Abstract
Context:
Polycystic ovary syndrome (PCOS) and adolescent hyperandrogenism (HA) are characterized by rapid luteinizing hormone (LH) pulse frequency. This partly reflects impaired gonadotropin-releasing hormone pulse generator (hypothalamic) sensitivity to progesterone (P4) negative feedback. We assessed whether metformin may improve P4 sensitivity in adolescent HA, for which it is prescribed widely.
Objective:
To test the hypothesis that metformin improves hypothalamic P4 sensitivity in adolescent HA.
Design:
Nonrandomized, interventional trial.
Setting:
Academic clinical research unit.
Participants:
Ten adolescent girls with HA.
Intervention:
The girls underwent LH sampling every 10 minutes for 11 hours, at study baseline and after 7 days of oral P4 and estradiol (E2). Participants then took metformin (1 g twice daily) for 9.4 to 13.7 weeks, after which participants again underwent frequent LH sampling before and after 7 days of oral P4 and E2 (while continuing metformin). Total and free testosterone (T) and fasting insulin were assessed at each admission. At admissions 1 and 3, 2-hour oral glucose tolerance tests were performed.
Main Outcome Measure:
Metformin-related change in hypothalamic P4 sensitivity index [percent change in LH pulse frequency (before vs after P4 and E2) divided by day 7 P4 level].
Results:
Free T levels decreased by 29% with metformin (P = 0.0137). Measures of hyperinsulinemia and P4 sensitivity index did not significantly change with metformin use.
Conclusion:
Short-term metformin use improved biochemical hyperandrogenemia, but did not improve hypothalamic sensitivity to P4 suppression, in adolescent girls.
Ten girls with hyperandrogenism received metformin for 3 months. Hyperandrogenemia decreased without improvement in hypothalamic sensitivity to progesterone or in measures of insulin resistance.
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism (HA; clinical and/or biochemical), oligo- or anovulation, and polycystic ovarian morphology (1). Women with PCOS demonstrate elevated luteinizing hormone (LH) pulse frequency, which promotes LH excess and relative follicle-stimulating hormone (FSH) deficiency; these gonadotropin abnormalities, in turn, contribute to hyperandrogenemia and ovulatory dysfunction (2). Similar neuroendocrine abnormalities have been described in adolescent HA (3), which can represent a precursor to adult PCOS.
Progesterone (P4) is the primary inhibitor of gonadotropin-releasing hormone (GnRH) pulse frequency (4). Prior studies have demonstrated relative GnRH pulse generator resistance to P4 negative feedback in PCOS. For example, Pastor et al. (5) demonstrated that 7 days of exogenous P4 and estradiol (E2), which achieved physiologic luteal concentrations, suppressed LH pulse frequency by 60% in normal women but by only 25% in adult PCOS. Similar abnormalities have been observed in adolescent HA (6, 7). This defect appears to reflect hyperandrogenemia, because sensitivity to P4 negative feedback can be normalized in adult PCOS with androgen-receptor blockade by flutamide (8).
Metformin has been extensively studied as a treatment of PCOS in animal models and humans. In prenatally androgenized female mice, metformin restored regular menses and normalized both GnRH neuronal firing rates and LH levels (9). Our group previously assessed the effect of short-term metformin use on hypothalamic P4 sensitivity in adults with PCOS (10); despite reductions in both testosterone (T) and insulin, metformin did not improve hypothalamic P4 sensitivity. However, results of a study in adolescent girls with HA suggested hypothalamic insensitivity to P4 suppression correlated with higher fasting insulin levels (7). Moreover, it is possible that metformin is more effective when administered earlier in life, before the pathophysiology of PCOS is fully established. Therefore, we posited metformin would improve hypothalamic sensitivity to P4-negative feedback when administered to adolescent girls with HA.
Participants and Methods
Participants
Postmenarcheal adolescent girls (N = 10) were studied at the University of Virginia (UVA). Eligible participants were healthy girls aged 10 to 17 years with HA (defined by hirsutism and/or morning free T level more than two standard deviations above the mean for our previously studied cohort of normal-weight [body mass index (BMI)-for-age percentile, 5 to 85] control participants of the same Tanner stage) (11). Specific free T cutoffs relevant to the current study were 29.8 pmol/L (at Tanner 4) and 31.2 pmol/L (at Tanner 5). Exclusion criteria included congenital adrenal hyperplasia, hyperprolactinemia, and hypothyroidism; abnormal screening laboratory study results; and use of medications known to affect the reproductive axis within 90 days before the study. Participant characteristics are shown in Table 1.
Table 1.
Participant Baseline Characteristics
| Participant No. | Self-Identified Race or Ethnicity | Age (y) | BMI% | Weight (kg) | Tanner Breast Stage | Years After Menarche | Total T (ng/dL) | Free T (pmol/L) | Fasting Insulin (uIU/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | W | 15.0 | 93 | 63.0 | 5 | 4.0 | 53.9 | 25.9 | 2.0 |
| 2 | W | 17.5 | 97 | 92.0 | 5 | 8.0 | 32.9 | 27.5 | 20.0 |
| 3 | W | 15.7 | 98 | 118.0 | 5 | 2.0 | 29.0 | 26.6 | 50.0 |
| 4 | B/W | 15.0 | 98 | 77.0 | 5 | 2.0 | 82.2 | 84.4 | 22.5 |
| 5 | B/W | 14.5 | 98 | 96.0 | 5 | 3.0 | 63.8 | 67.0 | 22.8 |
| 6 | W | 16.8 | 98 | 108.0 | 5 | 2.0 | 44.5 | 45.0 | 39.2 |
| 7 | H | 13.0 | 99 | 96.4 | 5 | 0.8 | 28.0 | 29.8 | 24.8 |
| 8 | W | 16.0 | 98 | 84.1 | 4 | 6.0 | 16.4 | 5.0 | 20.3 |
| 9 | W | 15.1 | 96 | 69.5 | 5 | 4.0 | 58.9 | 35.8 | 7.5 |
| 10 | W | 14.9 | 98 | 92.0 | 5 | 2.0 | 35.0 | 33.5 | 52.6 |
| Mean ± SD | 15.3 ± 1.2 | 97.3 ± 1 | 89.6 ± 16.0 | 4.9 ± 0.3 | 3.3 ± 2.3 | 44.5 ± 20.0 | 38.0 ± 22.6 | 26.2 ± 16.6 |
Abbreviations: B, black; BMI%, BMI percentile for age; H, Hispanic; SD, standard deviation; W, white.
Study procedures
Study procedures were approved by the Institutional Review Board of UVA. Informed assent and consent were obtained from all participants and their custodial parents, respectively. To ensure general good health and the absence of unexpected hormone levels, all participants underwent a detailed, physician-obtained medical history and physical examination, which included breast examination for pubertal Tanner staging, in addition to fasting laboratory evaluation, as previously described (12).
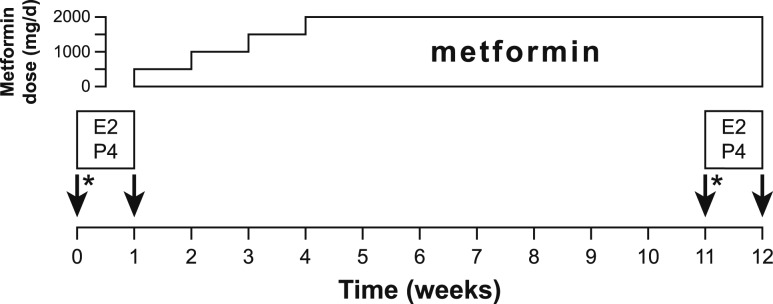
A study schematic is presented in Fig. 1. Immediately before all overnight admissions, participants’ urine samples were determined to be negative for human chorionic gonadotropin. Girls with regular menses were admitted for initial overnight study from day 7 to 11 (inclusive) of the menstrual cycle, and girls with fewer than 10 menses per year were admitted on or after day 7 of the cycle. Serum P4 was assessed within 3 days before admission; if the P4 level was >1.5 ng/mL, the admission was rescheduled. Participants were given instructions to eat a diet containing >150 g of carbohydrate per day for the 3 days preceding admission. Participants were admitted to the clinical research unit (CRU) at 5:00 pm. Frequent blood sampling began at 7:00 pm through an indwelling intravenous catheter in the forearm. LH was measured every 10 minutes; FSH was measured hourly; and P4, E2, and total T were measured every 2 hours. Lights were extinguished and participants were encouraged to sleep from 11:00 pm to 7:00 am. Participants wore a wrist actigraph to estimate periods of sleep (Motionlogger Basic-L; Ambulatory Monitoring, Ardsley, NY). Participants fasted from 11:00 pm until completion of blood sampling at 9:00 am. Every 10-minute blood sampling concluded at 6:00 am, but blood was obtained at 7:00 am for P4, E2, total T, sex hormone–binding globulin (SHBG), and dehydroepiandrosterone (DHEAS). At 7:00 am, a 2-hour oral glucose tolerance test (OGTT) began with administration of 75 g of glucose; blood was sampled to measure glucose and insulin levels at 0, 10, 20, 30, 60, 90, and 120 minutes.
Figure 1.
Study schematic. Arrows indicate overnight CRU admissions for frequent blood sampling to measure LH levels. Participants took E2 (1 mg daily) and P4 (0.5 mg/kg three times daily) between admissions 1 and 2 and again between admissions 3 and 4. After admission 2, the metformin dose was increased weekly by 500 mg/d until participants were using 1000 mg twice daily. *75 g OGTT performed at 7:00 am on the days of discharge from admissions 1 and 3.
At discharge from admission 1, participants were supplied with an oral P4 suspension (0.5 mg/kg three times daily at 7:00 am, 3:00 pm, and 11:00 pm) and oral micronized E2 (Estrace, 1 mg once daily; Apotex Corp., Weston, FL) to be used for 7 days. E2 was administered to standardize serum E2 levels and ensure adequate hypothalamic P4 receptor expression. The P4 suspension was prepared by the UVA investigational pharmacy, as previously described (10). Participants were instructed to take P4 with food to help standardize absorption patterns. On days 3 and 5 of P4 and E2 use, participants had blood drawn at 5:00 pm to assess serum P4 and E2 levels. On day 3, hemoglobin also was assessed to ensure safety of a frequent sampling protocol on day 7.
The second CRU admission occurred on day 7 of P4 and E2 administration, with procedures identical to those of admission 1, with the exception that OGTT was not performed.
On the day of discharge from admission 2, participants began use of metformin 500 mg daily (Barr Pharmaceuticals, Inc., Montvale, NJ) and were instructed to escalate the total daily dose by 500 mg every 7 days until achieving the target dose of 1000 mg twice daily. During the titration process, participants were contacted for a weekly reminder of each dose increase and to assess for adverse events. Between 35 and 42 days after discharge from admission 2, participants came to the clinic to have blood drawn for a comprehensive metabolic panel (as a safety measure).
The third CRU admission occurred after an average of 11.7 (range, 9.4 to 13.7) weeks of metformin use. The CRU protocol for admission 3 was identical to that of admission 1, including cycle stage requirements, preadmission P4 assessments and dietary instructions, and OGTT.
Upon discharge from admission 3, participants resumed the same doses of P4 and E2 that were given after admission 1, and metformin use was continued. Again on days 3 and 5 of P4 and E2 use, participants had blood drawn at 5:00 pm for P4 and E2 measurements, and hemoglobin was measured on day 3 for anemia screening. The fourth CRU admission occurred on day 7 of P4 and E2 use and followed a protocol identical to that of admission 2. Participants were instructed to discontinue all study medications at the conclusion of admission 4.
Hormonal measurements
UVA’s Center for Research in Reproduction Ligand Core Laboratory performed all hormone assays. Each participant’s samples were analyzed in duplicate in the same hormonal assay. Assay sensitivities, intra- and interassay coefficients of variation, and manufacturers for all hormonal measurements have been previously reported (13). For each time point, we used both LH values for the purposes of LH pulse detection (described later in this article); otherwise, the mean of the duplicate assays was used for data analysis. When measured values were below assay sensitivity, they were assigned the value of the assay’s sensitivity. Free T was calculated from total T and SHBG levels (14).
Formulae to convert conventional to international units are as follows: total T = (ng/dL) × 3.467 (nmol/L); free T = (pg/mL) × 3.467 (pmol/L); P4 = (ng/mL) × 3.18 (pmol/L); E2 = (pg/mL) × 3.671 (pmol/L); DHEAS = (µg/dL) × 27.211 (nmol/L); SHBG = (µg/mL) × 8.896 (nmol/L); insulin = (µIU/mL) × 7.175 (pmol/L); and glucose = (mg/dL) × 0.0555 (mmol/L).
Data analysis
BMI-for-age percentiles were calculated as previously described (15). Obesity was defined as BMI-for-age percentile ≥95, and overweight was defined as BMI-for-age percentile ≥85 but <95. LH pulses were identified using the computerized pulse detection algorithm Cluster 7, as previously described (12, 16, 17).
Statistical analyses
The primary outcome variable was the P4 sensitivity index, which we defined as the percent change in LH pulse frequency (i.e., 11-hour LH pulse count before vs after P4 and E2 administration for 7 days), divided by the day 7 mean serum P4 concentration, as assessed during the overnight CRU admission (7). As the primary analysis, we compared the P4 sensitivity index after metformin administration to the baseline P4 sensitivity index, using the Wilcoxon signed-rank test, which is a paired difference test based on ranks of observations and requires no assumptions about underlying data distribution.
Secondary analyses included Spearman (nonparametric) correlation procedures to evaluate whether baseline characteristics correlated with baseline P4 sensitivity index. We also used Wilcoxon signed-rank tests to assess for metformin-related changes (i.e., between CRU admissions 1 and 3) in free and total T levels, SHBG levels, fasting insulin levels, and fasting homeostatic method assessment of insulin resistance (HOMA-IR), in addition to metformin-related changes in OGTT-related parameters such as average insulin and glucose levels during OGTTs. Last, we used Spearman correlation to assess whether metformin-related changes in selected characteristics (e.g., free T level) correlated with metformin-related changes in P4 sensitivity index.
Two participants (participants 2 and 7) reported incomplete adherence to metformin, missing >10% of doses. We repeated analyses after exclusion of these two participants. Results were not altered, so we only report results from analyses including all participants.
Unless otherwise stated, data are presented as mean ± standard deviation. For all tests, a two-sided P value ≤ 0.05 was used as the null hypothesis rejection rule. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Participants’ baseline characteristics are presented in Table 1. Nine girls were obese and one was overweight. Only one participant (participant 2) reported regular menses. Two participants (1, 9) were not hyperinsulinemic at baseline; of note, these participants also had the lowest body weights of the group (63 and 69 kg).
Effect of metformin on hypothalamic sensitivity to P4 negative feedback
Before metformin treatment, LH pulse frequency decreased from 9.5 ± 1.4 [median, 10; interquartile range (IQR), 9 to 10] pulses per 11 hours in admission 1 to 6.4 ± 2.2 (median, 7; IQR, 5 to 8) pulses per 11 hours in admission 2 (P = 0.0020, admission 1 vs 2). Achieved P4 and E2 concentrations during admission 2 were 8.9 ± 6.3 (median, 6.1; IQR, 5.5 to 8.3) ng/mL and 173 ± 71 (median, 161; IQR, 120 to 214) pg/mL, respectively. Baseline (premetformin) P4 sensitivity index did not significantly correlate with the following baseline characteristics: weight; BMI; BMI-for-age percentile; total T, free T, and fasting insulin levels; HOMA-IR; or mean insulin levels during OGTT.
Participants used metformin for a total of 11.7 (range, 9.4 to 13.7) weeks between the second and third admissions. After metformin treatment, LH pulse frequency decreased from 8.5 ± 2.5 (median, 9; IQR, 8 to 10) pulses per 11 hours in admission 3 to 6.0 ± 2.4 (median, 6; IQR, 5 to 7) pulses per 11 hours in admission 4 (P = 0.0293, admission 3 vs 4). Achieved P4 and E2 concentrations during admission 4 were 7.5 ± 4.7 (median, 5.0; IQR, 3.7 to 11.3) ng/mL and 131 ± 59 (median, 114; IQR, 87 to 168) pg/mL, respectively. LH pulse frequencies were similar in admissions 1 and 3 (P = 0.3750), suggesting that metformin alone did not alter basal LH pulse frequency. In addition, achieved P4 and E2 levels were not demonstrably different between admissions 2 and 4 (P = 0.2031 and 0.0840, respectively).
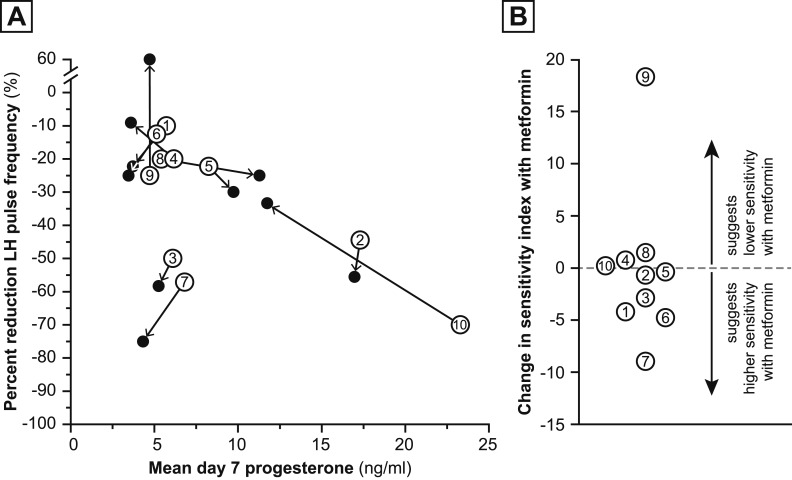
Figure 2A presents the percent changes in LH pulse frequency (before vs after P4 and E2 administration for 7 days) as a function of achieved plasma P4 level on day 7, which reflects GnRH pulse generator sensitivity to P4 negative feedback. In Fig. 2A, pre- and postmetformin P4 sensitivity indices for a single individual are connected by arrows pointing from baseline to postmetformin assessments. Overall, the P4 sensitivity index (percent change in LH pulse frequency divided by P4 concentration) before metformin use was −4.12 ± 2.39 (median, −3.13; IQR, −5.29 to −2.57], and P4 sensitivity index after metformin use was −4.29 ± 7.66 (median, −3.18; IQR, −7.20 to −2.51). The metformin-related change in P4 sensitivity index was not significant [−0.16 ± 7.11 (median,−0.55; IQR, −4.23 to 0.74); P = 0.4922]. Figure 2B presents the metformin-related change in P4 sensitivity index for each participant.
Figure 2.
Metformin-related changes in P4 sensitivity index. (A) Open circles represent the numbered participant’s hypothalamic sensitivity to P4 suppression at baseline (premetformin); that is, percent reduction in LH pulse frequency at admission 2 compared with admission 1 (y-axis) as a function of achieved P4 concentration assessed during admission 2 (x-axis). Closed circles represent the participant’s hypothalamic sensitivity to P4 suppression after metformin; that is, percent reduction in pulse frequency (admission 4 vs admission 3) as a function of achieved P4 concentration during admission 4. Arrows connecting these circles indicate the directionality of the change in pulse frequency after metformin treatment of an individual participant. Participant 9 had a 60% increase in LH pulse frequency. (B) Change in P4 sensitivity index with metformin treatment. Each participant’s metformin-related change in P4 sensitivity index (pre- vs postmetformin treatment) is represented by an open circle containing the participant’s number. The dashed line indicates no change in sensitivity.
Metformin-related changes in P4 sensitivity index did not correlate with metformin-related changes in weight, BMI; BMI-for-age percentile; total T, free T, and fasting insulin levels; HOMA-IR; or mean insulin level during OGTT.
Effect of metformin on secondary outcomes
Metformin-related changes (admission 1 vs admission 3) in mean LH concentrations, LH pulse amplitude, and mean FSH concentrations were not significant (P > 0.3 for all).
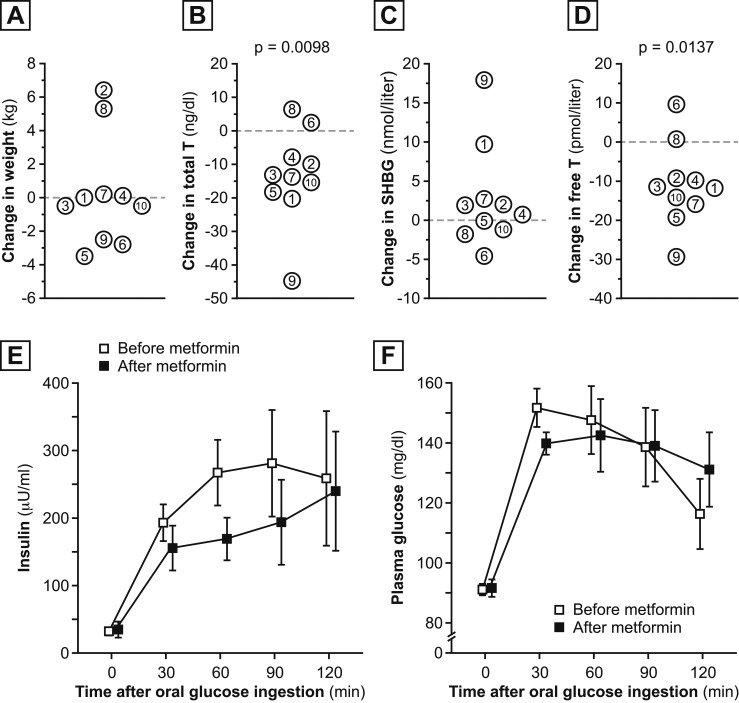
Participant weight was 89.6 ± 16.8 kg at baseline and 89.8 ± 16.8 kg after metformin use [Fig. 3(A)]. Metformin use was associated with significant decreases in total T level (44 ± 20 ng/dL in admission 1 vs 31 ± 19 ng/dL in admission 3; P = 0.0098; Fig. 3(B)] and free T level [38 ± 23 pmol/L in admission 1 vs 27 ± 23 pmol/L in admission 3; P = 0.0137; Fig. 3(D)]. SHBG [Fig. 3(C)], androstenedione, and DHEAS levels did not change with metformin use (P > 0.25 for all).
Figure 3.
Metformin-related changes in selected parameters. Metformin-related changes in (A) weight, (B) total T, (C) SHBG, and (D) free T levels. Metformin-related changes in (E) insulin and (F) glucose responses to oral glucose ingestion. Data from admission 1 (mean ± standard error of the mean) are shown in open squares; data from admission 3 are shown in closed squares.
Although Fig. 3E suggests a possible metformin-related decrease in average insulin levels during OGTT, metformin use was not associated with a significant change in average insulin level (200 ± 117 U/mL vs 162 ± 127 U/mL; P = 0.3008) or average glucose (125 ± 12 mg/dL vs 127 ± 17 mg/dL; P = 0.6523) during OGTT. Similarly, metformin use was not associated with significant changes in fasting insulin level (26 ± 16 vs 30 ± 23 U/mL) or HOMA-IR (7.3 ± 4.4 vs 8.2 ± 9.7; P > 0.3 for both).
Miscellaneous data
Between admissions 1 and 2, P4 concentrations on days 3 and 5 of P4 and E2 administration were 4.0 ± 2.6 and 4.6 ± 2.5 ng/mL, respectively; whereas E2 levels were 70.4 ± 22.5 and 61.3 ± 17.2 pg/mL, respectively. Between admissions 3 and 4, day 3 and 5 P4 levels were 4.3 ± 2.0 and 2.9 ± 1.3 ng/mL, respectively; whereas day 3 and 5 E2 concentrations were 92.7 ± 33.2 and 71.7 ± 31.2 pg/mL, respectively. Sleep efficiency (i.e., percentage of total sleep time—from first to last sleep epoch—spent asleep) was 89% ± 9%, 84% ± 15%, 89% ± 10%, and 85% ± 8% for admissions 1 through 4, respectively.
Discussion
Relative hypothalamic (GnRH pulse generator) insensitivity to P4 suppression is an important mechanism underlying the gonadotropin abnormalities that contribute to PCOS pathophysiology. Although multiple studies suggest that hypothalamic insensitivity to P4 suppression is largely related to hyperandrogenemia (5, 8, 18), an earlier study in adolescent girls with hyperandrogenemia suggested that hyperinsulinemia may also play a role (7). Accordingly, we assessed the effect of metformin—a common treatment of adolescent PCOS—on hypothalamic sensitivity to P4 suppression in adolescent girls with HA. Although short-term metformin use significantly improved hyperandrogenemia, it did not improve hypothalamic sensitivity to P4. These results are in keeping with our prior study of metformin in adult PCOS (10), in which we assessed hypothalamic P4 sensitivity after treatment with metformin 500 mg three times daily for at least 4 weeks. In that study, we reported E2- and P4-mediated reductions in LH pulse frequency by 61% in control participants and 25% in women with PCOS, despite similar reductions in total T level (18% in control participants and 15.9% in women with PCOS). These metformin-treated women with PCOS demonstrated similar P4-induced changes in LH pulse frequency compared with previously studied women with PCOS without metformin pretreatment (5). Overall, we concluded that metformin use failed to improve hypothalamic sensitivity to P4 in women with PCOS, despite modest improvement in hyperandrogenemia and hyperinsulinemia.
Compared with our prior study (10), the current study involved a higher dose of metformin (1 g twice daily vs 500 mg thrice daily) administered for a longer time (mean, 12.7 weeks vs 4 weeks), an administration pattern that more closely aligns with typical clinical practice. Perhaps most importantly, the current study involved assessments of hypothalamic P4 sensitivity both before and after metformin treatment, which allowed participants to serve as their own controls. This differs from our prior study of adult women, in which historical control participants were used for comparison. Overall, the procedural differences in the current study would be expected to enhance our ability to detect an improvement in hypothalamic P4 sensitivity, if a difference exists.
We previously reported that use of flutamide, an androgen blocker, normalized hypothalamic P4 sensitivity in adult PCOS (8). The current study represents the second report suggesting that, although short-term metformin improves biochemical HA, it does not improve hypothalamic P4 sensitivity. We suggest that metformin may not reduce HA sufficiently to reverse hypothalamic sensitivity to P4 suppression, at least in the short term. That is, it is possible that, in contrast to potent androgen-receptor blockade, the modest reductions of circulating androgens observed with metformin are inadequate to reverse hypothalamic resistance to P4 negative feedback. It seems likely that most of our participants had relatively high androgen levels for several years, which perhaps promoted more durable changes that are resistant to metformin treatment. It is possible that the results of our study may have differed had we studied a group of girls with HA who were younger and nearer to menarche.
Despite therapeutic-dose metformin use for approximately 3 months, we did not observe a demonstrable improvement in hyperinsulinemia in the current study. Theoretically, a marked amelioration of hyperinsulinemia may have normalized hypothalamic sensitivity to P4 negative feedback. Prior studies using metformin in adolescents with PCOS have had variable findings. Arslanian et al. (19) found improvement in fasting and OGTT area-under-the-curve (AUC) insulin and glucose levels in 15 adolescents with PCOS who were treated with metformin (850 mg twice daily) for 3 months; these participants additionally had significant reductions in BMI and both total and free T levels, without changes in SHBG. Hoeger et al. (20) randomly assigned adolescents with PCOS to treatment of 6 months with placebo, metformin (850 mg twice daily), oral contraceptive pills (OCPs), or lifestyle changes. The six participants in the metformin group did not have a significant improvement in AUC glucose or insulin levels during OGTTs, nor in total T or SHBG levels; they did exhibit a significant improvement in fasting glucose levels. Bridger et al. (21) observed in 22 hyperinsulinemic adolescents with PCOS a nonsignificant trend toward improvement in insulin AUC and HOMA-IR with use of metformin (750 mg, twice daily) vs placebo for 3 months; BMI did not significantly improve, whereas total T level decreased significantly. Al-Zubeidi and Klein (22) studied 22 adolescents with PCOS who were randomly assigned to receive metformin (1000 mg twice daily) or OCPs for 6 months. The researchers found no significant improvement in insulin resistance measures with either therapy, significantly decreased BMI with both therapies, and improvement in free T levels only with OCPs.
Of note, in the current study, we did not provide structured education on lifestyle modifications to encourage weight loss; a multifaceted approach to hyperinsulinemia may have been associated with improved hypothalamic P4 sensitivity. It is possible that the lack of improvement in hyperinsulinemia could reflect participants’ unreported nonadherence to metformin therapy. Additionally, our tests for insulin resistance and hyperinsulinemia are relatively imprecise and, therefore, may have failed to capture a decrease in insulin levels over the course of therapy. Furthermore, serum insulin levels may not be representative of the potentially more pertinent hypothalamic insulin exposure.
We note several additional limitations of our study. First, our study involved a relatively small study population—a limitation inherent to such studies in adolescents; accordingly, we may have had insufficient statistical power to detect modest changes in hypothalamic P4 sensitivity related to metformin use. Second, although the racial and ethnic diversity of the studied cohort reflects UVA’s patient population, we recognize that this is unlikely to reflect the racial and ethnic composition of the general population with HA; findings may differ in a more diverse population. Participant 7, who is Hispanic, was the most sensitive to P4 suppression pre- and postmetformin therapy. This aligns with our prior observation of a trend toward normal P4 sensitivity in Hispanic study participants with HA (7). Finally, we noted that two participants (participant 2 at admissions 2 and 4, and participant 9 at admission 2) demonstrated day 7 P4 levels >15 ng/mL. This may be sufficient to overcome relative hypothalamic resistance to P4 suppression. The elevation in day 7 P4 concentrations may reflect participants’ failing to use P4 as instructed.
Numerous studies, both in vitro and in vivo, have indicated a relationship between hyperinsulinemia and hyperandrogenemia. Insulin augments ovarian androgen production and additionally reduces SHBG, enabling higher free T levels (23, 24). Despite ongoing research that has built on these concepts, the appropriate role for metformin in PCOS management remains unclear. Per the Endocrine Society’s guidelines, metformin should be used in women with PCOS and type 2 diabetes or impaired glucose intolerance when these conditions cannot be managed with lifestyle changes alone (25). For adolescents with suspected PCOS, the guidelines suggest consideration of metformin for treatment of glucose intolerance or metabolic syndrome. Some experts suggest that, compared with adults, adolescents with PCOS are more likely to benefit from metformin use (26). Although numerous studies have demonstrated reduction in biochemical hyperandrogenemia with insulin sensitizers, there is no clear evidence to suggest that metformin substantially improves clinical HA in adults (e.g., hirsutism) (27). Metformin appears to improve ovulation rates (28); however, clomiphene and letrozole appear to be superior to metformin in this regard (29, 30), and the Endocrine Society’s guidelines do not recommend metformin as first-line treatment of anovulatory infertility (25).
In conclusion, the findings of this study of adolescent girls with HA suggest improvement in hyperandrogenemia, but not hyperinsulinemia or hypothalamic P4 sensitivity, after several months of metformin treatment. Interpreted within the context of the findings of our prior study of adult PCOS, this study further supports that metformin affects ovulatory function though a non-neuroendocrine mechanism, though future studies are needed.
Acknowledgments
We thank research coordinators Lauren Lockhart, Michelle Abshire, and Anne Gabel, prior clinical research fellows who contributed to study implementation and oversight, the nurses and staff of the University of Virginia’s Clinical Research Unit, and Daniel Haisenleder and the Center for Research in Reproduction Ligand Core Laboratory for performance of study assays.
Financial Support: This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants P50 HD28934 (J.C.M.), 1 F32 HD091951-01 (J.A.L.), 1 F32 HD088047-01 (S.H.K.), and K23 HD070854 (C.M.B.S.).
Clinical Trial Information: ClinicalTrials.gov no. NCT01427595 (registered 30 August 2011).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- CRU
- clinical research unit
- DHEAS
- dehydroepiandrosterone
- E2
- estradiol
- FSH
- follicle-stimulating hormone
- GnRH
- gonadotropin-releasing hormone
- HA
- hyperandrogenism
- HOMA-IR
- homeostatic method assessment of insulin resistance
- LH
- luteinizing hormone
- OCP
- oral contraceptive pill
- OGTT
- oral glucose tolerance test
- PCOS
- polycystic ovary syndrome
- P4
- progesterone
- SHBG
- sex hormone–binding globulin
- T
- testosterone
- UVA
- University of Virginia.
References
- 1.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29(2):181–191. [Google Scholar]
- 2.Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57(5):1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apter D, Bützow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79(1):119–125. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE. Neuroendocrine control of the menstrual cycle In: Strauss JF, ed. Yen & Jaffe’s Reproductive Endocrinology. 7th ed. Philadelphia, PA: Elsevier Saunders; 2014:131–154. [Google Scholar]
- 5.Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. [DOI] [PubMed] [Google Scholar]
- 6.Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. [DOI] [PubMed] [Google Scholar]
- 7.Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls--implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94(7):2360–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. [DOI] [PubMed] [Google Scholar]
- 9.Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152(2):618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC. Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab. 2003;88(11):5158–5162. [DOI] [PubMed] [Google Scholar]
- 11.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burt Solorzano CM, Helm KD, Patrie JT, Shayya RF, Cook-Andersen HL, Chang RJ, McCartney CRMJ. Increased adrenal androgens in overweight peripubertal girls. J Endocr Soc. 2017;1(5):538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 15.McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006;91(5):1714–1722. [DOI] [PubMed] [Google Scholar]
- 16.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250(4 Pt 1):E486–E493. [DOI] [PubMed] [Google Scholar]
- 17.McCartney CR, Gingrich MB, Hu Y, Evans WS, Marshall JC. Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase reflects the gradual loss of the restraining effects of progesterone. J Clin Endocrinol Metab. 2002;87(5):2194–2200. [DOI] [PubMed] [Google Scholar]
- 18.Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab. 1997;82(12):4179–4183. [DOI] [PubMed] [Google Scholar]
- 19.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87(4):1555–1559. [DOI] [PubMed] [Google Scholar]
- 20.Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo-controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridger T, MacDonald S, Baltzer F, Rodd C. Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2006;160(3):241–246. [DOI] [PubMed] [Google Scholar]
- 22.Al-Zubeidi H, Klein KO. Randomized clinical trial evaluating metformin versus oral contraceptive pills in the treatment of adolescents with polycystic ovarian syndrome. J Pediatr Endocrinol Metab. 2015;28(7-8):853–858. [DOI] [PubMed] [Google Scholar]
- 23.Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62(5):904–910. [DOI] [PubMed] [Google Scholar]
- 24.Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994;43(5):647–654. [DOI] [PubMed] [Google Scholar]
- 25.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecht Baldauff N, Arslanian S. Optimal management of polycystic ovary syndrome in adolescence. Arch Dis Child. 2015;100(11):1076–1083. [DOI] [PubMed] [Google Scholar]
- 27.Cosma M, Swiglo BA, Flynn DN, Kurtz DM, Labella ML, Mullan RJ, Elamin MB, Erwin PJ, Montori VM. Clinical review: insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 28.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012;(5):CD003053. [DOI] [PubMed]
- 29.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Giudice LC, Leppert PC, Myers ER; Cooperative Multicenter Reproductive Medicine Network . Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–566. [DOI] [PubMed] [Google Scholar]
- 30.Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H; NICHD Reproductive Medicine Network . Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371(2):119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]