Abstract
Medicinal plants, vegetables and fruits are the sources of huge number of bioactive lead/scaffolds with therapeutic and nutraceutical importance. Bioautography is a means of target-directed isolation of active molecules on chromatogram. Organic solvents employed in chromatographic separation process can be completely removed before biological detection because these solvents cause inactivation of enzymes and/or death of living organisms. They offer a rapid and easy identification of bioactive lead/scaffolds in complex matrices of plant extracts. Bioautography is a technique to isolate hit(s)/lead(s) by employing a suitable chromatographic process followed by a biological detection system. This review critically describes the methodologies to identify antimicrobial, antioxidant, enzyme inhibitor lead/scaffolds by employing bioautography. A significant number of examples have been incorporated to authenticate the methodologies.
Keywords: Bioactivity, Bioassay, Bioautography, Detection principle, Thin layer chromatography
1. Introduction
Planar chromatographic analysis hyphenated with the biological detection method is termed as bioautography [1]. It is an effective and inexpensive technique for the phytochemical analysis of plant extracts to identify bioactive lead/scaffolds. It can thus be performed both in highly developed laboratories as well as in small research laboratories which have minimum access to sophisticated equipments [2]. Despite having sophisticated on-line high-performance liquid chromatography coupled bioassays, bioautography offers a simple, rapid and inexpensive method for the chemical and biological screening of complex plant extracts, with subsequent bioassay-guided isolation [3]. In 1946, Goodall and Levi [4] introduced paper chromatography (PC)-based bioautography for the first time to estimate the purity of penicillin. In 1961, Fisher and Lautner [5] and Nicolaus et al. [6] introduced thin layer chromatography (TLC)-based bioautography. The first review on bioautography was written by Betina in 1973 [7]. Generally, planar chromatographic, viz. TLC and PC are used for bioautography, but the detection method can be successfully improved by the application of advanced chromatographic tools, namely, high performance thin layer chromatography (HPTLC), over-pressured layer chromatography (OPLC), and planar electro chromatography (PLC). The major applications of bioautography are the fast screening of a large number of samples for bioactivity, namely, antibacterial, antifungal, antioxidant, enzyme inhibition, etc. and in the target-directed isolation of active compounds [8], [9], [10], [11]. In this review, the techniques and application of bioautography are discussed in details with suitable examples.
2. Detection of anti-microbial agents by bioautography
PC and TLC have become tools in the screening of antimicrobial agents through bioautography. Three bioautographic methods, namely, (i) agar diffusion or contact bioautography, (ii) direct TLC bioautographic detection and (iii) immersion or agar overlay bioautography are used to detect antimicrobial agents in a mixture of compounds [12], [13].
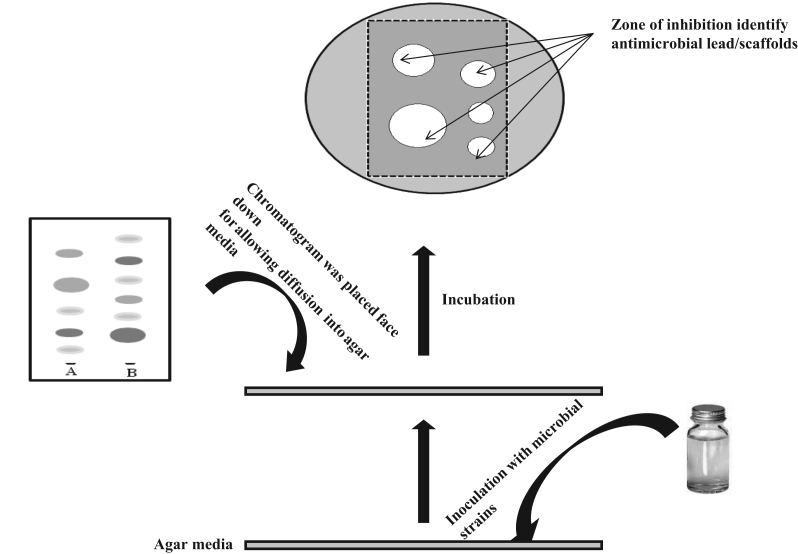
2.1. Agar diffusion or contact bioautography
In contact bioautography, antimicrobial agents diffuse from a developed TLC plate or paper to an inoculated agar plate [14], [15], [16]. The chromatogram is placed face down onto the inoculated agar layer for a specific period to enable diffusion. Then the chromatogram is removed and the agar layer is incubated. The zones of inhibition on the agar surface, corresponding to the spots in chromatographic plates, are indicative of the antimicrobial substances. An overall view of contact bioautography has been depicted in Fig. 1. Incubation time for the growth ranges between 16 and 24 h but it can be reduced to 5–6 h by spraying with 2,6-dichlorophenol-indophenol or 2,3,5-tetrazoliumchloride [17]. The disadvantages of contact bioautography are difficulties in obtaining complete contact between the agar and the plate and adherence of the adsorbent to the agar surface. Another problem may arise due to the differential diffusion of components, especially water-insoluble, from the chromatogram to the agar plate. To overcome these difficulties, Wagman and Bailey [12] introduced Chrom-AR and silicic acid/glass fiber sheets for bioautography of antimicrobial compounds. The principle of the method was the same and antimicrobials had to be transferred from the chromatographic plates to agar causing their loss and dilution. Another special case is bioautographic detection of 6-aminopenicilanic acid which is a very weak antibiotic and must be converted through phenyl acetylation to benzyl penicillin by spraying chromatographic plates or paper with acetyl chloride in mild alkaline condition before bioautography [18]. This is a technique familiar to the microbiologists in search for antibiotics from microorganisms, and different procedures have been used to improve its performance [19]. Sphaerococcenol A, a bromoditerpene antibiotic, was isolated by contact bioautography [20]. Three carboxylic polyether antibiotics, namely, monensin, lasalocid and salinomycin, were isolated using contact bioautography by VanderKop et al. [21]. Eight different antifungal agents were isolated from the bark of Bridelia retusa using contact bioautography [22]. The development of HPTLC has improved resolution and sensitivity over TLC [23]. Application of HPTLC also reduced the consumption of time and solvents. Through HPTLC-contact bioautography, Ramirez et al. [24] showed multiple antibiotic residues in cow׳s milk. TLC contact bioautographic assay was introduced by Shahverdi et al. [25] for the detection of antibiotic resistance reversal agents.
Fig. 1.
Schematic overview of contact bioautography.
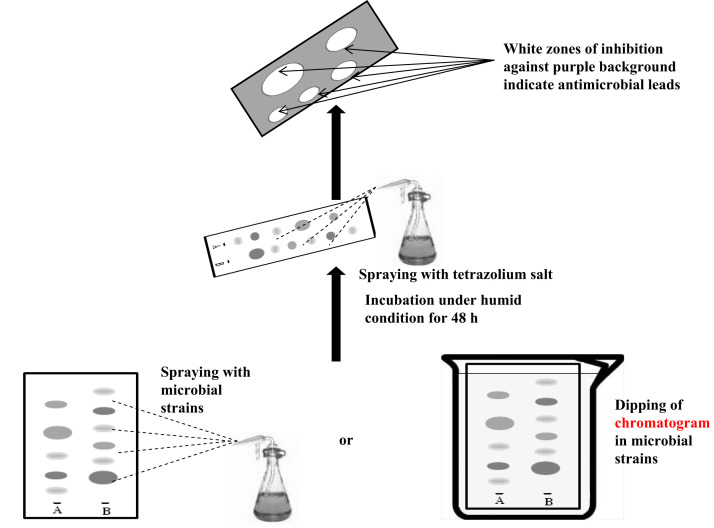
2.2. Direct TLC bioautographic detection
In direct TLC bioautography, the developed TLC plate is sprayed with or dipped into a fungal or bacterial suspension (Fig. 2). A suspension of test bacteria or fungi is used for the spraying or dipping purpose. An inoculam of absorbance of 0.84 at 560 nm was suggested for bacteria like Staphylococcus aureus [26], while using a suspension of 106 CFU/mL could be employed for both bacteria and fungi [27]. The bioautogram is then incubated at 25 °C for 48 h under humid condition. For visualization of microbial growth, tetrazolium salts are used. These salts are converted by the dehydrogenases of living microorganisms to intensely colored formazen [28]. These salts are sprayed onto the bioautogram and are reincubated at 25 °C for 24 h [28] or at 37 °C for 3–4 h [29], [30]. Clear white zones against a purple background on the TLC plate indicate antimicrobial activity of the sample [31]. Okusa et al. [32] incorporated a new medium for direct TLC bioautography which is fluid enough to disperse microorganisms and viscous enough to adhere to the TLC plates; according to them a mixture of Muller–Hinton (MH) broth and MH agar in the ratio of 90:10 fulfils this requirement. Homans et al. [33] found that direct spraying of the thin-layer chromatograms with a spore suspension of the test fungus in glucose–mineral salts medium was the easiest technique for the detection of fungitoxic substance, and it also gave the most reliable results [33]. Direct bioautographic methods have been described for spore-producing fungi such as Aspergillus, Penicillium and Cladosporium [33] and also for bacteria [34], [35]. Bacillus subtilis, S. aureus and Escherichia coli are used to identify the active compounds. p-Iodonitrotetrazolium violet is the most suitable detection reagent [35]. TLC bioautography showed Pseudomonas cepacia B37W produces an antifungal compound pyrronitrin which acts against potato׳s dry root invasion in nanogram level at the wound sight [36]. A cyclopeptide antibiotic named condaline-A has been isolated from the bark of Condalia buxifolia along with the other antibiotics adoutine-Y/, scutianine-B and scutianine-C, and has been established using direct bioautography [37]. Separation of erythromycin from its base to identify systemic concentration of erythromycin was established through direct TLC and PC bioautography by Esterbrook et al. [38]. A simple bioautographic technique has been used in laboratories for many years to detect fungitoxic substances according to Weltzine et al. [39], and modified by Dekhuijzen et al. [40]. In this technique, chromatograms are developed on Whatman no. 3 mm paper with propanol–water (85:15) and after drying are sprayed with a conidial suspension of Glomerella cingulata. After incubation clearly visible inhibition zones indicate the presence of fungitoxic compounds. TLC-direct bioautography is also useful for the rapid chemical and biological screening of plant extracts [41]. Once an activity has been located at the TLC plate, the sample can be analyzed by liquid chromatography-mass spectrometry to establish whether known or new compounds and/or substance classes are involved. This screening strategy concerns rapid detection of antibacterial and antifungal compounds [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]. Choma et al. [52], [53], [54] in his review on TLC-bioautography indicated plenty of examples of analysis on bacteria and fungi for the detection of antimicrobial activity of antimicrobial agent through bioautography and bioluminicence [52], [53], [54], [55], [56]. The BioLuminizer™ system (commercialized by Camag, Muttenz, Switzerland), which is a general detection system for bioactive substances, uses Vibrio fischeri, a non-pathogenic gram negative marine bacterium. This is an intrinsic bioluminescence, emitting a greenish light as a product of cellular respiration at a critical cellular density. Luciferase, the bioluminescence catalyst, is expressed and catalyzes an oxidation reaction that releases excess energy in the form of light [57]. After migration, the TLC plate is coated with the bioluminescent bacteria which produce dark spots on a luminescent background with toxic or bioactive compounds [58]. This method is suitable for the detection of toxins and chemical adulterants in food, beverages, cosmetics, waste water and drinking water in picogram quantities. Du and Li [59] used this luciferase assay for evaluation of estrogenic activity of Mucuna sempervirens extracts. The bioautographic method can be extended for use in 2D-TLC experiments [60]. In 2D-TLC direct bioautography, TLC plates are developed once with a polar solvent, turned 90°, and then developed again with a non-polar solvent system. The TLC plates are then dried and sprayed with a nutrient broth seeded with microbial suspension, and the microbial culture is grown directly on the silica gel surface of the TLC plate. The advantage of this method is that migration in each of the two dimensions can be brought about with solvents of very different polarities. 2D-TLC direct bioautography has been found to be useful for the detection of inhibitors of the plant pathogens, viz. Colletotrichum acutatum, Colletotrichum fragariae and Colletotrichum gloeosporioides [60]. Antifungal activity of chromenes from Peperomia serpens (Sw.) Loudon (Piperaceae) was established by the direct bioautographic assay technique against Cladosporium cladosporioides and Cladosporium sphaerospermum [61]. Tyiháka et al. [62] introduced a new system of bioautography known as bio-arena where they combined OPLC with bioautography. Separation in an OPLC system results in better-defined spots than in conventional TLC and HPTLC separations. A number of applications have been reported, including the investigation of chelidonium alkaloids [63].
Fig. 2.
Schematic diagram indicates the direct bioautographic process.
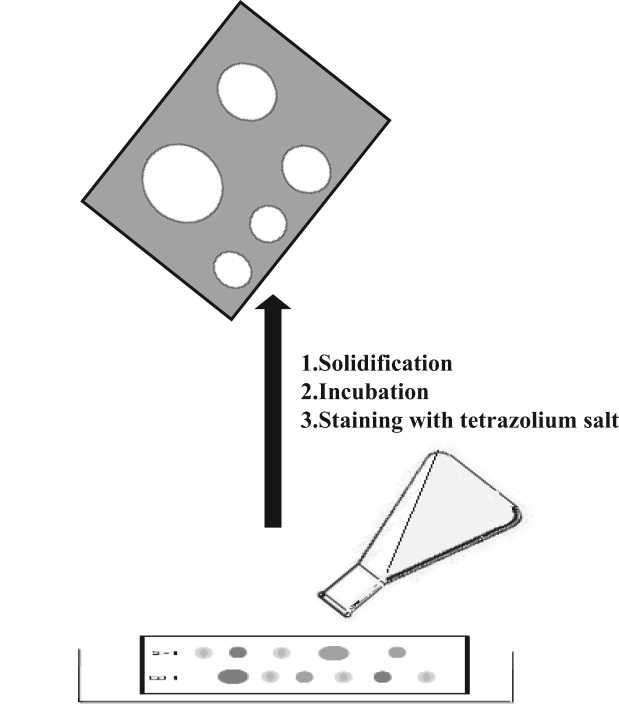
2.3. Immersion or agar overlay bioautography
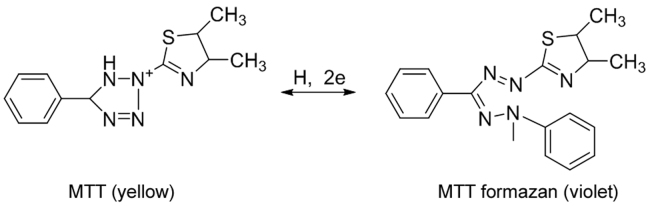
Agar overlay is a combination of contact and direct bioautography. In this method, the chromatogram is covered with a molten, seeded agar medium. After solidification, incubation and staining (usually with tetrazolium dye), the inhibition or growth bands are visualized (Fig. 3) [64], [65], [66]. For Gram-negative bacteria, an agar solution containing the red-colored bacterium Serracia marcescens can be employed. The red-colored gel is incubated overnight at room temperature and inhibition zones appear as white or pale yellow areas on a red background [67]. With other, colorless, microorganisms, zones of microbial growth inhibition are visualized with the aid of a dehydrogenase activity-detecting reagent (tetrazolium salt). Metabolically active microorganisms convert the tetrazolium salt (MTT) into the corresponding intensely colored formazan (Fig. 4).
Fig. 3.
Schematic diagram of immersion or agar overlay bioautography.
Fig. 4.
Reduction of tetrazolium salts.
The agar overlay assay has been used for yeasts such as Candida albicans and can also be applied to bacteria such as B. subtilis, E. coli, Pseudomonas aeruginosa and S. aureus [48], [68]. The antibacterial activity of isoflavonoid and sesquiterpenoid phytoalexins has been evaluated by an overlay method using Pseudomonas syringae pv. phaseolicola, with 2,3,5-triphenyl-tetrazolium chloride (TZC) as visualizating reagent. Addition of glycerol to the overlay nutrient medium, as a carbon source, facilitated the reduction of TZC to pink colored formazans by bacterial dehydrogenases [69]. Antimicrobial carotenoids have been characterized in Bixa orellana seed extracts [70]. The antibacterial activity was detected by overlaying TLC plates with agar containing S. aureus and then spraying with tetrazolium salt (INT) [70]. Cytrynska et al. [71] characterized eight antimicrobial peptides from Galleria mellonella larvae immune hemolymph using tricine SDS-PAGE bioautography. Several antimicrobial compounds were isolated from Tylosema esculentum husks, cotyledons, and tubers by employing agar overlay bioautography using S. aureus, E. coli, B. subtilis, P. aeruginosa and C. albicans as test microorganisms [72].
3. Detection of antioxidant agents by bioautography
3.1. Bioautography using DPPH as detection reagent
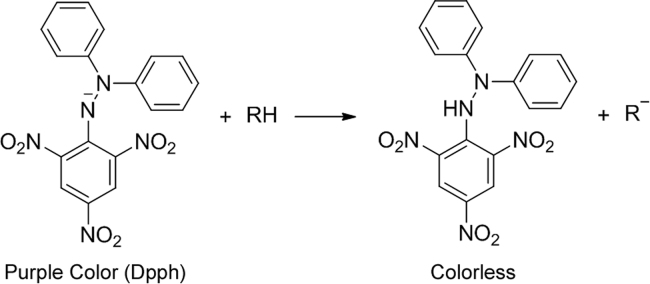
The stable 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) has an absorption maximum at 517 nm, which decreases upon reduction through reaction with a radical scavenger [73]. The corresponding color change can thus be observed in a TLC bioassay [74]. The reaction has been depicted in Fig. 5. The developed chromatogram is sprayed with a solution of 0.2% DPPH in methanol/ethanol. The plate is examined in daylight after 30 min. Free-radical scavengers appear as cream/yellow spots against a purple background. The intensity of the yellow color can be measured with a chromameter. Rossi et al. [75] identified the radical scavenging molecules in essential oil from Croton lechleri and successfully compared the antioxidant potential with thyme oil. Four antioxidant compounds, viz. rosmarinic acid, luteolin, apigenin, and chrysoeriol, were the isolated fruit of Perilla frutescens var. acuta by bioautography on TLC using DPPH as a detection reagent [76].
Fig. 5.
Reaction of DPPH radical with radical scavengers.
3.2. Bioautography using ABTS as detection reagent
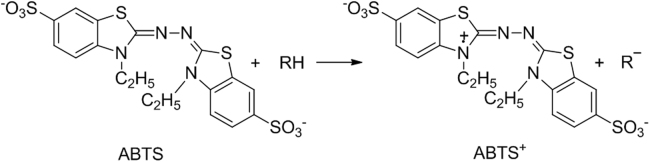
2,2-Azino-bis(3-ethylbenzthiazoline-6-sulfonic) acid (ABTS) radical scavenging method can be extended to TLC plates [77]. The reaction involved is shown in Fig. 6. Layers stained with ABTS show a green background and radical scavengers give colorless or pink spots. However, DPPH is more stable on the plates and gives a more homogeneous coloring of spots [74].
Fig. 6.
Reaction of ABTS radical with radical scavengers.
3.3. Bioautography using β-carotene as detection reagent
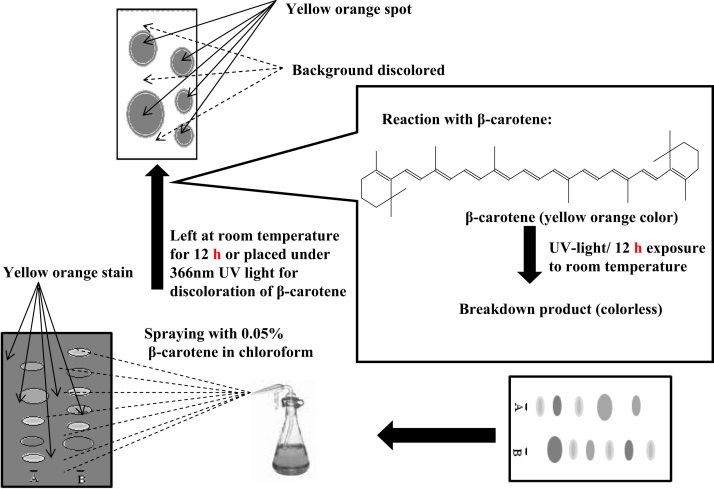
Completely dried TLC chromatograms are sprayed with a 0.05% solution of β-carotene in chloroform. They can be left at room temperature for decolorization of the background or they can be placed under 366 nm UV light. Active compounds remain as yellow-orange spots on a white background [78]. A schematic overview is depicted in Fig. 7.
Fig. 7.
Schematic diagram of inhibition of bleaching of β-carotene.
3.4. Inhibition of bleaching of β-carotene induced by auto-oxidation of linoleic acid
Dry TLC plates are sprayed with a mixture of linoleic acid in ethanol and β-carotene in chloroform. After exposing the plate to sunlight, antioxidant activity is shown by the presence of orange spots on a white background [79].
4. Enzyme inhibition
Enzymes are important molecular targets for lead discovery in primary screening assays. The use of a TLC support to screen for potential plant-derived enzyme inhibitors is a rapid method which is relatively free of disturbances due to the solvent.
4.1. Bioautographic detection of acetyl cholinesterase (AchE) inhibitor
4.1.1. Detection by diazotization
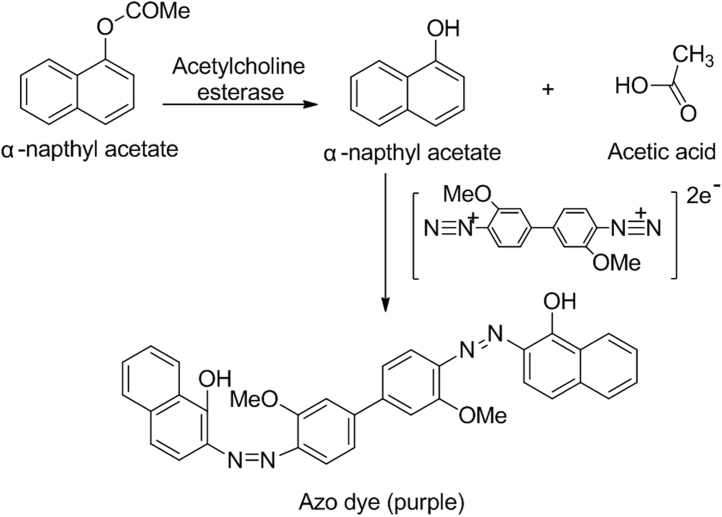
The basic principle of this method is that the enzyme converts α-naphthyl acetate (substrate) into α-naphthol. α-Naphthol reacts with fast blue B salt (chromogenic agent) to make a purple colored background on the TLC plates, while AchE inhibitors produce white spots. Enzyme inhibitors block the formation of α-naphthol and hence no purple coloration is produced (Fig. 8). Samples were applied to silica gel TLC plate and migrated in a suitable solvent. After complete removal of solvents, the AchE solution is sprayed. The enzyme is allowed to incubate for 20 min at 37 °C and is then sprayed with a mixture of 1-naphthyl acetate and fast blue B salt. After 1–2 min, AchE inhibitors show white spots on a purple background [80]. Detection limits for AchE inhibitors remain within the nanogram level [9]. This bioautographic method offers a rapid identification of a large number of AchE inhibitors.
Fig. 8.
Reaction between acetylcholine esterase with α-napthyl acetate, produced α-napthol again reacts with fast blue B salt to produce purple colored azo dye.
Another variant of the bioassay [81], [82] uses β-naphthyl acetate and enzyme solution in the TLC elution solvent and incubated with enzyme for 10 min. This method supposedly gives a deeper violet background color and higher sensitivity of detection. However, β-naphthyl acetate is considerably more expensive than α-naphthyl acetate. To overcome the cost of the experiment, Yang et al. [83] uses 4-methoxy phenyl acetate as substrate for AchE and a mixed solution of K3(FeCN)6 and FeCl3·6H2O as chromogenic agent. In this method, the spot appeared as a light yellow spot and the other parts are aquamarine blue. The consumption of the enzyme is satisfactory. A microplate assay has been developed for the diazotization method [84]. This gives a quantitative result (such as IC50 values), but TLC assays, which are useful as qualitative methods, are in general more sensitive than microplate assays. However, there was a good correlation between the TLC bioautographic assay and the solution assay. Queiroz et al. [85] showed that dichloromethane extract of the aerial parts of Blumea gariepina (Asteraceae) was found to be active against AchE. Seven new AchE inhibitors were isolated from this plant through bioautography. The original detection method by diazotization has been employed to screen Amaryllidaceae species for AChE inhibition [86].
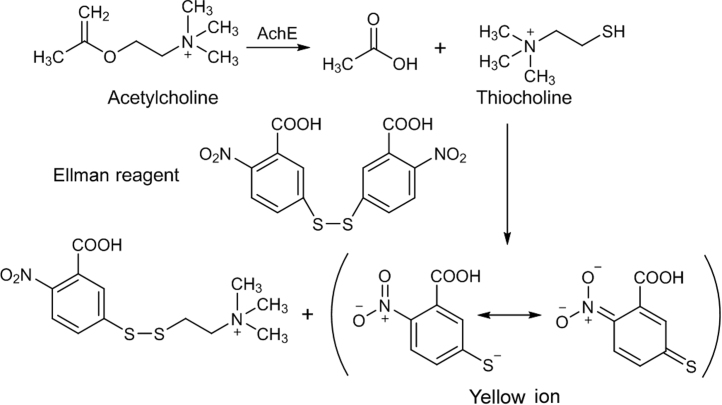
4.1.2. Detection by the Ellman reaction
The Ellman method for the colorimetric determination of AchE activity was first described in 1961 [87]. Acetylthiocholine (ATCI) is cleaved by AchE to form thiocholine which reacts with 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) to give the yellow 5-thio-2-nitrobenzoate anion (Fig. 9). It could be adapted for the TLC screening of AchE inhibitors [88]. In this method, a solution of DTNB and ATCI is sprayed on the chromatogram (after complete removal of solvents), followed by spraying with AchE. A pale yellow background forms within about 5 min. AchE inhibitors appear as white spots. The Ellman reaction is more difficult to visualize than the diazotization method. False positive effect is possible with the presence of aldehydes [89]. An indole alkaloid from Tabernaemontana australis showed considerable AchE inhibitory activity and it is evaluated through the Ellman reaction on the TLC plate [90].
Fig. 9.
Ellman reaction.
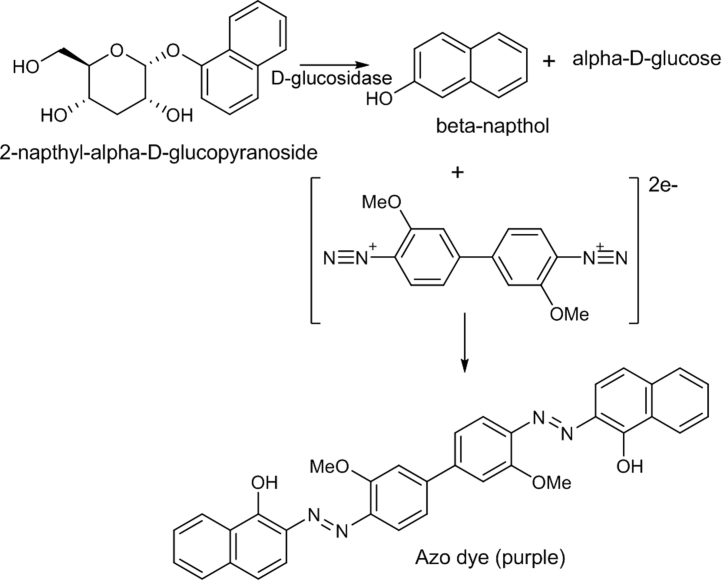
4.2. α and β-glucosidase inhibition
Glucosidase inhibitors are potentially useful as antidiabetic, anti-obesity, antiviral, antiadhesive, antibacterial or antimetastatic agents [91]. Screening methods for glucosidase inhibitors include spectrophotometry with o- or p-nitrophenyl-β-d-glucopyranoside as substrate [92], [93] or an agar plate method [94]. Both of these have limitations. With p-nitrophenyl-β-d-glucopyranoside, glucosidases cleave the sugar, to develop a yellow background due to the formation of p-nitrophenol. However, this is only observed on the TLC plate at pH 7.5 and the zone of inhibition remains poorly visible. No yellow background color is observed in the corresponding test with p-nitrophenyl-β-d-glucopyranoside [95]. In TLC-bioautography, the chromatogram is sprayed with enzyme solution in appropriate buffer. An alternative is a test which involves the cleavage of 2-naphthyl-β-d-glucopyranoside or 2-naphthyl-α-d-glucopyranoside by respective α- or β-glucosidase inhibitors [96]. The β-naphthol which is formed reacts with fast blue B salt, to give a purple colored diazo dye (Fig. 10) [96]. For the detection of the active enzyme, the solutions of 2-naphthyl-α-d-glucopyranoside (for α-glucosidase) or 2-naphthyl-β-d-glucopyranoside (for β-glucosidase) in ethanol and fast blue Bsalt in distilled water are prepared. The naphthyl-glucopyranoside solution and the fast blue B salt solution are mixed at a ratio of 1:1 (for α-glucosidase) or 1:4 (for β-glucosidase). The mixture is sprayed onto the plate to give a purple coloration within 2–5 min. A TLC assay has previously been established for the detection of β-glucosidase inhibitors. It is based on the hydrolysis of esculin into esculetin which reacts with FeCl3 to provide a brown complex [65], [97].
Fig. 10.
Diazotization reaction for the detection of α and β-glucosidase inhibition.
4.3. Xanthine oxidase inhibition
The enzyme xanthine oxidase (XO) catalyzes the oxidation of hypoxanthine and xanthine to uric acid and producing and H2O2. The inhibition of XO diminishes oxidative stress and exhibits prophylactic role on inflammation, arteriosclerosis, cancer, aging, etc. [65]. To identify XO inhibitors on TLC plates, the enzyme is suspended in agar and distributed on the TLC plate (direct measurement of enzyme activity on the TLC plate is not possible). After solidification, the plate is immersed into a solution of xanthine at 38 °C for 20 min in the dark. Enzymatic oxidation of xanthine produces which reduces the pale yellow tetrazolium salt (NBT) to a formazan and hence a purple background is obtained on the plate (Fig. 11). Allopurinol, an inhibitor of XO, is detected as a white spot on the purple background. This method could determine the nanogram level of XO inhibitor. A modification of the test, in which is generated chemically, allows the distinction between pure inhibitors of XO, such as allopurinol and radical scavengers [98].
Fig. 11.
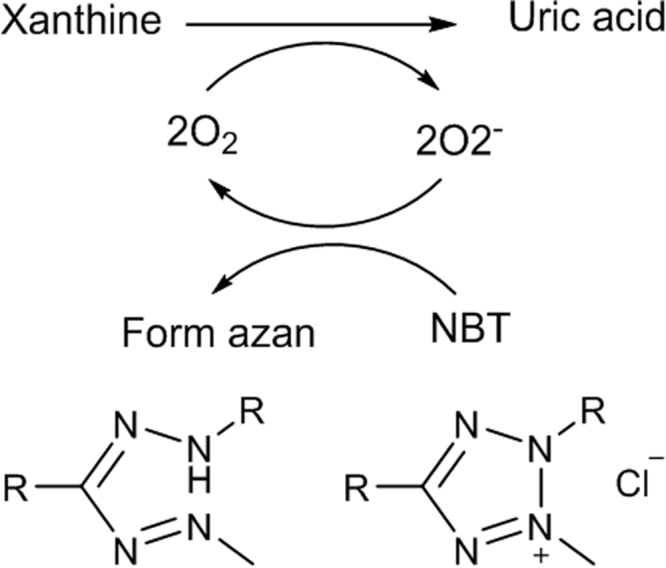
Reaction to detect xanthine oxidase inhibitors.
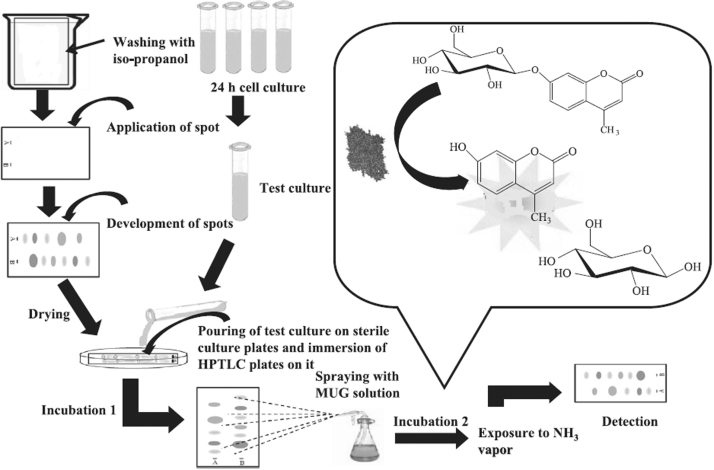
5. Detection of estrogenic compounds
Estrogen receptor agonists include natural and synthetic hormones, phytoestrogens and chemicals such as metabolites of alkyl-phenol extoxylate, bisphenol A, parabens, benzophenons and phthalates [99]. Muller et al. [1] first observed that an estrogenic compound can be bioautographed using production capacity of β-galactosidase enzyme from yeast cell. This process is also known as the HPTLC-YES (yeast estrogen screen) procedure [1]. This is a reporter gene assay which had been developed for the assessment of the estrogenic potency of individual substances. Here 4-methyl umbelliferyl β-d-galactopyranoside (MUG) is used as a fluorogenic substrate on which the enzyme β-galactosidase works and converts it into 4-methyl umbelliferone (Fig. 12). Chlorophenol red β-d-galactopyranoside a chromogenic substrate can also be used in place of MUG but the sensitivity of the test was the highest in the case of MUG.
Fig. 12.
Schematic diagram of the HPTLC-YES process.
6. Conclusion
In spite of wide employment of sophisticated chromatographic techniques coupled with on-line bioassays, bioautography is still proving its worth as a simple and inexpensive tool for simultaneous chemico-biological screening of natural sources. In other word, it offers the simplest mean of bioassay guided lead discovery from natural products. For the natural product the separation process is not easy, and if separated the amount is very less in maximum cases, so it is necessary to develop a process which can detect lead in a small amount and biological activity can also be measured successively. Considering these problems, we can say that bioautographic detection technique would create a new era in separation science.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Muller M.B., Dausend C., Weins C. A new bioautographic screening method for the detection of estrogenic compounds. Chromatographia. 2004;60:207–211. [Google Scholar]
- 2.Marston A., Maillard M., Hostettmann K. A TLC bioautographic method for the detection of α- and β-glucosidase inhibitors in plant extracts. GIT Lab. J. 1997;1:36–39. doi: 10.1002/pca.1154. [DOI] [PubMed] [Google Scholar]
- 3.Hostettmann K., Terreaux C., Marston A. The role of planar chromatography in the rapid screening and isolation of bioactive compounds from medicinal plants. J. Planar Chromatogr. 1997;10:251–258. [Google Scholar]
- 4.Goodall R.R., Levi A.A. A microchromatographic method for the detection and approximate determination of the different penicillins in a mixture. Nature. 1946;158:675–676. doi: 10.1038/158675a0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R., Lautner H. Zum papierchromatographischen Nachweis von Penicillinpräparaten. Arch. Der. Pharm. 1961;294:1–7. [Google Scholar]
- 6.Nicolaus B.J.R., Coronelli C., Binaghi A. Microbiological determination of antibiotics by thin layer chromatograms. Farmaco. Ed. Prat. 1961;16:349–370. doi: 10.1007/BF02158302. [DOI] [PubMed] [Google Scholar]
- 7.Betina V. Bioautography in paper and thin-layer chromatography and its scope in the antibiotic field. J. Chromatogr. 1973;78:41–51. doi: 10.1016/s0021-9673(01)99035-1. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann B., Howard A.J., Hollister Z.J. Application of paper chromatograms to the study of inducers of λ bacteriophage in Escherichia coli. Appl. Microbiol. 1967;15:723–725. doi: 10.1128/am.15.4.723-725.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choma I.M., Grzelak E.M. Bioautography detection in thin-layer chromatography. J. Chromatogr. A. 2011;1218:2684–2691. doi: 10.1016/j.chroma.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 10.Houghton P.J. Use of small scale bioassays in the discovery of novel drugs from natural sources. Phytother. Res. 2000;14:419–423. [PubMed] [Google Scholar]
- 11.Bohlin L., Bruhn J.G. Kluwer Academic Publishers; Dordrecht: 1999. Bioassay Methods in Natural Product Research and Drug Development. p. 67. [Google Scholar]
- 12.Wagman G.H., Bailey J.V. Use of silicic acid–glass fiber sheets for bioautography of antimicrobial substances. J. Chromatogr. 1969;41:263–264. [Google Scholar]
- 13.Rios J.L., Recio M.C., Villar A. Screening methods for natural products with antimicrobial activity: a review of the literature. J. Ethnopharmacol. 1988;23:127–149. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 14.Sherma J. Planar chromatography. Anal. Chem. 2008;80:4253–4267. doi: 10.1021/ac7023415. [DOI] [PubMed] [Google Scholar]
- 15.Meyers E., Smith D.A. Bioautography of antibiotic spread-layer chromatograms. J. Chromatogr. 1964;14:129–132. doi: 10.1016/s0021-9673(00)86603-0. [DOI] [PubMed] [Google Scholar]
- 16.Narasimhachari N., Ramachandran S. A simple bioatuographic technique for identifying biologically active material on thin-layer chromatograms. J. Chromatogr. 1967;27:494. doi: 10.1016/s0021-9673(01)85909-4. [DOI] [PubMed] [Google Scholar]
- 17.Shahat A.A., El-Barouty G., Hassan R.A. Chemical composition and antimicrobial activities of the essential oil from the seeds of Enterolobium contortisiliquum (Leguminosae) J. Environ. Sci. Health B. 2008;43:519–525. doi: 10.1080/03601230802174714. [DOI] [PubMed] [Google Scholar]
- 18.Betina V., Pilatova L. A paper chromatography method for the determination of suitable pH values for the extraction of antibiotics. Csl. Mikrobiol. 1958;3:202–204. doi: 10.1038/182796a0. [DOI] [PubMed] [Google Scholar]
- 19.Dawson R.M.C., Elliott D.C., Elliott W.H. 2nd edn. Oxford University Press; Oxford: 1969. Data for Biochemical Research. [Google Scholar]
- 20.Caccamese S., Cascio O., Compagnini A. Isolation of an antimicrobial bromoditerpene from a marine alga aided by improved bioautography. J. Chromatogr. A. 1989;478:255–258. [Google Scholar]
- 21.VanderKop P.A., MacNeil J.D. Separation and detection of monensin, lasalocid and salinomycin by thin-layer chromatography/bioautography. J. Chromatogr. 1990;508:386–390. doi: 10.1016/s0021-9673(00)91282-2. [DOI] [PubMed] [Google Scholar]
- 22.Jayasinghe L., Kumarihamy B.M.M., Jayarathna K.H.R.N. Antifungal constituents of the stem bark of Bridelia retusa. J. Phytochem. 2003;62:637–647. doi: 10.1016/s0031-9422(02)00623-4. [DOI] [PubMed] [Google Scholar]
- 23.Touchstone J.C., Dobbins M.F. 2nd edn. Wiley; New York: 1983. Practice of Thin Layer Chromatography. [Google Scholar]
- 24.Ramirez A., Gutiérrez R., Diaz G. High-performance thin-layer chromatography-bioautography for multiple antibiotic residues in cow׳s milk. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2003;784:315–322. doi: 10.1016/s1570-0232(02)00819-x. [DOI] [PubMed] [Google Scholar]
- 25.Shahverdi A.R., Abdolpour F., Monsef-Esfahani H.R. A TLC bioautographic assay for the detectionof nitrofurantoin resistance reversal compound. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2007;850:528–530. doi: 10.1016/j.jchromb.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Meyer J.J.M., Dilika F. Antibacterial activity of Helichrysum pedunculatum used in circumcision rites. J. Ethnopharmacol. 1996;53:51–54. doi: 10.1016/0378-8741(96)01411-0. [DOI] [PubMed] [Google Scholar]
- 27.Schmourlo G., Mendonca-Filho R.R., Alviano C.S. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J. Ethnopharmacol. 2004;96:563–568. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Silva M.T.G., Simas S.M., Batista T.G.F.M. Studies on antimicrobial activity, in vitro, of Physalis angulata L. (Solanaceae) fraction and physalin B bringing out the importance of assay determination. Mem. Instit. Oswaldo Cruz. 2005;100:779–782. doi: 10.1590/s0074-02762005000700018. [DOI] [PubMed] [Google Scholar]
- 29.Dilika F., Afolayan A.J., Meyer J.J.M. Comparative antibacterial activity of two Helichrysum species used in male circumcision in South Africa. S. Afr. J. Bot. 1997;63:158–159. [Google Scholar]
- 30.Runyoro D.K.B., Matee M.I.N., Ngassapa O.D. Screening of Tanzanian medicinal plants for anti-candida activity. BMC Complement. Altern. Med. 2006;6:1–10. doi: 10.1186/1472-6882-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das K., Tiwari R.K.S., Shrivastava D.K. Techniques for evaluation of medicinal plant products as antimicrobial agent: current methods and future trends. J. Med. Plants Res. 2010;4:104–111. [Google Scholar]
- 32.Okusa P.N., Stevigny C., Devleeschouwer M. Optimization of the culture medium used for direct TLC-bioautography. Application to the detection of antimicrobial compounds from Cordia gilletii De Wild (Boraginaceae) J. Planar Chromatogr. 2010;23:245–249. [Google Scholar]
- 33.Homans A.L., Fuchs A. Direct bioautography on thin-layer chromatograms as a method for detecting fungitoxic substances. J. Chromatogr. 1970;51:327–329. doi: 10.1016/s0021-9673(01)96877-3. [DOI] [PubMed] [Google Scholar]
- 34.Hamburger M.O., Cordell G.A. A direct bioautographic TLC assay for compound possessing antibacterial activity. J. Nat. Prod. 1987;50:19–22. doi: 10.1021/np50049a003. [DOI] [PubMed] [Google Scholar]
- 35.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 36.Burkhead K.D., Schisler D.A., Slininger P. Pyrrolnitrin production by biological control agent Pseudomonas cepacia B37w in culture and in colonized wounds of potatoes. J. Appl. Environ. Microbiol. 1994;60:2031–2039. doi: 10.1128/aem.60.6.2031-2039.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morel A.F., Araujo C.A., da Silva U.F. Antibacterial cyclopeptide alkaloids from the bark of Condalia buxifolia. Phytochemistry. 2002;61:561–566. doi: 10.1016/s0031-9422(02)00287-x. [DOI] [PubMed] [Google Scholar]
- 38.Esterbrook S.M., Hersey J.A. Bioautography of erythromycin and its esters. J. Chromatogr. 1976;121:390–394. doi: 10.1016/s0021-9673(00)85039-6. [DOI] [PubMed] [Google Scholar]
- 39.Weltzien H.C. Ein biologischer Test für fungizide Substanzen auf dem Paper chromatogram. Naturwiss. 1958;45:288–289. [Google Scholar]
- 40.Dekhuijzen H.M. The transformation in plants of sodium dimethyldithiocarbamate into other fungitoxic compounds. Staat. Gent. 1961;26:1542–1543. [Google Scholar]
- 41.Paxton J.D. Assays for antifungal activity. In: Hostettmann K., editor. vol. 6. Academic Press; London: 1991. (Methods in Plant Biochemistry—Assays for Bioactivity). [Google Scholar]
- 42.Hostettmann K., Marston A. vol. 17th. Elsevier; Amsterdam: 1990. Studies in Natural Products Chemistry. [Google Scholar]
- 43.Hostettmann K., Potterat O. Strategy for the isolation and analysis of antifungal, molluscicidal and larvicidal agents from tropical plants. ACS Symp. Ser. 1997;658:14–26. [Google Scholar]
- 44.Meyers E., Erickson R.C. Bioautography of antibiotics on thin layer chromatograms. J. Chromatogr. 1967;26:531–532. doi: 10.1016/s0021-9673(01)98921-6. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton P.B., Cook C.E. Some techniques for bioautography of antimicrobial substances on thin-layer chromatograms. J. Chromatogr. 1968;35:295–296. doi: 10.1016/s0021-9673(01)82388-8. [DOI] [PubMed] [Google Scholar]
- 46.Islam N., Parveen S.A., Nakazawa N. Bioautography with the fungus Valsa ceratosperma in the search for antimycotic agents. Pharm. Biol. 2003;41:637–640. [Google Scholar]
- 47.Billow J., Speaker T.J. Bioautography of antibiotic compounds: a simplification and improvement. J. Chromatogr. 1972;67:191–192. doi: 10.1016/s0021-9673(01)97170-5. [DOI] [PubMed] [Google Scholar]
- 48.Rahalison L., Hamburger M., Hostettmann K. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochem. Anal. 1991;2:199–203. [Google Scholar]
- 49.Rocha L., Marston A., Potterat O. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense. J. Phytochem. 1995;40:1447–1452. doi: 10.1016/0031-9422(95)00507-4. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez S., Wolfender J.L., Hakizamungu E. An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina. Planta Med. 1995;61:362–364. doi: 10.1055/s-2006-958102. [DOI] [PubMed] [Google Scholar]
- 51.Terreaux C., Maillard M., Hostettmann K. Analysis of the fungicidal constituents from the bark of Ocotea usambarensis Engl. (Lauraceae) Phytochem. Anal. 1994;5:233–238. [Google Scholar]
- 52.Choma I.M., Choma A., Staszczuk K. Direct bioautography – thin layer chromatography of flumequine and doxycycline in milk. J. Planar Chromatogr. 2002;15:187–191. [Google Scholar]
- 53.Choma I.M., Komaniecka I. Matrix solid-phase dispersion combined with thin-layer chromatography-direct bioautography for determination of enrofloxacin and ciprofloxacin residues in milk. J. Liq. Chromatogr. Relat. Technol. 2005;28:2467–2478. [Google Scholar]
- 54.Choma I.M., Kowalski C., Lodkowski R. TLC-DB as an alternative to the HPLC method in the determination of cefacetril residues in cow׳s milk. J. Liq. Chromatogr. Relat. Technol. 2008;31:1903–1912. [Google Scholar]
- 55.Choma I.M. Thin-layer chromatography—direct bioautography of flumequine residues in milk. J. Liq. Chromatogr. Relat. Technol. 2006;29:2083–2093. [Google Scholar]
- 56.Choma I.M. Screening of enrofloxacin and ciprofloxacin residues in milk by HPLC and by TLC with direct bioautography. J. Planar Chromatogr. 2006;19:104–108. [Google Scholar]
- 57.Eberz G., Rast H.G., Burger K. Bioactivity screening by chromatography—bioluminescence coupling. Chromatographia. 1996;43:5–9. [Google Scholar]
- 58.Weins C., Jork H. Toxicological evaluation of harmful substances by in situ enzymatic and biological detection in high-performance thin-layer chromatography. J. Chromatogr. A. 1996;750:403–407. doi: 10.1016/0021-9673(96)00601-2. [DOI] [PubMed] [Google Scholar]
- 59.Du Q., Li B. Identification of antioxidant compounds of Mucuna sempervirens by high-speed counter-current chromatographic separation—DPPH radical scavenging detection and their oestrogenic activity. Food Chem. 2012;131:1181–1186. [Google Scholar]
- 60.Wedge D.E., Nagle D.G. A new 2D-TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J. Nat. Prod. 2000;63:1050–1054. doi: 10.1021/np990628r. [DOI] [PubMed] [Google Scholar]
- 61.Kitamura R.O.S., Romoff P., Young M.C.M. Chromenes from Peperomia serpens (Sw.) Loudon (Piperaceae) Phytochemistry. 2006;67:2398–2402. doi: 10.1016/j.phytochem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 62.Tyiháka E., Mincsovics E., Móricza Á.M. Over pressured layer chromatography: from the pressurized ultramicro chamber to BioArena system. J. Chromatogr. A. 2012;1232:3–18. doi: 10.1016/j.chroma.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 63.Sarkozi A., Moricz A.M., Ott P.G. Investigation of chelidonium alkaloids by use of a complex bioautographic system. J. Planar Chromatogr. 2006;19:267–272. [Google Scholar]
- 64.Harborne J.B. Chapman and Hall Ltd.; London: 1973. Phytochemical Methods. pp. 49–188. [Google Scholar]
- 65.Marston A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatogr. A. 2011;1218:2676–2683. doi: 10.1016/j.chroma.2010.12.068. [DOI] [PubMed] [Google Scholar]
- 66.Harborne J.B. Phenolic compounds. In: Heftmann E., editor. Chromatography. 5th edn. Elsevier; Amsterdam: 1992. [Google Scholar]
- 67.Williams L., Bergersen O. Towards an integrated platform for combinatorial library synthesis and screening. J. Planar Chromatogr. 2001;14:318–321. [Google Scholar]
- 68.Saxena G., Farmer S., Towers G.H.N. Use of specific dyes in the detection of antimicrobial compounds from crude plant extracts using a thin layer chromatography agar overlay technique. Phytochem. Anal. 1995;6:125–129. [Google Scholar]
- 69.Slusarenko A.J., Longland A.C., Whitehead I.M. A convenient sensitive and rapid assay for antibacterial activity of phytoalexins. Bot. Helv. 1989;99:203–207. [Google Scholar]
- 70.Galindo-Cuspinera V., Rankin S.A. Bioautography and chemical characterization of antimicrobial compound(s) in commercial water-soluble annatto extracts. J. Agric. Food Chem. 2005;53:2524–2529. doi: 10.1021/jf048056q. [DOI] [PubMed] [Google Scholar]
- 71.Cytrynska M., Mak P., Zdybicka-Barabas A. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides. 2007;28:533–546. doi: 10.1016/j.peptides.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Mazimba O., Majinda R.R.T., Modibedi C. Tylosema esculentum extractives and their bioactivity. Bioorg. Med. Chem. 2011;19:5225–5230. doi: 10.1016/j.bmc.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Olech M., Komsta Ł., Nowak R. Investigation of antiradical activity of plant material by thin-layer chromatography with image processing. Food Chem. 2012;132(1):549–553. doi: 10.1016/j.foodchem.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 74.Takao T., Kitatani F., Watanabe N. Simple screening method for antioxidants and isolation of several antioxidant produced by marine bacteria from fish and shellfish. Biosci. Biotechnol. Biochem. 1994;58:1780–1783. [Google Scholar]
- 75.Rossi D., Guerrini A., Maietti S. Chemical fingerprinting and bioactivity of Amazonian Ecuador Croton lechleri Müll. Arg. (Euphorbiaceae) stem bark essential oil: a new functional food ingredient? Food Chem. 2011;126:837–848. [Google Scholar]
- 76.Gu L., Wua T., Wang Z. TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT – Food Sci. Technol. 2009;42:131–136. [Google Scholar]
- 77.Miller N.J., Rice-Evans C.A. Factors influencing the antioxidant activity determined by the ABTS radical cation assay. Free. Radic. Res. 1997;26:195–199. doi: 10.3109/10715769709097799. [DOI] [PubMed] [Google Scholar]
- 78.Pratt D.E., Miller E.E. A flavonoid anti-oxidant in Spanish pea nuts. Am. J. Oil Chem. Soc. 1984;61:1064–1067. [Google Scholar]
- 79.Whittern C.C., Miller E.E., Pratt D.E. Cotton seed flavonoids as lipid antioxidants. J. Am. Oil Chem. 1984;61:1075–1078. [Google Scholar]
- 80.Yang Z., Zhang X., Duan D. Modified TLC bioautographic method for screening acetylcholinesterase inhibitors from plant extracts. J. Sep. Sci. 2009;32:3257–3259. doi: 10.1002/jssc.200900266. [DOI] [PubMed] [Google Scholar]
- 81.Mroczek T. Highly efficient, selective and sensitive molecular screening of acetylcholinesterase inhibitors of natural origin by solid-phase extraction-liquid chromatography/electrospray ionisation-octopole-orthogonal acceleration time-of-flight-mass spectrometry and novel thin-layer chromatography-based bioautography. J. Chromatogr. A. 2009;1216:2519–2528. doi: 10.1016/j.chroma.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 82.Mroczek T., Mazurek J. Pressurized liquid extraction and anticholinesterase activity-based thin-layer chromatography with bioautography of Amaryllidaceae alkaloids. Anal. Chim. Acta. 2009;633:188–196. doi: 10.1016/j.aca.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 83.Yang Z.D., Song Z.W., Ren J. Improved thin-layer chromatography bioautographic assay for the detection of actylcholinesterase inhibitors in plants. Phytochem. Anal. 2011;22:509–515. doi: 10.1002/pca.1310. [DOI] [PubMed] [Google Scholar]
- 84.Marston A., Kissling J., Hostettmann K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002;13:51–54. doi: 10.1002/pca.623. [DOI] [PubMed] [Google Scholar]
- 85.Queiroz E.F., Ioset J.R., Ndjoko K. On-line identification of the bioactive compounds from Blumea gariepina by HPLC-UV-MS and HPLC-UV-NMR, combined with HPLC-micro-fractionation. Phytochem. Anal. 2005;16:166–174. doi: 10.1002/pca.839. [DOI] [PubMed] [Google Scholar]
- 86.Kissling J., Ioset J.R., Marston A. Bio-guided isolation of cholinesterase inhibitors from the bulbs of Crinum x powellii. Phytother. Res. 2005;19:984–987. doi: 10.1002/ptr.1770. [DOI] [PubMed] [Google Scholar]
- 87.Ellman G.L., Courtney K.D., Andres V. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 88.Rhee I.K., van de Meent M., Ingkaninan K. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A. 2001;915:217–223. doi: 10.1016/s0021-9673(01)00624-0. [DOI] [PubMed] [Google Scholar]
- 89.Rhee I.K., van de Meent M., Ingkaninan K. Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem. Anal. 2003;14:127–131. doi: 10.1002/pca.675. [DOI] [PubMed] [Google Scholar]
- 90.Andrade M.T., Lima J.A., Pinto A.C. Indole alkaloids from Tabernaemontana australis (Müell. Arg) Miers that inhibit acetylcholinesterase enzyme. Bioorg. Med. Chem. 2005;13:4092–4095. doi: 10.1016/j.bmc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 91.Mehta A., Zitzmann N., Rudd P.M. Alpha-glucosidase inhibitors as potential broad based anti-viral agent. FEBS Lett. 1998;430:17–22. doi: 10.1016/s0014-5793(98)00525-0. [DOI] [PubMed] [Google Scholar]
- 92.Kwon O.S., Park S.H., Yun B.S. Cyclo (D-Pro-L-Val), a specific beta-glucosidase inhibitor produced by Aspergillus sp. F70609. J. Antibiot. 2001;54:179–181. doi: 10.7164/antibiotics.54.179. [DOI] [PubMed] [Google Scholar]
- 93.Ali M.S., Jahangir M., Hussan S.S. Inhibition of alpha-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry. 2002;60:295–299. doi: 10.1016/s0031-9422(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 94.Kurihara H., Sasaki M., Hatano M. A new screening method for glucosidase inhibitors and its application to algal extracts. Fish. Sci. 1994;60:759–761. [Google Scholar]
- 95.Simoes-Pires C.A., Hmicha B., Marston A. A TLC bioautographic method for the detection of α- and β-glucosidase inhibitors in plant extracts. Phytochem. Anal. 2009;20:511–515. doi: 10.1002/pca.1154. [DOI] [PubMed] [Google Scholar]
- 96.Salazar M.O., Furlan R.L.E. A rapid TLC autographic method for the detection of glucosidase inhibitors. Phytochem. Anal. 2007;18:209–212. doi: 10.1002/pca.971. [DOI] [PubMed] [Google Scholar]
- 97.Lund B.M., Lyon G.D. Detection of inhibitors of Erwinia carotovora and E. herbicola on thin-layer chromatograms. J. Chromatogr. 1975;110:193–196. doi: 10.1016/s0021-9673(00)91229-9. [DOI] [PubMed] [Google Scholar]
- 98.Ramallo I.A., Zacchino S.A., Furlan R.L.E. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem. Anal. 2006;17:15–19. doi: 10.1002/pca.874. [DOI] [PubMed] [Google Scholar]
- 99.Routledge E.J., Sumpter J.P. Structural features of alkylphenolic chemicals associated with estrogenic activity. J. Biol. Chem. 1997;272:3280–3288. doi: 10.1074/jbc.272.6.3280. [DOI] [PubMed] [Google Scholar]