Abstract
A sensitive and selective liquid chromatography–tandem mass spectrometric (LC−MS/MS) method was established to determine 2-oxo-clopidogrel, a crucial intermediate metabolite in human plasma. A chromatographic separation was performed on a Sapphire C18 column following a liquid–liquid extraction sample preparation with methyl t-butyl ether. Detection was carried out on a triple quadrupole mass spectrometer operated in multiple reaction monitoring (MRM) with an electrospray ionization (ESI) mode. The method was validated in terms of specificity, accuracy, precision and limit of quantification. The calibration curves ranged from 0.50 to 50.0 ng/mL with good linearity. The stability was fully validated with addition of 1,4-dithio-DL-threitol (DTT) into the plasma sample prior to and in the preparation procedure. The validated method was proved to be suitable for use in pharmacokinetic study after single oral administration of 75 mg clopidogrel tablets in human subjects, which could make contribution to intensive study of the clinical drug–drug interactions of clopidogrel and individual treatment.
Keywords: 2-Oxo-clopidogrel, LC−MS/MS, Human plasma, Pharmacokinetics
1. Introduction
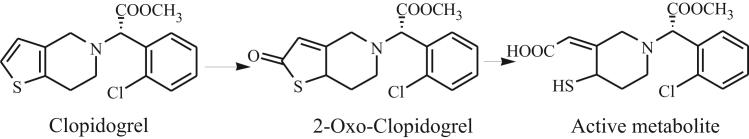
Clopidogrel, like prasugrel and ticlopidine, is a member of the thienopyridine class. As prodrugs, the responses to these thienopyridine prodrugs depend on exposure to their respective active metabolites, which are significantly attributed to thiolactone intermediate concentration [1]. For clopidogrel, a large inter-individual variability of platelet aggregation was observed in patients and this variation was relevant to CYP2C19 genetic polymorphisms, which was involved in CYP-dependent metabolism [2]. Clopidogrel is metabolized through two major pathways. Approximately 85% of a clopidogrel dose is hydrolyzed rapidly to form an inactive acid metabolite [3], [4], which will conjugate to the inactive acyl glucuronide [5]. A small portion of clopidogrel is oxidized to 2-oxo-clopidogrel (Fig. 1) by CYP3A, CYP2B6 and CYP2C19, followed by the formation of an active metabolite by CYP2C19 or by PON1 [2]. Compared with prasugrel, little metabolism of clopidogrel to the thiolactone has been observed [6], which led to less potent and consistent inhibition of platelet function of clopidogrel [7]. Therefore, the thiolactone intermediate 2-oxo-clopidogrel plays an important role in clopidogrel metabolism. To predict human response to clopidogrel and make individualized treatment and to make intensive research study of the clinical drug–drug interactions of clopidogrel on the basis of the fact that 2-oxo-clopidogrel inhibits the activity of CYP2C19 strongly [8], the detailed pharmacokinetics of 2-oxo-clopidogrel needs to be revealed.
Fig. 1.
Active metabolic pathway of clopidogrel.
Some LC−MS/MS assays for quantification of clopidogrel or its metabolites have been reported. Several methods were developed to simultaneously quantify clopidogrel and its main metabolite clopidogrel carboxylic acid [9], [10], [11], [12], [13], [14]: one work was established to quantify clopidogrel and its derivatized active thiol metabolite [15]; another study was reported for the simultaneous determination of clopidogrel, its main metabolite and derivatized isomers of thiol metabolite [16]; and one paper presented the simultaneous quantification of clopidogrel, its main metabolite and the newly described acyl glucuronide metabolite [17]. To the best of our knowledge, no methods have been reported for quantification of 2-oxo-clopidogrel in human plasma yet. However, the high activity of thiolactone poses great challenge for the establishment of analytic method. For the lucubration of clopidogrel metabolism and individual treatment, this study focused on developing an LC−MS/MS method [18], [19] to determine the concentration of 2-oxo-clopidogrel. To achieve the goal, the method was validated and applied to a pharmacokinetic study.
2. Materials and methods
2.1. Chemicals and reagents
Plavix tablets were purchased from Sanofi Pharmaceuticals Co., Ltd. (Hangzhou, China). 2-Oxo-clopidogrel hydrochloride was offered by Absin Bioscience Inc. (Shanghai, China). Mifepristone (Internal Standard, IS) was supplied by Xianju Pharmaceutical Co., Ltd. (Zhejiang, China). DTT (purity ≥98%) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Acetonitrile and methyl tert-butyl ether (MTBE) of HPLC/Spectro grade were obtained from Tedia Company Inc. (Fairfield, OH, USA). Other chemicals were all of analytical grade and purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China), and de-ionized water was generated with a Hydro-Reverse Osmosis system (Durham, NC, USA) connected to a Milli-Q UV Plus purifying system (Billerica, MA, USA).
2.2. Instrumentation
A Waters Alliance 2695 LC system with a column oven (Milford, MA, USA) was coupled with a Micromass Quattromicro tandem MS system (Micromass, Manchester, UK), which was equipped with an ESI source and operated with MassLynx 4.0 software.
2.3. Chromatographic and MS/MS conditions
HPLC separation was carried out on a Sapphire C18 analytical column (250 mm ×4.6 mm×5 μm) with mobile phase A-acetonitrile and B-water containing 0.1% (v/v) formic acid (A:B=95:5) at a flow rate of 1 mL/min and the column temperature was maintained at 40 °C. The sample injection volume was 30 μL and the analytical run time was 5.5 min. The eluent from the HPLC column was introduced directly to the Micromass using ESI source in the positive ion mode.
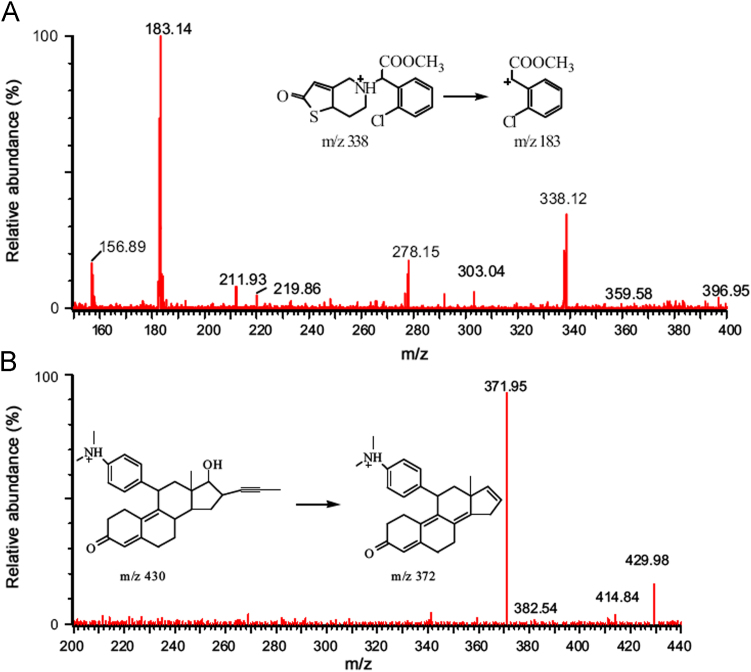
The Micromass Quantum parameters were optimized and set as follows: source temperature 120 °C, nitrogen desolvation gas 370 °C with a flow rate of 500 L/h, cone voltage at 24 V and 28 V, and the collision energies at 17 eV and 21 eV for 2-oxo-clopidogrel and mifepristone, respectively. Quantifications were performed by using MRM with ion transitions of m/z 338.1→183.1 for 2-oxo-clopidogrel and m/z 430.0→372.0 for mifepristone. The product ion spectra of these analytes are shown in Fig. 2.
Fig. 2.
Product ion spectra of (A) 2-oxo-clopidogrel and (B) IS.
2.4. Preparation of stock and working standard solutions
Stock solutions of 2-oxo-clopidogrel (10 μg/mL) and the IS (1 mg/mL) were separately prepared in acetonitrile, and then stored at −20 °C. Appropriate dilutions were made in acetonitrile to produce a series of working standard solutions of 5, 10, 20, 80, 100, 200, 300, 400 and 500 ng/mL for 2-oxo-clopidogrel and 10 μg/mL for IS. All the working standard solutions for 2-oxo-clopidogrel and IS were freshly prepared when needed.
2.5. Sample preparation
An aliquot of 400 μL human blank plasma in a 10 mL glass centrifugation tube containing 50 μL DTT (0.02 M) was spiked with 40 μL of 2-oxo-clopidogrel and 40 μL of the IS solutions (10 μg/mL). After 100 μL of HCl (0.05 M) was added and the solution was vortex-mixed for 10 s, 1.5 mL MTBE was added and vortex-mixed for 3 min. An aliquot of 1.2 mL of the supernatant was taken after centrifuged at 2000g for 10 min and 50 μL DTT (0.02 M) was added again, and then, evaporated to dryness under vacuum. The residual was reconstituted in 150 μL of a mobile phase of A-acetonitrile and B-water containing 0.1% (v/v) formic acid (95:5) and centrifuged at 16,000g for 10 min. Then, 30 μL aliquot was injected onto the LC−MS/MS system. For optimal stability, the auto-sampler temperature was set at 4 °C.
2.6. Method validation
The LC−MS/MS method was validated according to the Food and Drug Administration guidelines for bioanalytical method validation. The method was validated in terms of selectivity, linearity, the lower limit of quantification (LLOQ), accuracy, precision, extraction efficiency, matrix effect and stability.
Selectivity was performed using six different sources of blank plasma. They were processed by the same extraction procedure and analyzed to determine the extent to which endogenous plasma components may contribute to the interference at the retention time of 2-oxo-clopidogrel and the IS.
Linearity of the calibration curves assessed in duplicate on three consecutive days was estimated for the ratio of peak area of 2-oxo-clopidogrel to that of the IS as a function of the concentration of 2-oxo-clopidogrel covering the range of 0.50−50.0 ng/mL in plasma using 1/x2 as a weighting factor (where x is the concentration of 2-oxo-clopidogrel). The correlation coefficients of the calibration curves had been determined. The equations of calibration curves were used to calculate concentration of 2-oxo-clopidogrel in human plasma.
LLOQ was determined by the method with the accuracy (expressed as percentage of the nominal concentration) within 80–120% and precision (expressed as relative standard deviation, %RSD) not exceeding 20%. The intra-day accuracy and precision were evaluated by analyzing six replicates at low QC (1.00 ng/mL), medium QC (20.0 ng/mL), and high QC (40.0 ng/mL) levels of the same day. The inter-day accuracy and precision were determined by analyzing the respective QCs in three different days.
Stability of 2-oxo-clopidogrel in plasma was evaluated at concentrations of 2.00 and 50.0 ng/mL in three replicates for each concentration under different storage conditions. Sample stability was tested by analyzing QCs after three freeze-thaw cycles as well as short-term storage (plasma samples with DTT added and post-preparative samples kept at room temperature for 8 h) and long-term storage (45 days at −80 °C). Moreover, the stability of 2-oxo-clopidogrel in post-preparative samples in the auto-sampler at 4 °C for 24 h and stability of stock solutions at −20 °C for 2 months were also analyzed.
The extraction efficiency was evaluated using plasma samples of the calibration curves at concentrations of 1.00, 20.0 and 40.0 ng/mL. The reference samples of extraction efficiency were prepared by adding working solutions of 2-oxo-clopidogrel and IS to blank plasma extracts. To investigate the matrix effect, the reference samples of matrix effect were compared to reference solutions without matrix.
2.7. Application of the method to pharmacokinetic study
A pharmacokinetic study was conducted in eight human subjects. This study complied with the revised Declaration of Helsinki for biomedical research involving human subjects and the rules of good clinical practice (GCP), and was approved by the Research Ethics Board of Xijing Hospital. Informed consent was obtained from all human subjects. After the human subjects were recruited, each was administered a tablet of Plavix containing 75 mg clopidogrel with 250 mL water within half an hour after breakfast. Ethylenediamineteraacetic acid (EDTA) solution was used as an anti-coagulant and DTT was used as a reducing agent. Three milliliters venous blood was collected before Plavix administration and at 0.5, 1, 2, 2.5, 3, 3.5, 6, 8, 12 and 24 h after dosing. The plasma samples were separated by centrifugation at 16,000g for 10 min and frozen at −80 °C until assayed for 2-oxo-clopidogrel.
3. Results and discussion
3.1. Sample preparation and LC−MS/MS analysis
2-Oxo-clopidogrel has the tendency to be oxidized to the sulfoxide or sulfone without a reducing agent owing to the nature of sulfur atom in 2-oxided thiolactone. Our study showed that it was necessary to add a reducing agent to maintain 2-oxo-clopidogrel in an un-oxidized state during sample collection and preparation. At first, 2-oxo-clopidogrel could not be detected even in standard samples without any antioxidant added in plasma. Then several antioxidants were tested, and ascorbate showed some antioxidant capability. However, the data were still not stable enough, which were the same as what was found in the homocysteine thiolactione stability study [20]. For the thiolactone bioanalytic assay, it was reported that adding DTT in sample preparation process could make the analytes erdosteine and its metabolite stable [21]. DTT was tried, and then no significant degradation was found in stability test. However, preliminary experiment data showed that 2-oxo-clopidogrel could be oxidized to a certain extent if not adding antioxidant immediately after collection or not freezing the plasma sample right away (date not shown). Consequently, to ensure the stability of 2-oxo-clopidogrel during sample processing and storage, the antioxidant DTT was added into the tubes prior to sample collection.
The liquid chromatography method development began with optimizing the mobile phase composition and column type. The Sapphire C18 column (250 mm ×4.6 mm×5 μm) provided good selectivity and peak shape for both 2-oxo-clopidogrel and IS, as compared to other columns. The mobile phase consisting of A-acetonitrile and B-water containing 0.1% (v/v) formic acid (A:B=95:5) with a flow rate of 1 mL/min was found to be suitable during LC optimization. The retention times for 2-oxo-clopidogrel and IS were observed at 3.8 min and 5.0 min, respectively. Mifepristone was chosen as the internal standard for the determination of 2-oxo-clopidogrel in human plasma thanks to its similar polarity and stable ionization rate without interfering.
In the MS method development, full-scan mass spectra of both 2-oxo-clopidogrel and IS were obtained in the positive ion mode with ESI source. Quantifications were performed by using MRM with ion transitions of m/z 338.1→183.1 for 2-oxo-clopidogrel and m/z 430.0→372.0 for IS, which could further improve the sensitivity.
3.2. Method validation
3.2.1. Selectivity
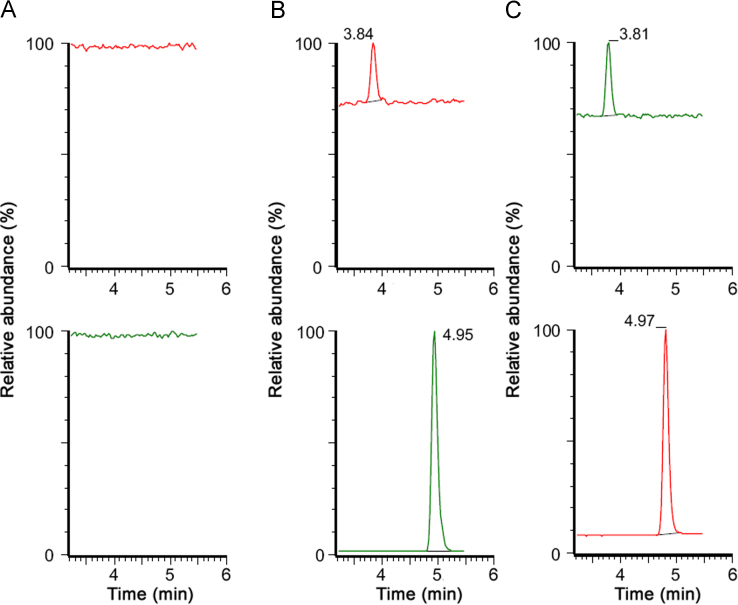
Fig. 3 depicts representative chromatograms resulting from the LC−MS/MS analysis of extracts of 400 μL plasma from: (A) blank plasma sample, (B) spiked LLOQ (0.50 ng/mL, tR=3.8 min) and IS (1 μg/mL, tR=5.0 min), and (C) plasma sample collected from a subject at 2 h elapsed from administration of a tablet with 75 mg clopidogrel. Furthermore, no carryover was observed for 2-oxo-clopidogrel or IS by running a blank sample following the upper limit of quantification (ULOQ: 50.0 ng/mL for 2-oxo-clopidogrel), without detecting 2-oxo-clopidogrel or IS peak at their respective retention.
Fig. 3.
LC−MS/MS of (A) Blank plasma extract, (B) Spiked LLOQ (0.5 ng/mL, tR=3.8 min) and mifepristone (1 μg/mL, tR=5.0 min), and (C) Plasma sample collected from a subject at 2 h elapsed from administration of 75 mg clopidogrel(6.50 ng/mL 2-oxo-clopidogrel and 1 μg/mL mifepristone).
3.2.2. Linearity
The calibration curve ranged from 0.50 to 50.0 ng/mL, with a typical equation for the calibration curve being y=0.00648x+0.000257 (r>0.997). For each calibration standard level, the concentration was back calculated from the calibration equation. The mean accuracy and precision for back calculated concentrations of each standard calculated from calibration curves are tabulated in Table 1.
Table 1.
The accuracy and precision from calibration curvesa.
| Nominal conc. (ng/mL) | Mean accuracy (%) | Precision (%RSD) |
|---|---|---|
| 0.50 | 102.7 | 2.9 |
| 1.00 | 93.0 | 3.4 |
| 2.00 | 104.6 | 7.9 |
| 8.00 | 97.0 | 11.0 |
| 10.0 | 96.2 | 6.1 |
| 20.0 | 100.5 | 2.5 |
| 30.0 | 102.8 | 7.5 |
| 40.0 | 103.3 | 6.7 |
| 50.0 | 96.1 | 4.3 |
n=6.
3.2.3. LLOQ
Independent LLOQ experiments were performed by preparing the LLOQ in six different lots of plasma. The LLOQ was 0.50 ng/mL of 2-oxo-clopidogrel. The accuracy for back calculated concentrations of LLOQ is tabulated in Table 2.
Table 2.
The accuracy and precision from LLOQsa.
| LLOQ conc. (ng/mL) | Measured conc. (ng/mL) | Accuracy (%) |
|---|---|---|
| 0.50 | 0.584 | 116.8 |
| 0.50 | 0.518 | 103.6 |
| 0.50 | 0.539 | 107.8 |
| 0.50 | 0.570 | 114.0 |
| 0.50 | 0.575 | 115.0 |
| 0.50 | 0.525 | 105.0 |
n=6.
3.2.4. Accuracy and precision
The intra- and inter-day accuracies were 102.7–105.8% and 97.2–106.0%, respectively, and the intra- and inter-day assay precisions were 4.0–5.8% and 6.4–14.2%, respectively.
3.2.5. Extraction recovery and matrix effect
The extraction recoveries in human plasma were 92.5±3.9%, 114.0±3.5% and 110.9±7.8% at 1.00, 20.0 and 40.0 ng/mL (n=6), respectively. For the IS, the extraction recovery at 1 μg/mL was 111.4±8.2% (n=18). The mean matrix effects of 2-oxo-clopidogrel from six different plasma sources at three concentration levels were 63.7%, 71.6% and 63.7%, respectively. For the IS, the matrix effect was 77.0±9.4% (n=18). The extraction recovery and matrix effect for 2-oxo-clopidogrel were consistent when examined repeatedly at the same concentration or at variant concentrations.
3.2.6. Stability
Stock solutions of 2-oxo-clopidogrel and IS in acetonitrile were stable at −20 °C for at least 2 months. In human plasma, no significant degradation of 2-oxo-clopidogrel occurred under the following conditions: three freeze–thaw cycles, plasma samples with DTT on the bench top at room temperature for 8 h and at −80 °C for 45 days, post-preparative samples at room temperature for 8 h, in auto-sampler at 4 °C for 24 h. The stability test results are displayed in Table 3.
Table 3.
Stability of 2-oxo-clopidogrel in human plasma.
| Stability experiments | Mean measured conc. (n=3) |
Mean % RE from theoretical |
||
| La (ng/mL) | Hb (ng/mL) | La(ng/mL) | Hb (ng/mL) | |
| Long-term stability (45 days) | 1.96 | 51.9 | −2.3 | 3.8 |
| Freeze-thaw stability (3rd cycles) | 1.84 | 50.9 | −8.0 | 1.8 |
| Bench top stability(8 h) | 2.29 | 52.9 | 14.5 | 5.8 |
| Post-preparative stability(8 h) | 2.12 | 48.1 | 6.0 | −3.8 |
| Auto-sampler stability(24 h) | 2.25 | 47.4 | 12.5 | −5.2 |
L means low concentration in human plasma (nominal concentration is 2.00 ng/mL).
H means high concentration in human plasma (nominal concentration is 50.0 ng/mL).
3.3. Application of the developed LC−MS/MS method
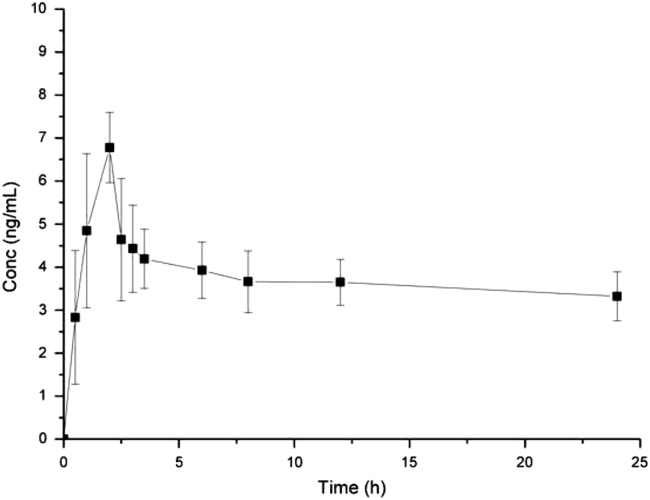
In order to verify the sensitivity and selectivity of the developed method in a real-time situation, the method described in this paper was applied to a human pharmacokinetic that generated over 8 human plasma samples. After oral administration of a tablet containing 75 mg clopidogrel to the eight subjects, the mean plasma concentration-time profile of 2-oxo-clopidogrel is depicted in Fig. 4 and the pharmacokinetic data of 2-oxo-clopidogrel are presented in Table 4.
Fig. 4.
Plasma concentration-time profile (mean±SD, n=8) of 2-oxo-clopidogrel after an administration of 75 mg Plavix in human subjects.
Table 4.
Pharmacokinetic data of 2-oxo-clopidogrel after their oral administration in 8 subjects.
| Parameter | 2-Oxo-clopidogrel |
|---|---|
| Cmax (ng/mL) | 6.8±0.9 |
| Tmax (h) | 1.8±3.4 |
| t1/2 (h) | 65±40 |
| AUC0–24(ng h/mL) | 89±10 |
| AUC0−∞ (ng h/mL) | 383±180 |
Cmax: maximum plasma concentration.
Tmax: time point of maximum plasma concentration.
t1/2: half life of drug elimination during the terminal phase.
AUC0–24: area under the plasma concentration–time curve from zero to 24 h.
AUC0–∞: area under the plasma concentration–time curve from zero hour to infinity.
4. Conclusion
The LC−MS/MS method has been successfully set up for the pharmacokinetic study of Plavix tablets in human subjects. The established LC−MS/MS method is sensitive for plasma 2-oxo-clopidogrel determination and suitable for the pharmacokinetic study of Plavix tablets. Because of the relative short chromatographic run time (5.5 min), the method is easy to follow and can be adopted for clinical drug monitoring.
Footnotes
Peer review under responsibility of Xi׳an Jiaotong University.
References
- 1.Braun O.Ö., Angiolillo D.J., Ferreiro J.L. Enhanced active metabolite generation and platelet inhibition with prasugrel compared to clopidogrel regardless of genotype in thienopyridine metabolic pathways. Thromb. Haemost. 2013;6:1223–1231. doi: 10.1160/TH13-03-0263. [DOI] [PubMed] [Google Scholar]
- 2.Kim M.J., Jeong E.S., Park J.S. Multiple cytochrome P450 isoforms are involved in the generation of a pharmacologically active thiol metabolite, whereas paraoxonase 1 and carboxylesterase 1 catalyze the formation of a thiol metabolite isomer from ticlopidines. Drug Metab. Dispos. 2014;42:141–152. doi: 10.1124/dmd.113.053017. [DOI] [PubMed] [Google Scholar]
- 3.Heestermans A.A.C.M., Werkum J.M.V., Schömig E. Clopidogrel resistance caused by a failure to metabolize clopidogrel into its metabolites. Thromb. Haemost. 2006;4:1143–1145. doi: 10.1111/j.1538-7836.2006.01891.x. [DOI] [PubMed] [Google Scholar]
- 4.Tang M., Mukundan M., Yang J. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J. Pharmacol. Exp. Ther. 2006;319:1467–1476. doi: 10.1124/jpet.106.110577. [DOI] [PubMed] [Google Scholar]
- 5.P. Yerino, P. Toren, B. Ogilvie, et al., Unlike gemfibrozil glucuronide, clopidogrel glucuronide is not a potent inhibitor of CYP2C8, in: Proceedings of the 14th North American ISSX Meeting, Rio Grande, Puerto Rico, 2006.
- 6.Hagihara K., Kazui M., Ikenaga H. Comparison of formation of thiolactones and active metabolites of prasugrel and clopidogrel in rats and dogs. Xenobiotica. 2009;39:218–226. doi: 10.1080/00498250802650077. [DOI] [PubMed] [Google Scholar]
- 7.Brandt J.T, Payne C.D., Wiviott S.D. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am. Heart J. 2007;153:66.e9–66.e16. doi: 10.1016/j.ahj.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Hagihara K., Nishiya Y., Kurihara A. Comparison of human cytochrome P450 inhibition by the thienopyridines prasugrel, clopidogrel, and ticlopidine. Drug Metab. Pharmacokinet. 2007;23:412–420. doi: 10.2133/dmpk.23.412. [DOI] [PubMed] [Google Scholar]
- 9.Lainesse A., Ozalp Y., Wong H. Bioequivalence study of clopidogrel bisulfate film-coated tablets. Arzneimittelforschung. 2004;54:600–604. doi: 10.1055/s-0031-1297056. [DOI] [PubMed] [Google Scholar]
- 10.Taubert D., Kastrati A., Harlfinger S. Pharmacokinetics of clopidogrel after administration of a high loading dose. Thromb. Haemost. 2004;92:311–316. doi: 10.1160/TH04-02-0105. [DOI] [PubMed] [Google Scholar]
- 11.Patel N.K., Subbaiah G., Shah H. Rapid LC–ESI-MS–MS method for the simultaneous determination of clopidogrel and its carboxylic acid metabolite in human plasma. J. Chromatogr. Sci. 2008;46:867–875. doi: 10.1093/chromsci/46.10.867. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.D., Kang W., Lee H.W. Bioequivalence and tolerability of two clopidogrel salt preparations, besylate and bisulfate: a randomized, open-label, crossover study in healthy Korean male subjects. Clin. Ther. 2009;31:793–803. doi: 10.1016/j.clinthera.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Zou J.J., Fan H.W., Guo D.Q. Simultaneous determination of clopidogrel and its carboxylic acid metabolite (SR26334) in human plasma by LC–ESI-MS–MS: application to the therapeutic drug monitoring of clopidogrel. Chromatographia. 2009;70:1581–1586. [Google Scholar]
- 14.Reddy S.R., Divi K.R., Sarath C.I. Development and validation of high-throughput liquid chromatography–tandem mass spectrometric method for simultaneous quantification of Clopidogrel and its metabolite in human plasma. J. Chromatogr. B. 2010;878(3):502–508. doi: 10.1016/j.jchromb.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Peer C.J., Spencer S.D., VanDenBerg D.A. A sensitive and rapid ultra HPLC–MS/MS method for the simultaneous detection of clopidogrel and its derivatized active thiol metabolite in human plasma. J. Chromatogr. B. 2012;880:132–139. doi: 10.1016/j.jchromb.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karaz´niewicz-Łada M., Danielak D., Teźyk A. HPLC–MS/MS method for the simultaneous determination of clopidogrel, its carboxylic acid metabolite and derivatized isomers of thiol metabolite in clinical samples. J. Chromatogr. B. 2012;911:105–112. doi: 10.1016/j.jchromb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Silvestro L., Gheorghe M., Iordachescu A. Development and validation of an HPLC–MS/MS method to quantify clopidogrel acyl glucuronide, clopidogrel acid metabolite, and clopidogrel in plasma samples avoiding analyte back-conversion. Anal. Bioanal. Chem. 2011;401:1023–1034. doi: 10.1007/s00216-011-5147-4. [DOI] [PubMed] [Google Scholar]
- 18.Chen M., Zhang Y., Que X.T. Pharmacokinetic study of inosiplex tablets in healthy Chinese volunteers by hyphenated HPLC and tandem MS techniques. J. Pharm. Anal. 2013;3:387–393. doi: 10.1016/j.jpha.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X.J., Sun Y.T., Yin L. Quantitation of bivalirudin, a novel anticoagulant peptide, in human plasma by LC–MS/MS: method development, validation and application to pharmacokinetics. J. Pharm. Anal. 2013;3:1–8. doi: 10.1016/j.jpha.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora B., Narayanasamy A., Nirmal J. Development and validation of a LC–MS/MS method for homocysteine thiolactone in plasma and evaluation of its stability in plasma samples. J. Chromatogr. B. 2014;944:49–54. doi: 10.1016/j.jchromb.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Jin J., Chen X.Y., Zhang Y.F. Simultaneous determination of erdosteine and its active metabolite in human plasma by liquid chromatography−tandem mass spectrometry with pre-column derivatization. Yaoxue Xuebao. 2013;48(3):395–400. [PubMed] [Google Scholar]