for raltegravir as a single analyte in human plasma with mass spectrometric detection.
| Extraction technique | Sample volume (µL) | Linear range (ng/mL) | Retention time (min); run time (min) | Application | Refs. |
|---|---|---|---|---|---|
| LLE with hexane–DCM | 200 | 2.0–1000 | 1.56; 3.5 | To support 18 clinical studies during Phase I through Phase III | [8] |
| LLE with hexane–DCM | 200 | 1.0–3000 | 3.80; 7.0 | Determination of raltegravir in a single HIV-infected patient | [12] |
| LLE with hexane–DCM | 500 | 10.0–7680 | 8.23; 16.0 | – | [13] |
| PP with ACN–methanol | 25 | 10–3000 | 1.65; 4.0 | Analysis of human plasma samples | [14] |
| PP with ACN–methanol | 50 | 50–10000 | 4.20; 10.0 | Pharmacokinetic study in one HIV-infected patient | [15] |
| LLE | 100 | 5.0–2560 | –; 1.0 | Pharmacokinetic study in human plasma samples | [20] |
| PP with ACN | 50 | 2.0–2000 nmol/L | 4.90; 9.0 | Pharmacokinetic study with 400 mg raltegravir in 6 healthy subjects | [21] |
| LLE with hexane–DCM | 100 | 2.0–6000 | 1.35; 2.0 | Bioequivalence study with 400 mg raltegravir in 18 healthy subjects; Reanalysis of 87 incurred samples (% change within±17%) | PW |
LLE: liquid–liquid extraction; PP: protein precipitation; SPE: solid phase extraction; DCM: dichloromethane; ACN: acetonitrile; PW: present work.
4. Conclusions
The proposed validated LC–MS/MS assay provided a reliable and rugged approach for the quantitation of RAL in human plasma in the negative ionization mode. The LLE procedure afforded highly selective separation of the analytes from endogenous components enabling quantification of 0.01–40 ng on-column per sample injection employing 100 µL plasma samples. The method was extensively validated for matrix effect and stability under different storage conditions. It was successfully applied in a clinical study and the reproducibility of the assay was demonstrated by incurred sample reanalysis.
Acknowledgments
The authors would like to thank Veeda Clinical Research, Ahmedabad, India, for supporting this work.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2014.10.002.
Appendix A. Supplementary materials
Supplementary data
References
- 1.Liedtke M.D., Tomlin C.R., Lockhart S.M. Long-term efficacy and safety of raltegravir in the management of HIV infection. Infect. Drug Resist. 2014;7:73–84. doi: 10.2147/IDR.S40168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merck Sharp & Dohme Corporation, ISENTRESS® (raltegravir) oral tablets. Prescribing Information, Whitehouse Station, NJ 08889, USA, April 2012. 〈https://www.merck.com/product/usa/pi_circulars/i/isentress/isentress_pi.pdf〉
- 3.Andrews E., Glue P., Fang J. Assessment of the pharmacokinetics of co-administered maraviroc and raltegravir. Br. J. Clin. Pharmacol. 2010;69:51–57. doi: 10.1111/j.1365-2125.2009.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savarino A. In-silico docking of HIV-1 integrase inhibitors reveals a novel drug type acting on an enzyme/DNA reaction intermediate. Retrovirology. 2007;4:1–15. doi: 10.1186/1742-4690-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey K.K. Raltegravir in HIV-1 infection: safety and efficacy in treatment-naive patients. Clin. Med. Rev. Ther. 2012;4:13–30. doi: 10.4137/CMRT.S5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molto J., Sanz-Moreno J., Valle M. Minimal removal of raltegravir by hemodialysis in HIV-infected patients with end-stage renal disease. Antimicrob. Agents Chemother. 2010;54:3047–3048. doi: 10.1128/AAC.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocohoba J., Dong B.J. Raltegravir: the first HIV integrase inhibitor. Clin. Ther. 2008;30:1747–1765. doi: 10.1016/j.clinthera.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Merschman S.A., Vallano P.T., Wenning L.A. Determination of the HIV integrase inhibitor, MK-0518 (raltegravir), in human plasma using 96-well liquid-liquid extraction and HPLC-MS/MS. J. Chromatogr. B. 2007;857:15–24. doi: 10.1016/j.jchromb.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Kassahun K., McIntosh I., Cui D. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab. Dispos. 2007;35:1657–1663. doi: 10.1124/dmd.107.016196. [DOI] [PubMed] [Google Scholar]
- 10.Rezk N.L., White N., Kashuba A.D.M. An accurate and precise high-performance liquid chromatography method for the rapid quantification of the novel HIV integrase inhibitor raltegravir in human blood plasma after solid phase extraction. Anal. Chim. Acta. 2008;628:204–213. doi: 10.1016/j.aca.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirier J.M., Robidou P., Jaillon P. Quantification of the HIV-integrase inhibitor raltegravir (MK-0518) in human plasma by high performance liquid chromatography with fluorescence detection. J. Chromatogr. B. 2008;867:277–281. doi: 10.1016/j.jchromb.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Long M.C., Bennetto-Hood C., Acosta E.P. A sensitive HPLC–MS–MS method for the determination of raltegravir in human plasma. J. Chromatogr. B. 2008;867:165–171. doi: 10.1016/j.jchromb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M., Konishi M., Kudaka Y. A conventional LC–MS method developed for the determination of plasma raltegravir concentrations. Biol. Pharm. Bull. 2008;31:1601–1604. doi: 10.1248/bpb.31.1601. [DOI] [PubMed] [Google Scholar]
- 14.Jourdil J.F., Bartoli M., Stanke-Labesquea F. Lack of specificity for the analysis of raltegravir using online sample clean-up liquid chromatography–electrospray tandem mass spectrometry. J. Chromatogr. B. 2009;877:3734–3738. doi: 10.1016/j.jchromb.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Ter Heine R., Hillebrand M.J.X., Rosing H. Quantification of the HIV-integrase inhibitor raltegravir and detection of its main metabolite in human plasma, dried blood spots and peripheral blood mononuclear cell lysate by means of high-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2009;49:451–458. doi: 10.1016/j.jpba.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz A., Gisslen M., Spudich S. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS ONE. 2009;4:1–5. doi: 10.1371/journal.pone.0006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talameh J.A., Rezk N.L., Kashuba A.D.M. Quantifying the HIV-1 integrase inhibitor raltegravir in female genital tract secretions using high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B. 2010;878:92–96. doi: 10.1016/j.jchromb.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldwirt L., Barrail-Tran A., Cruz M.D. Quantification of raltegravir (MK0518) in human plasma by high-performance liquid chromatography with photodiode array detection. J. Chromatogr. B. 2010;878:456–460. doi: 10.1016/j.jchromb.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Robbins B.L., Nelson S.R., Fletcher C.V. A novel ultrasensitive LC–MS/MS assay for quantification of intracellular raltegravir in human cell extracts. J. Pharm. Biomed. Anal. 2012;70:378–387. doi: 10.1016/j.jpba.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortuna S., Ragazzoni E., Lisi L. Validation of an UPLC–MS/MS method for quantitative analysis of raltegravir in human plasma samples. Ther. Drug Monit. 2013;35:258–263. doi: 10.1097/FTD.0b013e318280110d. [DOI] [PubMed] [Google Scholar]
- 21.Wang L.Z., Lee L.S., Thuya W.L. Simultaneous determination of raltegravir and raltegravir glucuronide in human plasma by liquid chromatography-tandem mass spectrometric method. J. Mass Spectrom. 2011;46:202–208. doi: 10.1002/jms.1874. [DOI] [PubMed] [Google Scholar]
- 22.D׳Avolio A., Baietto L., Siccardi M. An HPLC-PDA method for the simultaneous quantification of the HIV integrase inhibitor raltegravir, the new nonnucleoside reverse transcriptase inhibitor etravirine, and 11 other antiretroviral agents in the plasma of HIV-infected patients. Ther. Drug Monit. 2008;30:662–669. doi: 10.1097/FTD.0b013e318189596d. [DOI] [PubMed] [Google Scholar]
- 23.Notari S., Tommasi C., Nicastri E. Simultaneous determination of maraviroc and raltegravir in human plasma by HPLC–UV. IUBMB Life. 2009;61:470–475. doi: 10.1002/iub.181. [DOI] [PubMed] [Google Scholar]
- 24.Fayet A., Béguin A., Zanolari B. A LC–tandem MS assay for the simultaneous measurement of new antiretroviral agents: Raltegravir, maraviroc, darunavir, and etravirine. J. Chromatogr. B. 2009;877:1057–1069. doi: 10.1016/j.jchromb.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 25.Djerada Z., Feliu C., Tournois C. Validation of a fast method for quantitative analysis of elvitegravir, raltegravir, maraviroc, etravirine, tenofovir, boceprevir and 10 other antiretroviral agents in human plasma samples with a new UPLC–MS/MS technology. J. Pharm. Biomed. Anal. 2013;86:100–111. doi: 10.1016/j.jpba.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Jourdil J.F., Tonini J., Stanke-Labesquea F. Simultaneous quantitation of azole anti-fungals, antibiotics, imatinib, and raltegravir in human plasma by two-dimensional high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B. 2013;919-920:1–9. doi: 10.1016/j.jchromb.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Guidance for Industry, Bionanalytical Method Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Veterinary Medicine (CVM), 2001.
- 28.Gupta A., Guttikar S., Shrivastav P.S. Simultaneous quantification of prodrug oseltamivir and its metabolite oseltamivir carboxylate in human plasma by LC–MS/MS to support a bioequivalence study. J. Pharm. Anal. 2013;3:149–160. doi: 10.1016/j.jpha.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P., Contractor P., Guttikar S. Development of a sensitive and rapid method for quantitation of (S)-(−)- and (R)-(+)-metoprolol in human plasma by chiral LC–ESI–MS/MS. J. Pharm. Anal. 2014;4:63–79. doi: 10.1016/j.jpha.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guidance for Industry: ICH E6 Good Clinical Practice, U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Centre for Biologics Evaluation and Research (CBER), 1996.
- 31.Yadav M., Shrivastav P.S. Incurred sample reanalysis (ISR): a decisive tool in bio-analytical research. Bioanalysis. 2011;3:1007–1024. doi: 10.4155/bio.11.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data