Abstract
Context:
Offspring exposed in utero to maternal obesity have an increased risk of later obesity; however, the underlying mechanisms remain unknown.
Objective:
To assess the effect of an antenatal lifestyle intervention in obese women on the offspring’s cord blood metabolic profile and to examine associations of the cord blood metabolic profile with maternal clinical characteristics and offspring anthropometry at birth and age 6 months.
Design:
Randomized controlled trial and cohort study.
Setting:
The UK Pregnancies Better Eating and Activity Trial.
Participants:
Three hundred forty-four mother-offspring pairs.
Intervention:
Antenatal behavioral lifestyle (diet and physical activity) intervention.
Main Outcome Measures:
Targeted cord blood metabolic profile, including candidate hormone and metabolomic analyses.
Results:
The lifestyle intervention was not associated with change in the cord blood metabolic profile. Higher maternal glycemia, specifically fasting glucose at 28 weeks gestation, had a linear association with higher cord blood concentrations of lysophosphatidylcholines (LPCs) 16.1 (β = 0.65; 95% confidence interval: 0.03 to 0.10) and 18.1 (0.52; 0.02 to 0.80), independent of the lifestyle intervention. A principal component of cord blood phosphatidylcholines and LPCs was associated with infant z scores of birth weight (0.04; 0.02 to 0.07) and weight at age 6 months (0.05; 0.00 to 0.10). Cord blood insulin growth factor (IGF)-1 and adiponectin concentrations were positively associated with infant weight z score at birth and at 6 months.
Conclusions:
Concentrations of LPCs and IGF-1 in cord blood are related to infant weight. These findings support the hypothesis that susceptibility to childhood obesity may be programmed in utero, but further investigation is required to establish whether these associations are causally related.
Investigation of the cord blood metabolic profile was undertaken in offspring born to obese women identifying a novel role of lipid subspecies as a potential determinant of early infancy weight.
The increasing incidence of childhood obesity is a major public health concern. Recent global estimates from the WHO suggest that 41 million children under the age of 5 years are overweight or obese (1). Observational cohort studies and experimental animal studies have strongly suggested that both the pre- and postnatal environments modulate developmental pathways that increase susceptibility to later obesity (2). Offspring exposed to maternal obesity, excessive gestational weight gain (GWG), and/or gestational diabetes (GDM) in utero are at an increased risk of obesity and altered glucose metabolism throughout the life course (2, 3). Exposure to maternal obesity in utero is proposed to set offspring on a trajectory of increased adiposity throughout life due to persistent changes in metabolic function (4).
Metabolomics enables the investigation of low–molecular weight molecules such as intermediate metabolites and signaling molecules and can be used as a tool to provide insight in the systemic perturbations of an individual as a result of pathophysiological in utero exposure (5). Investigations of cord blood metabolic profiles have previously been conducted in small case control studies assessing associations with birth weight or postnatal trajectories and with limited adjustment for in utero confounding variables. In a large birth cohort from Germany, certain cord blood metabolites were associated with birth weight (6). However, neonatal adiposity explains only 40% of the observed variation in birth weight.
To date, no investigations have addressed the relations between maternal clinical and biochemical characteristics in obese women and fetal metabolism, in association with neonatal and early infancy weight and anthropometric measures of adiposity. We examined these relations in a group of obese pregnant women and their offspring who had taken part in the UK Pregnancies Better Eating and Activity Trial (UPBEAT), a randomized controlled trial (RCT) assessing a behavioral lifestyle intervention in 1555 obese pregnant women (7). Although the trial intervention did not reduce the incidence of GDM and delivery of a large-for-gestational-age infant (primary outcomes), we have recently reported a reduction in infant subscapular skinfold thickness (SFT) at 6 months of age mediated through marked improvements in maternal antenatal diet, measures of adiposity, and GWG initiated by the UPBEAT intervention (8).
Our primary aim was to determine if the intervention resulted in changes in a targeted cord blood metabolic profile and candidate hormones previously implicated with obesity and fetal growth. The secondary aim was to explore the relations between maternal antenatal characteristics including total GWG, prepregnancy body mass index (BMI) and GDM, and cord blood metabolic profile. As weight and adiposity have been shown to track through childhood, further assessment was made for potential relations between metabolites in the cord blood and measures of weight and anthropometry in offspring at birth and at 6 months of age.
Subjects and Methods
Study design
This study was a secondary analysis from the UPBEAT trial (7). To assess the primary aim of this study, the influence of the UPBEAT lifestyle intervention on the cord blood metabolic profile, the UPBEAT study was treated as an RCT (7). As the secondary aim of the study was to assess the relationship of the cord blood metabolic profile with maternal clinical characteristics and neonatal and infant anthropometry, an analysis based on a cohort study approach was chosen using both active treatment and control groups, and taking into account the original randomization allocation.
Study population
Primary aim
Women over the age of 16 years were recruited to the UPBEAT trial between 15+0 and 18+6 weeks gestation. The participants were from inner-city populations with high socioeconomic deprivation. The detailed study design, including inclusion and exclusion, has been previously published (7).
In summary, the UPBEAT study recruited 1555 obese women from eight tertiary maternity units located within inner-city populations. A behavioral intervention was devised based on psychological models of health behavior, including control and social cognitive theory, delivered via weekly sessions to increase physical activity and reduce maternal glycemic load and saturated fat intake. The primary maternal outcome was a reduction in the incidence of GDM at 27 to 28+6 weeks gestation, and the neonatal outcome was a reduction in the delivery of a large-for-gestational-age infant. Women were randomized using an online database with minimization for ethnicity, parity, and BMI to ensure that the groups were comparable at baseline. At 6 months postpartum, 47.3% of offspring were followed up. In comparison with those who did not take part, the mothers followed up at 6 months postpartum older, more likely to be white, nulliparous, and less likely to be current smokers (Supplemental Table 2 (974.3KB, pdf) ). There was no significant difference in sessions covered between those who did and did not take part in the current study (P = 0.09).
Secondary aim
Mother-neonate pairs were included in the analyses if detailed neonatal anthropometric and cord blood metabolic data were available. Infants were included within the further analysis of data at 6 months of age if they attended that follow-up appointment and did not suffer from major ill health.
Cord blood analyses
Cord blood biomarkers
Candidate cord blood biomarkers assessed in this study include cord blood insulin, C-peptide, glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, adiponectin, leptin, insulin growth factor (IGF)-I and -II, interleukin (IL)-6, and tumor necrosis factor (TNF)-α (Supplemental Text 1 (974.3KB, pdf) ).
Metabolomic analyses
A targeted cord plasma metabolome was analyzed using mass spectroscopy, enabling the quantification of phospholipids, acylcarnitines, nonesterified fatty acids (NEFAs), carboxylic acids, and amino acids as described previously (Supplemental Text 1 (974.3KB, pdf) ) (9, 10). Analysis was undertaken in eight batches.
Maternal variables
Maternal clinical characteristics that were investigated included maternal early-pregnancy BMI (kg/m2), total GWG (kg) defined from prepregnancy to 34 to 36 weeks gestation, GDM defined using the IADPSG’s diagnostic criteria at 24 to 28+6 weeks gestation, and the fasting glucose and 1- and 2-hour glucose concentrations at the time of the oral glucose tolerance test (OGTT) (11).
Offspring anthropometry
Neonate
Anthropometric measurements were made within 72 hours of birth by a trained midwife. Birth weight was recorded from maternal medical records and birth weight z scores calculated using a UK reference population adjusted for sex and gestation at delivery (12). Neonatal subscapular and triceps SFTs were measured using Harpenden skinfold calipers in triplicate, and the sum of SFTs (SSFTs) was calculated. Neonatal length was assessed using a neonatometer. Abdominal and mid upper arm circumferences were assessed.
Infant
Anthropometric measurements were collected at 6 months of age by a trained midwife. Weight was assessed using SECA scales, and length assessed in the supine position using an infantometer. Triceps and subscapular SFTs were measured in triplicate using Holtain calipers. Where reference WHO population data were available, z scores were calculated, adjusting for infant sex and age at measurement (13). Early catch-up growth was defined using the WHO definition of catch-up growth, defined as an increase ≥0.67 standard deviations (SDs) in weight z scores from birth to 6 months of age (13). Similarly catch-down growth was defined as a decrease in weight z scores from birth to 6 months of age of ≥0.67 SDs (13).
Statistical analyses
Cord blood metabolic profile
To correct for potential batch effects with the cord blood metabolomics analysis, linear regression models were applied and the residuals were used for further statistical analysis. Metabolites were standardized to average metabolite concentrations and SD over all eight batches for statistical analysis. Metabolites were included in the analyses if they had >70% complete data. Cord blood biomarkers and metabolomic variables were assessed for normality and transformed appropriately. Variables were summarized using mean (SD) and median interquartile range (IQR) where appropriate.
Principal component analysis (PCA) was undertaken for the metabolomic data only to reduce the number of metabolites based on a series of uncorrelated linear combinations of variables containing the most variance. Following orthogonal rotation, metabolites with a loading ≥ 0.1 were considered to have a strong association with the cluster.
Effect of a lifestyle intervention on the cord blood metabolic profile
Assessment was made for any differences in maternal characteristics and birth outcomes between those included vs those excluded from the analysis. Adjustment was made for any apparent differences in maternal characteristics at trial entry (15+0 to 18+6 weeks gestation) between the two arms. The effect of the UPBEAT intervention was assessed using linear regression adjusting for minimization variables used at trial randomization (ethnicity, parity, and maternal early-pregnancy BMI).
Maternal associations with cord blood metabolic profile
Maternal antenatal variables (including early-pregnancy BMI, GWG, and GDM) were assessed in relation to the cord blood metabolic profile. To assess for potential relationships, multivariable linear regression was undertaken, where components of the cord blood metabolic profile were treated as the outcome and maternal antenatal variables as the exposure adjusting for offspring sex, gestational age at delivery, and randomization to the UPBEAT intervention.
Cord blood metabolic profile and offspring anthropometry
To assess the association between the cord blood metabolic profile (exposure) and subsequent offspring anthropometry (outcome) at birth and at 6 months, multivariable linear or logistic regression was undertaken where appropriate.
Adjustment was made for confounders, selected a priori based on clinical knowledge with the aid of directed acyclic graphs (Supplemental Text 2 (974.3KB, pdf) ). Unless a systematic approach is taken, adjusting for potential confounders may increase bias; therefore, the proper use of directed acyclic graphs in selecting covariates is likely to reduce the degree of bias (14). Selected confounders included age at anthropometric measurement, offspring sex where appropriate, and randomization to the UPBEAT intervention (Model 1). Further adjustment was made for maternal parity, ethnicity (reference category, white ethnicity), current smoker in early pregnancy, GDM, and GWG (Model 2). For potential associations of metabolic profile at birth and infant anthropometry at 6 months of age, further adjustment was made for mode of feeding (reference category, exclusive breastfeeding ≥4 months of age).
All linear regression models were further assessed for data points exhibiting high leverage by using Cook’s Distance (defined as Di > 4/n), heteroscedasticity, and linearity. Correction for multiple testing was undertaken using a false discovery rate utilizing the Benjamin and Hochberg procedure. Presented significance levels were corrected for multiple testing (statistical significance, P < 0.05) (15).
Sensitivity analyses
Sensitivity analyses were undertaken by assessing demographic characteristics for those included within the analyses vs the mother-offspring pairs excluded. Sensitivity analyses were performed excluding offspring born <34 weeks gestation. A further sensitivity analysis was undertaken excluding mothers diagnosed with GDM. As mode of delivery has been shown to influence the cord blood metabolic profile, a fourth sensitivity analysis was undertaken with statistical models further adjusted for mode of delivery (reference category, unassisted vaginal delivery).
All statistical analyses were performed using Stata Version 14.1.
Results
Demography
Of the 608 cord samples available from neonates born to women randomized to the UPBEAT trial, 343 mother-offspring pairs were included (Supplemental Fig. 2 (974.3KB, pdf) ). Median maternal BMI was 35.6 kg/m2 (IQR 33.0, 38.9 kg/m2), 71.7% were of a white ethnic group, and 87.8% were in the highest quintiles of socioeconomic deprivation. Median neonatal birth weight was 3.5 kg (IQR 3.21, 3.82 kg), and 26.0% of offspring demonstrated significant catch-up growth as defined as >0.67 SD increase in infant weight z scores between offspring birth weight and assessment at 6 months of age. Further maternal, neonatal, and infant demographics and anthropometric characteristics are provided in Table 1. To assess for potential selection bias, comparisons were made between mother-offspring pairs included and excluded from the analysis. The incidence of black ethnicity, neonatal birth weight, and subscapular SFT was different between the two groups (Supplemental Table 2 (974.3KB, pdf) ). There was no difference in the incidence of GDM, total GWG, or infant anthropometric measures between the two groups (Supplemental Table 2 (974.3KB, pdf) ).
Table 1.
Maternal, Neonatal, and Infant Demographic, Anthropometry, and Clinical Characteristics of Mother-Offspring Pairs by Randomization Allocation (N = 343)
| Intervention |
Control |
All |
Mean (SD)/Median (IQR)/N (%) | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD)/Median (IQR)/N (%) | N | Mean (SD)/Median (IQR)/N (%) | N | |||
| Maternal | |||||||
| Age (y) | 169 | 31.0 (28.0, 35.0) | 174 | 31.0 (27.0, 35.0) | 343 | 31.0 (27.0, 35.0) | |
| BMI (kg/m2) | 169 | 35.5 (33.0, 39.1) | 174 | 35.7 (33.0, 38.5) | 343 | 35.6 (33.0, 38.9) | |
| Ethnicity | Asian | 169 | 12 (7.1) | 174 | 7 (4.0) | 343 | 19 (5.5) |
| Black | 32 (18.9) | 31 (17.8) | 63 (18.4) | ||||
| Other | 7 (4.1) | 8 (4.6) | 15 (4.4) | ||||
| White | 118 (69.8) | 128 (73.6) | 246 (71.7) | ||||
| Multiparous | 169 | 86 (50.9) | 174 | 79 (45.4) | 343 | 165 (48.1) | |
| Smoker in early pregnancy | 169 | 7 (4.1) | 174 | 12 (6.9) | 343 | 19 (5.5) | |
| Socioeconomic deprivation | 169 | 145 (85.7) | 174 | 151 (86.8) | 343 | 295 (87.8) | |
| GWG (kg) | 169 | 6.94 (4.27) | 174 | 8.00 (3.79) | 343 | 7.78 (4.07) | |
| GDM | 169 | 59 (34.9) | 174 | 52 (29.9) | 343 | 111 (32.4) | |
| Neonate | |||||||
| Mode of delivery | Vaginal | 169 | 73 (43.2) | 174 | 65 (37.4) | 343 | 138 (40.2) |
| Operative vaginal | 25 (14.8) | 19 (10.9) | 44 (12.8) | ||||
| Emergency C-section | 28 (16.6) | 50 (28.7) | 78 (22.7) | ||||
| Elective C-section | 43 (25.4) | 40 (23.0) | 83 (24.2) | ||||
| Gestation at delivery (wk) | 169 | 39.7 (38.7, 40.7) | 174 | 40.0 (38.7, 41.0) | 343 | 39.9 (38.7, 40.9) | |
| Birth weight (kg) | 169 | 3.51 (3.22, 3.79) | 174 | 3.59 (3.19, 3.86) | 343 | 3.6 (3.2, 3.8) | |
| Birth weight z scores | 169 | 0.12 (0.99) | 174 | 0.13 (0.99) | 343 | 0.21 (0.76) | |
| SFT-subscapular (mm) | 169 | 5.81 (1.46) | 174 | 5.75 (1.37) | 343 | 5.78 (1.42) | |
| SFT-triceps (mm) | 169 | 5.33 (1.42) | 174 | 5.34 (1.49) | 343 | 5.34 (1.45) | |
| SSFTs (mm) | 169 | 11.14 (2.61) | 174 | 11.09 (2.58) | 343 | 11.12 (2.59) | |
| Mid arm circumference (cm) | 169 | 11.57 (0.98) | 174 | 11.59 (0.97) | 343 | 11.58 (0.97) | |
| Abdominal circumference (cm) | 169 | 32.71 (2.11) | 174 | 32.57 (1.99) | 343 | 32.63 (2.05) | |
| Infant | |||||||
| Weight for age z score | 125 | 0.17 (1.04) | 123 | 0.42 (0.97) | 247 | 0.29 (1.01) | |
| Length for age z score | 120 | 0.36 (1.71) | 119 | 0.54 (1.99) | 238 | 0.44 (1.85) | |
| BMI for age z score | 120 | −0.00 (1.62) | 119 | 0.21 (2.02) | 238 | 0.10 (1.84) | |
| Arm circumference z score | 125 | 1.11 (0.92) | 122 | 1.40 (1.86) | 246 | 1.25 (1.47) | |
| Triceps SFT z score | 124 | 0.17 (1.59) | 119 | 0.44 (1.42) | 242 | 0.29 (1.51) | |
| Subscapular SFT z score | 107 | 0.23 (1.42) | 103 | 0.37 (1.49) | 209 | 0.30 (1.45) | |
| Catch-up growtha | 125 | 31 (24.8) | 122 | 34 (27.9) | 246 | 64 (26.0) | |
| Catch-down growthb | 125 | 36 (28.8) | 122 | 24 (19.7) | 246 | 60 (24.4) | |
Catch-up growth defined as a ≥0.67 SD increase in weight z score from birth to 6 months of age.
Catch-down growth defined as a ≥0.67 SD decrease in weight z scores from birth to 6 months of age.
One hundred ninety-one cord blood metabolites and 12 candidate biochemical markers were included in the analyses. Summary statistics of cord biochemical analyses including candidate biomarkers and metabolomic analyses are shown in Supplemental Table 3 (974.3KB, pdf) . Following PCA, four distinct principal components of metabolites were identified, which were phosphatidylcholines, NEFAs, long-chain acylcarnitines and tricarboxylic acid metabolites, and amino acids (Supplemental Fig. 3A–3D (974.3KB, pdf) ).
Effect of the UPBEAT intervention
Mothers included in this analysis were older, more likely to be nulliparous, and less likely to be of black ethnic origin compared with those without a cord blood sample (Supplemental Table 4 (974.3KB, pdf) ). Following correction for multiple testing, there were no significant differences in the cord blood metabolic profile, including clusters derived from PCA, between intervention and control arms (Supplemental Fig. 4 (974.3KB, pdf) ).
Relationships between maternal exposures and cord blood metabolic profile
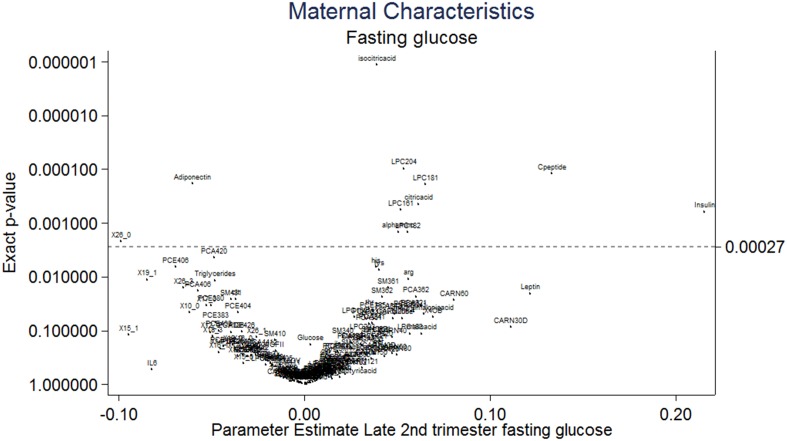
Diagnosis of maternal GDM was associated with reduced cord blood adiponectin and increased isocitric acid and lysophosphatidylcholine (LPC) 18.1 concentrations following correction for multiple testing by using a false discovery rate (Supplemental Fig. 5 (974.3KB, pdf) ). Both maternal early-pregnancy BMI and total GWG were not associated with the cord blood metabolic profile (Supplemental Figs. 6 and 7 (974.3KB, pdf) ). Maternal fasting glucose collected at the time of the OGTT (28 weeks gestation) was positively associated with higher cord insulin, C-peptide, LPC 18.1, 18.2, and 20.4, α aminoadipic acid, and citric acid following correction for multiple testing. Maternal fasting glucose was also associated with lower cord adiponectin and NEFA 26.0 (Fig. 1). Associations between maternal glucose at 1 and 2 hours post OGTT are illustrated in Supplemental Figs. 8 and 9 (974.3KB, pdf) .
Figure 1.
Volcano plot demonstrating the association of maternal fasting glucose at the time of the OGTT with the cord blood metabolic profile from infants born to obese pregnant women (n = 607). Parameter estimates are graphically represented for each biochemical variable in relation to maternal clinical characteristics following adjustment using a false discovery rate (Benjamin & Hochberg procedure) (17). Statistical significance, P < 0.0027. NEFAs are described using the nomenclature CX:Y, where X is the length of the carbon chain and Y is the number of double bonds. OH in the formula means the molecule contains a hydroxyl-group. CARN, carnitine; LPCE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PCE, acylalkylphosphatidylcholine; SM, sphingomyelin; X30B, x3methyl2oxobutanoicacid; X30V, x4methyl2oxovalvericacid.
Cord blood metabolic profile and neonatal anthropometry
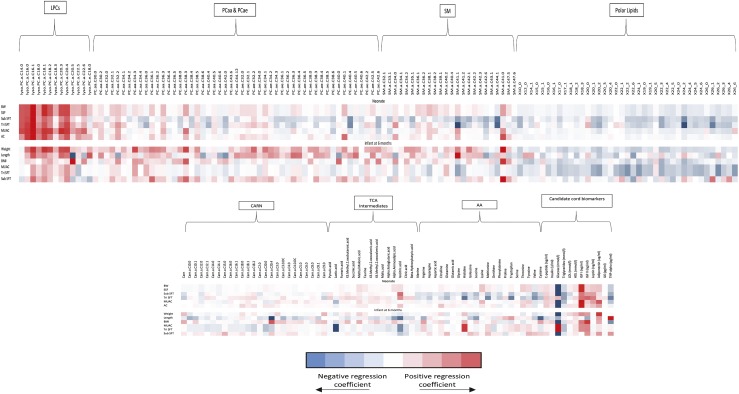
There was a positive linear relationship between cord C-peptide, insulin, IGF-1, leptin, and neonatal birth weight z scores, SSFT, subscapular SFT, triceps SFT (except for insulin and C-peptide), and mid upper arm and abdominal circumferences (Table 2; Supplemental Fig. 10 (974.3KB, pdf) ). Principal components of NEFAs as assessed in the metabolome and cord blood triglycerides were inversely associated with neonatal birth weight z scores, SSFT, subscapular SFT, triceps SFT, and mid upper arm circumference at birth (Table 2; Supplemental Fig. 10 (974.3KB, pdf) ). high-density lipoprotein, adiponectin, and principal components of phosphatidylcholines were linearly associated with birth weight z score only. LPC 16.1 and 18.0 were positively associated with neonatal birth weight, SSFT, and subscapular and triceps SFTs following correction for multiple testing (Fig. 2). Cord blood cholesterol was not associated with any measure of neonatal anthropometry (Supplemental Fig. 10 (974.3KB, pdf) ). IL-6 and TNF-α were negatively associated with neonatal birth weight z scores. There were no associations between cord principal components of acylcarnitines, amino acids, and IGF-II with any measure of neonatal anthropometry (Table 2; Fig. 2; Supplemental Fig. 10 (974.3KB, pdf) ).
Table 2.
Associations of the Cord Blood Metabolic Profile With Neonatal Anthropomety in Infants Born to Obese Pregnant Women (N = 344)
| Birth Weight z Scores (SDS)/Coef (95% CI) | SSFT (mm)/Coef (95% CI) | Subscapular SFT (mm)/Coef (95% CI) | Triceps SFT (mm)/Coef (95% CI) | MUAC (cm)/Coef (95% CI) | Abdominal Circumference (cm)/Coef (95% CI) | |
|---|---|---|---|---|---|---|
| PCA-phosphatidylcholines | 0.04 (0.02 to 0.07)a | −0.11 (–0.25 to 0.03) | −0.06 (–0.12 to 0.01) | −0.05 (–0.15 to 0.04) | 0.03 (–0.02 to 0.07) | 0.01 (–0.10 to 0.11) |
| PCA-NEFA | −0.04 (–0.08 to 0.00)b | −0.28 (–0.49 to –0.08)b | −0.11 (–0.21 to 0.00)b | −0.18 (–0.32 to –0.03)b | 0.02 (–0.05 to 0.10) | −0.01 (–0.17 to 0.15) |
| PCA-long-chain acylcarnitines and tricarboxylic acid metabolites | 0.00 (–0.04 to 0.04) | −0.05 (–0.27 to 0.17) | −0.02 (–0.13 to 0.09) | −0.03 (–0.18 to 0.12) | −0.01 (–0.08 to 0.07) | 0.01 (–0.15 to 0.17) |
| PCA-amino acids | 0.05 (0.00 to 0.11) | −0.05 (–0.36 to 0.25) | 0.06 (–0.09 to 0.20) | −0.11 (–0.32 to 0.10) | 0.06 (–0.04 to 0.16) | 0.19 (–0.03 to 0.40) |
| C-peptide (log2) (ng/mL) | 0.27 (0.14 to 0.39)a | 0.86 (0.22 to 1.50)b | 0.52 (0.17 to 0.87)b | 0.34 (–0.02 to 0.69) | 0.25 (0.00 to 0.49)b | 0.73 (0.22 to 1.25)b |
| Insulin (log2) (U/mL) | 0.16 (0.09 to 0.23)a | 0.47 (0.12 to 0.82)b | 0.31 (0.11 to 0.50)b | 0.17 (–0.03 to 0.37) | 0.16 (0.02 to 0.29)b | 0.38 (0.10 to 0.67)b |
| Glucose (log2) (mmol/L) | −1.80 (–3.59 to 0.00)b | −2.94 (–12.96 to 7.08) | −3.68 (–9.32 to 1.96) | 0.48 (–4.94 to 5.90) | −2.13 (–6.01 to 1.75) | −6.19 (–14.48 to 2.09) |
| Triglycerides (mmol/L) | −0.47 (–0.74 to –0.20)a | −1.65 (–3.18 to –0.11)b | −0.95 (–1.82 to –0.08)b | −0.73 (–1.56 to 0.10) | −0.90 (–1.48 to –0.33)b | −1.05 (–2.32 to 0.22) |
| Cholesterol (mmol/L) | 0.13 (–0.06 to 0.32) | −0.75 (–1.68 to 0.17) | −0.45 (–0.97 to 0.07) | −0.31 (–0.81 to 0.19) | −0.11 (–0.48 to 0.25) | 0.40 (–0.38 to 1.17) |
| HDL (mmol/L) | 0.22 (0.06 to 0.38)b | −0.11 (–0.89 to 0.67) | −0.25 (–0.68 to 0.17) | 0.16 (–0.28 to 0.60) | 0.02 (–0.27 to 0.32) | 0.38 (–0.25 to 1.01) |
| IGF-I (log2) (ng/mL) | 1.13 (0.97 to 1.29)a | 1.80 (0.89 to 2.72)a | 1.08 (0.58 to 1.59)a | 0.72 (0.20 to 1.24)b | 1.19 (0.87 to 1.51)a | 2.41 (1.70 to 3.12)a |
| IGF-II (log2) (ng/mL) | 0.31 (–0.02 to 0.65) | 1.57 (–0.15 to 3.29) | 0.68 (–0.27 to 1.63) | 0.89 (–0.08 to 1.85) | 0.29 (–0.37 to 0.96) | 0.54 (–0.86 to 1.93) |
| Leptin (log2) (ng/mL) | 0.38 (0.30 to 0.46)a | 1.59 (1.21 to 1.97)a | 0.83 (0.62 to 1.04)a | 0.76 (0.54 to 0.98)a | 0.50 (0.35 to 0.66)a | 0.80 (0.47 to 1.13)a |
| Adiponectin (log2) (ug/mL) | 0.40 (0.12 to 0.68) | 0.64 (–0.79 to 2.08) | 0.48 (–0.31 to 1.27) | 0.07 (–0.73 to 0.87) | 0.84 (0.31 to 1.37)b | 1.16 (0.00 to 2.31)b |
| IL-6 (log2) (pg/mL) | −0.04 (–0.07 to 0.00)b | 0.06 (–0.11 to 0.23) | −0.02 (–0.11 to 0.08) | 0.08 (–0.02 to 0.17) | −0.04 (–0.11 to 0.03) | −0.14 (–0.28 to 0.00)b |
| TNFα (log2) (pg/mL) | −0.58 (–1.12 to –0.03)b | −1.44 (–4.21 to 1.32) | −0.72 (–2.25 to 0.80) | −0.86 (–2.41 to 0.69) | −0.84 (–1.87 to 0.19) | −0.61 (–2.82 to 1.60) |
Regression coefficients with corresponding 95% confidence intervals presented are adjusted for maternal parity, ethnicity, smoker in early pregnancy, GDM, GWG, offspring sex, gestation at delivery, and randomization to UPBEAT Intervention.
Abbreviations: CI, confidence interval; Coef, regression coefficient; HDL, high-density lipoprotein; MUAC, mid upper arm circumference; SDS, SD scores.
P < 0.001.
P < 0.05.
Figure 2.
Heat map demonstrating associations between cord blood metabolites with anthropometric measurements in neonates (n = 344) and 6-month-old infants (n = 209). Data from infants born to obese pregnant women in the UPBEAT study. Regression coefficient plots with adjustment made for maternal parity, ethnicity, smoker in early pregnancy, GDM, GWG, offspring sex, gestation at delivery, and randomization to UPBEAT intervention. Additional adjustment is made for early mode of infant feeding for infant anthropometry data at 6 months of age. NEFAs are described using the nomenclature CX:Y, where X is the length of the carbon chain and Y is the number of double bonds. OH in the formula means the molecule contains a hydroxyl-group. BW, neonatal birth weight z scores (SDs); CARN, carnitine; LPCE, lysophosphatidylethanolamine; MUAC, mid upper arm circumference (cm); PC, phosphatidylcholine; PCaa, diacylphosphatidylcholine; PCae, acylalkylphosphatidylcholine; SM, sphingomyelin; tri SFT, triceps SFT (mm); sub SFT, subscapular SFT (mm).
Cord blood metabolic profile and infant anthropometry at 6 months of age
Of those biochemical variables, which were significantly associated with neonatal body composition, clusters of phosphatidylcholines and adiponectin were linearly associated with infant weight and length z scores at 6 months of age (Fig. 2; Supplemental Table 5 (974.3KB, pdf) ). In particular, LPC 16.1 and 18.1 were linearly associated with infant weight z scores at 6 months of age (Fig. 2; Supplemental Table 5 (974.3KB, pdf) ). Cord IGF-I was linearly associated with infant weight z scores, BMI z score, and mid upper arm circumference z score (Fig. 2; Supplemental Table 5 (974.3KB, pdf) ). Cord leptin and triglycerides were negatively associated with infant mid upper arm circumference z scores following adjustment for maternal and infant confounding (Fig. 2; Supplemental Table 5 (974.3KB, pdf) ). There were no associations between cord insulin, glucose, C-peptide, and IL-6 with infant anthropometry at 6 months of age (Fig. 2; Supplemental Table 5 (974.3KB, pdf) ).
For every unit increase in principal components of phosphatidylcholines, the odds of catch-up growth at 6 months of age increased by 1.35 (1.04 to 1.75), whereas leptin decreased by 0.33 (0.17 to 0.52) (Supplemental Table 6 (974.3KB, pdf) ). IGF-1 and leptin were positively associated, with increased odds of catch-down growth at 6 months of age (Supplemental Table 6 (974.3KB, pdf) ).
Sensitivity analyses
The associations between cord blood metabolic profile and neonatal or infant body composition remained unchanged following removal of offspring born <34 weeks gestation (n = 36) (Supplemental Figs. 11 and 12 (974.3KB, pdf) ) and those participants exposed to GDM (n = 111) (Supplemental Figs. 13 and 14 (974.3KB, pdf) ) and following further adjustment for mode of delivery (Supplemental Figs. 15 and 16 (974.3KB, pdf) ).
Discussion
This study reports a comprehensive cord blood metabolic profile, including candidate biochemical markers and metabolome, in offspring born to obese mothers. By demonstrating associations with fasting glucose, it has been shown that in utero exposure to maternal dysglycemia in obese pregnancies has the potential to modify the cord blood metabolic profile at birth. Although there was no effect of the UPBEAT antenatal lifestyle intervention on the cord blood metabolic profile, when treating the data as a cohort, associations were observed between the cord blood metabolic profile and offspring growth in early life. The unique associations between cord LPCs and neonatal adiposity, together with the relation with maternal hyperglycemia, may provide mechanistic insight into the early-life origins of obesity.
The lack of effect of the UPBEAT intervention on the cord metabolic profile may suggest that the differences observed in the maternal secondary outcomes, including reduction in adiposity and GWG, were inadequate to have a major impact on fetal metabolism, although more subtle molecular effects could have occurred to influence adiposity in the 6-month infants as recently reported (8). Principal components of phosphatidylcholines and LPCs were found to be positively associated with early growth velocities and weight z scores, providing possible mechanistic insight of the mechanisms contributing to early postnatal growth in offspring born to obese women. The finding that principal components of cord LPCs, primarily LPCs 16.1 and LPCs 18.1, were associated with not only neonatal weight z scores, but also infant growth and catch-up growth within the first 6 months of age provides unique evidence suggesting a role in the early life growth velocities. Of relevance, associations with cord LPCs and birth weight were recently reported in a birth cohort from Germany (6). In the current study, LPCs were associated with neonatal adiposity as well as birth weight, supporting a role in body fat accretion. It may be of relevance to these observations that the infant growth trajectory in the first 6 months of life has been shown to be predictive of adolescence and early-adulthood obesity (16) and that The European Childhood Obesity Program has shown that LPC 14.0 correlates with rapid growth in infancy and subsequent obesity at 6 years of age (17). Together these findings would suggest a possible role for LPCs in the early life “programming” of obesity risk (18).
Further interrogation of the data set demonstrated a significant linear relationship between cord blood insulin, C-peptide, and IGF-1 with cord LPCs (Supplemental Figs. 17 and 18 (974.3KB, pdf) ), supporting the suggestion of an interaction between fetal glucose homeostasis and these molecules (19). This observation is in part supported by one small case control study (N = 46) that identified an inverse relationship between maternal GDM and placental uptake of LPC 22:6 in women of heterogeneous BMI (20). A role in fetal metabolism for LPCs has also been suggested in the nonpregnant state with the development of visceral fat obesity, unrelated to genetic origin but associated with nutritional status (21). A study from the US Project Viva cohort demonstrated that associations between cord blood metabolites from a metabolome, particularly those related to one-carbon metabolism, may contribute to rapid postnatal weight gain in offspring born to women of heterogeneous BMI (22). Taken together, studies of the cord blood metabolic profile suggest that obesity risk may be determined at birth (4). Antenatal interventions directed toward optimizing adverse fetal exposures may therefore contribute to curbing the incidence of childhood obesity.
The positive associations between cord blood IGF-1 with neonatal measures of growth and body composition together with infant weight and mid upper arm circumference z scores at 6 months also suggests a persistent influence of in utero exposures on early growth. Although the relationship between the cord blood metabolic profile and differential growth in early infancy suggests a potential persistent effect on growth at 6 months of age, mechanisms must remain conjectural and causal inference should be made with caution. However, several studies have suggested that the IGF-1 gene may be prone to epigenetic modification in utero (23–25), with animal studies shedding some light on this in providing evidence of an interaction with maternal glycemia status. For example, Zinkhan et al. (26) demonstrated that in utero exposure to maternal glycemia in rats led to decreased hepatic H3Me3K36 and messenger RNA variants of the IGF-1 gene in the offspring. Others have implicated a role of these variants to a predisposition to later obesity and insulin resistance (25). Whether epigenetic modification may also influence lipid metabolism, including that of LPCs, remains conjectural.
The linear associations with cord adiponectin and measures of weight, length, and subscapular z scores at 6 months are in keeping with recent evidence from a prospective cohort study from Germany in children born to women of heterogeneous BMI (n = 141), suggesting a potential long-term influence in children at 5 years of age (27). We also found that cord blood leptin was associated with measures of neonatal growth and body composition and increased odds of catch-up growth from birth to 6 months of age, suggesting a potential mediatory role of early infancy growth. Cord blood leptin has been implicated as a proxy for neonatal fat mass as it is synthesized by the adipocyte Ob gene and is proportional to adipose tissue mass (28). This study has demonstrated an inverse relationship with cord leptin and catch-up growth independent of birth weight, which may be explainable by a state of leptin resistance in early infancy, as observed in previous studies (29).
The linear associations between cord blood anabolic hormones, including cord blood C-peptide, insulin, and IGF-1 with measures of growth and body composition at birth, agree with previous studies in offspring born to women of heterogeneous BMI (30, 31) and concur with the knowledge that insulin and IGF-1 are the most important regulators of fetal growth in the second and third trimester (32). IGF-1 has been shown consistently to be raised in cord blood of offspring born to obese women, predominately as a consequence of maternal dysglycemia (33).
Triglycerides and NEFAs in the maternal circulation have been widely implicated as determinants of fetal growth in obese and diabetic women (32). However, in this study, an inverse relationship between clusters of NEFAs and triglycerides with neonatal growth and adiposity was observed, which has also been reported by others (34, 35). This association with low rather than high birth weight could reflect mobilization of lipids as an alternative fuel source (34). Importantly, this study adds to others that have questioned the role of triglycerides in the determination of neonatal adiposity.
Despite the suggestion that inflammatory mediators (IL-6 and TNF-α) may contribute to the development of neonatal adiposity in utero, through regulation of central pathways of satiety and appetite (36, 37), we found an inverse association with neonatal body composition. There is no obvious explanation for this observation.
Strengths of this study include an extensive assessment of the cord blood metabolic profile at birth, detailed neonatal and infant anthropometric data collection and prospective collection of maternal early pregnancy BMI, total GWG, and measures of maternal insulin resistance. Using data reduction techniques for the cord blood metabolome, metabolite clusters of biological importance associated with measures of neonatal and infant anthropometry were identified.
Limitations include the collection of mixed cord blood (umbilical artery and vein), which weakens conclusions regarding fetal or maternal origin of the metabolites in this study, as well as previously published reports (22). Although treatment of GDM has the potential to influence cord insulin, C-peptide, and IGF-1 concentrations, this was not adjusted for within this analysis; however, a sensitivity analysis removing women with GDM did not modify the observed relationships. It must be recognized that the metabolome and the candidate markers measured provide only an incomplete profile of the late-pregnancy in utero fetal exposures, as unmeasured micronutrients, essential fatty acids, and steroid hormones may also contribute to neonatal and early life growth and body composition.
In summary, this study of more than 300 infants describes for the first time to our knowledge, a comprehensive cord blood metabolic profile in offspring born to obese women. Known associations of metabolic variables with infant adiposity were confirmed and questions raised regarding previous associations derived from smaller cohorts. Importantly we have highlighted unique associations with lipid subspecies and early postnatal growth and provide supporting evidence that IGF-1 at birth may be a determinant of later growth trajectories. Current investigation of the maternal metabolome and neonatal epigenome may shed light on the causative mechanisms and further insight into growth trajectories. Ongoing studies of the cord epigenome may provide further mechanistic insight into potential pathways. Replication in other cohorts including the use of Mendelian randomization methods are required to determine causality. Ongoing follow-up of the UPBEAT offspring will address the long-term implications of these observed associations.
Acknowledgments
Financial Support: This work was supported by the European Union’s 7th Framework Programme (FP7/2007-2013), project EarlyNutrition under grant 289346, the Action Medical Research Council (GN2456), National Institute for Health Research Programme Grants for Applied Research Programme (RP-0407-10452), the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG; no. 322605), the Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London, the Chief Scientist Office Scotland, Guy’s and St. Thomas’ Charity and Tommy’s Charity (registered charity no. 1060508), and the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre (to K.G.).
Clinical Trial Information: Current Controlled Trials no. ISCRTN89971375 (registered 28 November 2008).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- GDM
- gestational diabetes
- GWG
- gestational weight gain
- IGF
- insulin growth factor
- IL
- interleukin
- IQR
- interquartile range
- LPC
- lysophosphatidylcholine
- NEFA
- nonesterified fatty acid
- OGTT
- oral glucose tolerance test
- PCA
- principal component analysis
- SD
- standard deviation
- SFT
- skinfold thickness
- SSFT
- sum of skinfold thickness
- TNF
- tumor necrosis factor
- UPBEAT
- UK Pregnancies Better Eating and Activity Trial.
References
- 1.WHO. Ending childhood obesity report. Available at: http://apps.who.int/iris/bitstream/10665/204176/1/9789241510066_eng.pdf. Accessed 24 June 2015.
- 2.Patel N, Pasupathy D, Poston L. Determining the consequences of maternal obesity for offspring health. Exp Physiol. 2015;100(12):1421–1428. [DOI] [PubMed] [Google Scholar]
- 3.Fraser A, Lawlor DA. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diab Rep. 2014;14(5):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giles LC, Whitrow MJ, Davies MJ, Davies CE, Rumbold AR, Moore VM. Growth trajectories in early childhood, their relationship with antenatal and postnatal factors, and development of obesity by age 9 years: results from an Australian birth cohort study. Int J Obes. 2015;39(7):1049–1056. [DOI] [PubMed] [Google Scholar]
- 5.Hivert MF, Perng W, Watkins SM, Newgard CS, Kenny LC, Kristal BS, Patti ME, Isganaitis E, DeMeo DL, Oken E, Gillman MW. Metabolomics in the developmental origins of obesity and its cardiometabolic consequences. J Dev Orig Health Dis. 2015;6(2):65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmuth C, Uhl O, Standl M, Demmelmair H, Heinrich J, Koletzko B, Thiering E. Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obes Facts. 2017;10(2):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E, Pasupathy D, Patel N, Robson SC, Sandall J, Sanders TAB, Sattar N, Seed PT, Wardle J, Whitworth MK, Briley AL. Effect of a behavioural intervention in obese pregnant women (the UK Pregnancies Better Eating and Activity Trial study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–777. [DOI] [PubMed] [Google Scholar]
- 8.Patel N, Godfrey KM, Pasupathy D, Levin J, Flynn AC, Hayes L, Briley AL, Bell R, Lawlor DA, Oteng-Ntim E, Nelson SM, Robson SC, Sattar N, Singh C, Wardle J, White SL, Seed PT, Poston L. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes. 2017;41(7):1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harder U, Koletzko B, Peissner W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(7-8):495–504. [DOI] [PubMed] [Google Scholar]
- 10.Hellmuth C, Weber M, Koletzko B, Peissner W. Nonesterified fatty acid determination for functional lipidomics: comprehensive ultrahigh performance liquid chromatography-tandem mass spectrometry quantitation, qualification, and parameter prediction. Anal Chem. 2012;84(3):1483–1490. [DOI] [PubMed] [Google Scholar]
- 11.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright CM, Booth IW, Buckler JMH, Cameron N, Cole TJ, Healy MJR, Hulse JA, Preece MA, Reilly JJ, Williams AF. Growth reference charts for use in the United Kingdom. Arch Dis Child. 2002;86(1):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO World Health Organisation Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. [DOI] [PubMed] [Google Scholar]
- 14.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser A Stat Soc. 1995;1:289–300. [Google Scholar]
- 16.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–908. [DOI] [PubMed] [Google Scholar]
- 17.Rzehak P, Hellmuth C, Uhl O, Kirchberg FF, Peissner W, Harder U, Grote V, Weber M, Xhonneux A, Langhendries J-P, Ferre N, Closa-Monasterolo R, Verduci E, Riva E, Socha P, Gruszfeld D, Koletzko B; European Childhood Obesity Trial Study Group . Rapid growth and childhood obesity are strongly associated with lysoPC(14:0). Ann Nutr Metab. 2014;64(3-4):294–303. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133. [DOI] [PubMed] [Google Scholar]
- 19.Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong C, Halliday HL, Hennis AJ, Liley H, Ng PC, Coustan DR, Hadden DR, Hod M, Oats JJN, Trimble ER; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126(6):e1545–e1552. [DOI] [PubMed] [Google Scholar]
- 20.Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, Peissner W, Ruiz-Alcaraz AJ, Parrilla JJ, Koletzko B, Larque E. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr. 2016;1:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Kim M, Jung S, Lee S-H, Lee JH. Altered plasma lysophosphatidylcholines and amides in non-obese and non-diabetic subjects with borderline-to-moderate hypertriglyceridemia: a case-control study. PLoS One. 2015;10(4):e0123306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isganaitis E, Rifas-Shiman SL, Oken E, Dreyfuss JM, Gall W, Gillman MW, Patti ME. Associations of cord blood metabolites with early childhood obesity risk. Int J Obes. 2015;39(7):1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao PC, Matheny AP Jr, Lang CA. Insulin-like growth factor-I comparisons in healthy twin children. J Clin Endocrinol Metab. 1994;78(2):310–312. [DOI] [PubMed] [Google Scholar]
- 24.Baker J, Liu J-P, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 25.Fu Q, Yu X, Callaway CW, Lane RH, McKnight RA. Epigenetics: intrauterine growth retardation (IUGR) modifies the histone code along the rat hepatic IGF-1 gene. FASEB J. 2009;23(8):2438–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinkhan EK, Fu Q, Wang Y, Yu X, Callaway CW, Segar JL, Scholz TD, McKnight RA, Joss-Moore L, Lane RH. Maternal hyperglycemia disrupts histone 3 lysine 36 trimethylation of the IGF-1 gene. J Nutr Metab. 2012;2012:930364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer DM, Brei C, Stecher L, Much D, Brunner S, Hauner H. Cord blood and child plasma adiponectin levels in relation to childhood obesity risk and fat distribution up to 5 y. Pediatr Res. 2017;81(5):745–751. [DOI] [PubMed] [Google Scholar]
- 28.Zimmet P, Hodge A, Nicolson M, Staten M, de Courten M, Moore J, Morawiecki A, Lubina J, Collier G, Alberti G, Dowse G. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. BMJ. 1996;313(7063):965–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, Dunger DB; ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood . Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. J Clin Endocrinol Metab. 1999;84(3):1145–1148. [DOI] [PubMed] [Google Scholar]
- 30.Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. J Endocrinol. 1991;129(3):459–464. [DOI] [PubMed] [Google Scholar]
- 31.Carlsen EM, Renault KM, Jensen RB, Nørgaard K, Jensen J-EB, Nilas L, Cortes D, Michaelsen KF, Pryds O. The association between newborn regional body composition and cord blood concentrations of C-Peptide and insulin-like growth factor I. PLoS One. 2015;10(7):e0121350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32(6):1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawlor DA, West J, Fairley L, Nelson SM, Bhopal RS, Tuffnell D, Freeman DJ, Wright J, Whitelaw DC, Sattar N. Pregnancy glycaemia and cord-blood levels of insulin and leptin in Pakistani and white British mother-offspring pairs: findings from a prospective pregnancy cohort. Diabetologia. 2014;57(12):2492–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelishadi R, Badiee Z, Adeli K. Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatr Perinat Epidemiol. 2007;21(6):518–524. [DOI] [PubMed] [Google Scholar]
- 35.Geraghty AA, Alberdi G, O’Sullivan EJ, O’Brien EC, Crosbie B, Twomey PJ, McAuliffe FM. Maternal blood lipid profile during pregnancy and associations with child adiposity: findings from the ROLO study. PLoS One. 2016;11(8):e0161206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”. Exp Diabetes Res. 2011;2011:218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cesar HC, Pisani LP. Fatty-acid-mediated hypothalamic inflammation and epigenetic programming. J Nutr Biochem. 2017;42:1–6. [DOI] [PubMed] [Google Scholar]