Abstract
Context:
Aerobic exercise training can increase brain volume and blood flow, but the impact on brain metabolism is less known.
Objective:
We determined whether high-intensity interval training (HIIT) increases brain metabolism by measuring brain glucose uptake in younger and older adults.
Design:
Brain glucose uptake was measured before and after HIIT or a sedentary (SED) control period within a larger exercise study.
Setting:
Study procedures were performed at the Mayo Clinic in Rochester, MN.
Participants:
Participants were younger (18 to 30 years) or older (65 to 80 years) SED adults who were free of major medical conditions. Group sizes were 15 for HIIT (nine younger and six older) and 12 for SED (six younger and six older).
Intervention:
Participants completed 12 weeks of HIIT or SED. HIIT was 3 days per week of 4 × 4 minute intervals at over 90% of peak aerobic capacity (VO2peak) with 2 days per week of treadmill walking at 70% VO2peak.
Main Outcome Measures:
Resting brain glucose uptake was measured using 18F-fluorodeoxyglucose positron emission tomography scans at baseline and at week 12. Scans were performed at 96 hours after exercise. VO2peak was measured by indirect calorimetry.
Results:
Glucose uptake increased significantly in the parietal-temporal and caudate regions after HIIT compared with SED. The gains with HIIT were not observed in all brain regions. VO2peak was increased for all participants after HIIT and did not change with SED.
Conclusion:
We demonstrate that brain glucose metabolism increased after 12 weeks of HIIT in adults in regions where it is reduced in Alzheimer’s disease.
Twelve weeks of HIIT was sufficient to increase glucose uptake in select regions of the brain in younger and older adults.
Aerobic exercise can potently increase substrate oxidation pathways in exercising tissue (1), but less is known about metabolic benefits on nonexercising tissue, such as the brain. Acute studies indicate that cerebral blood flow is increased to the brain during aerobic exercise (2), and longitudinal studies show that aerobic exercise training improves brain volume and memory (3). Such increases in blood flow and brain volume indicate the potential for aerobic exercise training to improve brain metabolism. Glucose is the primary fuel source for the brain, and therefore we hypothesized that cerebral glucose uptake would improve after aerobic exercise training.
High-intensity interval training (HIIT) involves repeating short bouts of exercise for 30 seconds to 4 minutes with workloads near or above peak aerobic capacity (VO2peak). Training programs that include HIIT can produce large improvements in cardiorespiratory fitness (∼20% to 30% increases in VO2peak) and skeletal muscle mitochondria (∼50% to 70% increases in respiration) (1), and these robust gains occur within relatively shorter periods compared with moderate-intensity continuous exercise (4, 5). Greater exercise intensity helps promote cardiorespiratory fitness (6), which is an important consideration for brain health given that higher cardiorespiratory fitness is associated with greater brain plasticity at baseline and after aerobic training in older adults (7). Because HIIT can robustly improve cardio-metabolic outcomes, we hypothesized that HIIT would increase brain glucose uptake in previously sedentary (SED) adults.
Materials and Methods
We performed a 12-week training study with a SED control period in younger and older adults and reported the primary results on skeletal muscle adaptations (1). A subset of participants volunteered for analysis of brain glucose uptake using 18fluorodeoxyglucose positron emission tomography (18FDG-PET) scans at baseline and after the HIIT or SED periods. Participants provided written informed consent for the additional measurements. The study design was approved by the Institutional Review Board at Mayo Clinic.
Study participants
Participants were either 18 to 30 years or 65 to 80 years old and were SED as defined by engaging in structured activity for less than two sessions of 30 minutes per week. Exclusion criteria included pregnancy, cardiovascular disease, diabetes, kidney disease, or untreated thyroid disease. Exclusion criteria for medication included anticoagulants, insulin, corticosteroids, sulfonylureas, barbiturates, β-blockers, insulin sensitizers, opiates, and tricyclic antidepressants. The older women were postmenopausal, and all younger women had a form of hormonal contraceptive. Body composition was measured by dual-energy X-ray absorptiometry. The homeostatic model of insulin resistance (HOMA-IR) was calculated from fasting blood glucose (FPG, mmol/mL) and insulin (FPI, μIU/ml) as HOMA-IR = (FPG × FPI)/22.5 as described by Matthews et al. (8).
Aerobic capacity
VO2peak was measured using indirect calorimetry (Medgraphics Diagnostics, MN) and an electronically braked cycle ergometer (Lode Medical Technologies, Netherlands) using 2-minute stages (young male, 50W + 30W; young female, 50W + 20W; older male, 50W + 20W; older female, 25W + 20W). A high respiratory exchange ratio of >1.0 was achieved at VO2peak for all participants with an average ± standard deviation at baseline of 1.23 ± 0.08 for SED and 1.23 ± 0.09 for HIIT and then at follow-up of 1.22 ± 0.1 for SED or 1.18 ± 0.08 for HIIT.
Exercise training
HIIT was performed on a cycle ergometer 3 days per week of 4 × 4 minute intervals at 90% VO2peak with 3 minutes of rest (little to no workload) between intervals and 2 days per week of walking on a treadmill at 70% VO2peak for 45 minutes. The cycling intervals and treadmill walking were performed on separate days. Intensity was verified by monitoring heart rate, and all exercise sessions were supervised by an exercise physiologist. The SED group performed no structured exercise for 12 weeks, and activity was monitored by accelerometers, which were exchanged at a weekly visit that included recording of body weight.
Brain metabolism
Brain glucose uptake was measured by 18FDG-PET after an overnight fast as described (9) at baseline and after HIIT or SED. The post-HIIT PET scans were performed 96 hours after the final exercise bout to avoid potential acute effects of exercise, which has been shown to decrease brain glucose uptake within 30 minutes of exercise (10). The FDG scan was performed 30 minutes after injection with an 8-minute acquisition. Scans were analyzed using Cortex ID (GE Health Care, Inc., Milwaukee, WI) to produce FDG surface projections and Z-score maps (11).
Cortex ID generates standardized three-dimensional stereotactic surface projection datasets for individual subjects and performs statistical analysis as compared with healthy control subjects. For this, each individual PET scan was first realigned to the midsagittal plane. The AC-PC line (a line passing through the anterior and posterior commissures) was estimated by iterative matching between the individual image set and a standard atlas template. This standard atlas template was produced as an average of FDG images from 66 healthy volunteers. The individual image sets were realigned to the standard stereotactic coordinate system based on the estimated AC-PC line, and the images were warped to match the stereotactic atlas coordinate system proposed by Talairach and Tournoux (12). Individual landmarks for cortical projections were searched iteratively between centers and cortical landmarks predetermined on the template brain using a profile curve analysis. Detected individual landmarks were then warped to predefined landmarks, resulting in a standardized image set with a uniform voxel size of 2.25 mm, interpolation to 60 slices, and a matrix size of 128 × 128. This enabled reliable pixel-by-pixel comparisons of these anatomically standardized brain images. To determine projection map values, the program used a predetermined vector that is 6 pixels (13.5 mm) long and oriented perpendicular to the outer and medial surfaces of the right- and left-brain hemispheres for each surface pixel. The surface pixel was assigned the highest pixel value found along this vector. We normalized surface pixel values to the pons (option within Cortex ID). The normalized cortical pixel values were used to calculate a statistical map, which showed surface pixel-by-pixel z-scores derived from comparing an individual’s scan with results from normal control subjects. These z-score maps and values were used for comparison of baseline and post-test scans to detect changes in brain metabolism.
Statistics
The current study is a subset of a larger study (1) and was intended as an initial investigation into changes in brain glucose uptake with HIIT in younger and older adults. Statistics were analyzed using JMP 10 (SAS Institute Inc., Cary, NC) and graphed using Prism 6 (GraphPad Software Inc., La Jolla, CA). Changes in regional brain glucose uptake were calculated as z-scores for each person with the individual’s baseline value as the reference. Changes from pre to post were calculated for each outcome variable and then compared with two-way ANOVA (age × training) between the SED and HIIT groups. The current analysis was not powered to detect potential interactions between age and training. Statistical significance was set at P = 0.05 for main effects of ANOVA.
Results
Baseline characteristics are displayed in Table 1 and indicate that the absolute VO2peak was not different between SED and HIIT but was lower in older adults. Baseline fasting blood glucose was slightly higher in older adults, but fasting insulin or HOMA-IR were not different between age groups. Plasma glucose [mean (standard deviation) in mmol/L] was not different between groups prior to PET scans at baseline [SED: 5.2 (0.9); HIIT: 5.0 (0.4)] and at follow-up [SED: 5.4 (0.5); HIIT: 5.0 (0.5)]. HOMA-IR did not change with HIIT and SED.
Table 1.
Baseline Characteristics of Participants Studied for Brain Glucose Uptake Before and After SED Period or HIIT
| SED (n = 12) |
HIIT (n = 15) |
ANOVA P Value |
|||||
|---|---|---|---|---|---|---|---|
| Young | Older | Young | Older | Age | Training | Age × Training | |
| M/F | 3/3 | 1/5 | 4/5 | 3/3 | |||
| Age, y | 27 (2) | 69 (4) | 25 (5) | 69 (4) | |||
| BMI, kg/m2 | 26.7 (3) | 25.9 (4) | 23.6 (3) | 25.9 (3) | 0.4762 | 0.222 | 0.225 |
| DEXA fat, % | 33.6 (2) | 39.3 (6) | 32.1 (8) | 33.8 (3) | 0.067 | 0.233 | 0.576 |
| Fasting glucose, mmol/L | 5.3 (0.3) | 5.6 (0.6) | 5.3 (0.3) | 5.7 (0.3) | 0.012 | 0.886 | 0.861 |
| Fasting insulin, μIU/mL | 5.6 (3) | 4.4 (2) | 5.4 (2) | 5.3 (4) | 0.477 | 0.873 | 0.697 |
| HOMA-IR | 1.3 (0.8) | 1.1 (0.6) | 1.3 (0.5) | 1.4 (1) | 0.775 | 0.86 | 0.657 |
| VO2peak, mL/min | 2713 (652) | 1510 (580) | 2225 (514) | 1634 (384) | 0.0003 | 0.397 | 0.159 |
Data are mean (standard deviation) for change from baseline to post measurement; P values are reported for two-way ANOVA using Training and Age groups.
Abbreviations: BMI, body mass index; DEXA, dual-energy X-ray absorptiometry.
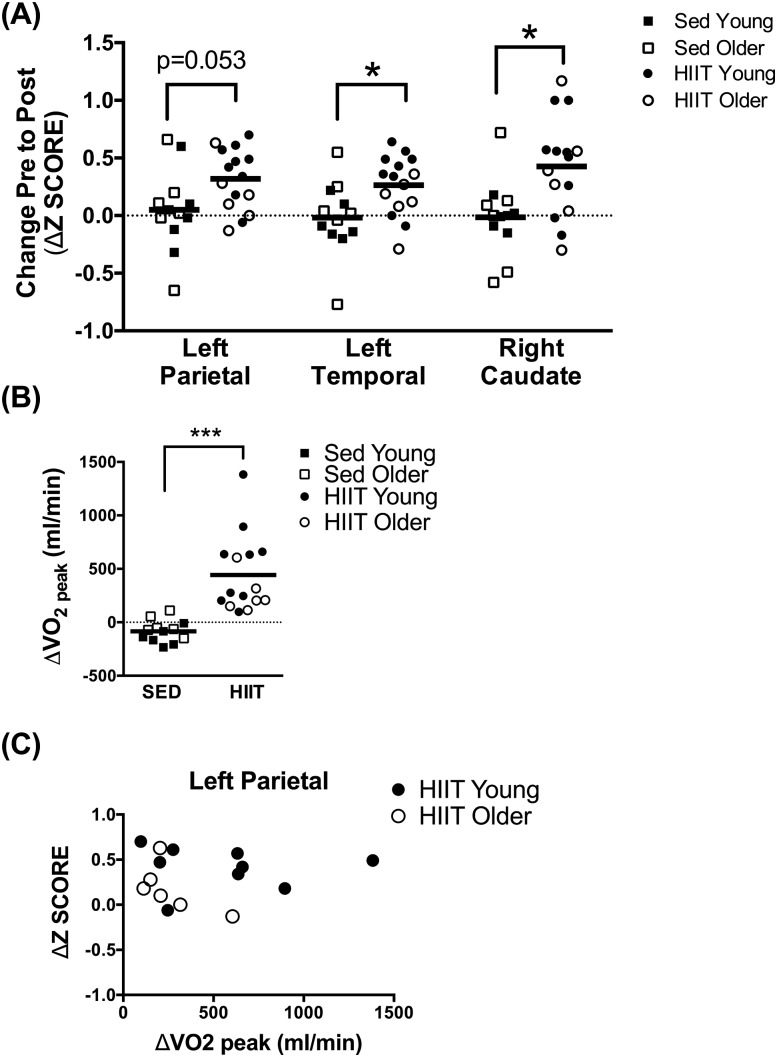
Resting glucose uptake was increased in parietal and temporal regions after HIIT compared with SED, and gains were observed in both younger and older adults [left parietal and left temporal lobes and right caudate nucleus displayed in Fig. 1(A)]. HIIT was effective at increasing absolute VO2peak in all participants compared with SED [Fig. 1(B)]. Absolute VO2peak did not change from baseline in the SED group. There were no correlations between changes in regional brain glucose uptake with HOMA-IR or VO2peak [example of left parietal region in Fig. 1(C)]. Increased glucose uptake was also noted in the caudate region of the brain but was not observed across all measured regions (Table 2).
Figure 1.
(A) Individual data points for change from premeasurement to postmeasurements of brain glucose uptake by 18FDG-PET in left parietal and temporal lobes and the right caudate nucleus after 12 weeks of SED or HIIT in young and older adults. (B) The change in ΔVO2peak from baseline was greater after HIIT than after SED. (C) There was no correlation between ΔVO2peak after HIIT and change in regional brain glucose uptake (left parietal region displayed). For panels (A) and (B), the horizontal bars indicate mean for SED or HIIT, and the dotted line at zero indicates no change from baseline. *P < 0.05; ***P < 0.001 for the main effect of training from two-way ANOVA (age × training).
Table 2.
Change in z-Score From Baseline in Brain Metabolism Using 18FDG-PET
| Region | SED |
HIIT |
ANOVA P Value |
||||
|---|---|---|---|---|---|---|---|
| Younger | Older | Younger | Older | Training | Age | Training × Age | |
| Right parietal | 0.075 (0.28) | 0.042 (0.5) | 0.443 (0.3) | 0.153 (0.37) | 0.105 | 0.257 | 0.376 |
| Left parietal | 0.048 (0.31) | 0.053 (0.42) | 0.413 (0.23) | 0.177 (0.26) | 0.053 | 0.343 | 0.323 |
| Right temporal | −0.025 (0.25) | −0.023 (0.44) | 0.297 (0.27) | 0.103 (0.3) | 0.085 | 0.449 | 0.442 |
| Left temporal | −0.045 (0.17) | 0.008 (0.44) | 0.358 (0.25) | 0.122 (0.23) | 0.029 | 0.418 | 0.204 |
| Right frontal | 0.047 (0.17) | 0.047 (0.58) | 0.406 (0.34) | 0.102 (0.4) | 0.189 | 0.331 | 0.331 |
| Left frontal | 0.185 (0.4) | 0.103 (0.49) | 0.411 (0.31) | 0.12 (0.4) | 0.439 | 0.238 | 0.503 |
| Right occipital | 0.408 (0.37) | −0.003 (0.63) | 0.331 (0.28) | 0.047 (0.4) | 0.935 | 0.047 | 0.705 |
| Left occipital | 0.488 (0.4) | 0.022 (0.56) | 0.354 (0.32) | 0.145 (0.37) | 0.974 | 0.046 | 0.43 |
| Right posterior cingulate | 0.093 (0.17) | 0.148 (0.4) | 0.29 (0.24) | 0.113 (0.37) | 0.501 | 0.612 | 0.337 |
| Left posterior cingulate | 0.105 (0.18) | 0.103 (0.48) | 0.3 (0.23) | 0.148 (0.31) | 0.331 | 0.532 | 0.541 |
| Right anterior cingulate | 0.015 (0.17) | 0.125 (0.24) | 0.286 (0.25) | 0.077 (0.18) | 0.202 | 0.565 | 0.072 |
| Left anterior cingulate | 0.068 (0.13) | 0.067 (0.29) | 0.138 (0.5) | 0.093 (0.14) | 0.72 | 0.863 | 0.873 |
| Right medial frontal | 0.063 (0.17) | 0.057 (0.49) | 0.269 (0.36) | 0.097 (0.34) | 0.388 | 0.528 | 0.558 |
| Left medial frontal | 0.088 (0.21) | 0.048 (0.47) | 0.228 (0.56) | 0.087 (0.24) | 0.597 | 0.59 | 0.763 |
| Right medial parietal | 0.267 (0.28) | 0.015 (0.4) | 0.366 (0.26) | 0.278 (0.43) | 0.185 | 0.214 | 0.541 |
| Left medial parietal | 0.25 (0.22) | −0.017 (0.48) | 0.264 (0.18) | 0.188 (0.22) | 0.338 | 0.14 | 0.405 |
| Right sensorimotor | 0.148 (0.41) | −0.112 (0.53) | 0.326 (0.26) | 0.027 (0.39) | 0.311 | 0.08 | 0.89 |
| Left sensorimotor | 0.158 (0.24) | −0.043 (0.52) | 0.266 (0.13) | 0.003 (0.47) | 0.583 | 0.107 | 0.829 |
| Right visual | 0.682 (0.55) | 0.033 (0.81) | 0.509 (0.5) | 0.177 (0.29) | 0.947 | 0.035 | 0.476 |
| Left visual | 0.652 (0.64) | −0.005 (0.85) | 0.497 (0.53) | 0.167 (0.32) | 0.972 | 0.048 | 0.497 |
| Right caudate | −0.008 (0.11) | −0.022 (0.47) | 0.473 (0.4) | 0.355 (0.5) | 0.012 | 0.679 | 0.741 |
| Left caudate | 0.015 (0.38) | −0.02 (0.6) | 0.472 (0.45) | 0.203 (0.73) | 0.122 | 0.481 | 0.587 |
| Right cerebellum | −0.043 (0.41) | 0.133 (0.41) | 0.183 (0.34) | 0.028 (0.31) | 0.673 | 0.94 | 0.256 |
| Left cerebellum | −0.003 (0.3) | 0.08 (0.39) | 0.23 (0.32) | 0.667 (1.71) | 0.23 | 0.442 | 0.6 |
| Right vermis | 0.247 (0.23) | 0.212 (0.23) | 0.289 (0.32) | 0.047 (0.19) | 0.546 | 0.18 | 0.312 |
| Left vermis | 0.242 (0.31) | 0.107 (0.32) | 0.229 (0.35) | 0.155 (0.18) | 0.883 | 0.392 | 0.801 |
| Pons | 0 (0.02) | −0.025 (0.03) | 0 (0.03) | −0.008 (0.02) | 0.425 | 0.118 | 0.425 |
| Right atrioventricular association | 0.028 (0.2) | 0.025 (0.52) | 0.379 (0.3) | 0.11 (0.35) | 0.13 | 0.337 | 0.349 |
| Left atrioventricular association | 0.01 (0.14) | 0.063 (0.46) | 0.396 (0.27) | 0.13 (0.31) | 0.075 | 0.39 | 0.201 |
| Atrioventricular cerebral | 0.107 (0.18) | 0.032 (0.44) | 0.308 (0.21) | 0.063 (0.3) | 0.312 | 0.169 | 0.459 |
Data are mean (standard deviation) for change from baseline to post measurement; P values are reported for two-way ANOVA using Training and Age groups.
Abbreviation: BMI, body mass index.
Discussion
We completed a 12-week aerobic training protocol that included HIIT in younger and older adults compared with SED control subjects and measured brain glucose uptake using 18FDG-PET scans. The major finding is that aerobic training with HIIT increased resting glucose uptake in select regions of the brain, but not globally, in younger and older adults compared with the SED groups. We observed improved glucose uptake in regions such as the caudate nucleus and parietal and temporal lobes but not across all regions. These results provide evidence that HIIT can increase brain glucose uptake in a small, but well-characterized, cohort of younger and older adults. Such gains in glucose uptake are consistent with improvements to brain energy metabolism and gains in mitochondria after aerobic training.
We demonstrated an increase of brain glucose uptake in parietal and temporal regions, which are regions that decline in glucose uptake with cognitive impairment and Alzheimer’s disease (13). These regions help integrate and store visual information as well as process language and emotional associations (14). The caudate nucleus is involved in performing executive action, memory, sleep, language, and emotions, and hypometabolism of caudate nucleus has been detected in degenerative brain diseases (15). Improvements in glucose uptake indicate enhanced metabolic activities of these regions and may contribute to the benefits of aerobic exercise, such as increased brain volume and blood flow and a decreased risk for cognitive disorders (3). Consistently, rodent models have demonstrated that exercise can remodel the brain for improved metabolism, including increased mitochondrial content (16) and respiration (17). The underlying mechanism on how nonexercising tissue such as brain can receive training benefits remains to be fully understood. Potential signals include peroxisome proliferator–activated receptor γ coactivator-1α and downstream stimulation of irisin, a myokine secreted after endurance exercise that stimulates release of hippocampal-derived neurotropic factor (18). In support of this finding, irisin was previously reported to be higher in plasma from young participants who completed HIIT compared with SED subjects (19).
Greater aerobic fitness appears to be beneficial for protecting against loss of brain tissue, specifically within the frontal, temporal, and parietal regions (20). A 9-year follow-up study indicated that more active lifestyles, measured by walking distance, were associated with greater gray matter volume that correlated with greater cognitive performance (21). Our results provide evidence that cerebral adaptations can be observed over relatively short periods of HIIT in younger and older adults who are SED but otherwise healthy.
Other researchers have demonstrated beneficial effects of moderate-intensity aerobic exercise (50% to 70% maximal capacity for 30 to 50 minutes over several months) on improving brain ketone uptake in patients with Alzheimer’s disease (22) and functional magnetic resonance imaging testing in healthy older adults (7). Our current results indicate that older adults have similar improvements in brain glucose uptake compared with younger adults, although our study was not designed to detect age and training interactions. Previous work has shown similar brain glucose uptake, measured by arterio-venous difference, during exercise in older adults compared with younger subjects even at increasing exercise intensities (23), but the current study showed this increase in the specific areas of the brain. The previous study demonstrating arterio-venous differences in glucose after acute exercise that do not appear to be impaired during exercise in healthy older people, even when exercising at a lower absolute workload than young adults. Our data after 12 weeks of training indicate that younger and older people improved brain glucose uptake and presumably its metabolism after aerobic training that incorporated HIIT. An important consideration is whether gains in brain glucose uptake reach a plateau or may continue to improve with extended exercise training, such as can occur in VO2peak during a rigorous training program (24). Aerobic exercise training can increase cerebral blood flow in aged mice, which is consistent with angiogenesis after exercise training (25). Furthermore, aerobic exercise stimulated neurogenesis in aged mice (26). Such gains in angiogenesis may support improvements to cerebral metabolism with aerobic training and protect against cognitive decline with aging.
We cannot exclude the possibility of increases in cerebral metabolic activity contributing to greater production of reactive oxygen species and oxidative stress. Previous reports in younger and older mice indicate that exercise training can stimulate oxygen consumption and reactive oxygen species production in the brain (27). Yet, exercise training can also improve the capacity to quench reactive oxygen species and contribute to lower oxidative stress. Compared with SED control rats, aging rats that performed treadmill training had greater antioxidant enzymes and lower accumulation of oxidative damage to proteins in the brain (28). We speculate that neuroprotective effects of long-term exercise training may result from a combination of increases in metabolism alongside antioxidant defenses and/or cellular repair pathways.
Brain glucose uptake was measured in the fasted state in healthy adults and is therefore interpreted in the presence of low insulin and euglycemia. In whole-body studies in healthy adults, the dose-response curve for insulin stimulation of brain glucose uptake may be left-shifted to lower insulin concentrations. Accordingly, increasing insulin concentrations above fasting does not stimulate brain glucose uptake in healthy adults (29). Lowering insulin concentrations using somatostatin infusion led to decreased brain glucose uptake compared with replacement insulin conditions (near fasting); this result indicates that brain glucose uptake may be saturated at low insulin concentrations in healthy middle-aged adults (30). However, the relationship between changes to peripheral insulin sensitivity and cerebral insulin sensitivity is less known. Obese adults with peripheral insulin resistance had similar fasting brain glucose uptake to lean adults, which was increased during insulin infusion (29). Improvements to insulin sensitivity appear to increase insulin stimulation of brain glucose uptake, such as shown after bariatric surgery (31). The insulin-sensitizing effects of exercise appear predominantly during insulin-stimulated conditions, but brain glucose uptake was measured in the fasted state in our current study. We previously reported that HIIT increased peripheral insulin sensitivity, but not endogenous glucose output, during higher insulin concentrations (1). Our current results indicate no correlation between changes in brain glucose uptake and HOMA-IR in healthy adults after HIIT.
HIIT is a potent stimulus for cardiorespiratory adaptations. We incorporated HIIT with moderate-intensity treadmill walking for 2 days per week. The increase in brain glucose metabolism in the current study is likely a chronic training effect given that the PET scans were performed at 96 hours after exercise. Brain glucose uptake has been reported to decrease during and immediately after aerobic exercise, and the declines are greater at higher intensity (10). The reason for this phenomenon remains to be fully understood but may be related to a decline in plasma glucose. We cannot exclude the possibility that lower-intensity aerobic training, or other forms of training such as resistance, could alter brain metabolism. For example, lower-intensity aerobic training for 1 year has been shown to stimulate brain hypertrophy and cognitive benefits in older adults (3). Also, patients with mild Alzheimer’s disease have shown improvements to brain ketone uptake after 3 months of moderate-intensity treadmill walking (22). We did not assess cognitive function, and the potential effects of cognitive decline associated with aging are less known.
In summary, aerobic training that included HIIT improved glucose uptake in parietal-temporal and caudate regions in younger and older adults. Our current results provide indication that healthy adults can improve brain metabolism in a relatively short time period of 12 weeks. These improvements occurred alongside robust gains in cardiorespiratory fitness and indicate that aerobic exercise can stimulate metabolic adaptations in the brain.
Acknowledgments
We thank the participants for their time and enthusiasm during the project. We acknowledge the skillful assistance of Kera Hoff along with the staff of the Dan Abraham Healthy Living Center and Clinical Research Unit at Mayo Clinic.
Financial Support: Funding was provided by National Institutes of Health Grants R01AG09531 (to K.S.N.) and T32DK7352 (M.M.R.) and by the Elsie and Marvin Dekelboum Family Foundation and institutional funds (to V.J.L.). Additional support was provided by the Mayo Foundation and the Murdock-Dole Professorship (to K.S.N.). This publication was made possible by the Mayo Clinic Metabolomics Resource Core through Grant U24DK100469 from the National Institute of Diabetes and Digestive and Kidney Diseases and Mayo Clinic Clinical and Translational Sciences Award (CTSA) Grant UL1TR000135 from the National Center for Advancing Translational Sciences (NCATs).
Clinical Trial Information: ClinicalTrials.gov no. NCT01738568 (registered 30 November 2012).
Author Contributions: M.M.R. supervised exercise training, analyzed data, and wrote the manuscript. V.J.L. provided support for PET scans, reviewed and interpreted data, and edited the manuscript. K.S.N. designed the study, obtained funding, interpreted data, and edited the manuscript.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 18FDG-PET
- 18F-fluorodeoxyglucose positron emission tomography
- HIIT
- high-intensity interval training
- HOMA-IR
- homeostatic model of insulin resistance
- SED
- sedentary
- VO2peak
- peak aerobic capacity.
References
- 1.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25(3):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiura M, Nariai T, Ishii K, Sakata M, Oda K, Toyohara J, Ishiwata K. Changes in cerebral blood flow during steady-state cycling exercise: a study using oxygen-15-labeled water with PET. J Cereb Blood Flow Metab. 2014;34(3):389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(1):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillen JB, Martin BJ, MacInnis MJ, Skelly LE, Tarnopolsky MA, Gibala MJ. Twelve weeks of sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS One. 2016;11(4):e0154075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross R, de Lannoy L, Stotz PJ. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin Proc. 2015;90(11):1506–1514. [DOI] [PubMed] [Google Scholar]
- 7.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101(9):3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 9.Lowe VJ, Kemp BJ, Jack CR Jr, Senjem M, Weigand S, Shiung M, Smith G, Knopman D, Boeve B, Mullan B, Petersen RC. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50(6):878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Långsjö J, Oikonen V, Rinne J, Nuutila P, Knuuti J. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568(Pt 1):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowe VJ, Peller PJ, Weigand SD, Montoya Quintero C, Tosakulwong N, Vemuri P, Senjem ML, Jordan L, Jack CR Jr, Knopman D, Petersen RC. Application of the National Institute on Aging-Alzheimer’s Association AD criteria to ADNI. Neurology. 2013;80(23):2130–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart, New York: Georg Thieme; 1988. [Google Scholar]
- 13.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. [DOI] [PubMed] [Google Scholar]
- 14.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27(1):279–306. [DOI] [PubMed] [Google Scholar]
- 15.Dukart J, Kherif F, Mueller K, Adaszewski S, Schroeter ML, Frackowiak RS, Draganski B; Alzheimer’s Disease Neuroimaging Initiative . Generative FDG-PET and MRI model of aging and disease progression in Alzheimer’s disease. PLOS Comput Biol. 2013;9(4):e1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marques-Aleixo I, Santos-Alves E, Balça MM, Rizo-Roca D, Moreira PI, Oliveira PJ, Magalhães J, Ascensão A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience. 2015;301:480–495. [DOI] [PubMed] [Google Scholar]
- 17.Herbst EA, Holloway GP. Exercise increases mitochondrial glutamate oxidation in the mouse cerebral cortex. Appl Physiol Nutr Metab. 2016;41(7):799–801. [DOI] [PubMed] [Google Scholar]
- 18.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015;22(4):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. [DOI] [PubMed] [Google Scholar]
- 21.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellano CA, Paquet N, Dionne IJ, Imbeault H, Langlois F, Croteau E, Tremblay S, Fortier M, Matte JJ, Lacombe G, Fülöp T, Bocti C, Cunnane SCA. A 3-month aerobic training program improves brain energy metabolism in mild Alzheimer’s disease: preliminary results from a neuroimaging study. J Alzheimers Dis. 2017;56(4):1459–1468. [DOI] [PubMed] [Google Scholar]
- 23.Fisher JP, Hartwich D, Seifert T, Olesen ND, McNulty CL, Nielsen HB, van Lieshout JJ, Secher NH. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol. 2013;591(7):1859–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickson RC, Bomze HA, Holloszy JO. Linear increase in aerobic power induced by a strenuous program of endurance exercise. J Appl Physiol. 1977;42(3):372–376. [DOI] [PubMed] [Google Scholar]
- 25.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gusdon AM, Callio J, Distefano G, O’Doherty RM, Goodpaster BH, Coen PM, Chu CT. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp Gerontol. 2017;90:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. [DOI] [PubMed] [Google Scholar]
- 29.Hirvonen J, Virtanen KA, Nummenmaa L, Hannukainen JC, Honka MJ, Bucci M, Nesterov SV, Parkkola R, Rinne J, Iozzo P, Nuutila P. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes. 2011;60(2):443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes. 2002;51(12):3384–3390. [DOI] [PubMed] [Google Scholar]
- 31.Tuulari JJ, Karlsson HK, Hirvonen J, Hannukainen JC, Bucci M, Helmiö M, Ovaska J, Soinio M, Salminen P, Savisto N, Nummenmaa L, Nuutila P. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese [published correction appears in Diabetes 2017;66(10):2724–2724]. Diabetes. 2013;62(8):2747–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]