Abstract
Context:
Monogenic diabetes is thought to account for 2% of all diabetes cases, but most patients receive misdiagnoses of type 1 or type 2 diabetes. To date, little is known about the histopathological features of pancreata from patients with monogenic diabetes.
Objective:
Retrospective study of the JDRF Network for Pancreatic Organ Donors with Diabetes biorepository to identify possible cases of monogenic diabetes and to compare effects of genetic variants on pancreas histology.
Methods:
We selected cases of diabetes for genetic testing on the basis of criteria that included young age at diagnosis, low body mass index, negative autoantibody status, and/or detectable C-peptide level. Samples underwent next-generation−targeted sequencing of 140 diabetes/diabetes-related genes. Pancreas weight and histopathology were reviewed.
Results:
Forty-one of 140 cases of diabetes met the clinical inclusion criteria, with 38 DNA samples available. Genetic variants of probable clinical significance were found in four cases: one each in KCNJ11, HNF1A, GATA6, and LMNA. The KCNJ11 and HNF1A samples had significantly decreased pancreas weight and insulin mass similar to that of type 1 diabetes but had no insulitis. The GATA6 sample had severe pancreatic atrophy but with abundant β cells and severe amyloidosis similar to type 2 diabetes. The LMNA sample had preserved pancreas weight and insulin mass but abnormal islet architecture and exocrine fatty infiltrates.
Conclusions:
Four cases of diabetes had putative causal variants in monogenic diabetes genes. This study provides further insight into the heterogeneous nature of monogenic diabetes cases that exhibited clinical and pathophysiological features that overlap with type 1/type 2 diabetes.
Genetic testing of 38/140 human pancreas samples revealed causal mutations in KCNJ11, LMNA, HNF1A, and GATA6 in four cases with atypical histologic features overlapping with type 1 or type 2 diabetes.
Monogenic forms of diabetes have been estimated to account for 2% of all cases of diabetes (1). However, the true prevalence is uncertain because genetic testing is rarely performed. As a result, most patients with monogenic diabetes are misdiagnosed as having type 1 or type 2 diabetes and are treated empirically (2). Monogenic diabetes is broadly classified into three forms: neonatal diabetes (diabetes diagnosed in the first 6 months of life), maturity-onset diabetes of the young (MODY; typically non−insulin-requiring diabetes usually diagnosed in nonobese adolescents or young adults), and syndromic forms of diabetes that include other clinical features (3). More than 20 genes are associated with monogenic diabetes, with the majority encoding proteins that play a critical role in pancreatic β-cell development and/or function (4).
Our current understanding of the pathogenesis is primarily derived from the function of the gene involved, as animal models of most forms of monogenic diabetes fail to recapitulate the human phenotype. Few data are available on the effect of mutations in monogenic diabetes genes on human pancreatic islet histology [i.e., other than for ABCC8 (5) and KCNJ11 (6)]. Studies characterizing pancreatic histopathological features including pancreas size, islet morphology, and insulin mass in conjunction with age at diagnosis, patient age, and diabetes duration would provide important clues about the mechanism(s) by which specific gene variants cause loss of functional β-cell mass and subsequent diabetes.
The JDRF Network for Pancreatic Organ Donors with Diabetes (nPOD) program was established to recover pancreata and related organs from patients with diabetes (e.g., type 1 diabetes, type 2 diabetes, gestational diabetes, and cystic fibrosis−related diabetes) for matching with those of donors without diabetes to facilitate studies of the pathogenic mechanisms of β-cell dysfunction and loss in the disease (7). Studies of the histopathology of pancreata from patients with type 1 diabetes have been well described and show a classic marked loss of insulin mass with variable numbers of residual β cells regardless of diabetes duration and age of onset (8, 9). In a recent study of nPOD donors with type 1 diabetes, islet inflammation (insulitis) was observed in less than 10% of examined islets of cases with residual β cells present, with the numbers of islets with insulitis (frequency) inversely related to diabetes duration irrespective of age at onset (8). Marked regional heterogeneity in islet β-cell loss was observed and is well known (8, 10).
The pancreatic histopathology of type 2 diabetes is also well described and generally shows minimal changes in insulin mass, a relative decrease in numbers of β cells to α cells, and variable degrees of islet amyloidosis (1% to 80%) (11). Islet amyloidosis is also reported in older patients without a history of diabetes (12).
Although the nPOD biorepository was established primarily for recovery of organ donors during the preclinical and clinical phases of type 1 diabetes, we hypothesized that it would include cases with monogenic diabetes at a frequency similar to that reported in the literature or higher on the basis of frequent clinical misdiagnosis (1, 13). In such cases, molecular genetic testing could provide a more definitive clinical diagnosis and thus facilitate classification of pancreatic islet morphology in monogenic diabetes compared with type 1 and type 2 diabetes. Here we describe four cases involving putative causal variants in KCNJ11, HNF1A, GATA6, and LMNA and their pancreas histopathology.
Cases and Methods
Pancreas recovery
All procedures for pancreas recovery were previously published, and standard procedures are available online (7, 14) (jdrfnpod.org). Clinical diagnosis was determined by pediatric endocrinologists (D.S. and L.J.) using data obtained from the admission chart, donor questionnaire, and laboratory test data [glycated hemoglobin (HbA1c) value, C-peptide levels, high resolution human leukocyte antigen (HLA) genotyping for type 1 diabetes alleles] according to American Diabetes Association guidelines (15). Islet autoantibody screening was done using enzyme-linked immunosorbent assay and confirmed via radioimmunoassay (glutamic acid decarboxylase autoantibodies, insulin autoantibodies, insulinoma-associated-2 autoantibodies, and zinc transporter 8 autoantibodies) (14). In most cases, the terminal hospitalization chart was available; however, records from initial diagnosis of diabetes and longitudinal data were not available. All procedures were conducted in accordance with federal organ donation guidelines and the University of Florida Institutional Review Board.
Pancreas histopathology
Pancreata were processed, and the relative pancreas weight (RPW) was calculated from the ratio of whole pancreas weight (g) to donor body weight (kg) (16). Formalin-fixed paraffin blocks and fresh frozen blocks were sectioned from pancreas regions (head, body, tail) according to standard operating procedures (7). Serial paraffin sections were stained by hematoxylin and eosin and immunolocalization to identify β cells (insulin), α cells (glucagon), CK19 (duct cells), CD3+ T cells, HLA-ABC, and cell replication determined by Ki67 staining (Supplemental Table 1 (29.3MB, pdf) ). Proportions of insulin area (%), insulin mass (mg), and Ki67+ total cells (%) or Ki67+ β cells (%) were calculated using an image analysis colocalization program (Aperio; Leica, Buffalo Grove, IL) as previously described (8).
Case selection
A retrospective study of 318 nPOD case histories was conducted for all cases recovered as of April 2015. Donors were excluded for genetic testing when they had no diabetes, cystic fibrosis−related diabetes, gestational diabetes, or a body mass index (BMI) >35 kg/m2. From the remaining 140 donors, we selected cases for targeted sequencing when they fulfilled one of the following criteria: (1) neonatal diabetes diagnosed at <1 year of age irrespective of autoantibody status, (2) diabetes diagnosed at <35 years of age without islet autoantibodies, or (3) insulin-dependent diabetes with C-peptide level above the minimum threshold (>0.05 ng/mL) and diabetes duration >5 years. The US organ donation system precludes sample collection from family members; thus, no additional family information was available.
DNA isolation, next-generation sequencing, and variant interpretation
DNA from snap-frozen spleen or pancreas tissue was isolated using the Gentra Puregene Tissue Kit (Qiagen, Valencia, CA). DNA samples were sequenced using a custom-designed next-generation sequencing (NGS) panel that included 140 genes implicated in monogenic diabetes (neonatal diabetes and MODY), insulin resistance, lipodystrophy, obesity, rare syndromic forms of diabetes, and diabetes candidate genes (17). All variants were interpreted according to the guidelines of the American College of Medical Genetics and Genomics (18). Variants were classified as most likely pathogenic when they were scored as “probably damaging” by two variant interpretation software programs (PolyPhen and SIFT), were not present in ExAc (http://exac.broadinstitute.org) or were extremely rare (allele frequency <0.01%), and/or when they were previously reported in the literature as causal (19). All likely pathogenic variants identified by NGS were confirmed by Sanger sequencing.
Results
Cases
Forty-one of the 140 cases met the clinical and sample inclusion criteria for genetic testing. Three samples from patients whose pancreata were obtained at autopsy rather than by organ donation were excluded because of inadequate DNA quality. Of the 38 samples sequenced, 25 (66%) were negative for putative pathophysiological variants in the panel of 140 genes. Four cases (11%) had likely pathogenic sequence changes in KCNJ11, HNF1A, GATA6, and LMNA (Tables 1 and 2), and two cases (5%) had sequence changes in GLIS3 and INSR that could have affected the clinical phenotype. Seven other cases (18%) had eight variants that were carefully reviewed and considered to be of unlikely significance: common in the public databases (BBS5, CEL, BLK), present in genes associated with recessive syndromes or hyperinsulinism (MNX1, BBS2, BBS5, UCP2), and reported or predicted to be benign (BLK, HNF4A, AKT2) (Supplemental Tables 2 (29.3MB, pdf) and 3 (29.3MB, pdf) ).
Table 1.
Gene Variant Characterization and Clinical Features of Donors
|
Case ID |
Gene |
Function |
Variant (NCBI
RefSeq) |
AA
Change |
Previous
Reports/dbSNP |
ExAC MAFa
(Allele Count) |
PolyPhenb
(Score) |
Age at Dx
(y) |
Age at Death
(y) |
C-Peptide
(ng/mL) |
HbA1c
(%) |
BMI
(kg/m2) |
HLA (T1D
Risk) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Causal Gene Variants | |||||||||||||
| 6033 | KCNJ11 | Ion channel | c.868G>A NM_000525.3 | Val290Met | 20 | 0.00001648 (2/121368) | PD (1) | 12 | 40 | <0.05 | N/A | 22.8 | DR7/12 (No risk) |
| rs750414160 | |||||||||||||
| 6176 | HNF1A | Transcription factor | c.29C>T NM_000545.5 | Thr10Met | 21, 22 | 0.00003463 (4/115514) | PD (0.965) | 25 | 40 | N/A | 8.3 | 25.3 | DR3/8 (Susceptible) |
| rs774637975 | |||||||||||||
| 6320 | GATA6 | Transcription factor | c.1366 C>T NM_005257.4 | Arg456Cys | 23 | 0.000008237 (1/121410) | PD (0.9) | 16 | 19 | 0.02 | 10.4 | 25.1 | DR7/15 (Protective) |
| rs387906818 | |||||||||||||
| 6166 |
LMNA |
Nuclear lamina matrix |
c.898G>A
NM_005572.3 |
Asp300Asn |
25 | 0 |
PD (0.955) |
11 |
26 |
1.18 |
N/A |
20.7 |
DR9/13 (Susceptible) |
|
rs267607591 | |||||||||||||
|
Variants of Possible Clinical Relevance | |||||||||||||
| 6243 | GLIS3 | Transcription factor | c.1863C>G NM_152629.3 | His621Gln | rs761845139 | 0.00001446 (4/276690) | PD (0.999) | 8 | 13 | 0.42 | 13.1 | 21.3 | DR3/3 (High risk) |
| 6264 | INSR | Insulin receptor | c.3034G>A NM_000208.2 | Val1012Met | 37, 38 | 0.008997 (1090/121148) | PD (0.992) | 3 | 12 | <0.05 | 8.9 | 22 | DR3/4 (High risk) |
| rs1799816 | |||||||||||||
All donors were treated with insulin and were autoantibody negative (microinsulin autoantibody excluded because of history of insulin use).
Abbreviations: AA, amino acid; dbSNP, database of single nucleotide polymorphisms; DR, antigen D-related; dx, diagnosis; MAF, minor allele frequency; N/A, not available; NCBI, National Center for Biotechnology Information; PD, probably damaging.
MAF from ExAC was calculated using allele count/allele number.
PolyPhen-2 scores for all four variants were considered to be PD. Scores range from 0.0 to 1.0 and can be interpreted as follows: 0.0 to 0.15, benign; 0.15 to 0.85, possibly damaging; 0.85 to 1.0, probably damaging.
Table 2.
Pancreas and Histopathological Data of Donors With Gene Variants of Interest
| Case ID |
Gene |
Pancreas Weight
(g) |
RPW
(g/kg)a |
H&E Block
Number |
Insulin IHC Block
Number |
Insulin Area (%)b |
Insulin Mass (mg)c |
Ki67+ Cells
(%)d |
Section Islet
Numbere |
Insulin+
Islet Number |
Insulin+
Islet (%) |
Total Section Area
(mm2) |
Islet Density
(number/mm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Causal Gene Variants | |||||||||||||
| 6033 | KCNJ11 | 35 | 0.49 | 18 | 15 | 0 | 1.5 | 0.02 | 130 | 10 | 0.08 | 25 | 5.24 |
| 6176 | HNF1A | 33.9 | 0.46 | 13 | 6 | 0.09 | 31.2 | 0.01 | 148 | 148 | 1 | 41 | 3.61 |
| 6320 | GATA6 | N/A | N/A | 6 | 6 | 0.41 | N/A | 0 | 153 | 153 | 1 | 58 | 2.64 |
| 6166 |
LMNA |
50.3 |
0.87 |
9 |
6 |
1.61 |
809.8 |
0.01 |
435 |
435 |
1 |
139 |
3.13 |
|
Variants of Possible Clinical Relevance | |||||||||||||
| 6243 | GLIS3 | 29.4 | 0.51 | 17 | 18 | 0.5 | 147.5 | 0.01 | 137 | 39 | 0.28 | 62 | 2.21 |
| 6264 | INSR | 20.4 | 0.6 | 16 | 16 | 0.06 | 12.7 | 0 | 163 | 8 | 0.05 | 95 | 1.72 |
Abbreviations: H&E, hematoxylin and eosin; IHC, immunohistochemistry; N/A, not available; T1D, type 1 diabetes; T2D, type 2 diabetes.
RPW: relative pancreas weight [whole pancreas weight (g)/body weight (kg): control, 1.04 ± 0.32; T1D, 0.55 ± 0.17; T2D, 0.92 ± 0.30 (16)].
Insulin area (%; n = 6−12 blocks per donor): control, 1.18 ± 0.72 (n = 50); T1D, 0.15 ± 0.17 (n = 72) (8).
Insulin mass (mg; insulin area % times pancreas weight): control, 797 ± 505; T1D, 47.9 ± 53.0 (8).
Ki67+ (%) for manual counts of Ki67+ acinar or β cells.
Paraffin section with insulin+ islets was analyzed for total number of islets and number of insulin+ islets with proportion of insulin+ islets (%) and islet density (total number of islets/mm2 section area).
KCNJ11 nPOD case 6033
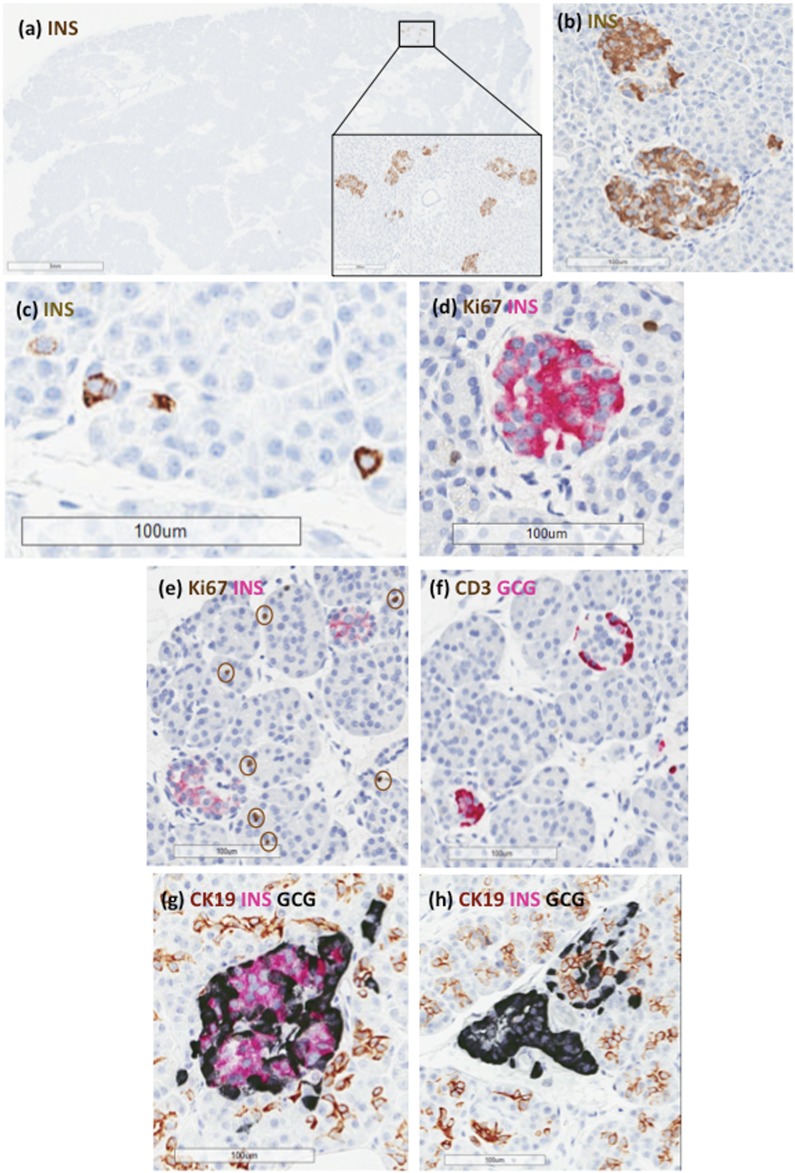
This donor was a 40-year-old Caucasian male with a 28-year history of insulin-dependent diabetes who also had hypertension, peripheral vascular disease, diabetic retinopathy, diabetic nephropathy, and a prior myocardial infarction. C-peptide levels were below the lower limit of detection (Table 1). The initial clinical diagnosis was type 1 diabetes. NGS analysis of the monogenic diabetes gene panel identified a rare heterozygous sequence change in KCNJ11 (p.Val290Met) that was previously reported in two families with congenital hyperinsulinism (20). The pancreas weight was decreased with an RPW of 0.49, similar to the RPW of donors with type 1 diabetes (0.55 ± 0.17) (Table 2) (16). Histological analysis was performed on hematoxylin and eosin−stained slides from 18 blocks and immunohistochemistry for insulin from 15 blocks and showed a marked loss of β cells, resulting in extremely low insulin area (0%) and mass (1.5 mg) with no evidence of β cell proliferation [Fig. 1(a−h); Supplemental Fig. 1(a−c) (29.3MB, pdf) ;jc.2017-01159.sm1.pdf (29.3MB, pdf) Table 2]. The tail region was further assessed for insulin+ islets using single insulin immunohistochemistry, and four of five blocks (80%) showed lobules with small- to medium-sized insulin+ islets and rare single β cells [Fig. 1(a−c); Supplemental Fig. 1(a) (29.3MB, pdf) ]. Insulitis was not observed. Additional histopathological findings included moderate acinar atrophy and acinar and periductular fibrosis with moderate interlobular fat [Supplemental Fig. 1(c) (29.3MB, pdf) ]. Additional immunohistochemistry studies showed a generalized increase in macrophages (CD68+) and CD8+ T lymphocytes in exocrine regions. Rare periductal CD3+ infiltrates were also observed. Multifocal mild squamous metaplasia was observed in several intralobular pancreatic ducts. Mild arteriosclerosis was observed in small vessels in several regions.
Figure 1.
Representative islet images from donor 6033. Paraffin sections were stained by immunohistochemistry for (a−c) insulin, (d, e) Ki67 and insulin, (f) CD3 and glucagon, or (g, h) CK19, insulin, and glucagon. (a, inset) Lobules in the tail region only contained (a, b) rare islets with β cells or (c) single β cells in the exocrine regions. (d, e) Decreased insulin-staining intensity was observed with no Ki67+ β cells (circles indicate Ki67+ acinar nuclei). (b, d, e, g) β cells comprised the majority of islet endocrine cells in insulin+ islets. (g) β cells showed no apparent localization with CK19+ cells. GCG, glucagon; INS, insulin.
HNF1A nPOD case 6176
This case involved a 45-year-old Hispanic male with a 20-year duration of diabetes that was initially treated with oral antihyperglycemic agents followed by insulin injections twice a day for the last 2 years. He also had a history of hypertension, acute renal failure, and drug abuse and presented with diabetic ketoacidosis. His HbA1c level was elevated at 8.3%, and his BMI was slightly high (25.3 kg/m2) (Table 1). Clinical diagnosis was type 2 diabetes. NGS gene panel analysis identified a rare heterozygous sequence change in HNF1A (p.Thr10Met) that was previously reported in two families with MODY3 (21, 22). Pancreas weight and RPW were low, as for donors with type 1 diabetes (16) (Table 2). Histological analysis of 13 pancreas blocks and insulin immunohistochemistry from six blocks showed small insulin+ islets without insulin-negative islets (Fig. 2). β-Cell numbers per islet were qualitatively reduced, with weak insulin-staining intensity and variable nuclear sizes [Fig. 2(a), 2(c), and 2(e)]. Insulitis and Ki67+ β cells were not observed. Insulin area and insulin mass were markedly reduced (Table 2). Exocrine atrophy with inter- and intraductal fibrosis was also observed with an intraductal papillary mucinous neoplasm (1.6 mm).
Figure 2.
Representative islet images from donor 6176. Paraffin sections were stained by immunohistochemistry for (a, c, e) Ki67 and insulin and (b, d, f) CD3 and glucagon. (a, b) All islets were insulin+ and ranged in size from small to medium. (c, e) β-Cell numbers within islets were decreased compared with (d, f) more numerous α cells. (c) Large nuclear sizes were observed in some β cells (arrows). GCG, glucagon; INS, insulin.
GATA6 nPOD case 6320
This case represented a 19-year-old Caucasian female with a 3-year history of insulin-dependent diabetes treated with an insulin pump. She also had a history of hypertension, hypercholesterolemia, hypothyroidism, and congenital heart disease (truncus arteriosus type 4A, interrupted aortic arch, truncal valve regurgitation, ventricular septal defect, and multiple corrective surgeries). She had a family history of type 2 diabetes. She had a low but detectable C-peptide level and a high HbA1c value (Table 1). Because criteria for either type 1 or type 2 diabetes were not fulfilled, the clinical diagnosis was assigned to the category of “Other diabetes.” An NGS gene panel analysis identified a heterozygous sequence change in GATA6 (p.Arg456Cys) that was previously described in two patients with neonatal diabetes, pancreatic agenesis, and heart defects (tetralogy of Fallot and truncus arteriosus/perimembranous ventricular septal defect, respectively) (23).
During the pancreas organ recovery, the surgeon noted severe pancreatic atrophy. The sample received, which was attached to the duodenum, weighed 21.3 g. The RPW and insulin mass were not calculated because of questions regarding whole pancreas weight. Histological and insulin analysis from six blocks showed numerous and variably sized islets with all islets containing β cells [Fig. 3(a−h)]. Scattered small pancreatic polypeptide-positive islets were observed in the ventral lobe. The insulin area was within normal limits (Table 2). Several islets also showed severe amyloidosis [Supplemental Fig. 2(a) and 2(b) (29.3MB, pdf) ]. Moderate to severe islet degeneration was observed, often in parallel with amyloidosis severity as well as glycogen accumulation (hydropic degeneration) [Fig. 3(c)]. Islets had inflammatory infiltrates, defined as six or more CD3+ cells immediately adjacent to endocrine cells (5.9% insulitis; nine of 153 islets) [Fig. 3(f); (Table 2)]. The classic definition of insulitis was not used because insulin-negative (pseudoatrophic) islets were not present (24). Additional findings included endocrine cells within ducts [Supplemental Fig. 2(c−f) (29.3MB, pdf) ] and moderate intralobular and interlobular fatty infiltration [Supplemental Fig. 2(a) (29.3MB, pdf) ].
Figure 3.
Representative islet images from donor 6320. Paraffin sections were stained by immunohistochemistry for (a, c, e) Ki67 and insulin and (b, d, f) CD3 and glucagon. (c) Severe glycogen deposition (hydropic degeneration) was observed in β cells. Islets were insulin+ yet could have (c, e) decreased numbers of β cells compared with (d, f) α cells. (f) Increased peri-islet and intraislet CD3+ cells were observed in islets indicative of islet inflammation (isletitis). GCG, glucagon; INS, insulin.
LMNA nPOD case 6166
This case involved a 26-year-old African American female with a 15-year history of insulin-dependent diabetes who also experienced asthma, hypertension, cardiomyopathy, and congestive heart failure (Table 1). Her C-peptide level was within normal limits (Table 1). Clinical criteria for classification as type 1 or type 2 diabetes were not fulfilled; hence, she was assigned “Other diabetes” for donor type. An NGS gene panel analysis identified a heterozygous sequence change in LMNA (p.Asp300Asn), which was reported in two patients with generalized lipoatrophy and severe coronary and peripheral artery disease (25). Pancreas weight and RPW were within the range for control donors and donors with type 2 diabetes (Table 2) (16). Histological analysis of nine blocks and immunohistochemistry in six blocks showed that all islets were insulin+ with insulin area (1.61%) and insulin mass (810 mg) within normal limits [Fig. 4(a−f); Table 2]. However, islet architecture was slightly abnormal, with some islets having distorted angular shapes rather than a typical spherical morphology [Fig. 4(e) and 4(f)]. Colocalization of insulin and glucagon staining was observed in these irregularly shaped islets (Supplemental Fig. 3 (29.3MB, pdf) ). Mild fatty infiltrates were observed in exocrine lobules, and moderate arteriopathy was also present.
Figure 4.
Representative islet images from donor 6166. Serial paraffin sections were stained by immunohistochemistry for (a, c, e) Ki67 and insulin and (b, d, f) CD3 and glucagon. Overall islet density was within normal limits with (c, d) spherical as well as (e, f) irregular islet outlines. (c, d) Colocalization of insulin and glucagon was also observed in disorganized islets (Supplemental Fig. 3 (29.3MB, pdf) ). GCG, glucagon; INS, insulin.
Other gene variants of possible clinical significance
NGS analyses of two nPOD donors identified variants that may have had an effect on clinical phenotype (Tables 1 and 2). nPOD 6243 had a rare heterozygous GLIS3 (p.His621Gln) variant that is predicted to be damaging. nPOD 6264 had a type 2 diabetes−associated variant in INSR (p.Val1012Met-rs1799816) that is also predicted to be damaging (26). Both donors were included in a previous analysis of insulin mass and insulitis frequency and are briefly described next for this report (8).
nPOD 6243 was a 13-year-old Caucasian male with a clinical diagnosis of type 1 diabetes of 5 years’ duration (Table 1). Islet autoantibodies were negative, C-peptide was positive (0.42 ng/mL), and HLA genotyping was consistent with a high-risk haplotype for type 1 diabetes. The pancreas weight was 29.4 g with a low RPW of 0.51 (Table 2). Insulin+ islets were frequent, with lobular heterogeneity, and included islets with insulitis and high HLA-ABC expression [Supplemental Fig. 4(a−c) (29.3MB, pdf) ]. Insulin area and insulin mass were 0.50% and 147.46 mg, respectively, and insulitis frequency was 7.8% as previously reported (8) (Table 2).
nPOD 6264 was a 12-year-old Caucasian female with a clinical diagnosis of type 1 diabetes (duration, 9 years) (Table 1). Islet autoantibodies were negative, C-peptide level was undetectable, and HLA genotyping also conferred a high risk for type 1 diabetes. The pancreas weight was 20.4 g, and she had a low RPW of 0.60 (Table 2). Insulin+ and insulin-negative islets were observed with a low insulitis frequency (0.3%) as previously reported (8) [Supplemental Fig. 4(d) and 4(e) (29.3MB, pdf) ]. Insulin+ islets were infrequent in 6264 compared with 6243, as reflected in extremely low insulin area (0.06%) and insulin mass (12.7 mg) (Table 2).
Discussion
We describe islet histopathology and architecture in addition to clinical features in organ donors with monogenic diabetes gene variants. We identified mutations in four genes (i.e., KCNJ11, HNF1A, GATA6, and LMNA) that are predicted to negatively influence β-cell function and survival.
The KCNJ11 mutation in nPOD 6033 is an inactivating mutation that was previously reported to reduce KATP channel activity in β cells and lead to insulin hypersecretion (20). One proband carried the p.Val290Met in the homozygous state, whereas the other proband was heterozygous and was presumed to have had hyperinsulinism due to somatic loss of the maternal allele in the pancreas (20). Two heterozygous carriers exhibited abnormally enhanced glucose tolerance but did not have overt hyperinsulinism or diabetes. Heterozygous loss-of-function mutations that result in a partial decrease in KATP channel activity have been associated with diabetes later in life, presumably because of years of insulin oversecretion leading to β-cell “burnout” (27). The pancreas in this donor showed substantially reduced insulin mass and pancreas exocrine atrophy reflected by the low RPW, mirroring several features of long-standing type 1 diabetes. We suggest that this pancreas is an example of the effect of a loss-of-function mutation in KCNJ11 on β cells and was a major contributor to the development of diabetes in this subject as a result of insufficient β-cell mass.
Mutations in HNF1A cause MODY3; patients with this condition exhibit decreased glucose-stimulated insulin secretion even before the onset of diabetes (28). To the best of our knowledge, pancreatic histopathology has not been reported in patients with HNF1A-MODY, such as nPOD 6176. His clinical diagnosis was assigned as type 2 diabetes; however, markedly reduced insulin mass and low pancreas weight are more compatible with histopathological features of type 1 diabetes donors. Nevertheless, because all islets contained residual β cells, his histopathological diagnosis is consistent with HNF1A-MODY.
GATA6 mutations were first identified as a cause of congenital heart defects and subsequently as a cause of permanent neonatal diabetes with pancreatic agenesis in association with heart defects (23). nPOD 6320 had a GATA6 mutation (p.Arg456Cys) and multiple congenital heart defects, which are present in the majority of previously described cases with pathologic GATA6 mutations. However, most GATA6 mutation reports described patients with neonatal diabetes, whereas nPOD 6320’s diagnosis of diabetes at 16 years of age is clearly atypical (29, 30). The pancreas obtained by the surgeon (consistent with the pancreas head) was very small, though the insulin area (%) of this atrophic sample was within the normal range. Studies of other monogenic causes of pancreatic agenesis suggest that some patients have a pancreatic remnant that often includes only the head (31). Multiple islets with amyloidosis were observed, which is compatible with a histopathological diagnosis of type 2 diabetes. Although animal models of Gata6 do not recapitulate the human phenotype, pancreatic Gata6 null mice have fatty infiltration similar to that of the current case (32). The residual β cells in our case were apparently capable of sufficient function until adolescence, which is a common age for diagnosis of many forms of monogenic diabetes involving reduced β-cell function. This case suggests that genetic testing should be considered in any young patient who has a congenital heart defect and is diagnosed as having diabetes.
Laminopathies have been associated with a wide range of heritable metabolic disorders including lipodystrophy, cardiomyopathy, muscular dystrophies, diabetes, and progeria (33). nPOD 6166, who had an LMNA mutation, was diagnosed as having diabetes at the young age of 11 years; however, at her death 26 years later, she still had a detectable C-peptide level and normal RPW, insulin area, and insulin mass. Her severe hypertension, cardiomyopathy, and congestive heart failure are characteristic features of patients with LMNA mutations (34). There were no clinical data regarding the presence of insulin resistance in this patient, but her medical history did indicate that she was insulin dependent. However, a normal BMI despite fatty infiltrates within the pancreas could be consistent with a lipodystrophy-like syndrome. We posit that the LMNA mutation was the primary contributor to the development of insulin resistance and clinical manifestation of diabetes because there was no evidence of loss of insulin mass, which would be expected in a donor with long-standing type 1 diabetes.
Two patients with variants of possible significance demonstrated clinical and histological features of early type 1 diabetes: GLIS3 with frequent insulin-positive islets and insulitis and INSR with lower insulin mass and insulitis frequency based on diabetes duration (5 and 9 years, respectively). GLIS3 plays a critical role in normal β-cell function and insulin gene expression (35). Mutations in GLIS3 can cause a recessive form of neonatal diabetes and congenital hypothyroidism (36); however, there are no reports of dominant GLIS3 mutations. Interestingly, GLIS3 has been associated with increased risk for both type 1 and type 2 diabetes (37). The clinical importance of the INSR Met-1012 substitution at present is uncertain because it has been reported in people with non−insulin-dependent diabetes and healthy controls (38). However, one large population study found that the Met-1012 variant was significantly associated with type 2 diabetes and may represent a risk factor for developing diabetes (26). These two and the other seven variants detected in genes related to insulin action or secretion may have predisposed these patients to abnormal β-cell development or β-cell failure, increasing the risk of diabetes.
Making a correct clinical diagnosis of diabetes type is the keystone to proper treatment, yet monogenic diabetes can be confirmed only by genetic testing (3, 39). Histological analysis can enhance our understanding of these various forms of diabetes but is not clinically feasible or necessary. Our ability to predict monogenic diabetes solely on the basis of phenotypic presentation is limited, and individuals with a genetic diagnosis of MODY often do not fit all the classic criteria (2, 40). Mutations in genes causing neonatal diabetes may be diagnosed with diabetes well after 6 months of age (41). Similarly, organ donors with diabetes can present with complicated clinical and histopathological findings.
Our study suggests that wider use of comprehensive genetic testing, such as our NGS panel, both in routine clinical use and among archival collections of diabetes cases would most likely reveal genetic variants and allow for better characterization of the genetic basis of diabetes and differential histopathological spectrum of human diabetes. Further studies will shape our understanding of how β cells, islets, and the surrounding exocrine pancreas interact and lead to diabetes. They will also improve understanding of the heterogeneous nature of diabetes and the role that genes play in β-cell survival or destruction by immune cells, whether through autosomal dominant mutations seen in monogenic diabetes or the effects of single or multiple genes in type 1 diabetes, type 2 diabetes, or other forms of diabetes.
Acknowledgments
The authors thank Dr. Graeme Bell (University of Chicago, IL) for his comments and thoughtful discussions and acknowledge nPOD and Organ Procurement Organizations and tissue recovery institution staff members that partner with nPOD. Whole slide scans of images used in this publication are available through the JDRF nPOD Online Pathology database and additional donor details are available through nPOD DataShare.
Financial Support: This work was supported by the JDRF nPOD (JDRF research grants 25-2013-268, 17-2012-3, and 25-2012-516) and by the National Institutes of Health under award numbers K23DK094866, R01DK104942, UC4 DK 104155 01 (M.C.-T.), and P30DK020595.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- HbA1c
- glycated hemoglobin
- HLA
- human leukocyte antigen
- MODY
- maturity-onset diabetes of the young
- NGS
- next-generation sequencing
- nPOD
- Network for Pancreatic Organ Donors with Diabetes
- RPW
- relative pancreas weight.
References
- 1.Pihoker C, Gilliam LK, Ellard S, Dabelea D, Davis C, Dolan LM, Greenbaum CJ, Imperatore G, Lawrence JM, Marcovina SM, Mayer-Davis E, Rodriguez BL, Steck AK, Williams DE, Hattersley AT; SEARCH for Diabetes in Youth Study Group . Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab. 2013;98(10):4055–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53(12):2504–2508. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99(1):1–16. [DOI] [PubMed] [Google Scholar]
- 4.De Franco E, Flanagan SE, Houghton JA, Lango Allen H, Mackay DJ, Temple IK, Ellard S, Hattersley AT. The effect of early, comprehensive genomic testing on clinical care in neonatal diabetes: an international cohort study. Lancet. 2015;386(9997):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busiah K, Verkarre V, Cavé H, Scharfmann R, Polak M. Human pancreas endocrine cell populations and activating ABCC8 mutations. Horm Res Paediatr. 2014;82(1):59–64. [DOI] [PubMed] [Google Scholar]
- 6.Greeley SA, Zielinski MC, Poudel A, Ye H, Berry S, Taxy JB, Carmody D, Steiner DF, Philipson LH, Wood JR, Hara M. Preservation of reduced numbers of insulin-positive cells in sulfonylurea-unresponsive KCNJ11-related diabetes. J Clin Endocrinol Metab. 2017;102(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G, Crawford J, Schatz D, Pugliese A, Atkinson M. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28(7):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poudel A, Savari O, Striegel DA, Periwal V, Taxy J, Millis JM, Witkowski P, Atkinson MA, Hara M. Beta-cell destruction and preservation in childhood and adult onset type 1 diabetes. Endocrine. 2015;49(3):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, Millis JM, Witkowski P, Hara M. Regional differences in islet distribution in the human pancreas--preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013;8(6):e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in type 2 diabetes? Diabetologia. 2004;47(2):157–169. [DOI] [PubMed] [Google Scholar]
- 12.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol. 1984;15(7):677–683. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd M, Shields B, Hammersley S, Hudson M, McDonald TJ, Colclough K, Oram RA, Knight B, Hyde C, Cox J, Mallam K, Moudiotis C, Smith R, Fraser B, Robertson S, Greene S, Ellard S, Pearson ER, Hattersley AT; UNITED Team . Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the U.K. pediatric diabetes population with monogenic diabetes. Diabetes Care. 2016;39(11):1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserfall C, Montgomery E, Yu L, Michels A, Gianani R, Pugliese A, Nierras C, Kaddis JS, Schatz DA, Bonifacio E, Atkinson MA. Validation of a rapid type 1 diabetes autoantibody screening assay for community-based screening of organ donors to identify subjects at increased risk for the disease. Clin Exp Immunol. 2016;185(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. [DOI] [PubMed] [Google Scholar]
- 16.Campbell-Thompson ML, Kaddis JS, Wasserfall C, Haller MJ, Pugliese A, Schatz DA, Shuster JJ, Atkinson MA. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59(1):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkorta-Aranburu G, Carmody D, Cheng YW, Nelakuditi V, Ma L, Dickens JT, Das S, Greeley SAW, Del Gaudio D. Phenotypic heterogeneity in monogenic diabetes: the clinical and diagnostic utility of a gene panel-based next-generation sequencing approach. Mol Genet Metab. 2014;113(4):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loechner KJ, Akrouh A, Kurata HT, Dionisi-Vici C, Maiorana A, Pizzoferro M, Rufini V, de Ville de Goyet J, Colombo C, Barbetti F, Koster JC, Nichols CG. Congenital hyperinsulinism and glucose hypersensitivity in homozygous and heterozygous carriers of Kir6.2 (KCNJ11) mutation V290M mutation: K(ATP) channel inactivation mechanism and clinical management. Diabetes. 2011;60(1):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellanné-Chantelot C, Carette C, Riveline JP, Valéro R, Gautier JF, Larger E, Reznik Y, Ducluzeau PH, Sola A, Hartemann-Heurtier A, Lecomte P, Chaillous L, Laloi-Michelin M, Wilhem JM, Cuny P, Duron F, Guerci B, Jeandidier N, Mosnier-Pudar H, Assayag M, Dubois-Laforgue D, Velho G, Timsit J. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes. 2008;57(2):503–508. [DOI] [PubMed] [Google Scholar]
- 22.Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013;34(5):669–685. [DOI] [PubMed] [Google Scholar]
- 23.Allen HL, Flanagan SE, Shaw-Smith C, De Franco E, Akerman I, Caswell R, Ferrer J, Hattersley AT, Ellard S; International Pancreatic Agenesis Consortium . GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet. 2011;44(1):20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell-Thompson ML, Atkinson MA, Butler AE, Chapman NM, Frisk G, Gianani R, Giepmans BN, von Herrath MG, Hyöty H, Kay TW, Korsgren O, Morgan NG, Powers AC, Pugliese A, Richardson SJ, Rowe PA, Tracy S, In’t Veld PA. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56(11):2541–2543. [DOI] [PubMed] [Google Scholar]
- 25.Renard D, Fourcade G, Milhaud D, Bessis D, Esteves-Vieira V, Boyer A, Roll P, Bourgeois P, Levy N, De Sandre-Giovannoli A. Novel LMNA mutation in atypical Werner syndrome presenting with ischemic disease. Stroke. 2009;40(2):e11–e14. [DOI] [PubMed] [Google Scholar]
- 26.Hart LM, Stolk RP, Dekker JM, Nijpels G, Grobbee DE, Heine RJ, Maassen JA. Prevalence of variants in candidate genes for type 2 diabetes mellitus in The Netherlands: the Rotterdam study and the Hoorn study. J Clin Endocrinol Metab. 1999;84(3):1002–1006. [DOI] [PubMed] [Google Scholar]
- 27.Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, Ganguly A, Shyng SL, Stanley CA. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118(8):2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne MM, Sturis J, Menzel S, Yamagata K, Fajans SS, Dronsfield MJ, Bain SC, Hattersley AT, Velho G, Froguel P, Bell GI, Polonsky KS. Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on chromosome 12. Diabetes. 1996;45(11):1503–1510. [DOI] [PubMed] [Google Scholar]
- 29.Chao CS, McKnight KD, Cox KL, Chang AL, Kim SK, Feldman BJ. Novel GATA6 mutations in patients with pancreatic agenesis and congenital heart malformations. PLoS One. 2015;10(2):e0118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Franco E, Shaw-Smith C, Flanagan SE, Shepherd MH, Hattersley AT, Ellard S; International NDM Consortium . GATA6 mutations cause a broad phenotypic spectrum of diabetes from pancreatic agenesis to adult-onset diabetes without exocrine insufficiency. Diabetes. 2013;62(3):993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haldorsen IS, Ræder H, Vesterhus M, Molven A, Njølstad PR. The role of pancreatic imaging in monogenic diabetes mellitus. Nat Rev Endocrinol. 2011;8(3):148–159. [DOI] [PubMed] [Google Scholar]
- 32.Martinelli P, Cañamero M, del Pozo N, Madriles F, Zapata A, Real FX. Gata6 is required for complete acinar differentiation and maintenance of the exocrine pancreas in adult mice. Gut. 2013;62(10):1481–1488. [DOI] [PubMed] [Google Scholar]
- 33.Chojnowski A, Ong PF, Dreesen O. Nuclear lamina remodelling and its implications for human disease. Cell Tissue Res. 2015;360(3):621–631. [DOI] [PubMed] [Google Scholar]
- 34.Lu JT, Muchir A, Nagy PL, Worman HJ. LMNA cardiomyopathy: cell biology and genetics meet clinical medicine. Dis Model Mech. 2011;4(5):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Chang BH, Samson SL, Li MV, Chan L. The Krüppel-like zinc finger protein Glis3 directly and indirectly activates insulin gene transcription. Nucleic Acids Res. 2009;37(8):2529–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senée V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, Charon C, Nicolino M, Boileau P, Cavener DR, Bougnères P, Taha D, Julier C. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38(6):682–687. [DOI] [PubMed] [Google Scholar]
- 37.Nogueira TC, Paula FM, Villate O, Colli ML, Moura RF, Cunha DA, Marselli L, Marchetti P, Cnop M, Julier C, Eizirik DL. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. 2013;9(5):e1003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elbein SC, Sorensen LK, Schumacher MC. Methionine for valine substitution in exon 17 of the insulin receptor gene in a pedigree with familial NIDDM. Diabetes. 1993;42(3):429–434. [DOI] [PubMed] [Google Scholar]
- 39.Naylor R, Philipson LH. Who should have genetic testing for maturity-onset diabetes of the young? Clin Endocrinol (Oxf). 2011;75(4):422–426. [DOI] [PubMed] [Google Scholar]
- 40.Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, Ellard S, Farmer AJ, McCarthy MI, Owen KR. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35(6):1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Støy J, Steiner DF, Park SY, Ye H, Philipson LH, Bell GI. Clinical and molecular genetics of neonatal diabetes due to mutations in the insulin gene. Rev Endocr Metab Disord. 2010;11(3):205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]