Abstract
Background
Glioma immunotherapy is an active area of clinical investigation. Glioma-associated immunosuppression remains an obstacle to efficacious immunotherapy, and combination approaches are likely necessary for durable success. OX40 is a member of the tumor necrosis factor receptor superfamily that is upregulated on activated lymphocytes, ligation of which results in enhanced activity and may be active against cancer. We sought to confirm the efficacy of agonist anti-OX40 immunotherapy against glioma and hypothesized that it is complementary with irradiated whole tumor cell vaccination.
Methods
GL261 tumor cells were implanted into the right frontal lobes of syngeneic mice, which were then treated with controls, agonist anti-OX40 monoclonal antibody, vaccination with subcutaneous injection of irradiated granulocyte macrophage colony stimulating factor (GM-CSF)–expressing GL261 cells (GVAX), or vaccination + agonist anti-OX40 therapy. Animals were followed for survival. On day 18, splenocytes were harvested for enzyme-linked immunosorbent spot analyses and brains were harvested for immunohistochemistry and flow cytometry analyses of infiltrating lymphocytes.
Results
Combination immunotherapy with GVAX and systemic agonist anti-OX40 monoclonal antibody improved survival by 14 days over controls (median survival 36 vs 22 days, P < 0.00005). Systemically, T helper cell type 1 (Th1) antitumor immunity was enhanced significantly by combination therapy. In the brain, combination immunotherapy increased the percentage of Th1 CD4+ T lymphocytes and reduced the fraction that were Th2. In the brain, vaccination improved the ratio of CD8+ to FoxP3+ T lymphocytes, while combination immunotherapy reversed intracranial T-lymphocyte exhaustion, reducing their coexpression of programmed cell death protein 1 (PD-1) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) as well as PD-1 and lymphocyte-activation gene 3 (LAG-3).
Conclusions
Anti-OX40 immunotherapy is active against intracranial glioma and synergizes with GVAX. Vaccination and anti-OX40 immunotherapy are mechanistically complementary, particularly in the glioma microenvironment.
Keywords: glioma, immunotherapy, lymphocyte exhaustion, OX40, tumor infiltrating lymphocyte (TIL)
Importance of the study
Efficacious immunotherapy against glial tumors will require combination approaches. Irradiated whole tumor cell vaccination provides a therapeutic backbone that expands the diversity of tumor-antigen–specific T lymphocytes. The following is one of a very few studies demonstrating both single-agent antiglioma activity of OX40 ligation and enhanced efficacy in combination with vaccination. We also demonstrate the mechanisms by which vaccination and OX40 stimulation overcome tumor suppressive networks in a complementary fashion, with synergistic impacts on systemic antitumor immunity, the cellular composition of the intratumoral inflammatory infiltrate, and the activation status of brain-infiltrating lymphocytes. Furthermore, this is just the second description of T-lymphocyte exhaustion—a relatively new concept in lymphocyte dysfunction—among glioma tumor-infiltrating lymphocytes, and the first report of the immunotherapy impact on expression of TIM-3 and LAG-3 on brain-infiltrating lymphocytes. Also, we are not aware of any other description of agonist anti-OX40 cancer immunotherapy preventing or reversing T-lymphocyte exhaustion in the tumor microenvironment.
Since 2010, cancer immunotherapy has made impressive inroads into the clinical armamentarium, most significantly with immune checkpoint inhibition by antibody-mediated blockade of cytotoxic T-lymphocyte associated antigen 4 (CTLA-4)1 and programmed cell death protein 1 (PD-1).2–5 Despite these successes, immunotherapy does not currently have proven efficacy in patients with glial tumors, though numerous clinical trials are under way. Prior to the current era, the field of brain tumor immunotherapy has been dominated by studies of cancer vaccination, including peptide vaccination, dendritic cell vaccination, and heat-shock protein vaccination. We have recently reported safety and feasibility of vaccination of patients with recurrent malignant glioma with irradiated autologous tumor cells mixed with irradiated granulocyte macrophage colony stimulating factor (GM-CSF)–expressing K562 cells—an example of a well-studied approach known as “GVAX.”6 GVAX refers to vaccination by the subcutaneous and/or intradermal injection of irradiated autologous tumor cells engineered to express CSF or coupled with a GM-CSF–expressing bystander line as a means of stimulating systemic antitumor immunity. The GVAX strategy has been employed in patients with advanced melanoma, non–small cell lung cancer, renal cell carcinoma, prostate carcinoma, and hematologic malignancies and consistently induces both cell-mediated and humoral antitumor immunity.7
In preclinical murine glioma models, GVAX consistently augments antitumor immunity and confers a modest survival advantage.8 In the subjects treated in our phase I study,6 GVAX drives specific antitumor responses and broad T-lymphocyte activation, characterized by increased expression of co-stimulatory molecules, including CTLA-4 and PD-1, as well as those associated with positive activation of T-cell responses such as 4-1BB and OX40.
The antitumor immune responses driven by whole tumor cell vaccination, while notable, do not afford cures or long-term survivors in glioma-bearing mice; however, we have shown that significantly improved outcomes can be achieved by combination immunotherapy with GVAX and immune checkpoint blockade using a monoclonal antibody against CTLA-4.9 This synergy is consistent with the hypothesis that while GVAX increases the number of activated glioma-specific circulating and tumor-infiltrating T lymphocytes (TILs), enhanced activation is required for these effectors to begin to overcome negative immune regulation, which can be heightened by both host- and tumor-related factors.
Similarly to CTLA-4, OX40 is expressed in a time-dependent fashion on the surface of T lymphocytes after antigenic stimulation via the T-cell receptor.10 OX40 ligation by agonistic monoclonal antibodies stimulates cluster of differentiation (CD)4+ and CD8+ T lymphocytes and has shown antitumor activity in several preclinical tumor models.10 In addition to enhancing proliferation and cytokine production, OX40 ligation promotes differentiation and expansion of memory CD8+ T lymphocytes and improves their function.11 We, therefore, hypothesized that agonist OX40 binding would synergize with GVAX.
Study of glioblastoma TILs has established that the intratumoral inflammatory cellular infiltrate may impact survival in glioma patients.12 Circulating effector lymphocytes and TILs must not only be present but must also properly function within and around the tumor. T-lymphocyte exhaustion is a recently described phenomenon in which chronic antigenic stimulation renders effector cells unable to constructively respond within their target microenvironment. Initially detailed in states of chronic viral infection, T-lymphocyte exhaustion is also operative in cancer, and is characterized by high cellular expression of PD-1 and the negatively regulating immune checkpoint molecules T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) and lymphocyte-activation gene 3 (LAG-3).13 Markers of lymphocyte exhaustion are little studied with respect to the glioma microenvironment—we aimed to describe the impact of effective immunotherapy on TIL status, hypothesizing that OX40 ligation, by prolonging antigen-specific activation, might reverse or prevent tumor-associated induction of exhaustion.
Here, we confirm that agonist anti-OX40 treatment of mice bearing intracranial glial tumors improves survival as monotherapy and is synergistic in combination with GVAX whole tumor cell vaccination. Vaccination and OX40 ligation both act to trend systemic antitumor immunity toward T helper cell 1 (Th1) polarization and have complementary impacts on the tumor microenvironment and the intratumoral inflammatory infiltrate. In particular, the GVAX/agonist anti-OX40 combination is associated with significantly reduced exhaustion of brain-infiltrating T lymphocytes (BILs).
Materials and Methods
Parental and Engineered Cell Lines
GL261, a syngeneic murine tumor cell line, was obtained from the National Cancer Institute. GL261 GM-CSF cells, which were used as a vaccine base, were engineered via retroviral transduction and infection as previously described.9 The expression and secretion of GM-CSF by GL261 GM-CSF cells were confirmed by enzyme-linked immunosorbent assay using manufacturer’s instructions (RayBiotech) and were measured as 442 ng (95% CI: 370–524) / 1 × 106 cells / 48 h. All GL261 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech) supplemented with 10% fetal bovine serum (HyClone) at 5% CO2 and 37°C.
Animals
Six- to 7-week-old female wild-type C57BL/6 mice were purchased from the National Cancer Institute. All mice were maintained and used in accordance with the animal protocol approved by our Institutional Animal Care and Use Committee (IACUC).
Intracranial Glioblastoma Cell Implantation
GL261 parental cells (75,000 cells per mouse) suspended in 3 µL DMEM were stereotactically implanted into the brains (right striatum, 2.5 mm lateral from bregma and 2.5 mm deep) of C57BL/6 mice (7 to 8 wk age) using a Hamilton 1701N Gastight syringe and a stereotactic mouse frame (Kopf Instruments).
Vaccination and Anti-OX40 Treatment
On days 3, 6, and 9 post tumor implantation, appropriate mice were subcutaneously injected with 1 × 106 irradiated (35 Gy) GL261 GM-CSF cells suspended in 100 µL of phosphate buffered saline (PBS). Subcutaneous PBS alone was used as vaccination control. At the same time, depending upon their study group, mice were also administered 250 µg of either anti-OX40 (OX86 clone, BioXCell) or isotype control (rat immunoglobulin G1) prepared in 100 µL PBS on days 3, 6, and 9 post tumor implantation. Mice were followed for survival and sacrificed when neurological symptoms became evident, per the IACUC protocol. Mice used for studies of immunity were euthanized 18 days following implantation of tumor.
Enzyme-Linked Immunosorbent Spot Assays
Mice receiving different treatments were sacrificed and their spleens were harvested 18 days post tumor implantation. Spleens were immediately processed to isolate splenocytes; 1 × 106 splenocytes were stimulated for 48 hours in vitro with 1 × 105 irradiated (35 Gy) GL261 cells or in Roswell Park Memorial Institute 1640 medium, supplemented with 10% inactivated fetal bovine serum, 50 μM 2-mercaptoethanol, 2 mM glutamine, 20 mM HEPES, and penicillin-streptomycin in 6-well tissue culture plates (BD Falcon). From mice in each treatment and control group, 1 × 105 splenocytes were loaded in duplicates onto 96-well polyvinylidene difluoride–backed microplates coated with anti-mouse interferon (IFN)-γ, anti-mouse interleukin (IL)-10, anti-mouse tumor necrosis factor (TNF)-alpha, anti-mouse IL-2, anti-mouse IL-13, and both anti-mouse CD4 and anti-mouse granzyme B monoclonal antibodies (R&D Systems). These plates were then incubated at 5% CO2 and 37°C for 24 hours. After incubation, the plates were washed 4 times with the wash buffer provided by the manufacturer, and biotinylated antibody specific for each coated antibody was added to each well and incubated overnight at 4°C. Next morning the plates were washed 4 times again; streptavidin–alkaline phosphatase was added into each well and the plates were incubated at room temperature for 2 hours. Plates were washed again, 5-bromo-4-chloro-3-indolyl phosphate/nitro-blue tetrazolium chromogen was added to each well, and the plates were incubated for an hour at room temperature. The contents of the plates were then discarded and plates were washed with distilled water, dried, and read using an ImmunoSpot version 5.1.36 enzyme-linked immunosorbent spot (ELISpot) reader (Cellular Technology Limited).
Tissue Processing and Immunohistochemistry
For immunohistochemistry studies, day 18 mice from each treatment group were sacrificed and their brains harvested and fixed in 10% formalin followed by 70% ethanol. Specimens were subsequently paraffin embedded and cut into 5-μm sections. Sections were deparaffinized in xylene and dehydrated in ethanol followed by microwave treatment in 10 mM sodium citrate buffer (pH 6.0) for 15 min for antigen retrieval. The sections were next treated with 3% H2O2 to block endogenous peroxidase. Bovine serum albumin and antibody-specific protein blocking buffer were then used to block nonspecific binding for 20 and 30 min, respectively. The sections were incubated overnight with anti-CD3 (ab5690, Abcam), anti-CD4 (4SM95, eBioscience), and anti-CD8a (4SM15, Affymetrix). Blocking buffer was used instead of the primary antibody for negative controls. The sections were then incubated for 30 min at room temperature with the appropriate peroxidase-labeled secondary antibody as instructed by the manufacturer in the kit (Vector Lab ImmPRESS polymer detection kit). Diaminobenzidine was used for color development, and the sections were counterstained with hematoxylin. Finally the sections were mounted with Cytoseal-XYL (8312-4, Thermo Scientific) and photographed under a light microscope (Nikon Optiphot 2) using spot software.
For quantitative analysis of the immune infiltrate (“histoscore”), we took 3 pictures with 20X magnification per cut section of the brain tumors. We counterstained cells from each picture and calculated an average from the three. This average represented the immunohistochemistry (IHC) score for that section. We studied 3 mice per treatment group in this fashion.
Flow Cytometry Analysis
Mice receiving different treatments were sacrificed and their brains were harvested 18 days post tumor implantations. Tumor tissues were processed from brains, and single cell suspension of the tumor cells was made for the analysis of TILs. Briefly, mouse brain containing the tumor was transferred in sterile fashion to a petri dish containing PBS. Using a sterile scalpel and forceps, the specimen was minced into equal sized pieces. The minced sample was transferred from the petri dish to a new 50 mL Falcon tube. The tube was then centrifuged at 1500 rpm for 5 min and the supernatant was removed. For dissociating the cells, 1–2 mL of Accutase (Sigma) containing 10 µL/mL DNase I (Roche) was added and incubated for 10 min at 37°C with gentle rocking of the tube. Fifteen milliliters of fluorescence activated cell sorting buffer was added and the contents of the tube were passed through a 40 µm cell strainer fixed on a 50 mL Falcon tube. The strainer was washed with PBS to get the maximum number of cells. The tube was then centrifuged at 1500 rpm for 5 min and the supernatant was removed. The cells were resuspended in 12 mL PBS and counted. Fluorescently labeled antibodies used for the analysis were purchased from Biolegend (BV 605-CD3, PerCP/Cy5.5-CD4, BV 510- CD8a, Alexa Fluor 647-FOXP3, PE/Cy7-CD279, BV 421-CD134, PE-CD366, BV 785-CD223, BV421-GATA3). Dead cells were excluded using the Zombie UV Fixable Viability Kit (Biolegend). For the analysis of T helper cell subsets, we used Multi-Color Flow Cytometry Kits (R&D Systems) for mouse Th1 cells (conjugated antibodies to T-bet-PerCP, IFN-gamma-fluorescein, IL-12 R beta 2-APC, and CD4-PE) and Th2 cells (conjugated antibodies to CD4-PerCP, IL-4 R-fluorescein, STAT6-APC, and IL-5-PE). Flow cytometry data were acquired using LSRII and analyzed using FlowJo software, and graphs were generated using GraphPad Prism 6 software.
For calculation of the CD8+/Forkhead box protein 3 (FoxP3) ratio, the percentage of the entire cellular population that was CD8+ was divided by the percentage of the entire cellular population that was CD4+ FoxP3+.
Statistical Analysis
Data were analyzed and graphed using GraphPad Prism 6. Data were expressed as mean ± SD, and differences were considered significant at P < 0.05. Individual data sets were compared using Student’s t-test when comparing 2 groups. Kaplan–Meier analysis was used for mouse survival studies, and the groups were compared using the log-rank test. Flow cytometry data were analyzed using FlowJo software.
Presented data are representative of experiments, each of which was performed at least 3 times. For survival studies, 8–10 animals per group were followed. For studies of immune function, we typically harvested splenocytes and TILs from 3 animals per group.
Results
Combination Immunotherapy with Vaccination and Anti-OX40 Antibody Improves Survival in Syngeneic Glioma-Bearing Mice
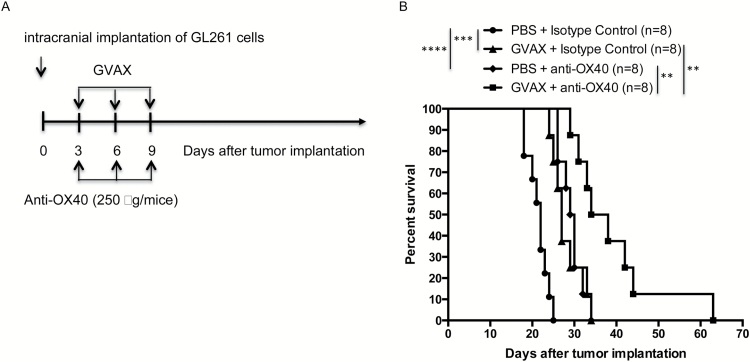
Given existing data that GVAX generates effective systemic antitumor immunity, we hypothesized that adding anti-OX40 antibody would strengthen and prolong the activation of tumor-specific lymphocytes. Following the paradigm of other experiments examining combining vaccination with immune checkpoint inhibitors,14 GL261 glioma cells were implanted intracranially on day 0, and vaccine and anti-OX40 antibody were delivered subcutaneously and intraperitoneally, respectively, on days 3, 6, and 9. While both vaccination and anti-OX40 antibody improved survival compared with controls as single agents (median survival 27 and 29.5 days respectively vs 22 days control), combination immunotherapy yielded improved survival compared with either as monotherapy (median survival 36 days) (Fig. 1).
Fig. 1.
(A) Schema for animal survival experiments. Irradiated autologous GL261 cells expressing GM-CSF were injected subcutaneously on days 3, 6, and 9 after intracranial implantation of tumor cells, synchronously with intraperitoneal injection of anti-OX40 antibody. (B) Animals treated with combination GVAX and anti-OX40 antibody lived significantly longer than animals receiving either as monotherapy. Animals from both GVAX and anti-OX40 monotherapy groups lived significantly longer than control-treated mice. (****P < 0.00005, ***P < 0.0005, **P < 0.005; absence of an asterisk denotes not significant comparison.)
Systemic Tumor-Specific Th1 Immune Responses Are Significantly Enhanced by Combination Therapy with Vaccination and Agonist Anti-OX40 Antibody
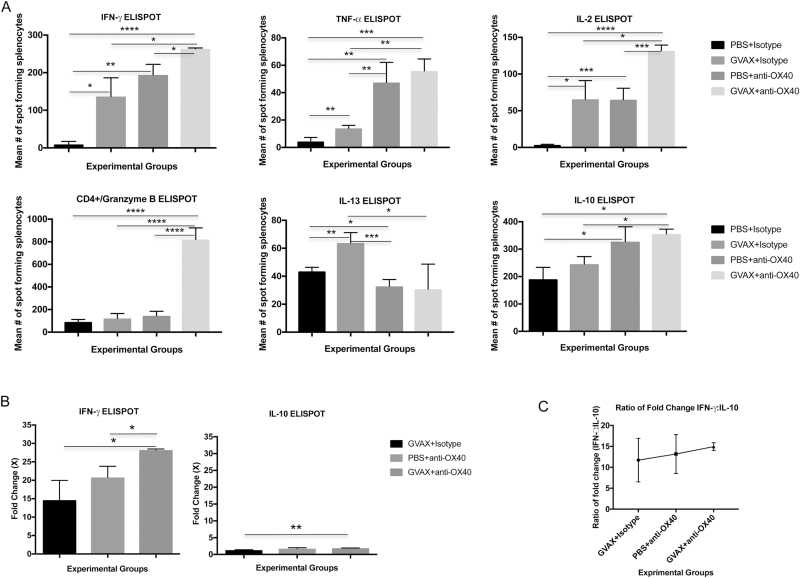
We used ELISpot assays for Th1- and Th2-associated cytokines to examine cellular expression profiles after splenocytes, harvested 18 days post intracranial GL261 implantation, were stimulated in vitro by irradiated GL261 cells (Fig. 2A). Relatively few splenocytes harvested from control-treated animals expressed Th1-associated cytokines, including IFN-γ, TNF-α, or IL-2 (mean numbers of spot-forming cells 9.3, 4.4, and 3.5, respectively). Likely reflective of more significant baseline Th2 immunity in glioma-bearing mice, higher numbers of splenocytes from control-treated animals expressed IL-13 or IL-10 after in vitro stimulation by tumor (mean numbers of spot-forming cells 43.5 and 190.3, respectively). Monotherapy with either vaccination or agonist anti-OX40 significantly increased the number of splenocytes that expressed IFN-γ, TNF-α, or IL-2. Likewise, splenocytes harvested from animals treated with combination GVAX and agonist anti-OX4O immunotherapy were significantly more likely to express each of these Th1-associated cytokines than those from monotherapy-treated animals (Fig. 2A).
Fig. 2.
ELISpot assay shows that combination immunotherapy skews systemic antitumor immunity toward Th1 polarization. (A) Splenocytes harvested from GVAX + agonist anti-OX40 treated glioma-bearing mice expressed significantly higher levels of Th1 cytokines IFN-γ, TNF-α, and IL-2 and granzyme B after combination immunotherapy compared with splenocytes harvested from either PBS or monotherapy-treated mice. Th2 cytokine (IL-13 and IL-10) was less impacted by treatment—GVAX monotherapy increased IL-13 expression levels compared with PBS treatment, and combination immunotherapy increased IL-10 expression from splenocytes, but with less significance. (B) The positive fold change of splenocyte expression of IFN-γ was higher than it was for IL-10. (C) The ratio between the fold change from IFN-γ vs the fold change for IL-10 was highly positive after any immunotherapy and highest after combination GVAX + agonist anti-OX40 treatment in glioma-bearing mice. Splenocytes were examined from 3 animals per group. (***P < 0.0005, **P < 0.005, *P < 0.05; absence of an asterisk denotes not significant comparison.)
Vaccination monotherapy significantly increased the number of splenocytes that expressed IL-13. Neither agonist anti-OX40 treatment nor combination immunotherapy altered the likelihood of IL-13 elaboration compared with untreated controls. IL-10 expression was positively impacted by treatment. Both agonist anti-OX40 monotherapy and combination immunotherapy drove significant increases in the number of IL-10+ spot-forming cells. However, due to the high baseline numbers of splenocytes from GL261-bearing mice that express IL-10, the relative positive change in the number of IL-10 expressing splenocytes was much lower than it was for the Th1 cytokines. For instance, for IFN-γ, the average number of spot-forming splenocytes harvested from combination-treated animals was 28.2-fold greater than the average number of spot-forming splenocytes harvested from control-treated mice (Fig. 2B). For IL-10, the number of spot-forming cells after combination immunotherapy was only 1.9-fold greater than after control treatment. Each treatment, and in particular combination immunotherapy, was associated with a greater shift toward Th1 antitumor immunity.
We calculated a comparative ratio of the changes in IFN-γ and IL-10 expression with the belief that it might provide a quantitative measure of a systemic shift from Th2-mediated antitumor immunity toward Th1. The ratios of IFN-γ change to IL-10 change for treatment with vaccination, agonist anti-OX40, and combination immunotherapy were 11.7, 13.2, and 15.1 (Fig. 2C).
Last, the expression of granzyme B by T cells usually reflects interaction between an antigen-specific effector cell and its target. ELISpot demonstrated that CD4+ splenocytes harvested from combination-treated animals were much more likely to express granzyme B after in vitro stimulation by tumor cells than splenocytes harvested from either control or monotherapy-treated groups (Fig. 2A). Here, the fold increases of granzyme B after combination immunotherapy were 9.0×, 6.8×, and 5.7× compared with saline controls, vaccination-treated animals, and agonist anti-OX40 monotherapy, respectively.
Combination Immunotherapy Increases Lymphocyte Infiltration in Intracranial Gliomas, Orients Intratumoral CD4+ Cells Toward Th1 Antitumor Responses, and Improves the Ratio of Cytotoxic CD8+ T Lymphocytes to Regulatory T Cells
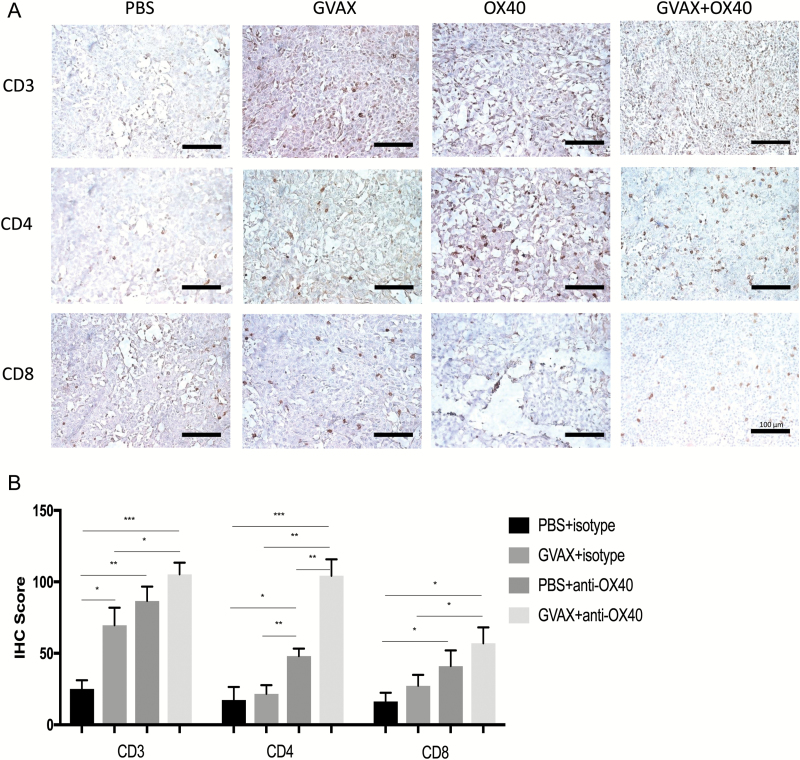
For a semiquantitative assessment of lymphocyte infiltration into the intracranial GL261 gliomas, we performed IHC of brains harvested on day 18 after implantation of tumors (Fig. 3A). Tumors from untreated mice had relatively scant lymphocytic infiltrates, as measured by IHC for CD3+, CD4+, and CD8+ expression. While vaccination increased the degree of lymphocytic infiltration into tumors, more evident lymphocytic cellularity was evident in the brain tumors of anti-OX40 treated mice, and the most densely infiltrated tumors had been treated with combination vaccination and anti-OX40 therapy. This was particularly clear for the CD4+ lymphocytic infiltrate.
Fig. 3.
(A) Immunohistochemistry with staining for CD3, CD4, and CD8. Brain and tumor specimens derived from untreated hosts show scant lymphocytic infiltration with relative increases in all 3 markers after either vaccination or anti-OX40 monotherapy. Qualitatively, it is apparent that inflammation is greatest in the tumors harvested from animals treated with combination GVAX and anti-OX40 immunotherapy, particularly in the CD4+ subset. All images are magnified 20× (bar within photomicrographs is 100 microns in length). (B) Histoscores derived from counts by blinded analysts confirm the impression that combination GVAX + agonist anti-OX40 immunotherapy increased the numbers of CD4+ and CD8+ intratumoral lymphocytes. Both the magnitude and the statistical significance were greatest in the CD4+ subset. (***P < 0.0005, **P < 0.005, *P < 0.05; absence of an asterisk denotes not significant comparison.)
Blinded analysts reviewed and counted IHC slides, from which we calculated “histoscores,” allowing fully quantitative comparisons across groups (Fig. 3B). For each of CD3+, CD4+, and CD8+ cells, the average amount of tumor infiltration increased from vaccine to agonist anti-OX40 treatment to combination immunotherapy. Relative increases in CD4+ cellular infiltrate was particularly marked after agonist anti-OX40 treatment in comparison with either control or vaccination monotherapy. Furthermore, the average number of counted CD4+ cells after combination GVAX and agonist anti-OX40 immunotherapy was more than twice as high as agonist anti-OX40 alone (104.33 ± 11.5 cells vs 48 ± 5.29 cells, P < 0.05). While both CD4+ and CD8+ cellular infiltrates go up with combination immunotherapy, the greater change is in the CD4+ subset.
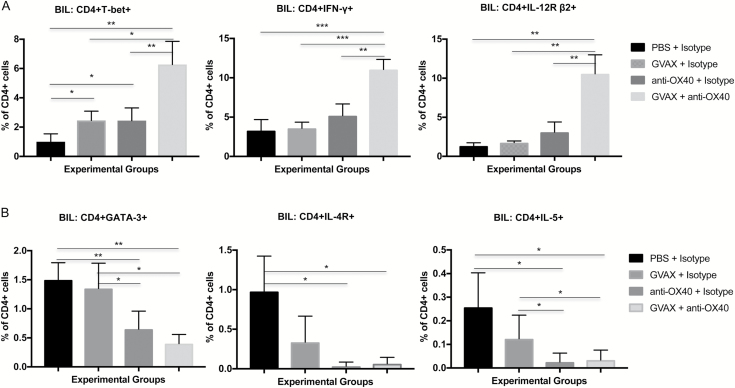
Having quantified the numbers of intratumoral inflammatory cells by “histoscore,” we used flow cytometry of the mouse brains to perform more detailed analyses of the lymphocyte subsets. Given the apparent importance of the CD4+ subset, we set up a panel of antibodies aimed at characterizing intratumoral Th1 versus Th2 immune responses. For Th1, we examined the percentages of CD4+ cells that expressed the transcription factor T-bet, IFN-γ, and the IL-12 receptor β2 subunit (IL-12R β2); for Th2, we stained with antibodies for transcription factor GATA-3, IL-4 receptor, and IL-5. While GVAX alone and agonistic anti-OX40 monotherapy tended to increase the likelihood of microenvironment Th1 orientation, combination immunotherapy resulted in more highly significant increases in expression of these markers when measured against the BILs from animals treated with monotherapies or controls (Fig. 4A). Similarly, combination immunotherapy leads to significantly diminished expression of Th2 markers by tumor-infiltrating CD4+ cells (Fig. 4B).
Fig. 4.
Th1 vs Th2 BILs. (A) Flow cytometry demonstrating percent expression of T-bet, IFN-γ, and IL-12R β2 on CD4+ lymphocytes 18 days after GL261 tumor implantation by treatment group. Combination immunotherapy is associated with significant increases in all 3 Th1 markers. (***P < 0.0005, **P < 0.005, *P < 0.05; absence of an asterisk denotes not significant comparison.) (B) Flow cytometry demonstrating percent expression of GATA3, the IL-4 receptor, and IL-5 on CD4+ lymphocytes 18 days after GL261 tumor implantation by treatment group. Combination immunotherapy is associated with significant decreases in all 3 Th2 markers. (**P < 0.005, *P < 0.05, absence of an asterisk denotes not significant comparison.)
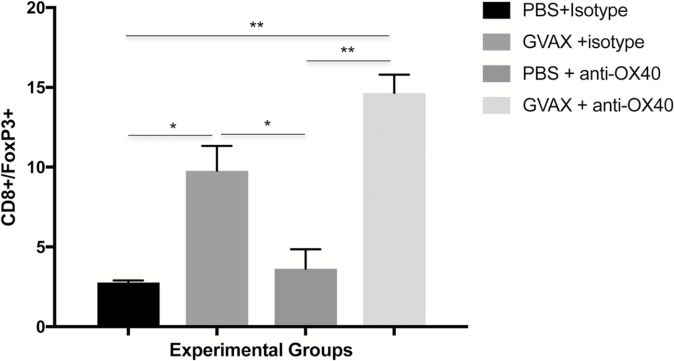
The intratumoral CD8+/FoxP3+ ratio is associated with outcome in several immunotherapy preclinical models and may be relevant for survival in human glioblastoma. We calculated the CD8+/FoxP3+ ratio in the brains of mice from all 4 treatment groups by performing flow cytometry of BILs, harvested on day 18 (Supplementary Figure S1). GVAX monotherapy consistently resulted in an increased CD8+/FoxP3+ ratio compared with controls (Fig. 5). Agonist anti-OX40 monotherapy did not significantly impact the CD8+/FoxP3+ ratio, compared with control treatments. The improved ratio accomplished by vaccination alone was slightly enhanced when adding systemic therapy with anti-OX40 antibody.
Fig. 5.
Day 18 flow cytometry of BILs shows that vaccination improves the intratumoral CD8+/FoxP3+ lymphocyte ratio, which is little affected by treatment with anti-OX40 immunotherapy. (**P < 0.005, *P < 0.05; absence of an asterisk denotes not significant comparison.)
Agonist Anti-OX40 Immunotherapy Is Associated with Reduced Exhaustion in Glioma-Infiltrating Lymphocytes
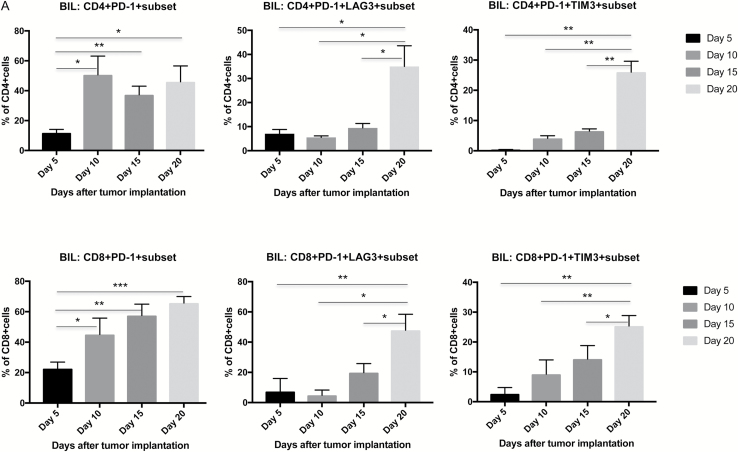
T-lymphocyte exhaustion is defined as a dysfunctional state representing a condition of hyporesponsiveness resulting from chronic exposure to antigen. To date, very few studies have examined the role of lymphocyte exhaustion in suppression of antiglioma immune responses. Phenotypically, exhausted T lymphocytes express high levels of PD-1, TIM-3, and LAG-3, and coexpression of PD-1 with either TIM-3 or LAG-3 is thought to be highly specific for exhaustion. One study to date has chronicled the high levels of PD-1/TIM-3 coexpression in advanced, near-terminal intracranial GL261 tumors.15 We first confirmed that both CD4+ and CD8+ lymphocytes harvested from the brains of either GL261 or CT2A tumor-bearing mice expressed relatively low levels of PD-1 five days after implantation. The percentage of T lymphocytes expressing PD-1 steadily increased over time and was highest when measured at day 20, just shy of reaching terminal status—46% and 66% for CD4+ and CD8+ T lymphocytes, respectively. As PD-1 expression can reflect either activation or exhaustion of lymphocytes, we also examined expression of LAG-3 or TIM-3 on PD-1+ T cells. Similarly, brain-infiltrating CD4+ and CD8+ cell coexpression of PD-1 and TIM-3 or LAG-3 was highest late in the natural history of GL261 tumors (day 20 vs days 5, 10, and 15; Fig. 6A), suggesting that significant lymphocyte exhaustion is a feature of advanced glioma.
Fig. 6.
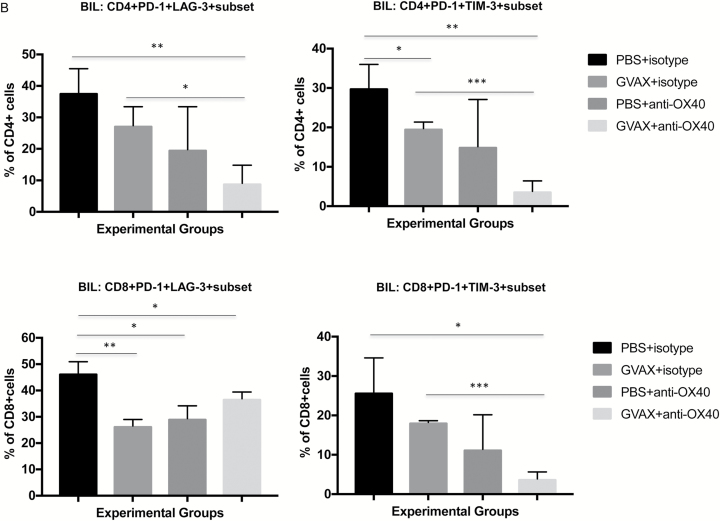
In mice bearing intracranial GL261 glioma, BIL coexpression of PD-1 with either TIM-3 or LAG-3 increases with tumor progression and is significantly decreased after GVAX + agonist anti-OX40 immunotherapy. (A) Time-course study showing that PD-1 expression on both CD4+ and CD8+ lymphocytes starts at relatively low levels 5 days after glioma implantation and is higher thereafter. At day 20 after implantation, both CD4+ and CD8+ T lymphocytes coexpress PD-1 with either TIM-3 or LAG-3 at significantly higher levels than at earlier measured timepoints, reflecting the exhausted status of BILs. (B) BIL coexpression of PD-1 with either TIM-3 or LAG-3 is diminished by immunotherapy, most significantly by the GVAX + agonist anti-OX40 combination. (***P < 0.0005, **P < 0.005, *P < 0.05; absence of an asterisk denotes not significant comparison.)
While GVAX immunotherapy for GL261 brain tumors modestly and, in some cases, significantly (Fig. 6B) reduced expression of LAG-3 or TIM-3 on PD-1–expressing BILs on day 19, agonist anti-OX40 monotherapy tended to effect this change with more vigor. Combination GVAX and agonist anti-OX40 immunotherapy leads to the lowest levels of lymphocyte coexpression of PD-1 and LAG-3 or TIM-3 in all possible combinations, driving the percentage of exhausted cells below 10% of CD4+ or CD8+ lymphocytes in most cases. In this regard, CD8+ PD-1+ LAG-3+ cells were somewhat of an exception, though all forms of immunotherapy tested here led to lower PD-1/LAG-3 coexpression than in lymphocytes from control-treated animals.
Discussion
In this study, we have shown that vaccination using irradiated syngeneic glioma cells engineered to express GM-CSF is complementary with systemic delivery of an activating antibody against OX40, a co-stimulatory receptor on T lymphocytes. This builds upon our previous work demonstrating that GVAX and CTLA-4 blockade synergize against intracranial murine glioma.9 Since then, immune checkpoint inhibition with molecules blocking negatively regulating receptors on T cells, such as CTLA-4 and PD-1, has yielded groundbreaking results in patients with a variety of subsets of advanced malignancy, including melanoma,1 non–small cell lung carcinoma,5 head and neck cancer,16 and renal cell carcinoma,5 and clinical studies for patients with glioblastoma are ongoing.17
OX40 is a member of the TNF receptor superfamily and is expressed by T lymphocytes in a time-dependent fashion after initial antigen-specific activation; however, as opposed to inhibitory immune checkpoint molecules like CTLA-4 and PD-1, when bound by ligand, OX40 drives enhanced stimulation, measured by proliferation, cytokine production, and effector memory responses.11 OX40 is expressed on both activated CD4+ and CD8+ T lymphocytes and can be expressed on regulatory T cells as well.18 Intratumoral CD8 T lymphocytes expressing OX40 may represent a subset that have received a strong signal through the T-cell receptor, have high affinity for tumor-associated antigens, and are thus suitable candidate targets for prolonged stimulation through immunotherapy.19
A number of preclinical studies have demonstrated activity of OX40 stimulation against cancer.20 The initial phase I experience in patients with advanced solid malignancies21 showed that high doses of a murine monoclonal anti-OX40 antibody were well tolerated and associated with increased proliferation of CD8+ T lymphocytes but not regulatory T lymphocytes. Twelve of 30 subjects had some degree of tumor response. A number of clinical studies are accruing patients currently—preliminary data confirm tolerance, both as monotherapy and in combination with other immune modulating agents (https://clinicaltrials.gov/ct2/results?term=OX40&Search=Search, accessed October 23, 2016).
To date, a single study has examined the role of OX40 expression, signaling, or stimulation in patients with brain tumors. Shihibara et al22 have revealed by quantitative PCR that high-grade glioma cells express OX40 ligand and that higher expression levels are associated with survival after gross total resection. In the same report, OX40 ligation was shown to positively impact survival in a murine glioma model in combination with subcutaneous injection of tumor lysate. Single-agent activity was not explored. Murphy et al23 demonstrated single-agent activity in mice bearing intracranial GL261 tumors, but not when an alternative syngeneic glioma line was implanted. PCR of a series of inflammatory genes showed that susceptibility of GL261 tumors to OX40 immunotherapy may relate to general tumor immunogenicity, reflected by increased PD-L1 and IFN-γ expression. OX40 immunotherapy for patients with glioma has yet to be examined.
We have previously reported a phase I clinical trial examining the safety and activity of vaccinating patients with recurrent glioblastoma with irradiated autologous tumor cells mixed with an irradiated GM-CSF–expressing bystander cell line, a vaccination approach analogous to the one used in this murine study.6 Flow cytometry of T lymphocytes in peripheral blood reflected consistent vaccination-induced activation, partly measured by induced expression of OX40 on CD4+ cells. This finding generated the initial rationale for this study, with the proposed mechanism being that autologous tumor cell vaccination both increases the diversity of antigen-specific T-cell responses and, by activating more lymphocytes, broadens the opportunity for OX40 ligation and enhanced stimulation of antitumor immunity.
In this murine study, systemic delivery of agonist anti-OX40 monoclonal antibody, alone and in combination with whole tumor cell vaccination, impacted both systemic antitumor immunity and the tumor microenvironment. In particular, OX40 agonist activity drove responses that helped to overcome glioma-associated immunosuppression. Our study both confirms and builds significantly upon the excellent work by Murphy et al,23 who initially reported activity of OX40 ligation in the GL261 glioma model. In fact, Murphy and colleagues report 70% long-term survival via combination therapy of tumor lysate vaccination and Fc-OX40 ligand after optimization of treatment schedules. Their study differs from ours in several ways, including upfront differences in the therapeutic agents. Furthermore, we have created a more formidable tumor challenge by intracranial implantation of 75,000 GL261 cells rather than 15,000. Also, while we initiate our combination treatment 4 days earlier than does Murphy and colleagues, their regimen is more intensive and longer (combination vaccine/Fc-OX40 ligand on days 7, 10, and 13, followed by Fc-OX40 ligand alone on days 15–19). Most importantly, we have been able to carry out a more contemporary analysis of the mechanisms by which GVAX and agonist OX40 synergize to slow the growth of intracranial tumors. Successful glioma immunotherapy combinations will both drive effector lymphocytes into the tumor and allow them to maintain activated status once there. In detail, we have demonstrated systemic shifts toward Th1 immunity, antigen-specific activation, increased numbers of effector lymphocytes in brain tumors, and their full activation in combination immunotherapy treated mice.
Baseline ELISpot analyses of murine peripheral lymphocytes confirmed that, while there is evidence of a tumor-specific immune response in glioma-bearing mice, the tumor drives a Th2-dominant orientation, reflected by relatively little expression of IFN-γ and high levels of IL-10 elaboration after in vitro stimulation by irradiated tumor cells. However, combination therapy with whole GL261 cell vaccination and OX40 stimulation drives a strong Th1 antiglioma response, as evidenced by significantly increased IFN-γ and TNF-α expression by restimulated splenocytes. While anti-OX40 treatment and vaccine combined with anti-OX40 immunotherapy lead to measurable increases in both IFN-γ and IL-10 expression by splenocytes, this effect was much stronger for IFN-γ—up to 10-fold. Combination immunotherapy, in this case, reverses the baseline glioma-associated Th2 > Th1 skew in peripheral immune responses. In future murine studies and in immunotherapy clinical trials, we will examine whether the ELISpot-derived ratio of fold-change in expression levels of IFN-γ to that of IL-10 is a biomarker of systemic response.
Regulatory T lymphocytes play a prominent role in suppressing antiglioma immunity, both systemically and in the tumor microenvironment. Clinically, the intratumoral CD8+/FoxP3+ ratio is predictive of survival, and post-immunotherapy, this relationship may also correlate with overall therapeutic impact.24,25 In our model, vaccination, either as monotherapy or in combination with OX40 stimulation, appears to positively impact the CD8+/FoxP3+ ratio.
Treatment with agonizing anti-OX40 antibody alone, on the other hand, does not affect the intra-glioma CD8+/FoxP3+ ratio. It does, however, impact the activation status of the glioma-infiltrating lymphocytes, an outcome not previously described, at least explicitly. To the best of our knowledge, this is the first study to confirm in murine glioma that, in near end-stage disease, baseline expression of PD-1, TIM-3, and LAG-3 on brain-infiltrating CD4+ and CD8+ T lymphocytes is very high—the lymphocytic infiltrate was both scant and reflective of deactivation or exhaustion. Anti-OX40 treatment, alone or in combination with vaccination, reduced the expression of PD-1, TIM-3, and LAG-3 on BILs examined 3 weeks after tumor cell implantation and roughly 2 weeks after completion of therapy. The impact of vaccination alone, which positively impacted the cellular composition of the tumor microenvironment, measured by the CD8+/FoxP3+ ratio, remained hindered by the exhausted state of the infiltrate; however, combination with agonist anti-OX40 therapy added the advantage of persistent T-cell activation in the tumor microenvironment, measured by stronger Th1 orientation and reduced exhaustion, leading to improved survival.
We strongly support examining and applying combination approaches to glioblastoma immunotherapy. Cancer generally, and glioblastoma in particular, drives immune tolerance via a number of mechanisms.26 Effective and durable therapy may require increasing antigen exposure, counteracting regulatory T cells, and reversing negative immune regulation and lymphocyte fatigue or exhaustion. We have shown that amplifying and shifting T-cell responses through binding of OX40 is promising and may ultimately play a role in effective glioma therapy. While combining OX40 stimulation with whole glioma cell vaccination may be synergistic, other double and even triple combinations should be considered as well, including with CTLA-4 and PD-1 inhibition. Also, OX40 is not the only stimulating receptor expressed on the surface of activated T lymphocytes. Theoretically, 4-1BB and inducible co-stimulator are attractive targets for the amplification of antigen-specific T-cell responses and are appropriate for preclinical study and clinical evaluation in patients with malignant glioma. In addition, the optimal timing for delivery of these agents with respect to each other requires further detailed investigation. Although our data indicate that while OX40 stimulation reduces the expression of markers of T-lymphocyte fatigue in the glioma microenvironment, these effects may also be mediated by strategies directly targeting these molecules, such as combination therapy with anti–PD-1, anti–TIM-3, or anti–LAG-3 antibodies, and further work is necessary.
In summary, in mice bearing established intracranial glial tumors, systemic delivery of agonist anti-OX40 monoclonal antibodies augments GVAX efficacy and survival impact. Mechanistically, GVAX plus agonist anti-OX40 drives enhanced peripheral and intracranial Th1 antitumor immunity and reverses T-cell exhaustion that is at baseline associated with the glioma lymphocytic infiltrate.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by a grant from the “A Shot for Life” cancer research nonprofit organization.
Conflict of interest statement
None.
Supplementary Material
References
- 1. Hodi FS, O’Day SJ, McDermott DF et al. . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamid O, Robert C, Daud A et al. . Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larkin J, Chiarion-Sileni V, Gonzalez R et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Topalian SL, Hodi FS, Brahmer JR et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curry WT Jr, Gorrepati R, Piesche M et al. . Vaccination with irradiated autologous tumor cells mixed with irradiated GM-K562 cells stimulates antitumor immunity and T lymphocyte activation in patients with recurrent malignant glioma. Clin Cancer Res. 2016;22(12):2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men. Int Rev Immunol. 2006;25(5-6):321–352. [DOI] [PubMed] [Google Scholar]
- 8. Herrlinger U, Kramm CM, Johnston KM et al. . Vaccination for experimental gliomas using GM-CSF-transduced glioma cells. Cancer Gene Ther. 1997;4(6):345–352. [PubMed] [Google Scholar]
- 9. Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT Jr. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35(5):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol Rev. 2011;244(1):218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redmond WL, Linch SN. Combinatorial immunotherapeutic approaches to restore the function of anergic tumor-reactive cytotoxic CD8(+) T cells. Hum Vaccin Immunother. 2016;12(10):2519–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sayour EJ, McLendon P, McLendon R et al. . Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother. 2015;64(4):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35(2):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim JE, Patel MA, Mangraviti A et al. . Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017;23(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferris RL, Blumenschein G Jr, Fayette J et al. . Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curry WT, Lim M. Immunomodulation: checkpoint blockade etc. Neuro Oncol. 2015;17(Suppl 7):vii26–vii31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schreiber TH, Wolf D, Bodero M, Gonzalez L, Podack ER. T cell costimulation by TNFR superfamily (TNFRSF)4 and TNFRSF25 in the context of vaccination. J Immunol. 2012;189(7):3311–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moran AE, Polesso F, Weinberg AD. Immunotherapy expands and maintains the function of high-affinity tumor-infiltrating CD8 T cells in situ. J Immunol. 2016;197(6):2509–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. [DOI] [PubMed] [Google Scholar]
- 21. Curti BD, Kovacsovics-Bankowski M, Morris N et al. . OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shibahara I, Saito R, Zhang R et al. . OX40 ligand expressed in glioblastoma modulates adaptive immunity depending on the microenvironment: a clue for successful immunotherapy. Mol Cancer. 2015;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy KA, Lechner MG, Popescu FE et al. . An in vivo immunotherapy screen of costimulatory molecules identifies Fc-OX40L as a potent reagent for the treatment of established murine gliomas. Clin Cancer Res. 2012;18(17):4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodi FS, Butler M, Oble DA et al. . Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105(8):3005–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–298. [DOI] [PubMed] [Google Scholar]
- 26. Mangani D, Weller M, Roth P. The network of immunosuppressive pathways in glioblastoma. Biochem Pharmacol. 2017;130:1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.