Abstract
Context:
Abnormal glucagon concentrations contribute to hyperglycemia, but the mechanisms of α-cell dysfunction in prediabetes are unclear.
Objective:
We sought to determine the relative contributions of insulin secretion and action to α-cell dysfunction in nondiabetic participants across the spectrum of glucose tolerance.
Design:
This was a cross-sectional study. A subset of participants (n = 120) was studied in the presence and absence of free fatty acid (FFA) elevation, achieved by infusion of Intralipid (Baxter Healthcare, Deerfield, IL) plus heparin, to cause insulin resistance.
Setting:
An inpatient clinical research unit at an academic medical center.
Participants:
A total of 310 nondiabetic persons participated in this study.
Interventions:
Participants underwent a seven-sample oral glucose tolerance test. Subsequently, 120 participants were studied on two occasions. On one day, infusion of Intralipid plus heparin raised FFA. On the other day, participants received glycerol as a control.
Main Outcome Measure(s):
We examined the relationship of glucagon concentration with indices of insulin action after adjusting for the effects of age, sex, and weight. Subsequently, we sought to determine whether an acute decrease in insulin action, produced by FFA elevation, altered glucagon concentrations in nondiabetic participants.
Results:
Fasting glucagon concentrations correlated positively with fasting insulin and C-peptide concentrations and inversely with insulin action. Fasting glucagon was not associated with any index of β-cell function in response to an oral challenge. As expected, FFA elevation decreased insulin action and also raised glucagon concentrations.
Conclusions:
In nondiabetic participants, glucagon secretion was altered by changes in insulin action.
This study examined glucagon secretion in nondiabetic participants. An acute or chronic decrease in insulin action raised glucagon concentrations.
In 1975, Unger and Orci (1) described type 2 diabetes mellitus as a bihormonal disease characterized by defective insulin secretion and hyperglucagonemia. The combination of these defects results in hyperglycemia. Indeed, in the presence of delayed and decreased insulin secretion, impaired glucagon suppression contributes to hyperglycemia in health (2) and in type 2 diabetes (3). People with type 2 diabetes also exhibit impaired suppression of glucagon in response to insulin administration (4). Despite substantial progress in understanding the changes in β-cell function that contribute to impaired glucose tolerance and the pathogenesis of type 2 diabetes (5), the mechanisms leading to α-cell dysfunction and their subsequent contribution to impaired glucose tolerance remains uncertain (6, 7).
In vitro studies have characterized a complex system of α-cell regulation that includes various paracrine mediators (8–12), autonomic input (13), and circulating hormones (14). In humans, hyperglucagonemia has been associated with impaired insulin secretion (15), although some evidence suggests that this is not a causal relationship (16). In vivo, experiments using an oral or an isoglycemic intravenous glucose challenge suggested that decreased insulin secretion, impaired insulin action (Si), impaired incretin effect, or α-cell insensitivity to incretin hormones contributes to impaired postprandial suppression of glucagon (17, 18). More recently, Færch et al. (19) reported that impaired Si is associated with higher fasting glucagon concentrations and higher postprandial (30 minutes after an oral challenge) glucagon concentrations.
With the increasing prevalence of type 2 diabetes mellitus and prediabetes, a greater understanding of the characteristics associated with abnormal α-cell function may provide insight into the contribution of glucagon to the progression of prediabetes to diabetes (20). This may be especially relevant given the advent of therapies that specifically target glucagon signaling for the treatment or prevention of type 2 diabetes (21). We therefore examined fasting and postprandial glucagon concentrations in a large cohort of nondiabetic participants to determine whether changes in fasting glucagon concentrations are associated with alterations in insulin secretion and action.
We report that fasting glucagon concentrations are inversely associated with Si and are not associated with indices of β-cell function; indeed, they are positively associated with fasting insulin and C-peptide concentrations. In a subset of 120 participants, induction of acute insulin resistance by elevation of circulating free fatty acids (FFAs) increased fasting and nadir glucagon concentrations. Taken together, these data suggest that defects in Si impair α-cell function and increase fasting glucagon concentrations.
Research Design and Methods
Participants
The volunteers in this study participated in previously published studies, during which they underwent a standardized seven-sample, 2-hour, 75-g oral glucose tolerance test, as previously described (22, 23), together with measurement of body composition using dual-energy x-ray absorptiometry (DPX scanner; Lunar, Madison, WI) at the time of screening. Glucagon was measured at the time of study in both cohorts (23, 24). The studies were performed after approval by the Mayo Institutional Review Board and informed, written consent was obtained. Participants were not taking medications that could affect glucose metabolism and had no history of chronic illness or upper gastrointestinal surgery. Participants were otherwise in good health, were at a stable weight, and did not engage in regular vigorous exercise. All participants were instructed to follow a weight-maintenance diet containing 55% carbohydrate, 30% fat, and 15% protein for at least 3 days before the study.
Experimental design: acute change in Si
Participants from one of the cohorts were also studied on two additional occasions in random order, as previously described (23). On one occasion, participants received an infusion of Intralipid (Baxter Healthcare, Deerfield, IL) and heparin to raise FFA concentrations; on the other occasion, glycerol was infused at a rate of 5 μmol/kg/min (to match the amount of glycerol present in the Intralipid infused during the FFA study day). On each occasion, participants were admitted to the Clinical Research Unit at 1700 on the day before study. At 1800 they consumed a standard 10-kcal/kg meal (55% carbohydrate, 30% fat, 15% protein), followed by an overnight fast. At 0630 (−210 min) the following morning, a forearm vein was cannulated to allow infusions to be performed. In addition, a cannula was inserted retrogradely into a vein of the contralateral dorsum of the hand. This was placed in a heated Plexiglas box maintained at 55°C to allow sampling of arterialized venous blood.
At 0700 (−180 min), an infusion of Intralipid (20%, 0.011 mL/kg/min) and heparin (200 U prime, 0.2 U/kg/min continuous) was started, as previously described (25). The infusion was continued till the end of study at 1600 (360 minutes). At 1000 (0 minutes), participants ingested a glucose drink (1 g/kg body weight). Blood samples were obtained at periodic intervals for hormone and substrate measurement during both the fasting and postprandial period.
Analytic techniques
Arterialized plasma samples were placed in ice, centrifuged at 4°C, separated, and stored at −20°C until assay. Plasma glucose concentrations were measured by using a glucose oxidase method Analox Instruments Inc., Lunenburg, MA; Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin concentrations were measured by using a chemiluminescence assay with reagents (Access Assay; Beckman, Chaska, MN). Plasma C-peptide and glucagon concentrations were measured by radioimmunoassay (EMD Millipore, Billerica; MA).
Calculations
Integrated postprandial hormone concentrations—area above basal or area below basal (ABB) in the case of glucagon—were calculated by using the trapezoidal rule. Net Si was calculated from plasma insulin and glucose concentrations using the unlabeled oral minimal model (26). β-Cell responsivity indexes were calculated from the plasma glucose and C-peptide concentrations by using the oral C-peptide minimal model, incorporating age-associated changes in C-peptide kinetics (27). The C-peptide minimal model assumes that insulin secretion is composed of a static (ϕs) and dynamic (ϕd) component. An index of total β-cell responsivity to glucose (Φ) is derived from both indices. Disposition indices were subsequently calculated as the product of Φ and Si, as reviewed previously (28).
Statistical analysis
Across the quartiles of fasting and 2-hour glucose, there were significant differences in age, sex, and weight (data not shown). In addition, fasting glucagon concentrations were higher in men (88 ± 3 in men vs 72 ± 1 ng/L in women; P < 0.01). Consequently, in our subsequent multivariate regression analyses we incorporated age, sex and weight as covariates.
In the experiment where participants were studied on two occasions in the presence and absence of FFA elevation, differences between study days were tested by using a paired t test (parametric) or a Wilcoxon matched-pairs signed-rank test (nonparametric). All data are presented as mean ± standard error of the mean unless otherwise noted. Multivariate analysis adjusting for the effects of age, sex, and weight (see the following sections) was performed in JMP Pro 11 (SAS Institute Inc., Cary, NC) and in Primer 5 (GraphPad Software, San Diego, CA). Residuals for the conditional logistic regression of a particular parameter with the covariates were used to confirm or refute the contribution of that parameter to variation in glucagon concentrations. A P value < 0.05 was considered to indicate a statistically significant difference.
Results
Participant characteristics
A total of 310 (134 men and 176 women) participants were studied. Their mean age was 57 ± 1 years, their mean weight was 79 ± 1 kg, and their mean fasting glucose level was 5.52 ± 0.03 mmol/L.
Correlation of fasting glucagon with integrated ABB glucagon concentrations
Integrated postprandial suppression (ABB) of glucagon concentration in response to a 75-g oral glucose challenge was inversely correlated with fasting glucagon concentrations after adjustment for covariates (R2 = 0.35; P < 0.01). On the other hand, ABB glucagon was not directly associated with fasting β-cell polypeptides or indexes of insulin secretion or action (independently of fasting glucagon). Hence, subsequent analyses focused on fasting glucagon concentrations.
Correlation of fasting glucagon with Si and fasting C-peptide concentrations
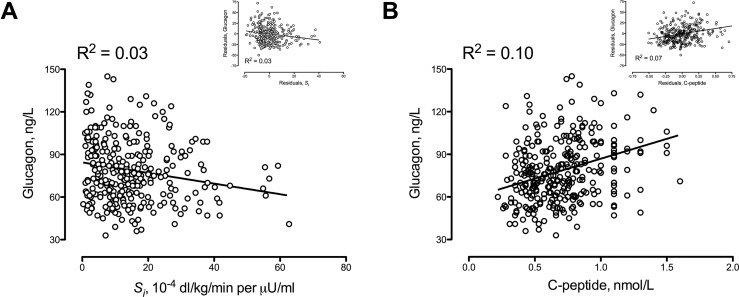
Fasting glucagon concentrations (Fig. 1A) were inversely associated with Si before and after adjustment for covariates. The correlation was weak but significant (R2 = 0.03; P < 0.01). Fasting insulin (data not shown) and C-peptide concentrations (Fig. 1B) were also weakly correlated (P < 0.01) with fasting glucagon concentrations both before and after adjustment for covariates.
Figure 1.
Relationship of fasting glucagon with (A) Si and (B) fasting C-peptide concentrations. The inset panels show the relationship of fasting glucagon with Si and with fasting C-peptide adjusted for the covariates of age, sex, and weight.
Glucagon concentrations and relationship of fasting glucagon concentrations with Si in presence or absence of increased FFA concentrations
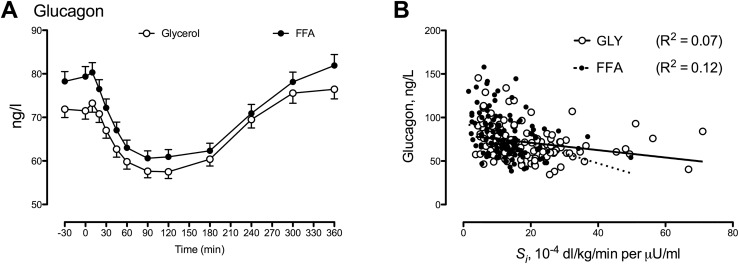
Intralipid and heparin infusion increased FFA approximately threefold (23). This resulted in a significant decrease in Si (18 ± 1 vs 13 ± 1 10−4 dL/kg/min/μU/mL; P < 0.01) with an accompanying increase in both fasting (72 ± 2 vs 79 ± 2 ng/L; P < 0.01) and nadir (52 ± 1 vs 56 ± 2 ng/L; P < 0.01) glucagon concentrations (Fig. 2A). Fasting glucagon was weakly but significantly (P < 0.01) correlated with Si during both the glycerol and FFA study days (Fig. 2B).
Figure 2.
(A) Glucagon concentrations in 120 nondiabetic participants in response to a 1-g/kg body weight glucose challenge with accompanying glycerol (GLY; open circles) and Intralipid and heparin (FFA; solid circles) infusion. Values plotted are means ± standard errors of the mean. (B) The relationship of fasting glucagon with Si during glycerol (open circles) and during Intralipid and heparin infusion (solid circles) is shown.
Discussion
People with type 2 diabetes have impaired postprandial suppression of glucagon (29). It generally has been assumed that this is a consequence of impaired insulin secretion (4). However, Færch et al. (19) recently reported that impaired Si (but not insulin secretion) is associated with both higher fasting and 30-minute postprandial concentrations of glucagon, suggesting that α-cell function may be modulated by alterations in Si. Si in that study was assessed qualitatively by using ratios of insulin and glucose, an approach that has significant limitations (30). Postprandial glucagon concentrations were measured only at 30 and 120 minutes.
We previously used the oral minimal model to measure insulin secretion and action in these 310 nondiabetic participants, which provided us with an opportunity to independently assess the relationship between fasting and postprandial glucagon concentrations with these indices. These data indicate that a decrease in Si is weakly associated with higher fasting glucagon concentrations (Fig. 1A). On the other hand, indices of β-cell function, such as responsivity to glucose (Φ), are not associated (R2 = 0.001) with glucagon concentrations. In fact, fasting glucagon concentrations are weakly, but positively, correlated with fasting insulin and C-peptide concentrations (Fig. 1B).
Fasting insulin concentrations or the ratio of fasting insulin to fasting glucose concentrations are often used as a surrogate of insulin resistance (19). Therefore, the observation that fasting glucagon is negatively correlated with Si and positively correlated with fasting insulin and C-peptide is consistent with surrogates of Si, such as the homeostatic model assessment of insulin resistance, which suggest that an alteration in Si impairs regulation of the α cell. This conclusion is consistent with the observation that accumulation of ceramide in α cells slows the reduction of preproglucagon messenger RNA by insulin (31). In addition, insulin-resistant mice exhibit high glucagon concentrations despite hyperinsulinemia (31).
An alternative explanation for these observations is that insulin secretion in the fasting state increases in response to rising glucagon concentrations so as to maintain euglycemia rather than solely due to a decrease in Si. To distinguish between these two possibilities, we used data from a subset of these participants (n = 120) who were studied on two occasions: in the presence or absence of acute FFA-induced insulin resistance. Despite increased insulin and C-peptide secretion, the FFA-induced decrease in Si resulted in an increase in fasting glucagon concentrations (Fig. 2A). Taken together, these data strongly imply that in nondiabetic humans, α-cell function is modulated directly or indirectly by the prevailing level of Si (Fig. 2). These observations are in keeping with the conclusions of Ahrén and Larsson (15), who reported that impaired glucagon suppression in people with impaired glucose tolerance correlated with decreased Si as measured by a euglycemic clamp. They are also congruent with those reported by Færch et al. (19).
Intriguingly, sex significantly influenced fasting and integrated postchallenge glucagon concentrations in this cohort. Men have consistently been shown to have higher fasting blood glucose concentrations compared with women (32, 33), as also was observed in this cohort (data not shown). Although the effects of estrogen on α-cell function are unclear, the age of our cohort suggests that estrogen is unlikely to explain the sex-based differences in glucagon concentrations observed. Previously we examined the role of glucagon-like peptide-1 (GLP-1) in the pathogenesis of type 2 diabetes and prediabetes (24). However, there was no relationship of glucagon-like peptide 1 concentrations with glucagon suppression. Intriguingly, fasting total and active glucagon-like peptide 1 were positively correlated with fasting glucagon concentrations, as has been reported previously (34).
Although the current study has strengths, such as quantitative measures of insulin secretion and action, as well as the frequent sampling of glucose, glucagon, insulin, and C-peptide concentrations before and after glucose ingestion, important limitations need to be considered. Part of this cohort was recruited on the basis of genotype at rs7903146 in the TCF7L2 locus, which is independently associated with impaired glucagon suppression [but not with changes in fasting glucagon concentrations (23)]. However, TCF7L2 does not alter Si and therefore would not explain the observed relationship of glucagon with Si (23, 35). Given the effect of impaired Si on fasting glucagon concentrations, it is intriguing to speculate that the increased postprandial glucagon concentrations attributable to diabetes-associated variation at the TCF7L2 locus would interact with an environmentally associated (e.g., obesity) decrease in Si to magnify any underlying defects of β-cell and α-cell function (23).
Absolute insulin deficiency as seen in type 1 diabetes is associated with hyperglucagonemia, and correction of insulin deficiency lowers glucagon concentrations (4). However, these conditions are not pertinent to people with prediabetes or type 2 diabetes. Previous work has suggested that in these populations impaired Si is associated with variation in glucagon concentrations (15, 19). This is again the case in the current study and is supported by similar findings in insulin resistant animals (31). The observation that fasting glucagon concentrations are inversely correlated with Si is bolstered by the increase in fasting glucagon concentrations in response to an FFA-induced decrease in Si. This strongly suggests that impaired Si is accompanied by, or causes dysregulation of, α-cell function in nondiabetic humans.
Acknowledgments
Financial Support: This study was funded by funds from the Mayo Clinic General Clinical Research Center (UL1 TR000135) and by the National Institutes of Health [DK116231 (to A.V.) and DK78646]. R.T.V. is supported by training grant 5T32DK007352-37.
Author Contributions: A.S., R.T.V., and M.S. researched data and ran the studies; C.D.M. undertook mathematical modeling of insulin secretion and action; C.C. and R.A.R. contributed to the discussion and reviewed/edited the manuscript; K.R.B. oversaw the statistical analysis, contributed to the discussion, and reviewed/edited manuscript. A.V. designed the study, oversaw its conduct, researched data, and wrote the manuscript. A.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Disclosure Summary: A.V. is an investigator in an investigator-initiated study sponsored by Novo Nordisk. He has consulted for XOMA, Sanofi-Aventis, Novartis, and Bristol-Myers Squibb in the past 5 years. The remaining authors have nothing to disclose.
Footnotes
- ABB
- area below basal
- FFA
- free fatty acid
- Si
- insulin action.
References
- 1.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14–16. [DOI] [PubMed] [Google Scholar]
- 2.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol. 1999;277(2 Pt 1):E283–E290. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85(11):4053–4059. [DOI] [PubMed] [Google Scholar]
- 4.Aydin I, Raskin P, Unger RH. The effect of short-term intravenous insulin administration on the glucagon response to a carbohydrate meal in adult onset and juvenile type diabetes. Diabetologia. 1977;13(6):629–636. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, Matsuda M, Jani R, Jenkinson CP, Coletta DK, Kaku K, DeFronzo RA. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2008;295(2):E401–E406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–3549. [DOI] [PubMed] [Google Scholar]
- 7.Quesada I, Tudurí E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199(1):5–19. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest. 1984;74(6):2296–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9(4):350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54(6):1808–1815. [DOI] [PubMed] [Google Scholar]
- 11.Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53(4):1038–1045. [DOI] [PubMed] [Google Scholar]
- 12.Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58(2):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63(8):2714–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, Habib AM, Moritoh Y, Zhang E, Reimann F, Rosengren A, Shibasaki T, Gribble F, Renström E, Seino S, Eliasson L, Rorsman P. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010;11(6):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahrén B, Larsson H. Impaired glucose tolerance (IGT) is associated with reduced insulin-induced suppression of glucagon concentrations. Diabetologia. 2001;44(11):1998–2003. [DOI] [PubMed] [Google Scholar]
- 16.Hare KJ, Vilsbøll T, Holst JJ, Knop FK. Inappropriate glucagon response after oral compared with isoglycemic intravenous glucose administration in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2010;298(4):E832–E837. [DOI] [PubMed] [Google Scholar]
- 17.Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;300(6):E1038–E1046. [DOI] [PubMed] [Google Scholar]
- 18.Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia. 2007;50(4):797–805. [DOI] [PubMed] [Google Scholar]
- 19.Færch K, Vistisen D, Pacini G, Torekov SS, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Jørgensen ME, Ahrén B, Holst JJ. Insulin Resistance Is Accompanied by Increased Fasting Glucagon and Delayed Glucagon Suppression in Individuals With Normal and Impaired Glucose Regulation. Diabetes. 2016;65(11):3473–3481. [DOI] [PubMed] [Google Scholar]
- 20.Siu AL; U.S. Preventive Services Task Force . Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(11):861–868. [DOI] [PubMed] [Google Scholar]
- 21.Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, Fu H, Watson DE, Lewin AJ, Landschulz WH, Deeg MA, Moller DE, Hardy TA. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies [published correction appears in Diabetes Care. 2017;40(6):808]. Diabetes Care. 2016;39(7):1241–1249. [DOI] [PubMed] [Google Scholar]
- 22.Sathananthan A, Dalla Man C, Zinsmeister AR, Camilleri M, Rodeheffer RJ, Toffolo G, Cobelli C, Rizza RA, Vella A. A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin Endocrinol (Oxf). 2012;76(2):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah M, Varghese RT, Miles JM, Piccinini F, Dalla Man C, Cobelli C, Bailey KR, Rizza RA, Vella A. TCF7L2 Genotype and α-cell function in humans without diabetes. Diabetes. 2016;65(2):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smushkin G, Sathananthan A, Man CD, Zinsmeister AR, Camilleri M, Cobelli C, Rizza RA, Vella A. Defects in GLP-1 response to an oral challenge do not play a significant role in the pathogenesis of prediabetes. J Clin Endocrinol Metab. 2012;97(2):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah P, Vella A, Basu A, Basu R, Adkins A, Schwenk WF, Johnson CM, Nair KS, Jensen MD, Rizza RA. Effects of free fatty acids and glycerol on splanchnic glucose metabolism and insulin extraction in nondiabetic humans. Diabetes. 2002;51(2):301–310. [DOI] [PubMed] [Google Scholar]
- 26.Dalla Man C, Campioni M, Polonsky KS, Basu R, Rizza RA, Toffolo G, Cobelli C. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 2005;54(11):3265–3273. [DOI] [PubMed] [Google Scholar]
- 27.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. [DOI] [PubMed] [Google Scholar]
- 28.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63(4):1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler PC, Rizza RA. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance of hepatic glucose cycling in glucose-intolerant or NIDDM patients. Diabetes. 1991;40(1):73–81. [PubMed] [Google Scholar]
- 30.Xiang AH, Watanabe RM, Buchanan TA. HOMA and Matsuda indices of insulin sensitivity: poor correlation with minimal model-based estimates of insulin sensitivity in longitudinal settings. Diabetologia. 2014;57(2):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y, Berglund ED, Yu X, Wang MY, Evans MR, Scherer PE, Holland WL, Charron MJ, Roth MG, Unger RH. Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci USA. 2014;111(36):13217–13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47(1):31–39. [DOI] [PubMed] [Google Scholar]
- 33.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. [DOI] [PubMed] [Google Scholar]
- 34.Vollmer K, Holst JJ, Baller B, Ellrichmann M, Nauck MA, Schmidt WE, Meier JJ. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57(3):678–687. [DOI] [PubMed] [Google Scholar]
- 35.Varghese RT, Viegas I, Barosa C, Marques C, Shah M, Rizza RA, Jones JG, Vella A. Diabetes-Associated Variation in TCF7L2 Is Not Associated With Hepatic or Extrahepatic Insulin Resistance. Diabetes. 2016;65(4):887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]