Abstract
The aim of this study was to develop and validate a simple, sensitive, precise and cost-effective one-level agar diffusion (5+1) bioassay for estimation of potency and bioactivity of Levofloxacin in pharmaceutical preparation which has not yet been reported in any pharmacopoeia. Among 16 microbial strains, Bacillus pumilus ATCC-14884 was selected as the most significant strain against Levofloxacin. Bioassay was optimized by investigating several factors such as buffer pH, inoculums concentration and reference standard concentration. Identification of Levofloxacin in commercial sample Levoflox tablet was done by FTIR spectroscopy. Mean potency recovery value for Levofloxacin in Levoflox tablet was estimated as 100.90%. A validated bioassay method showed linearity (r2=0.988), precision (Interday RSD=1.05%, between analyst RSD=1.02%) and accuracy (101.23%, RSD=0.72%). Bioassay was correlated with HPLC using same sample and estimated potencies were 100.90% and 99.37%, respectively. Results show that bioassay is a suitable method for estimation of potency and bioactivity of Levofloxacin pharmaceutical preparations.
Keywords: Levofloxacin, Antibiotic resistance, Microbiological bioassay, HPLC, Pharmacopoeia
1. Introduction
Levofloxacin is a synthetic broad-spectrum antibiotic of fluoroquinolone group and is used to treat severe bacterial infections which failed to respond to other antibiotic classes [1], [2]. Levofloxacin is chemically(S)-9-fluoro-2, 3-dihydro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-7 H-pyrido [1,2,3–de]-1, 4 benzoxazine-6-carboxylic acid hemihydrate (Fig. 1) with molecular formula C18H20FN3O4, and a molecular weight of 370.4 [3]. It is a yellowish white to yellow powder [3], [4].
Fig. 1.
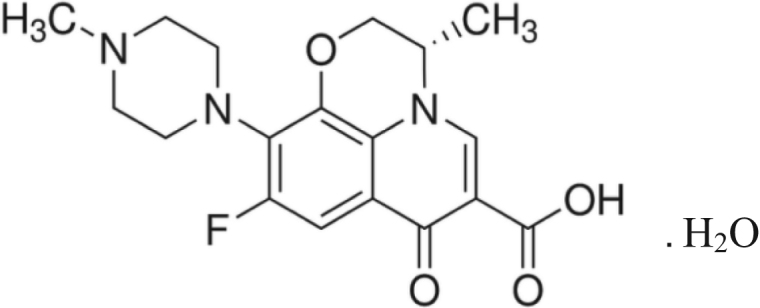
Chemical structure of Levofloxacin.
Levofloxacin is active against both Gram-positive and Gram-negative bacteria [5]. It is used in the treatment of bronchitis, urinary tract infections, pneumonia, skin and soft tissues infections [6]. This antibiotic can also be used to prevent infection after exposure to inhaled anthrax. Levofloxacin inhibits bacterial topoisomerases II, topoisomerases IV and DNA gyrase, which are important enzymes required for DNA replication, transcription, repair and recombination, thereby inhibiting cell division [6], [7].
Among all pharmaceutical products, the most commonly faked and adulterated ones are antibiotics probably because the frequency of their use is very high [8]. The misuse of antibiotics fosters the increase and spread of antibiotic resistance and may lead to superinfections [9]. An important factor in the development of drug-resistant strains of microorganisms is that many antibiotics are bacteriostatic rather than bactericidal [10]. In order to overcome the resistance problem and for the safe use of antibiotics, the correct measurement of potency and bioactivity of antibiotics is essential. Due to the increased resistance problem, the quantification of the actual concentration of active ingredients in antibiotic preparation is critical. A mild difference in the concentration of active ingredient in antibiotic preparations may have impact on actual efficacy. Therefore, quantification of active pharmaceutical ingredient (API) in antibiotic preparation is very necessary because most of the time these drugs are the lines that separate life from death [11]. These substances in very low concentrations are known to totally destroy or partially inhibit microorganisms [12].
The potency of antibiotics can be determined by chemical and biological methods. Chemical methods such as capillary electrophoresis, ultraviolet (UV) spectrophotometry, high performance liquid chromatography (HPLC) and high performance thin layer chromatography (HPTLC) have been used for the quantitative determination of Levofloxacin in formulations as well as in human urine, and serum [6], [13]. However, the microbiological assay for determination of potency of Levofloxacin has not yet been reported in any pharmacopoeia. Biological method is the most convenient way to determine the potency of antibiotics [14].
Determination of antimicrobial potency is extremely important for the quality control and quality assurance concerning pharmaceutical preparations, being thus necessary to develop practical and economical methods which can be applied in the validation and dosage of drugs [15], [16]. The application of microbiological assay has been recently developed for intravenously administered antibiotics. This method is highly acceptable by regulating authorities to control antibiotic potency [17], [18]. Microbiological bioassay plays an essential role in the manufacturing and quality control of antibiotic medicines and demands considerable skill and expertise to assure success [18], [19]. Microbiological assay helps in estimating active constituents, biological activity and in monitoring the stability of antibiotics. Any small change in the antibiotic molecule, which may not be detected by chemical methods, will be revealed by a change in antimicrobial activity [4]. Hence, microbiological assay is very useful for resolving doubts regarding possible change in potency of antibiotics and their preparations. A microbial bioassay requires effective and fully characterized microbial strains. The identification and characterization of microbial strain are performed by culturable and non-culturable techniques [20], [21].
The potency of antibiotics can be measured by microbial bioassay, in which their inhibitory effect on the growth of test microorganisms is evaluated [3], [4], [14], [22]. Bioassays do not require specialized equipment or toxic solvents [23]. The agar diffusion method widely used in antibiotic assay relates the size of the zone of inhibition to the dose of the antibiotic assayed. The relation of the diameter of inhibitory zones to concentration of antibiotic in a solution applied in cups has been considered theoretically [24], [25]. The ability of an antibiotic is to inhibit or to kill the growth of living microorganisms. The inhibition of microbial growth in standardized conditions may be utilized for demonstrating the therapeutic efficacy of antibiotics. The antimicrobial activity of Levofloxacin in ophthalmic solution was measured using Bacillus subtilis, ATCC-6633 [26]. The in vitro activity of Levofloxacin was evaluated against 234 strains of Mycobacterium tuberculosis and MIC50 and MIC90 were obtained as 0.25 mg/L and 0.5 mg/L, respectively [27].
The proposed article focuses on the development and validation of a simple, sensitive, accurate, precise and cost-effective one-level agar diffusion (5+1) bioassay for the quantification of potency and bioactivity of Levofloxacin in pharmaceutical preparations.
2. Materials and methods
2.1. Chemicals and reagents
Chemicals and reagents used were of analytical grade (Merck Ltd., Mumbai). Milli-Q water (Millipore) was used to prepare solutions. United States Pharmacopoeia (USP) reference standard of Levofloxacin was used for standard solution preparation. Commercial sample Levoflox tablet containing Levofloxacin 500 mg was obtained from the local market.
2.2. Equipment
All equipments used for the bioassay study were calibrated and validated. Sterilized glassware (Class B) such as Petri plates, test tubes, volumetric flasks, pipettes and sterile borer were used in the experiment. Steam Sterilizer/Autoclave (Make-Nat steel) was used to sterilize the media at 121 °C and 15 psi for 15 min. Glycerol stocks of microbial cultures stored at –80 °C Deep freezers (Make – Haier) were used as test strains. Identification of Levofloxacin was performed by an FTIR spectroscope (Perkin Elmer) and HPLC (Make-Agilent Technologies) was used for comparative study. Bioassay plates were incubated at 37 °C inside the incubator (Make-Thermolab) for bacterial growth. Zones of inhibition were measured by an antibiotic zone reader (Make-Aarachal Corporation).
2.3. Test microbial strains
Microbial cultures were procured from American Type Culture Collection (ATCC), USA, and National Collection of Type Cultures (NCTC), UK. The different Gram-positive bacteria Bacillus cereus (ATCC-11778), B. pumilus (ATCC-14884), B. subtilis (ATCC-6633), Staphylococcus aureus (ATCC-6538, 29737, 9144), Staphylococcus epidermidis (ATCC-12228), Kocuria rhizophila (ATCC- 9341), Micrococcus luteus (ATCC-10240) and Gram-negative bacteria Escherichia coli (ATCC-10536, 8739), Salmonella abony (NCTC-6017), Pseudomonas aeruginosa (ATCC-25619, 9027), Klebsiella pneumoniae (ATCC-10031), Bordetella bronchiseptica (ATCC-4617) were used in microbiological bioassay.
2.4. Preparation of phosphate buffer
pH has a major influence on the response of antibiotics upon indicator microorganisms. The experiments were performed with different pH buffers. Buffer solutions of different pH were prepared by dissolving various quantities of K2HPO4 and KH2PO4 in sufficient Milli-Q water. The pH was adjusted with 8 M phosphoric acid or 10 M potassium hydroxide and sterilized in autoclave [3].
2.5. Preparation of microbiological media
Dehydrated media were procured from Hi-Media Ltd., Mumbai (India). Primary objective of the media was to support the rapid growth of indicator microorganism being used in the bioassay. Antibiotic assay medium No.11 was used as a bioassay medium to prepare the base layer and the seed layer. Soyabean casein digest agar media were used for slant preparation for bacterial growth. Dehydrated media were dissolved in the distilled water and pH was adjusted as per instructions on the dehydrated media container. Media were sterilized in the autoclave at 121 °C and 15 psi for 15 min.
2.6. Preparation of standard solution
Accurately weighed quantity of reference standard of Levofloxacin 25.0 mg was dissolved in phosphate buffer and till the volume was 25 mL to obtain 1000 µg/mL of Levofloxacin. Five standard dilutions, i.e., S1 (2.56 µg/mL), S2 (3.20 µg/mL), S3 (4.00 µg/mL), S4 (5.00 µg/mL), and S5 (6.25 µg/mL), were prepared in stepwise increasing concentration of 4:5. Dilution S3 was considered as the reference concentration (mean concentration) level of standard.
2.7. Preparation of sample solution
Twenty tablets were weighed and pulverized. A quantity of powder equivalent to 25.0 mg of the commercial sample Levoflox tablet was accurately weighed and transferred into a 25 mL volumetric flask. The final volume was made up to 25 mL with phosphate buffer to obtain a concentration of 1000 µg/mL. From this stock, a solution with a concentration of 100 µg/mL was prepared and finally a dilution i.e., “T”, was made which was equivalent to the mean reference standard concentration (S3).
2.8. Inoculums preparation and its standardization
Effective and fully characterized microbial strain is required for the bioassay. Fresh microbial strains preserved on glycerol stock were revived and then sub-cultured on the slants of soybean casein digest agar media. Slants were incubated at 37 °C for 24 h for bacterial growth. Fresh culture slants were used throughout the study. About 3 mL sterilized saline solution (0.9%) was used to wash the microorganism from agar slant and then the dilution factor was determined, which gave 25% light transmission at about 530 nm.
2.9. Bioassay method
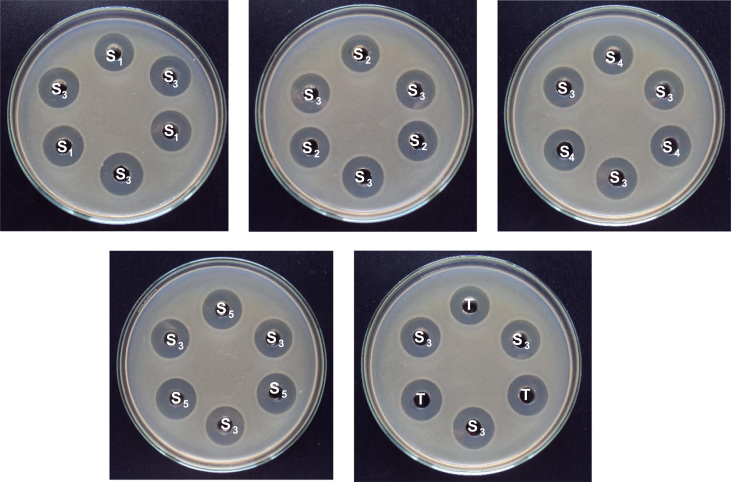
The 5+1 bioassay design with standard curve was carried out by the cylinder-plate method. This method depends upon diffusion of the antibiotic solution from a vertical cylinder or cavity through a solidified agar layer in a Petri plate to an extent such that growth of the added microorganism is prevented entirely in a zone around the cylinder or cavity containing a solution of the antibiotic [3]. Standardized microbial suspension was used to prepare double-layer plates of Assay medium No.11 by pouring 4 mL seed layer (inoculated with the desired strain) over a solidified 21 mL base layer of assay medium in a 100 mm×20 mm Petri dish [4]. These plates were left for 30 min for solidification. After media solidification, 5 mm-diameter wells were bored at six points for a 5+1 bioassay design (Fig. 2). 100 µL of each standard or test solution was pipetted into individual wells. Five Petri dishes were used for each assay in order to test the reference concentration (S3) concomitantly with each standard or sample concentration. The plates were left standing for 1–4 h at room temperature as a period of pre-incubation diffusion to minimize the effects of variation in time between the applications of the different solutions. The plates were incubated at 37 °C for 24 h.
Fig. 2.
One-level agar diffusion bioassay (5+1 assay): Five Petri dishes represent the reference solutions S1 (2.56 µg/mL), S2 (3.20 µg/mL), S3 (4.0 µg/mL), S4 (5.0 µg/mL) and S5 (6.25 µg/mL). “T” represents the sample solution (4.0 µg/mL).
After the complete incubation period, the diameters (in mm) of the inhibition zones were accurately measured by a zone reader and the results were observed. Assay plates were tested in triplicate, resulting in nine measures of each of the standards S1, S2, S4 and S5 and the test sample “T”. The reference concentration “S3” was tested 36 times with the lowest and the highest concentration of standards and 9 times with the test sample in order to fit the data obtained in all the dishes. The average of all readings of solution “S3” and the readings of the concentration tested on each of the sets of three plates and the average of all the 36 readings of “S3” were estimated. The average of the 36 reading of solution “S3” was the correction point for curve. Highest and lowest zone diameters for final potency calculation were obtained by the following equations:
where L is the zone diameter for the lowest concentration of the standard curve response line; H is the zone diameter for the highest concentration of the standard curve response line; c is the average zone diameter of 36 readings of the reference point standard solution; and a, b, d, and e are the corrected average values for the other standard solutions, lowest to highest concentrations.
2.10. FTIR spectroscopy analysis
Fourier transform infrared (FTIR) spectroscopy analysis was used to ascertain the presence or absence of Levofloxacin in the market tablet sample (Levoflox). Identification of Levofloxacin was performed by FTIR spectroscopy using Perkin Elmer system (Modal-Spectrum one). The commercial sample (Levoflox) and reference standard were prepared in the form of discs dispersed in potassium bromide (IR grade) and the spectra were recorded between 2000 cm−1 and 400 cm−1 under the same operational conditions.
2.11. HPLC assay
The specificity of the proposed bioassay was compared with HPLC. The HPLC assay was carried out on a system of Agilent Technologies, Series 1200, which is composed of a quaternary pump, an autosampler, a photodiode array detector (DAD) and EZ Chrome Elite software. The column used was a Cosmosil C18 MS II from Thermo Electron Corporation. The mobile phase consisted of a mixture of 85 volume of buffer solution prepared by dissolving 84 volumes of 0.05 M citric acid monohydrate and 1 volume of 1 M ammonium acetate; and 15 volumes of acetonitrile. A 0.1% (m/v) solution of Levofloxacin reference standard was prepared in 0.1 M hydrochloric acid and 5 mL of this solution was diluted to 50 mL with distilled water. The test solution was prepared by dissolving a quantity containing 100 mg of the commercial sample Levoflox tablet and dispersed in 100.0 mL of 0.1 M hydrochloric acid. 5 mL of this solution was diluted to 50 mL with distilled water. All solutions were filtered through 0.45 µm membrane filter before injection. The spectrophotometer was set at 293 nm. Flow rate was maintained as 1 mL/min and the injection volume was 10 µL.
3. Results
3.1. Selection of significant microbial strain
The criteria for selection of significant microbial strain were well-defined edges and large measurable zone diameter under antibiotic treatment. The 16 bacterial strains were tested for their response and susceptibility against Levofloxacin. Among all tested strains, B. pumilus ATCC-14884 showed the most significant result at the same conditions. Hence, B. pumilus ATCC-14884 strain was used further for one-level (5+1) bioassay study.
3.2. Effect of pH on zone diameter
The growth rate of microorganisms is highly influenced by buffer pH. The activity of Levofloxacin was studied in the range of phosphate buffer pH 6.0–8.0. The phosphate buffer pH 7.0 was selected as the most suitable for significant growth of B. pumilus ATCC-14884 and production of measurable sharp zone of inhibition. The effect of different buffer pH solutions on zone of inhibition is shown in Table 1.
Table 1.
Response of microbial strains to Levofloxacin (4.0 µg/mL) at different buffer pH.
| Name of organisms | Zone diameter (mm) |
Results | ||||
|---|---|---|---|---|---|---|
| pH 6.0 | pH 6.5 | pH 7.0 | pH 7.5 | pH 8.0 | ||
| Bacillus cereus (ATCC-11778) | 19.2 | 19.7 | 20.5 | 20.3 | 19.9 | Sharp zone |
| Bacillus pumilus (ATCC-14884) | 21.1 | 21.8 | 22.5 | 21.7 | 20.4 | Very sharp & clear zone |
| Bacillus subtilis (ATCC-6663) | 23.9 | 24.1 | 24.7 | 24.5 | 24.1 | Large zone |
| Staphylococcus aureus (ATCC-6538) | 19.5 | 19.7 | 20.1 | 20.4 | 19.9 | Sharp zone |
| Staphylococcus aureus (ATCC-29737) | 25.4 | 25.7 | 26.5 | 26.0 | 25.9 | Large zone |
| Staphylococcus aureus (ATCC-9144) | 19.6 | 19.8 | 20.4 | 20.2 | 19.7 | Sharp zone |
| Staphylococcus epidermidis (ATCC-12228) | 18.8 | 19.0 | 19.6 | 19.9 | 20.0 | Intermediate zone |
| Kocuria rhizophila (ATCC-9341) | 17.5 | 18.0 | 19.4 | 19.1 | 19.2 | Intermediate zone |
| Micrococcus luteus (ATCC-10240) | 17.9 | 18.2 | 18.5 | 18.7 | 19.4 | Intermediate zone |
| Escherichia coli (ATCC-10536) | 19.4 | 19.9 | 20.5 | 21.5 | 22.7 | Sharp zone |
| Escherichia coli (ATCC-8739) | 19.0 | 19.8 | 20.6 | 20.9 | 21.6 | Sharp zone |
| Salmonellae abony (NCTC-6017) | 25.7 | 26.2 | 26.8 | 27.0 | 27.1 | Large zone |
| Pseudomonas aeruginosa (ATCC-25619) | − | − | − | − | − | No inhibition zone |
| Pseudomonas aeruginosa (ATCC-9027) | − | − | − | − | − | No inhibition zone |
| Klebsiella pneumoniae (ATCC-10031) | − | − | − | − | − | No inhibition zone |
| Bordetella bronchiseptica (ATCC-4617) | 24.9 | 25.4 | 25.7 | 25.8 | 25.6 | Large zone |
3.3. Selection of optimum inoculums concentration
Wide variation in the number of microorganism produced different diameters of zone of inhibition. An experiment was performed to determine how critical the concentration of inoculums might be in the present system when other factors were constant. Optimum inoculum concentration was selected based on zone diameter with edge sharpness. Small zone diameter and overlapping of growth pattern were observed with high inoculum concentration, whereas larger zone diameter and very poor growth were observed with too diluted inoculums concentration. Optimum inoculum concentration should lie in between these two extremes. In the present study, six different inoculums concentrations, i.e., 0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0%, were tested and their effects on diameter of zone of inhibition were observed (Table 2). All the inoculums concentrations were optimized at 25% transmittance and optimum inoculum concentration of B. pumilus ATCC-14884 for microbial bioassay was selected as 2.0%.
Table 2.
Effect of different inoculum concentrations on the diameter of zone of inhibition.
| Inoculum concentration (%) | Dilution factor (µg/mL) | Zone diameter (mm) | Observation |
|---|---|---|---|
| 0.5 | 4 | 34.8 | Very light and large zone |
| 1.0 | 4 | 29.0 | Light and large zone |
| 1.5 | 4 | 26.9 | Light and large zone |
| 2.0 | 4 | 22.6 | Very sharp and clear zone |
| 2.5 | 4 | 19.8 | Overlapped zone with hazy growth |
| 3.0 | 4 | 16.8 | Overlapped zone with hazy growth |
3.4. Determination of optimum antibiotic concentration
The optimization of antibiotic concentration is very important to overcome the resistance problem and for safe use of antibiotics. The 5+1 bioassay design (One-level assay) was used to quantify the potency of Levofloxacin. One-level assay design involved the preparation of five sets of concentration for standard in stepwise increasing ratio of 4:5. The effect of all these standard concentrations on the zone of inhibition is depicted in Table 3. All concentrations were used in triplicate and assigned as S1, S2, S3, S4 and S5, which represent lower to higher concentration with increasing ratio 4:5. “S3” represents the reference concentration (mean concentration) of Levofloxacin reference standard and was used as a correction factor. The optimum antibiotic concentration “S3” was estimated as 4 µg/mL.
Table 3.
Effect of different concentrations of reference standard of Levofloxacin on zone of inhibition.
| Standard concentration (µg/mL) | Mean zone diameter (mm) |
|---|---|
| 2.56 | 19.6 |
| 3.20 | 21.8 |
| 4.00 | 22.9 |
| 5.00 | 24.5 |
| 6.25 | 26.7 |
3.5. Percentage potency calculation
The correction in zone diameter with standard was carried out by taking average zone diameter of the standard mean concentration “S3” separately for each of the standard response line concentrations S1, S2, S4, and S5. Similarly average zone diameter of standard response line concentrations (S1, S2, S4, and S5) was taken. All the 36 responses of “S3” were averaged for all the four sets of plates. The average of the 36 responses of “S3” was considered as the correction point of the response line and by putting these values in the standard equation the percentage potency was calculated as 100.90%.
3.6. Method validation
All the parameters of the bioassay were optimized prior to validation to accurately estimate the performance of the proposed bioassay method. The bioassay method was validated by evaluation of linearity, precision, accuracy and robustness according to the International Conference on Harmonization [28].
3.6.1. Linearity
To evaluate the linearity of assay, five concentrations of standards, i.e., 2.56, 3.20, 4.0, 5.0 and 6.25 µg/mL, were used. A calibration curve for log10 of concentrations (µg/mL) of Levofloxacin versus zone of inhibition (mm) was plotted and the obtained data were subjected to regression analysis by the least squares method. The representative linear equation was y=1.69x+18.03. The determination coefficient (r2=0.9883) obtained was highly significant for the method.
3.6.2. Precision
Precision was determined by repeatability and intermediate precision and was expressed as the relative standard deviation (RSD). The repeatability was examined by assaying the six replicates of commercial samples (Levoflox) at 100% concentration level, i.e., 4 µg/mL against the reference standard of Levofloxacin on the same day (intraday) by the same analyst under the same experimental condition (Table 4). The intermediate precision of bioassay was estimated by performing the analysis in the same laboratory on 2 days (interday) with different analysts (between analysts). The results are presented in Table 5.
Table 4.
Repeatability of the bioassay with a commercial sample of Levoflox tablet.
| Theoretical amount (mg) | Experimental amount (mg) | Potency (%) | Mean potency (%) | RSD (%) |
|---|---|---|---|---|
| 500 | 507.6 | 101.52 | 100.90 | 1.09 |
| 503.0 | 100.60 | |||
| 514.2 | 102.84 | |||
| 499.4 | 99.88 | |||
| 502.1 | 100.42 | |||
| 500.7 | 100.14 |
Table 5.
Intermediate precision data of bioassay of Levofloxacin in a commercial sample Levoflox tablet.
| Precision | Observed potency (%) | Mean potency (%) | RSD (%) |
|---|---|---|---|
| Inter-day precision | |||
| Day 1 | 100.84 | 100.48 | 1.05 |
| 101.82 | |||
| Day 2 | 99.62 | ||
| 99.66 | |||
| Inter-analyst precision | |||
| Analyst 1 | 100.06 | 100.66 | 1.02 |
| 99.56 | |||
| Analyst 2 | 101.84 | ||
| 101.18 | |||
3.6.3. Accuracy
Accuracy of the bioassay method was evaluated at 80%, 100% and 120% of the nominal analytical concentration in the specified range of 2.56–6.25 µg/mL. The mean accuracy was 101.23% with RSD 0.72%, which confirms the ability of the method to determine with accuracy the Levofloxacin concentration within the range of 80%−120%. The results are shown in Table 6.
Table 6.
Accuracy of microbial bioassay determined for Levofloxacin.
| Theoretical potency (%) | Observed potency (%) | Mean potency (%) | Accuracy (%) | RSD (%) |
| 80 | 81.78 | 81.62 | 101.23 | 0.72 |
| 80.92 | ||||
| 82.17 | ||||
| 100 | 100.58 | 100.59 | ||
| 99.96 | ||||
| 101.25 | ||||
| 120 | 121.08 | 121.29 | ||
| 120.84 | ||||
| 121.96 | ||||
3.6.4. Robustness
The robustness of the bioassay was determined by analyzing the same sample under a variety of conditions. To assess the robustness some parameters were modified from the normal tested conditions: solvent used for the standard and sample dilution (distilled water), inoculum concentration (1.5%) and incubation temperature (30 °C). Changing the experimental conditions to the specified parameters, no significant differences on the potencies were observed as shown in Table 7.
Table 7.
Factors investigated in the robustness test.
| Factors | Parameters | Potency (%) | RSD (%) |
|---|---|---|---|
| Solvent | Distilled water | 100.12 | 0.47 |
| 100.38 | |||
| 99.47 | |||
| Inoculum | 1.5% | 99.92 | 0.41 |
| concentration | 99.49 | ||
| 100.31 | |||
| Incubation | 30 °C | 99.96 | 0.55 |
| temperature | 100.29 | ||
| 101.04 | |||
3.7. Identification by FTIR spectroscopy
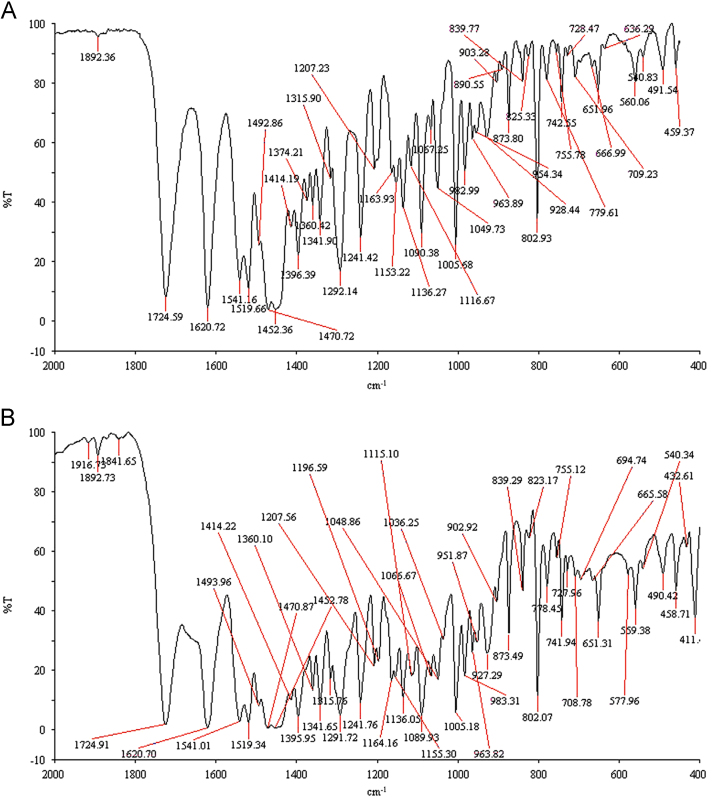
Spectrum obtained from a commercial sample Levoflox tablet was compared with a reference standard of Levofloxacin. The full IR spectrum of the commercial sample Levoflox showed characteristic peaks similar to those of the reference standard of Levofloxacin (Fig. 3A and B). These peaks are known to be used in the identification of Levofloxacin.
Fig. 3.
FTIR absorbtion spectra of Levofloxacin. (A) Reference standard; (B) commercial sample Levoflox.
3.8. Correlation of microbial bioassay results and HPLC
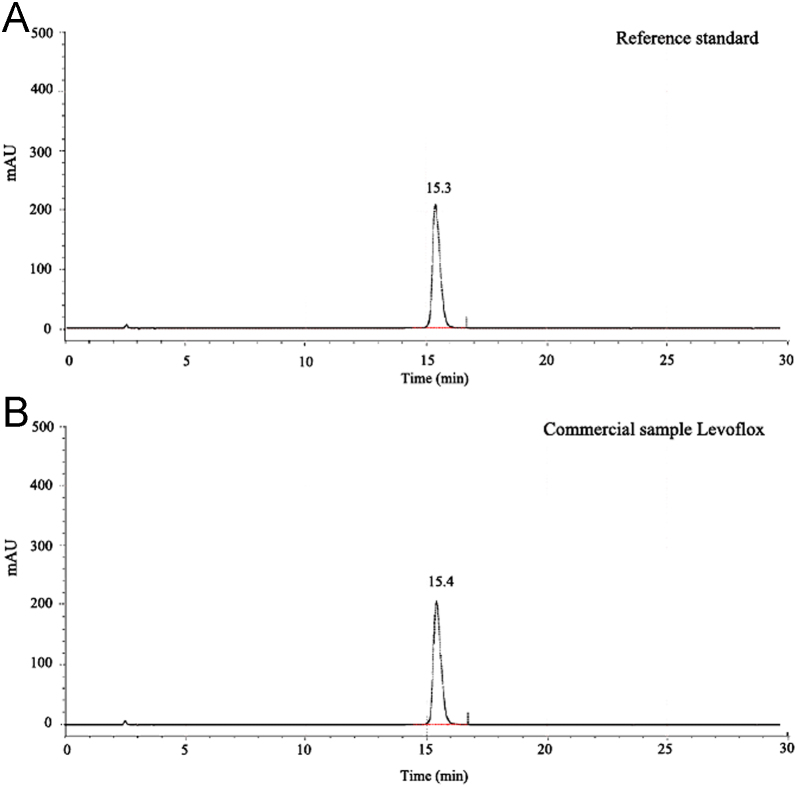
The correlation between microbial bioassay and HPLC methods was evaluated using the commercial sample Levoflox. The obtained chromatograms of the commercial sample (Levoflox) and reference standard of Levofloxacin showed symmetrical peaks having peak area 9,458,400 and 9,817,344, respectively (Fig. 4A and B). The data in Table 8 indicate percentage potency of Levofloxacin determined by the bioassay compared to the percentage potency assayed by the HPLC method. The potency of Levofloxacin in Levoflox tablet was determined as 99.37% through the HPLC method whereas potency observed through the bioassay was 100.90%. The content of Levofloxacin in Levoflox tablet obtained through both the methods was significantly identical. However, the observed minute deviation may be due to the considerable differences between the two distinct methods, such as experimental condition and detection technique.
Fig. 4.
HPLC chromatograms of Levofloxacin. (A) Reference standard; (B) commercial sample Levoflox.
Table 8.
Percentage potency of Levofloxacin in a commercial sample (Levoflox tablet) obtained by the bioassay and HPLC methods.
| Sample | Potency (%) |
|
|---|---|---|
| Bioassay | HPLC | |
| 1 | 101.52 | 100.08 |
| 2 | 100.60 | 98.11 |
| 3 | 102.84 | 100.27 |
| 4 | 99.88 | 98.45 |
| 5 | 100.42 | 99.79 |
| 6 | 100.14 | 99.52 |
| Mean potency (%) | 100.90 | 99.37 |
4. Discussion
The choice of a suitable analytical method is fundamental for quality control of the medicines and is based on several factors like drug source, its complexity, sample quantity, availability of equipments and reagents. Literature survey showed the use of HPLC or other chemical methods for potency estimation of Levofloxacin in pharmaceutical preparation. Yet no microbiological bioassay is available for potency determination of Levofloxacin in any pharmacopoeia. Although HPLC is a fast method for potency determination of an antibiotic, it cannot determine bioactivity. However, the microbiological assay estimates both potency and bioactivity of antibiotics. Additionally, bioassay can be used to estimate the effective dose against antibiotic-resistant microbes. So, through this article we made an attempt to develop and validate a microbiological bioassay as a suitable and simple method for the quantification of Levofloxacin in pharmaceutical preparations.
Compared to chemical methods, microbiological assay measures the true response of antibiotics on a biological system and it is used to obtain more realistic and precise measurements of potency. The bioassay methods used for potency determination are the key determinants in generating reproducible and reliable data, which are used in quality control of the medicine [29]. Microbiological bioassay is advantageous because the parameters that are measured with these techniques and the properties for the drug used are the same. Thus, impurities and the related substances do not interfere, maintaining the precision of the analytical method [30]. Therefore, microbiological bioassay remains, in general, the standard for resolving doubts with respect to possible loss of activity [4].
The use of bacterial strains was found in some articles to test the activity of Levofloxacin. However, technical details about the methodology and the validation of bioassay method were not described. Rodriguez et al. [27] evaluated the in vitro activity of Levofloxacin against different strains of M. tuberculosis. Similarly, the concentration of Levofloxacin in ophthalmic solution was measured using B. subtilis ATCC-6633 [26]. In the proposed study 16 strains of bacteria were tested for their response and susceptibility against Levofloxacin. Although most of the tested strains showed susceptibility against Levofloxacin, B. pumilus ATCC-14884 was selected as the most significant microbial strain because of its high response and capacity to form sharply defined zone of inhibition.
To quantify an antibiotic through microbial bioassay, the inoculum concentration should be validated, which showed sharp and clear antibiotic zone of inhibition [31]. An influence of inoculum concentration on resulting zone size is widely recognized and experiments were performed to determine how critical the concentration of inoculum might be, when other factors are constant [31], [32]. Approximately 2×105 CFU/mL of inoculum suspension of B. subtilis ATCC-6633 was used in a cylinder agar plate method to measure the concentration of Levofloxacin in ophthalmic solution [26]. Wide variation in the concentration of microorganism produces different zone diameters. High inoculum concentration of the test microorganism showed small zone diameter and hazy growth pattern, whereas low concentration of inoculum showed light and larger zone diameter. Thus, optimization of inoculum concentration is necessary for a bioassay. In the proposed study six different inoculums concentrations, i.e., 0.5%, 1.0%, 1.5%, 2.0%, 2.5% and 3.0%, of microbial strains were tested and the optimum inoculum concentration of B. pumilus ATCC-14884 was selected as 2.0% for the microbial bioassay.
One of the critical factors that influence the rate of microbial growth is buffer pH. In the current study, the activity of Levofloxacin was studied in a range of phosphate buffers pH 6.0–8.0 and phosphate buffer pH 7.0 was found suitable for the significant growth of B. pumilus ATCC-14884 and the production of measurable sharp zone of inhibition. Another important subject is the selection of antibiotic concentration range. Different concentrations of Levofloxacin, i.e., 0.06, 0.125, 0.25, 0.5, 1, 2, 4, 8 and 16 mg/L, were used against several strains of M. tuberculosis [27]. In the proposed bioassay, the zone of inhibition was measured for the range of selected concentrations of the reference standard. The concentrations of reference standard of Levofloxacin were selected as 2.56, 3.20, 4.0, 5.0 and 6.25 µg/mL against the tested microorganisms. Reasons for the selected range of concentration were the susceptibility of microorganism to low concentration, size of the zones of inhibition that was limited by the size of Petri dish for high concentration and linear relationship between the logarithm of concentration and mean zone diameters. The calibration value of Levofloxacin was constructed by plotting the logarithm of antibiotic concentration (µg/mL) versus mean diameter of inhibition zone (in mm) and good linearity was found in the range of the selected concentrations of the reference standard.
A comparative study of microbiological bioassay was also carried out with the HPLC method and the potency of Levofloxacin was estimated as 100.90% and 99.37%, respectively. The equivalence in results shows a strong correlation between these two methods. However, estimating the bioactivity microbiological assay is an effective method of determining the subtle change in the antibiotic.
5. Conclusions
A standard validated analytical method is mandatory for the maintenance of quality of pharmaceutical preparations. In the literature, mostly HPLC assay was found for the measurement of Levofloxacin concentration in different preparations. However, a validated microbial bioassay method for the potency assessment of Levofloxacin in pharmaceutical preparation has not yet been reported in any pharmacopoeias. Bioactivity of an antibiotic can be determined only by the microbial bioassay method, which is the main advantage over the HPLC method. Although both bioassay and HPLC methods are complementary to each other, due to the estimation of both potency and bioactivity by bioassay it seems to be the most suitable method.
Experimental results show the significance of the proposed bioassay method in estimating the potency and bioactivity of antibiotic by comparing their quantitative effect with a reference standard of defined potency. The optimization of the bioassay was performed using various conditions and B. pumilus ATCC-14884 was selected as the most susceptible organism against Levofloxacin. Several factors were also examined such as buffer pH, inoculums concentration and standard solution concentration. The commercial sample Levoflox tablet was analyzed by the bioassay and the percentage potency was determined as 100.90%. The specificity of bioassay was correlated with the HPLC method and the potency was estimated as 100.90% and 99.37%, respectively, which are significantly identical. The obtained results show that both methods are reliable for potency estimation of Levofloxacin. Moreover, bioassay is less expensive and is appropriate while investigating drug dynamics and bioactivity.
Acknowledgments
The authors would like to express their sincere thanks to the staff of Chemistry Division, IPC, for helping in the chemical analysis during this study.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Nelson J.M., Chiller T.M., Powers J.H. Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin. Infect. Dis. 2007;44:977–980. doi: 10.1086/512369. [DOI] [PubMed] [Google Scholar]
- 2.Kawahara S. Chemotherapeutic agents under study. Nippon Rinsho. 1998;56:3096–3099. [PubMed] [Google Scholar]
- 3.Indian Pharmacopoeia, Indian Pharmacopoeia Commission, Ghaziabad, India, 2010, pp. 1579–1581/49–56.
- 4.United States Pharmacopoeia, United States Pharmacopoeial Convention, Rockville, MD, USA, 2009, pp. 87–93.
- 5.Bertino J., Fish D. The safety profile of the fluoroquinolones. Clin. Ther. 2000;22:798. doi: 10.1016/S0149-2918(00)80053-3. [DOI] [PubMed] [Google Scholar]
- 6.Tsai Y.H., Bair M.J., Hu C.C. Determination of levofloxacin in human urine with capillary electrophoresis and fluorescence detector. J. Chin. Chem. Soc. 2007;54:991–995. [Google Scholar]
- 7.Kothekar K.M., Jayakar B., Khandhar A.P. Quantitative determination of levofloxacin and ambroxol hydrochloride in pharmaceutical dosage form by reversed − phase high performance liquid chromatography. Eurasian J. Anal. Chem. 2007;2:21–31. [Google Scholar]
- 8.Ejikeme U.C., Ademola O.J. Microbiological assay of the active component of ampicillin in ampicillin and ampicillin/cloxacillin suspensions using Bacillus megatharium NCTC10342A (76) as indicator organism. Afr. J. Microbiol. Res. 2010;4:51–54. [Google Scholar]
- 9.Prescott L.M., Harley J.P., Klein D.A. seventh ed. McGraw-Hill; New York: 2008. Microbiology. pp. 835–858. [Google Scholar]
- 10.Black J.G. sixth ed. John Wiley & Sons Inc.; USA: 2005. Microbiology: Principles and Explorations. pp. 352–384. [Google Scholar]
- 11.Hewitt W. first ed. Academic Press; New York: 1977. Microbiological Assay: An Introduction to Qualitative Principles and Evaluation. pp. 1–50. [Google Scholar]
- 12.Denyer S.P., Hodges N.A., Gorman S.P. seventh ed. Blackwell Publishing Company; UK: 2004. Hugo & Russell׳s Pharmaceutical Microbiology. [Google Scholar]
- 13.Avhad M., Bonde C.G. Development and validation of simultaneous UV spectrophotometric method for the determination of levofloxacin and ambroxol in tablets. Int. J. ChemTech Res. 2009;1:873–888. [Google Scholar]
- 14.Cazedey E.C.L., Salgado H.R.N. Development and validation of a microbiological agar assay for determination of Orbifloxacin in pharmaceutical preparations. Pharmaceutics. 2011;3:572–581. doi: 10.3390/pharmaceutics3030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto C.H., Pinto T.J.A. Rapid determination of neomycin by a microbiological agar diffusion assay using triphenyltetrazolium chloride. J. Assoc. Anal. Chem. 1996;79:434–440. [PubMed] [Google Scholar]
- 16.Lourenco F.R., Pinto T.J.A. Comparison of three experimental designs employed in Gentamycin microbiological assay through agar diffusion. Braz. J. Pharm. Sci. 2009;45:559–566. [Google Scholar]
- 17.Zuluaga A.F., Agudelo M., Rodriguez C.A. Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics. BMC Clin. Pharmacol. 2009;9 doi: 10.1186/1472-6904-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dafale N.A., Semwal U.P., Agarwal P.K. Quantification of ceftriaxone sodium in pharmaceutical preparations by a new validated microbiological bioassay. Anal. Methods. 2012;4:2490–2498. [Google Scholar]
- 19.Baired R.M., Hodges N.A., Denyer S.P. CRC Press; Boca Raton, FL, USA: 2000. Handbook of Microbiological Quality Control: Pharmaceuticals and Medical Devices. [Google Scholar]
- 20.Dafale N.A. Exploration of genetic information from dynamic microbial populations for enhancing the efficiency of azo-dye-degrading systems. Environ. Rev. 2011;19:310–332. [Google Scholar]
- 21.Dafale N., Agrawal L., Kapley A. Selection of indicator bacteria based on screening of 16 S rDNA metagenomic library from a two-stage anoxic–oxic bioreactor system degrading azo dyes. Bioresour. Technol. 2010;101:476–484. doi: 10.1016/j.biortech.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 22.British Pharmacopoeia, The Stationary Office, London, 2011, pp. A348−A355.
- 23.T.J.A. Pinto, F.R. Lourenco, T.M. Kaneko, Microbiological assay of Gentamycin employing an alternative experimental design. In: AOAC Annual Meeting and Exposition, 121, 2007; Anais. Anahein-California, 2007, p. 157.
- 24.Humphrey J.H., Lightbown J.W. A general theory for plate assay of antibiotics with some practical applications. J. Gen. Microbiol. 1952;7:120–143. doi: 10.1099/00221287-7-1-2-129. [DOI] [PubMed] [Google Scholar]
- 25.Cooper K.E., Lindon A.H. Importance of temperature during the early hour of incubation of agar plates in assay. J. Gen. Microbiol. 1952;7:8–17. doi: 10.1099/00221287-7-1-2-8. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda M., Sasaki K. General purpose antimicrobial ophthalmic solutions evaluated using new pharmacokinetic parameter of maximum drug concentration in aqueous. Jpn. J. Ophthalmol. 2002;46:384–390. doi: 10.1016/s0021-5155(02)00509-9. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez J.C., Ruiz M., Lopez M. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents. 2002;20:464–467. doi: 10.1016/s0924-8579(02)00239-x. [DOI] [PubMed] [Google Scholar]
- 28.ICH, Harmonised Tripartite Guideline, Validation of Analytical Procedures, Methodology, Geneva, 1996, pp. 1–8.
- 29.Shah V.P., Midha K.K., Findlay J.W.A. Bioanalytical method validation–a revisit with a decade of progress. Pharm. Res. 2000;17:1551–1557. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 30.Hodjes N.A. Pharmaceutical applications of microbiological techniques. In: Aulton M.E., editor. Pharmaceutics, The Science of Dosage Form Design. ninth ed. Churchill Livingstone; London: 2001. pp. 623–643. [Google Scholar]
- 31.Dafale N.A., Agarwal P.K., Semwal U.P. Development and validation of microbial bioassay for the quantification of potency of the antibiotic cefuroxime axetil. Anal. Methods. 2013;5:690–698. [Google Scholar]
- 32.Hewitt W. Interpharm/CRC Press LLC; Boca Raton, FL: 2009. Microbiological Assay for Pharmaceutical Analysis: A Rational Approach (1st Indian Reprint) [Google Scholar]