Abstract
A rapid, sensitive and selective pseudoMRM (pMRM)-based method for the determination of solutol HS15 (SHS15) in rat plasma was developed using liquid chromatography/tandem mass spectrometry (LC–MS/MS). The most abundant ions corresponding to SHS15 free polyethyleneglycol (PEG) oligomers at m/z 481, 525, 569, 613, 657, 701, 745, 789, 833, 877, 921 and 965 were selected for pMRM in electrospray mode of ionization. Purity of the lipophilic and hydrophilic components of SHS15 was estimated using evaporative light scattering detector (ELSD). Plasma concentrations of SHS15 were measured after oral administration at 2.50 g/kg dose and intravenous administration at 1.00 g/kg dose in male Sprague Dawley rats. SHS15 has poor oral bioavailability of 13.74% in rats. Differences in pharmacokinetics of oligomers were studied. A novel proposal was conveyed to the scientific community, where formulation excipient could be analyzed as a qualifier in the analysis of new chemical entities (NCEs) to address the spiky plasma concentration profiles.
Keywords: SHS15, LC–MS/MS, Spiky profiles, Validation
1. Introduction
In the early stages of drug discovery, pharmacokinetic, drug metabolism and disposition studies will be conducted in rodents as they are relatively inexpensive and can be easily acquired and handled [1]. In a typical pharmacokinetic study, new chemical entities (NCEs) are administered to rats via intravenous and oral routes. Serial blood samples are collected and analyzed by liquid chromatography/tandem mass spectrometry (LC–MS/MS). Few NCEs have spiky plasma concentration profiles and various reasons for such profiles could be due to enterohepatic circulation, or discrepancies in sample collection/sample processing. Spiky profiles in elimination phase will lead to inaccurate quantification of pharmacokinetic parameters. Extensive studies should be conducted to characterize enterohepatic circulation behavior of test compounds. Drugs that undergo enterohepatic cycling to a significant extent include colchicine, phenytoin, leflunomide and tetracycline antibiotics [2]. As formulation excipients have fixed plasma concentration profiles irrespective of the NCE dosed, monitoring the plasma concentration levels of excipient helps to take a decision on the spiky plasma concentration profiles of NCEs. A thoroughly developed and validated bioanalytical method is required to fix the plasma concentration profile and understand the pharmacokinetic disposition of formulation excipient studied. Integrity of results from pharmacokinetic studies can be cross verified if formulation excipients that have fixed plasma concentration profile/pharmacokinetic parameters are monitored along with the test compound studied. Solutol HS15 (SHS15) is the excipient of choice for chemotherapy drugs as severe toxicity concerns were raised with the use of cremophor EL as a formulation excipient [3]. SHS15 has better solubilizing capacity for a wide variety of compounds [4]. This excipient has been proved to be safe in preclinical species and recommended for preclincal pharmacokinetic studies [5]. SHS15 is a nonionic emulsifying agent obtained by reacting 15 mol of ethylene oxide with 1 mol of 12-hydroxystearic acid. SHS15 consists of about 70% of mono- and diesters of 12 hydroxystearic acid (lipophilic part) and 30% of free polyethyleneglycol (hydrophilic part) [6]. To our knowledge, there has been no LC–MS/MS method reported for the quantitative estimation of SHS15. A gel permeation chromatography method with selective wavelength detector was reported for the analysis of SHS15 [7]. But, the objective of this method was to check the reversal of multi-drug resistance for various fractions of SHS15 collected, instead of quantitative application. In the present work, an attempt was made to develop and validate a bioanalytical method for the quantitative estimation of SHS15 using LC–MS/MS and present the plasma concentration profile/pharmacokinetic parameters in male Sprague Dawley rats. Pharmacokinetic parameters for SHS15 and differences in pharmacokinetics of its oligomers were established.
2. Experimental
2.1. Materials
SHS15, dimethyl sulfoxide (DMSO) and telmisartan (internal standard) were procured from Sigma-Aldrich Co. (St. Louis, MO, USA). Acetonitrile, water and acetone (HPLC grade) were procured from Merck specialities Pvt. Ltd. (Mumbai, India). Formic acid (90% purified) was procured from Merck specialities Pvt. Ltd. (Mumbai, India). Sprague Dawley rats were obtained from Bioneeds Ltd. (Bangalore, India). Vacutainers (lithium heparin as anticoagulant) were sourced from BD (Franklin lakes, USA).
2.2. Estimation of hydrophilic and lipophilic components of SHS15
Percentage of hydrophilic and lipophilic components of SHS15 was estimated by using an HPLC system equipped with an evaporative light scattering detector (ELSD) (Agilent, Santaclara, USA). The HPLC system consisted of an Agilent 1200 RRLC (Agilent, Santaclara, USA). The stationary phase was XBridge C18 (250 mm×3.0 mm, 5 µm) (Waters, Milford, USA). The mobile phase consisted of 0.1% formic acid in water as aqueous component (A) and 100% acetonitrile as organic modifier (B). A generic gradient LC method (time (min)/%B=0.01/2, 25.00/50, 45.00/95, 50.00/95, 55.00/2, 60.00/2) with a run time of 60 min and a flow rate of 0.5 mL/min was developed for the purity analysis of SHS15. The column and autosampler were maintained at 40 and 4 °C, respectively. The ELSD was operated with typical settings as follows: evaporation temperature, 75 °C; nebulizer temperature, 80 °C; and gas, 1.65 standard liter per minute (SLM).
2.3. Plasma stability determination of SHS15
Master stock solution (40 mg/mL) of SHS15 was prepared in DMSO. 10 µL of the master stock was spiked in 390 µL of plasma to obtain a final concentration of 1 mg/mL. Samples were incubated for 1 h at 37 °C in rat plasma. Samples were incubated on thermomixer (Eppendorf, Hamburg, Germany) at vortex speed of 600 rpm. Reaction was terminated at different time intervals (5, 10, 20, 40, 60 min) by precipitating 50 µL of incubation mixture with 200 µL of acetonitrile containing telmisartan as an internal standard (IS). 0 min control samples were prepared in heat inactivated plasma (heated at 70 °C for 5 min) and 50 µL of the sample was precipitated with 200 µL of acetonitrile containing telmisartan as the IS. Samples were vortex mixed for 10 min at 1200 rpm and centrifuged at 3350g for 10 min. 50 µL of supernatant was transferred to a fresh analysis plate and diluted with 450 µL of methanol:water (1:1). Aliquots of 10 µL were injected for LC–MS/MS analysis.
2.4. Preparation of calibration standards and quality control (QC) samples
Master stock solutions of SHS15 (40 mg/mL) and telmisartan (1 mg/mL) were prepared in DMSO. Working standard solutions of SHS15 were prepared by serial diluting master stock with acetonitrile:DMSO:water (2:2:1). Working standard solutions were prepared at 25-fold higher concentration than plasma calibration standards and QC samples. A total of nine plasma calibration standards (0.40, 0.80, 4.01, 20.04, 57.26, 104.12, 148.74, 167.31 and 185.90 µg/mL) and quality control samples (1.64, 117.12 and 156.16 µg/mL) of SHS15 (free PEG oligomers) were prepared by spiking 2 µL of the working standard solutions into 48 µL of blank rat plasma. The working solution for internal standard (100 ng/mL) was prepared by diluting an aliquot of master stock solution with acetonitrile. Master and working stock solutions were stored at 4 °C when not in use.
2.5. Sample preparation
A 50 µL aliquot of plasma (blank control plasma, plasma samples from rats dosed with SHS15, blank plasma spiked with calibration standards and QC samples) was pipetted into a 96 well polypropylene plate and extracted with 200 µL of acetonitrile containing internal standard. Samples were vortex mixed for 10 min at 1200 rpm and centrifuged at 3350g for 10 min at 4 °C. 50 µL of supernatant was pipette transferred into a fresh analysis plate and diluted with 450 µL of methanol:water (1:1). 10 µL aliquots were injected for LC–MS/MS analysis.
2.6. LC–MS/MS analysis
All mass spectrometric estimations were performed on a Sciex 3200 QTrap triple quadrupole instrument with turboionspray (AB Sciex, Toronto, Canada) using C18 column (50 mm×4.6 mm, 2.5 µm) (Thermo, Waltham, USA). The HPLC system consisted of two LC20AD UFLC pumps and a SIL HTC autosampler (Shimadzu, Kyoto, Japan). The mobile phase consisted of 0.1% formic acid in water as aqueous component (A) and 100% acetone as organic modifier (B). A generic gradient LC method (time (min)/%B=0.01/5, 2.50/95, 3.50/95, 3.60/5, 5.00/5) with a short run time of 5 min and a flow rate of 1.00 mL/min was developed for the analysis of SHS15 in plasma samples. The column and autosampler were maintained at 40 and 4 °C, respectively. The turboionspray source was operated with typical settings as follows: ionization mode, positive; curtain gas, 20 psi; nebulizer gas (GS1), 50 psi; heater gas (GS2), 50 psi; ionspray voltage, 5500 V; temperature, 550 °C. The mass spectrometer was set up to perform in MS mode and to run in pseudoMRM mode. The molecular ions of SHS15 and telmisartan were formed using the declustering potentials of 140 and 40 V, respectively. In pseudoMRM mode the most abundant and informative molecular ions of free PEG oligomers were selected at m/z 481.5 (Oligomer 1), 521.5 (Oligomer 2), 569.5 (Oligomer 3), 613.5 (Oligomer 4), 657.5 (Oligomer 5), 701.5 (Oligomer 6), 745.5 (Oligomer 7), 789.5 (Oligomer 8), 833.5 (Oligomer 9), 877.5 (Oligomer 10), 921.5 (Oligomer 11), 965.5 (Oligomer 12) with medium CAD gas setting at a collision energy of 5 V. Molecular ion (m/z, 515.30) of telmisartan was fragmented to m/z 276.10 at collision energy of 65 V with medium CAD gas setting. High-purity nitrogen (99.99% pure) was used as nebulizer, curtain, heater and CAD gas. Peak areas for all components were automatically integrated using Analyst software version 1.5.
2.7. Method validation
Method validation was performed on the total SHS15 (free PEG oligomers), instead of each oligomer. Three precision and accuracy batches, consisting of calibration standards (0.40, 0.80, 4.01, 20.04, 57.26, 104.12, 148.74, 167.31, and 185.90 µg/mL), were analyzed on three different days to complete the method validation. In each batch, QC samples at 1.64, 117.12, 156.16 µg/mL were assayed in sets of six replicates to evaluate the intra- and inter-day precision and accuracy. Relative error (RE) and coeffecient of variation (CV) were served as the measure of accuracy and precision, respectively. The selectivity was evaluated by analyzing blank plasma samples obtained from different animals. Extraction efficiency of SHS15 was determined by comparing peak areas of analyte spiked before extraction into six different lots of plasma with those of the analyte post-spiked into plasma extracts. Matrix effect was evaluated from matrix factor values. Matrix factor was calculated by dividing mean peak areas of analyte post spiked into plasma extracts with those of analyte spiked into neat solutions at three QC levels. To assess post-preparative stability, six replicates of QC samples at each of the low, mid and high concentrations were processed and stored under autosampler conditions for 24 h before analysis. To assess bench-top stability, six replicates of QC samples at each of the low, mid and high concentrations were kept at room temperature for 8 h before analysis. Freeze-thaw stability was assessed at three QC levels for three freeze-thaw cycles. To assess long-term stability, six replicates of QC samples at each of the low, mid and high concentrations were kept at −80 °C for 60 days before analysis.
2.8. Application
SHS15 was administered intravenously (lateral tail vein) at 1.0 g/kg dose and orally (oral gavage needle) at 2.5 g/kg dose to fasted male Sprague Dawley rats. The volume of administered dose was 5 mL/kg. The composition of dosing vehicle used for intravenous route of administration was ethanol/SHS15/water (10:20:70, v/v/v) [8], [9]. The composition of dosing vehicle used for oral route of administration was ethanol/SHS15/water (10:50:40, v/v/v) [8], [9]. Serial blood samples were collected into vacutainers containing lithium heparin (anticoagulant) at 0.08, 0.25, 0.50, 1, 2, 4, 8 and 24 h [10] after intravenous administration and 0.25, 0.50, 1, 2, 4, 8 and 24 h [10] after oral administration. At each time point, 200 µL of blood was collected into vacutainers. Blood samples were collected using the retro orbital puncture method. Plasma was isolated by centrifugation at 10,000 rpm for 10 min and stored frozen at −80 °C until assay. Pharmacokinetic parameters including elimination rate constant (Kel), half life (T1/2), extrapolated drug concentration (C0), AUC0-last, AUC0-inf, AUC%Extrapolated, volume of distribution (Vd), clearance (Cl), Tmax, Cmax, MRTlast and absolute bioavailability (%F) were calculated using phoenix winnonlin software (v6.3). Absolute bioavailability was calculated using AUC0-inf values as AUC%Extrapolated was less than 20%.
3. Results and discussion
3.1. Percentage of hydrophilic and lipophilic components of SHS15
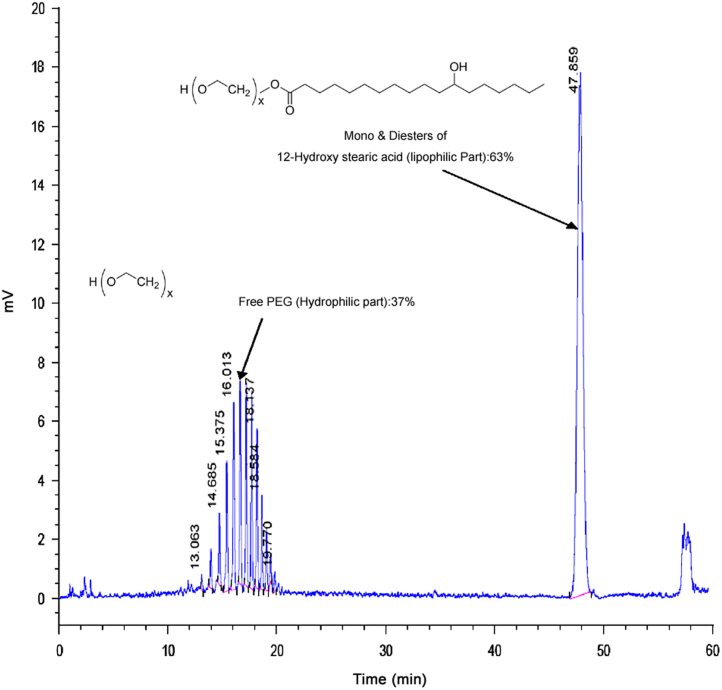
Hydrophilic and lipophilic components of SHS15 have very weak ultraviolet (UV) absorbance and need to be separated by gradient elution chromatography. This precludes their detection by UV and refractive index (RI). RI and low wavelength UV detection are highly subject to baseline drifts with gradients and have limited solvent selection. Conversely, ELSD allows direct detection of components of SHS15 without derivatisation and is compatible with gradient elution chromatography. Longer gradient program with maximum % acetonitrile ramped to 95% has achieved good separation between hydrophilic and lipophilic components of SHS15 (Fig. 1). Ramping to lower percentages of acetonitrile in the gradient program (75–80%) did not achieve complete elution of lipophilic component of SHS15. Percentage of each oligomer was estimated by area normalization method (percentage of each oligomer was calculated by summing up peak area of all the components to 100%). Percentage of oligomers 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 was 0.41%, 1.044%, 1.903%, 4.037%, 5.181%, 5.246%, 5.092%, 5.087%, 4.034%, 2.415%, 1.542% and 0.665%, respectively. Purity of lipophilic and hydrophilic components was found to be 63.344% and 36.656%, respectively. Estimated purity was in match with the supplier׳s specifications [6].
Fig. 1.
Chromatogram representing the elution pattern of hydrophilic and lipophilic components of SHS15 under reverse phase conditions in ELSD.
3.2. Plasma stability of SHS15
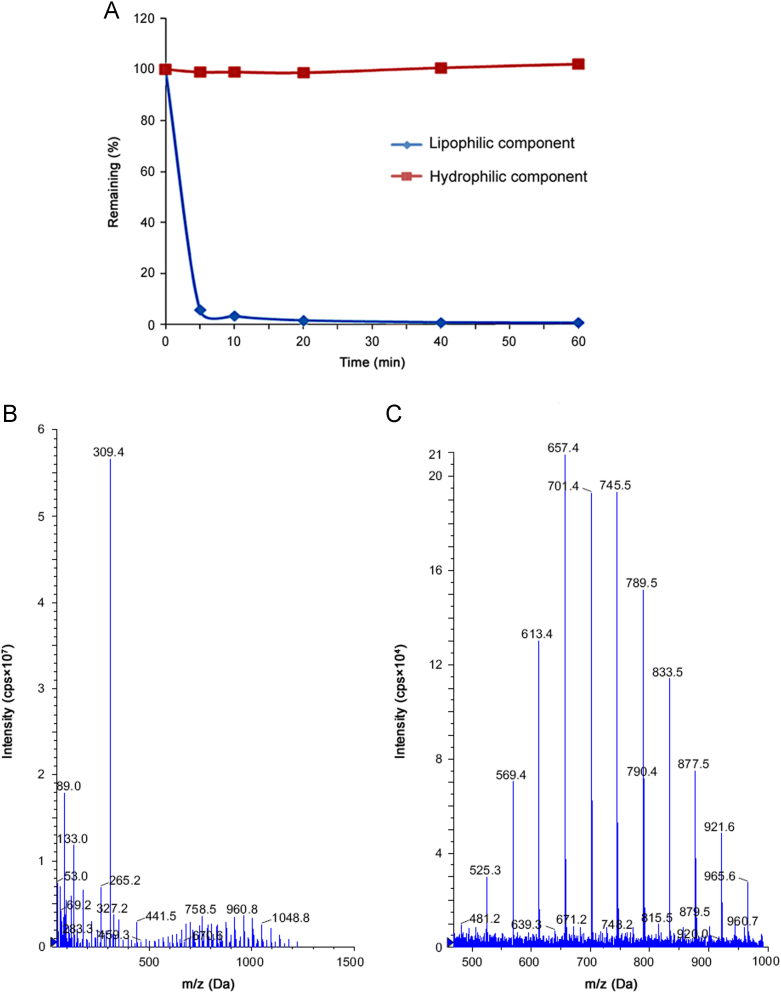
Lipophilic component of SHS15 was found very fast hydrolyzing (ester hydrolysis) in rat plasma with <1% remaining at 60 min (Fig. 2A). This could be due to esterases present at higher levels in rat species causing hydrolysis of lipophilic component of SHS15. Similarly hydrophilic component was found stable in 60 min incubation period. Insource fragment (m/z, 309.20) (Fig. 2B) was analyzed in pseudoMRM mode to quantify the % remaining of lipophilic component of SHS15. As lipophilic component was found highly unstable in the rat plasma, hydrophilic component was considered to study the pharmacokinetic disposition of SHS15 in Sprague Dawley rats.
Fig. 2.
(A) Remaining (%) vs time curve representing the plasma stability of hydrophilic and lipophilic components of SHS15. (B) Parent ion (full scan) scan of mono- and diesters of 12-OH stearic acid. (C) Parent ion (full scan) scan of free PEG oligomers.
3.3. LC–MS/MS analysis
The electrospray ionization of SHS15 produced the abundant molecular ions for free PEG component (hydrophilic) at m/z 481.50, 521.50, 569.50, 613.50, 657.50, 701.50, 745.50, 789.50, 833.50, 877.50, 921.50 and 965.50 (Fig. 2C) under positive ionization conditions. The molecular ions of free PEG component detected were sodium adducts. A total of 12 abundant and informative oligomers were identified. For calculating the plasma concentrations of SHS15 as a whole the analyte peak areas of each oligomer were summed up to develop the calibration curve. Calibration range of 0.40–185.90 µg/mL represents total free PEG component of SHS15. In order to characterize the pharmacokinetic differences of SHS15 oligomers, plasma concentrations for each oligomer were measured against identical calibration curves that are built based on purity of each oligomer. Electrospray ionization of telmisartan (internal standard) produced abundant protonated molecules ([MH]+) at m/z 515.20 and an intense fragment at m/z 276.10. The LC–MS/MS method was operated with the C18 column and a 5 min generic gradient LC method was developed for the analysis of SHS15 in rat plasma. Final mobile phase composition used for the analysis was 0.1% formic acid in water as aqueous phase and 100% acetone as organic modifier. Response saturation at higher calibration standards was observed with the use of acetonitrile or methanol as organic modifiers.
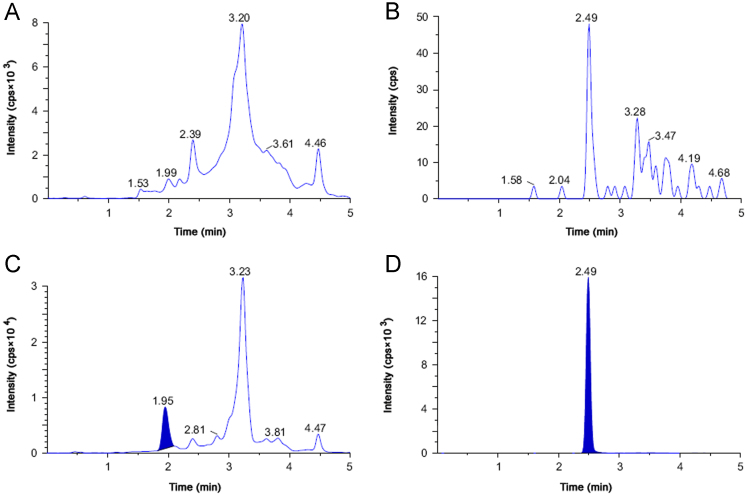
Because of the higher sensitivity of the LC–MS/MS method, lesser plasma sample volume (50 µL) was sufficient to obtain a lower limit of quantitation (LLOQ) of 0.40 µg/mL. Even though the calibration range of 0.40–185.90 µg/mL was higher for analysis on mass spectrometer, analysis of plasma samples revealed that the plasma concentrations of SHS15 were around 1–1.5 mg/mL in the initial sampling points from intravenous route. Therefore, if these study samples have to fit into the low ng/mL standard curve, very high dilution (100–1000 fold) is required, which is practically a limitation in drug discovery. Instead of developing a method with high sensitivity, we tried to develop a fit for purpose bioanalytical method, by (a) diluting the precipitated samples 10 fold after precipitation, (b) using less sensitive mass spectrometer (3200 Qtrap), and (c) injecting less volume of sample (10 µL). No interference at the retention times of SHS15 (1.85 min) (Fig. 3A) and telmisartan (2.51 min) (Fig. 3B) was observed in any of the lots screened as shown in representative chromatogram of the extracted blank plasma sample, confirming the selectivity of the present method. Representative chromatogram of SHS15 (chromatogram representing sum of peaks of all oligomers) at LLOQ is shown in Fig. 3C. Representative chromatogram of telmisartan at 100 ng/mL spiked concentration is shown in Fig. 3D. The LLOQ was set at 0.40 µg/mL for SHS15 using 50 µL of rat plasma. The retention times of SHS15 and telmisartan were reproducible throughout the experiment and no column deterioration was observed after analysis of plasma samples.
Fig. 3.
pMRM LC–MS/MS chromatograms of (A) SHS in blank rat plasma, (B) telmisartan in rat blank plasma, (C) rat plasma sample spiked with 0.40 μg/mL of SHS15 (summed peak area of 12 PEG oligomers) (LLOQ), and (D) telmisartan spiked at 100 ng/mL concentration in rat plasma.
3.4. Method validation
The developed method was validated as per the FDAs bioanalytical method validation guidelines [11]. Calibration curve was linear over the concentration range of 0.40–185.90 µg/mL with mean correlation coefficient of ≥0.9978. Quadratic regression analysis with a weighting of 1/(x×x) gave the optimum accuracy of the corresponding calculated concentrations at each level (Table 1). The low CV value for the slope indicated the repeatability of the method (Table 1). Table 2 shows a summary of intra- and inter-day precision and accuracy data for QC samples containing SHS15. Both intra- and inter-assay CV values ranged from 0.41% to 7.29% at three QC levels. The intra- and inter-assay RE values for SHS15 were −6.75% to 5.69% at three QC levels. These results indicated that the present method had an acceptable accuracy and precision. As shown in Table 3, the overall extraction efficiency of SHS15 was 99.24%, which was consistent with a total % CV less than 0.59% at three QC concentration levels. Mean matrix factor values of 1.00 (Table 3) at three QC levels showed that the developed method was totally free of matrix effects for the analysis of SHS15. Acceptable matrix factor range for qualifying the method to be free from matrix effects was 0.85–1.15. Protein precipitation was successfully applied to the extraction of SHS15 from rat plasma. Extracted QC samples were stable when stored at 4 °C for 24 h (autosampler stability) prior to injection, with <8.67% (Table 3) difference from theoretical concentration. Spiked QC samples were stable when stored at room temperature for 8 h (bench-top stability) prior to injection, with <6.16% (Table 3) difference from theoretical concentration. Spiked QC samples were stable for three freeze-thaw cycles (freeze-thaw stability) with <7.50% (Table 3) difference from theoretical concentration. Long-term stability at −80 °C was proved for a period of 60 days with <3.26% (Table 3) difference from theoretical concentration.
Table 1.
Calculated concentrations and statistical parameters of SHS15 (free PEG oligomers) calibration standards prepared in rat plasma (n=3).
| Concentration (µg/mL) |
SD | CV (%) | Relative error (%) | Accuracy (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Actual conc | Set-1 | Set-2 | Set-3 | Mean | ||||
| 0.40 | 0.38 | 0.40 | 0.39 | 0.39 | 0.01 | 3.06 | −2.81 | 97.19 |
| 0.80 | 0.91 | 0.80 | 1.00 | 0.90 | 0.10 | 11.09 | 13.03 | 113.03 |
| 4.01 | 3.54 | 3.93 | 4.41 | 3.96 | 0.44 | 11.07 | −1.29 | 98.71 |
| 20.04 | 20.96 | 19.32 | 19.17 | 19.82 | 0.99 | 5.01 | −1.11 | 98.89 |
| 57.26 | 54.67 | 62.24 | 53.71 | 56.87 | 4.67 | 8.22 | −0.67 | 99.33 |
| 104.12 | 114.11 | 105.69 | 104.71 | 108.17 | 5.17 | 4.78 | 3.89 | 103.89 |
| 148.74 | 144.27 | 140.07 | 161.84 | 148.73 | 11.55 | 7.76 | −0.01 | 99.99 |
| 167.31 | 164.65 | 168.81 | 158.37 | 163.94 | 5.26 | 3.21 | −2.01 | 97.99 |
| 185.90 | 179.71 | 184.18 | 166.97 | 176.95 | 8.93 | 5.05 | −4.81 | 95.19 |
Table 2.
Precision and accuracy of SHS15 (free PEG oligomers) in quality control samples.
| Type | Concentration (µg/mL) | Mean (µg/mL) | SD | CV (%) | Accuracy (%) | Relative error (%) |
|---|---|---|---|---|---|---|
| Intra-day set-1 (n=6) | LQCa | 1.67 | 0.11 | 6.83 | 101.98 | 1.98 |
| MQCb | 109.22 | 5.95 | 5.45 | 93.25 | −6.75 | |
| QCc | 14.98 | 8.89 | 5.93 | 96.04 | −3.96 | |
| Intra-day set-2 (n=6) | LQCa | 1.73 | 0.08 | 4.84 | 105.69 | 5.69 |
| MQCb | 113.81 | 8.30 | 7.29 | 97.18 | −2.82 | |
| HQCc | 150.11 | 9.58 | 6.38 | 96.12 | −3.88 | |
| Intra-day set-3 (n=6) | LQCa | 1.71 | 0.08 | 4.53 | 104.07 | 4.07 |
| MQCb | 110.81 | 5.53 | 4.99 | 94.61 | −5.39 | |
| HQCc | 149.00 | 9.66 | 6.48 | 95.41 | −4.59 | |
| Inter-day (n=18) | LQCa | 1.70 | 0.03 | 1.79 | 103.91 | 3.91 |
| MQCb | 111.28 | 2.33 | 2.10 | 95.01 | −4.99 | |
| HQCc | 149.69 | 0.61 | 0.41 | 95.86 | −4.14 | |
LQC=1.64 µg/mL.
MQC=117.12 µg/mL.
HQC=156.16 µg/mL.
Table 3.
Summary of validation parameters for SHS15 (free PEG oligomers) in rat plasma.
| Validation parameter | Mean (%) (n=6) | SD | CV (%) |
|---|---|---|---|
| Extraction recovery | 99.24 | 0.59 | 0.59 |
| Matrix factor (matrix effect) | 1.00 | 0.06 | 6.37 |
| Autosampler stability | 102.63 | 8.67 | 8.45 |
| Bench-top stability | 100.77 | 6.16 | 6.12 |
| Freeze-thaw stability | 100.93 | 7.50 | 7.44 |
| Long-term stability | 101.55 | 3.26 | 3.21 |
3.5. Application study
3.5.1. SHS15
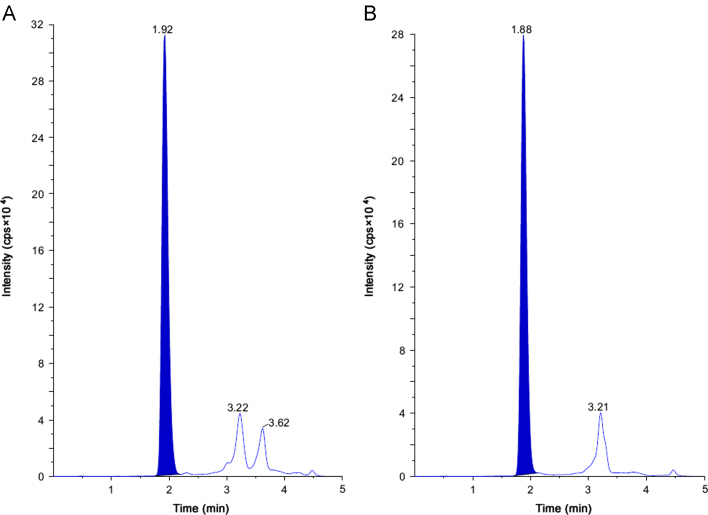
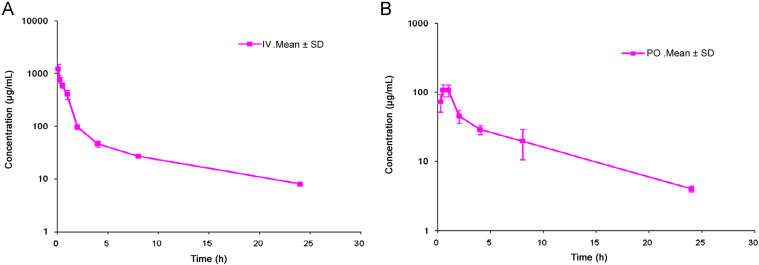
This method has been successfully applied to the bioanalysis of rat plasma samples in absolute bioavailability study of SHS15. Representative chromatograms of SHS15 from intravenous (1.00 h) and oral (1.00 h) study samples are shown in Fig. 4A and B, respectively. Intravenous and oral concentration/time profiles of SHS15 are represented in Fig. 5A and B, respectively. As SHS15 had a clear absorption and elimination phase in oral route of administration and clear elimination phase in intravenous route of administration, measuring plasma concentrations along with NCEs helped to take a decision on the spiky profile of NCEs. Monitoring formulation vehicles concentrations from pharmacokinetic study samples acted as quality control check starting from dose preparation to bioanalysis. Intravenous and oral pharmacokinetic parameters of SHS15 are listed in Table 4, Table 5 respectively. The mean oral bioavailability of SHS15 was measured as 13.74% with a mean terminal half life of 6.72 h. Mean Tmax and Cmax after oral administration of SHS15 to Sprague Dawley rats were 0.67 h and 109.02 µg/mL, respectively. Mean residence time after intravenous and oral administration of SHS15 to Sprague Dawley rats was 3.78 and 6.38 h, respectively.
Fig. 4.
pMRM LC–MS/MS chromatograms of (A) plasma sample obtained 1.00 h after intravenous administration of SHS15 to SD rats and (B) plasma sample obtained 1.00 h after oral administration of SHS15 to SD rats.
Fig. 5.
Mean concentration-time profile of SHS15 (free PEG oligomers) after (A) intravenous administration at 0.37 g/kg dose (free PEG component) to SD rats and (B) oral administration at 0.92 g/kg (free PEG component) dose to SD rats.
Table 4.
Pharmacokinetic parameters of SHS15 after intravenous administration at 1.00 g/kg dose in male Sprague Dawley rats (n=3).
| Subject | Kel (1/h) | T1/2 (h) | C0 (µg/mL) | AUClast (h µg/mL) | AUCINF_obs (h µg/mL) | AUC_%Extrap_obs (%) | Vz_obs (L/kg) | Cl_obs (mL/min kg) | MRTlast (h) |
|---|---|---|---|---|---|---|---|---|---|
| RAT-1 | 0.10 | 6.92 | 1999.02 | 1615.46 | 1702.53 | 5.11 | 2.15 | 3.59 | 3.52 |
| RAT-2 | 0.10 | 7.01 | 1148.51 | 1273.69 | 1349.79 | 5.64 | 2.75 | 4.53 | 4.06 |
| RAT-3 | 0.10 | 6.73 | 1490.49 | 1426.95 | 1504.51 | 5.16 | 2.37 | 4.06 | 3.76 |
| Mean | 0.10 | 6.89 | 1546.01 | 1438.70 | 1518.94 | 5.30 | 2.42 | 4.06 | 3.78 |
| SD | 0.00 | 0.14 | 427.96 | 171.19 | 176.81 | 0.29 | 0.30 | 0.47 | 0.27 |
| CV% | 2.07 | 2.05 | 27.68 | 11.90 | 11.64 | 5.49 | 12.45 | 11.55 | 7.16 |
Table 5.
Pharmacokinetic parameters of SHS15 after oral administration at 2.50 g/kg in male Sprague Dawley rats (n=3).
| Subject | Kel (1/h) | T1/2 (h) | Tmax (h) | Cmax (µg/mL) | AUClast (h µg/mL) | AUCINF_obs (h µg/mL) | AUC_%Extrap_obs (%) | Vz_F_obs (L/kg) | Cl_F_obs (mL/min kg) | MRTlast (h) | F (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RAT-1 | 0.11 | 6.56 | 0.50 | 87.81 | 533.54 | 573.51 | 6.97 | 15.12 | 26.63 | 6.95 | 15.10 |
| RAT-2 | 0.10 | 7.08 | 0.50 | 130.51 | 414.15 | 457.78 | 9.53 | 20.44 | 33.36 | 5.47 | 12.06 |
| RAT-3 | 0.11 | 6.51 | 1.00 | 108.73 | 500.06 | 534.14 | 6.38 | 16.10 | 28.59 | 6.72 | 14.07 |
| Mean | 0.10 | 6.72 | 0.67 | 109.02 | 482.58 | 521.81 | 7.63 | 17.22 | 29.53 | 6.38 | 13.74 |
| SD | 0.00 | 0.32 | 0.29 | 21.35 | 61.58 | 58.84 | 1.67 | 2.83 | 3.46 | 0.80 | 1.55 |
| CV% | 4.58 | 4.69 | 43.30 | 19.58 | 12.76 | 11.28 | 21.96 | 16.43 | 11.73 | 12.50 | 11.28 |
3.5.2. SHS15 oligomers
Mean intravenous and oral pharmacokinetic parameters of SHS15 oligomers are presented in Table 6, Table 7, respectively. It was found that upon increase in molecular weight of oligomers, there was decrease in absolute bioavailability [12], [13]. This could be attributed to decrease in permeability with increase in molecular weight of oligomer. All the oligomers of SHS15 have clear absorption and elimination phase in oral route of administration and clear elimination phase in intravenous route of administration. So for qualifying the analytical batches, any of the 12 oligomers can be studied along with NCEs or all the oligomers can be monitored and summed up for reflecting the total SHS15 pharmacokinetic profile.
Table 6.
Mean pharmacokinetic parameters of SHS15 oligomers after intravenous administration of SHS15 at 1.00 g/kg in male Sprague Dawley rats.
| Oligomer # | Kel (1/h) | T1/2 (h) | C0 (µg/mL) | AUClast (h µg/mL) | AUCINF_obs (h µg/mL) | AUC_%Extrap_obs (%) | Vz_obs (L/kg) | Cl_obs (mL/min kg) | MRTlast (h) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.12 | 5.64 | 15.24 | 15.89 | 16.41 | 3.20 | 2.06 | 4.21 | 3.37 |
| 2 | 0.11 | 6.16 | 43.03 | 43.12 | 44.93 | 4.05 | 2.08 | 3.90 | 3.51 |
| 3 | 0.10 | 6.60 | 73.79 | 73.02 | 76.63 | 4.73 | 2.38 | 4.16 | 3.67 |
| 4 | 0.10 | 6.76 | 165.86 | 151.19 | 159.04 | 4.99 | 2.51 | 4.27 | 3.70 |
| 5 | 0.09 | 7.30 | 228.10 | 195.68 | 208.24 | 6.07 | 2.65 | 4.18 | 3.91 |
| 6 | 0.09 | 7.47 | 237.12 | 203.86 | 217.48 | 6.28 | 2.63 | 4.06 | 3.93 |
| 7 | 0.09 | 8.07 | 205.87 | 187.75 | 203.52 | 7.68 | 2.93 | 4.22 | 4.12 |
| 8 | 0.09 | 7.60 | 190.31 | 191.00 | 204.33 | 6.51 | 2.75 | 4.19 | 4.06 |
| 9 | 0.09 | 7.50 | 173.62 | 152.37 | 162.57 | 6.29 | 2.70 | 4.15 | 3.93 |
| 10 | 0.10 | 7.06 | 115.81 | 88.34 | 93.35 | 5.39 | 2.67 | 4.37 | 3.74 |
| 11 | 0.10 | 6.89 | 75.10 | 67.47 | 71.18 | 5.18 | 2.18 | 3.67 | 3.72 |
| 12 | 0.10 | 6.99 | 32.61 | 30.09 | 31.78 | 5.31 | 2.12 | 3.51 | 3.74 |
Table 7.
Mean pharmacokinetic parameters of SHS15 oligomers after oral administration of SHS15 at 2.50 g/kg in male Sprague Dawley rats.
| Oligomer # | Kel (1/h) | T1/2 (h) | Tmax (h) | Cmax (µg/mL) | AUClast (h µg/mL) | AUCINF_obs (h µg/mL) | AUC_%Extrap_obs (%) | Vz_F_obs (L/kg) | Cl_F_obs (mL/min kg) | MRTlast (h) | F (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.12 | 5.88 | 0.50 | 1.92 | 9.08 | 9.56 | 5.12 | 9.25 | 18.09 | 6.47 | 23.30 |
| 2 | 0.11 | 6.55 | 0.50 | 4.65 | 20.82 | 22.42 | 7.34 | 11.33 | 19.79 | 6.57 | 19.96 |
| 3 | 0.10 | 6.76 | 0.83 | 6.72 | 29.56 | 31.98 | 7.64 | 14.66 | 25.06 | 6.34 | 16.69 |
| 4 | 0.10 | 6.87 | 0.83 | 12.68 | 54.14 | 58.79 | 7.92 | 17.12 | 28.84 | 6.33 | 14.78 |
| 5 | 0.10 | 6.80 | 0.67 | 14.29 | 62.95 | 68.56 | 8.27 | 18.82 | 31.88 | 6.33 | 13.17 |
| 6 | 0.10 | 6.77 | 0.83 | 14.66 | 58.67 | 63.74 | 8.01 | 20.27 | 34.59 | 6.21 | 11.72 |
| 7 | 0.10 | 6.95 | 0.83 | 13.27 | 54.09 | 59.10 | 8.63 | 21.76 | 35.99 | 6.37 | 11.62 |
| 8 | 0.09 | 7.50 | 0.83 | 10.21 | 41.37 | 45.67 | 9.63 | 30.57 | 46.82 | 6.50 | 8.94 |
| 9 | 0.09 | 7.55 | 1.00 | 5.69 | 21.91 | 24.33 | 10.10 | 45.57 | 69.45 | 6.42 | 5.99 |
| 10 | 0.10 | 7.37 | 1.00 | 2.50 | 10.02 | 11.09 | 9.81 | 58.61 | 91.12 | 6.54 | 4.75 |
| 11 | 0.08 | 9.06 | 1.00 | 1.64 | 6.97 | 8.15 | 14.39 | 62.21 | 79.70 | 7.17 | 4.58 |
| 12 | 0.07 | 9.80 | 1.00 | 0.63 | 2.70 | 3.27 | 17.50 | 72.65 | 85.42 | 7.48 | 4.11 |
4. Conclusion
A rapid, sensitive and reliable LC–MS/MS method for the determination of SHS15 in rat plasma has been successfully developed and validated using the protein precipitation extraction method. This method demonstrated acceptable sensitivity (LLOQ: 0.40 µg/mL), precision, accuracy, selectivity, recovery and stability. The validated method was successfully applied to assay rat plasma samples and represented the plasma concentration profiles/pharmacokinetic parameters of SHS15 after intravenous and oral administration. Pharmacokinetic parameters of SHS15 as a whole and difference in pharmacokinetics of oligomers were established.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Tse F.L.S., Jaffe J.M. Marcel Dekker Inc.; New York: 1991. Preclinical Drug Disposition—A Laboratory Handbook; p. 34. [Google Scholar]
- 2.Sloss A., Kluber P. Prescribing in liver disease. Aust. Prescr. 2009;32:32–35. [Google Scholar]
- 3.Medha J., Sulabha P., Shobhona S. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: nanoproject. Int. J. Pharm. 2008;364:119–126. doi: 10.1016/j.ijpharm.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Bittner B., Mountfield R.J. Intravenous administration of poorly soluble new drug entities in early drug discovery: the potential impact of formulation on pharmacokinetic parameters. Curr. Opin. Drug Discov. Dev. 2002;5:59–71. [PubMed] [Google Scholar]
- 5.Sherry K.U., Ranga V. Solutol HS15 as a novel excipient. Pharm. Technol. 2010:108–110. [Google Scholar]
- 6.Technical information, Kolliphor HS15, BASF, 2012, pp. 1–8. 〈http://www.pharma-ingredients.basf.com/Solubilizer/Kolliphor_range/kolliphor_HS15.aspx〉.
- 7.Coon J.S., Knudson W., Clodfelter K. Solutol HS15, nontoxic polyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance. Cancer Res. 1991;51:897–902. [PubMed] [Google Scholar]
- 8.Neervannan S. Preclinical formulations for drug discovery toxicology: physicochemical challenges. Expert Opin. Drug Metab. Toxicol. 2006;2(5):715–731. doi: 10.1517/17425255.2.5.715. [DOI] [PubMed] [Google Scholar]
- 9.Sheftel V.O. Indirect food additives and polymers: migration and toxicology. Lewis. 2000:1114–1116. [Google Scholar]
- 10.Kwon Y. Kluwer Academic Publishers; New York?>: 2002. Pharmacokinetic Study Design and Data Interpretation; pp. 21–46. [Google Scholar]
- 11.Guidance for Industry-Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, May 2001, Available from URL: 〈http://www.fda.gov/cder/guidance/index.htm〉.
- 12.He Y.L., Murby S., Warhurst G. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly(ethylene glycol) and d-peptides. J. Pharm. Sci. 1998;87:626–633. doi: 10.1021/js970120d. [DOI] [PubMed] [Google Scholar]
- 13.Bhaskar V.V., Middha A., Tiwari S. Liquid chromatography tandem mass spectrometry for quantitative estimation of polyethyleneglycol 400 and its applications. J. Chromatogr. B. 2013;926:68–76. doi: 10.1016/j.jchromb.2013.02.021. [DOI] [PubMed] [Google Scholar]