Abstract
Background
Acute kidney injury (AKI) after multiple Hymenoptera stings is well known but still a rare phenomenon.
Methods
We conducted a retrospective study of the clinicopathological spectrum of AKI due to multiple Hymenoptera stings over 13 years (July 2003–June 2016).
Results
A total of 35 patients were diagnosed with AKI due to multiple Hymenoptera stings. The mean age of the patients was 44.7 ± 17.4 years and the majority (60%) were men. Haematological and biochemical laboratory abnormalities included anaemia (97.1%), leucocytosis (54.3%), hyperkalaemia (68.6%), severe metabolic acidosis (51.4%), hepatic dysfunction (74.3%), haemolysis (91.4%) and rhabdomyolysis (62.9%). The main complications included acute respiratory distress syndrome (ARDS) and encephalopathy in four (11.4%) patients each; gastrointestinal bleeding, hypertension and panniculitis in two (5.7%) patients each and one (2.9%) patient each developed intra-abdominal bleeding, stroke and polyserositis. Twenty-nine (83%) patients required dialysis. Ten (29%) patients died. A higher white blood cell count (P = 0.05) and the complications of ARDS (P = 0.004) and encephalopathy (P = 0.004) were associated with mortality. The kidney functions normalized at 5.5 ± 2.6 weeks in patients who survived. Kidney biopsy was done in 13 patients. The predominant lesion was acute tubular necrosis (ATN) with or without pigmented granular cast in 10 (77%) patients. In four (30.8%) patients, the kidney biopsy showed severe ATN and in the other six (46.2%), the kidney biopsy showed features of ATN associated with mild to moderate acute interstitial nephritis (AIN). In three (23%) patients the histopathological examination revealed only moderate AIN and these patients were treated with a short course of steroids.
Conclusions
AKI due to multiple Hymenoptera stings is severe and is associated with high mortality. On renal histology, ATN and AIN are common.
Keywords: acute interstitial nephritis, acute kidney injury, acute tubular necrosis, envenomation, Hymenoptera stings
Introduction
Envenomation or poisoning by toxins from animals poses an important health hazard in the tropics. Approximately 5 million snake bites, scorpion stings and anaphylactic reactions to insect stings occur worldwide annually, causing >100 000 deaths each year, most of which happen in the tropics [1, 2]. Insects that sting to defend their colonies belong to the order Hymenoptera. Medically important groups of Hymenoptera are the Apoidea (bees), Vespoidea (wasps, hornets and yellow jackets) and Formicidae (ants). These insects deliver their venom by stinging their victims. Bees lose their barbed stinger after stinging and die. Wasps, hornets and yellow jackets can sting multiple times [3, 4].
Stinging events involving honeybees and wasps are rare; most deaths or clinically important incidents involve very few stings (<10) and anaphylactic shock. However, mass stinging events can prove life threatening via toxic action of the venom when injected in large amounts [3]. Hymenoptera toxins are complex mixtures of peptides, enzymes, proteins and chemicals and they cause cellular injury via several mechanisms [2]. Despite the differences in venom composition and quantity released per sting in the two insect groups, both lead to similar medical consequences, such as localized normal allergic reactions, mild to severe anaphylaxis and shock and multiple organ and tissue injury leading to multiple organ failure [4, 5].
As a highly vascularized and excretory organ, the kidney is particularly vulnerable to Hymenoptera toxins [2]. Acute kidney injury (AKI) after multiple Hymenoptera stings is well known but still a rare phenomenon. AKI is usually caused by intravascular haemolysis, rhabdomyolysis, shock and the direct toxic effects of the venom; the triad of AKI, haemolysis and rhabdomyolysis often occurs in patients subjected to multiple wasp or bee stings [6]. Renal biopsy usually reveals acute tubular necrosis (ATN) as well as occasional acute interstitial nephritis (AIN) [6, 7]. More than half of the victims who experience multiple wasp or bee stings develop AKI, and most of these patients require intermittent haemodialysis (IHD) or peritoneal dialysis (PD) [8–11]. The mortality rate of patients who experience AKI has been reported to be as high as 25% [6].
Himachal Pradesh is a mountainous state in the northern part of India. The state is covered with a large forest with abundant flora and fauna. The population is largely (>90%) rural, living on agriculture [12]. Farmers and people living close to forests are prone to insect stings. Indira Gandhi Medical College Hospital, Shimla is the only tertiary-care hospital in the state of Himachal Pradesh. We retrospectively analysed the records to study the clinicopathological spectrum of AKI due to multiple Hymenoptera stings seen during last 13 years.
Materials and Methods
This was a retrospective study. The secondary data from medical records of patients admitted at Indira Gandhi Medical College Hospital, Shimla with multiple Hymenoptera stings and AKI over a period of 13 years (July 2003–June 2016) were evaluated. The inclusion and exclusion criteria were defined as follows.
Inclusion criteria
Definitive history of bee or wasp sting.
Clinical picture consistent with multiple bee or wasp stings, i.e. the presence of sting marks on physical examinations.
Presence of AKI as defined using Kidney Disease: Improving Global Outcomes criteria based on serum creatinine (increase in serum creatinine by ≥0.3 mg/dL within 48 h or increase in serum creatinine to ≥1.5 times baseline), which is known or presumed to have occurred within the prior 7 days [13]. It was presumed that the patient had normal renal function if the serum creatinine was 1.5 mg/dL.
Exclusion criteria
Patients with pre-existing renal disease (serum creatinine > 1.5 mg/dL prior to bee or wasp sting or ultrasonography of the abdomen suggestive of bilateral small kidneys/loss of corticomedullary differentiation/obstructive nephropathy/other renal pathology).
Exposure to nephrotoxic drugs/toxins.
Medical records were evaluated for the patient’s information, including age, gender, month of the sting, time from stinging to arrival at the hospital, number of stings and the presence of oliguria or haematuria.
All the patients were subjected to detailed history and clinical examination. Haematological and biochemical investigations were performed in all patients. All the patients received supportive care (intravenous fluids, antihistamines, steroids, antibiotics and analgesics) and forced alkaline diuresis was given when patients presented early without volume overload or oliguria. The patients were subjected to dialysis as per standard hospital protocol. Ventilatory support was needed for patients with respiratory failure.
Laboratory results of haemoglobin (Hb), leucocyte count, platelet count, blood urea, serum creatinine, serum electrolytes of sodium and potassium, serum albumin, serum bilirubin, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT), alkaline phosphatase, creatine kinase (CK), lactate dehydrogenase (LDH) and arterial blood gas analysis and the prevalence of anaemia (Hb <12 g/dL), leucocytosis, thrombocytopenia, hyperkalaemia, hypoalbuminaemia, hepatic dysfunction, severe metabolic acidosis (pH 7.2), haemolysis and rhabdomyolysis were recorded. Peak values or the worst results of these laboratory parameters during the admission were used for analysis. Complications such as hypertension, acute respiratory distress syndrome (ARDS), gastrointestinal (GI) bleeding, encephalopathy, etc. seen during the hospitalization were recorded.
Outcomes of requirement of dialysis, number of HD sessions, survival, mortality and time to normalization of kidney function were analysed. The survival and non-survival groups were compared to determine the differences in demographic factors, laboratory results, clinical characteristics and complications between the two groups.
Hepatic dysfunction was defined as an AST or ALT >60 IU/L. Intravascular haemolysis was defined as the presence of anaemia, jaundice, reticulocytosis, abnormal peripheral blood smear and raised serum LDH levels. Rhabdomyolysis was characterized by elevated serum CK levels of five or more times the normal levels, with a suggestive clinical picture and without heart and cerebral injury [14]. Encephalopathy/delirium was defined as acute onset decline in cognitive functions in patients where meningitis or meningoencephalitis was excluded. Patients remaining oligoanuric or whose serum creatinine did not decrease satisfactorily at the end of 2 weeks of treatment underwent kidney biopsy that was examined with light and immunofluorescence microscopy. The findings of histopathological examination of the kidney biopsies were also analysed. Complete recovery of kidney function was defined as a decrease in the serum creatinine level to within a normal range. Permission from the Institutional Review Board and the hospital authorities was obtained for the study [HFW(MS)G-5(Ethic)/2015-9078].
Statistical analysis
The data collected were stored using a computer program. Continuous data are expressed as mean ± SD, and the means of the two study groups were compared using an unpaired t test. Nominal data are expressed as frequencies or proportions and the chi-square test and Fisher’s exact test, as appropriate, were used to compare the differences in frequency between the two study groups. For non-normal data, a Mann–Whitney U test was performed. A P-value <0.05 was considered statistically significant. All statistics were carried out using SPSS version 16 (SPSS, Chicago, IL, USA).
Results
A total of 35 patients were diagnosed with AKI due to multiple Hymenoptera stings over this period.
Baseline demographic and clinical characteristics
Table 1 shows the demographic and clinical characteristics of the study patients. The mean age of the patients was 44.7 ± 17.4 years. Of the 35 patients, 21 (60%) were male and 14 (40%) were female. The cause of injury was multiple wasp stings (Vespa magnifica) 32 (91.4%) and multiple bee stings (forest bees, Apis dorsata) 3 (8.6%) patients. Most (80%) patients had stinging events during the months August–November. All the Hymenoptera sting accidents occurred in rural areas. The mean number of stings was 57 ± 49 (range 15–200) and a time interval to arrival at the hospital after the sting was 5.1 ± 4.8 days (range 0.2–16). Of the patients, 29 (82.9%) had oliguria and 20 (57.1%) had a history of haematuria or having passed cola-coloured urine.
Table 1.
Demographic and clinical characteristics of the study patients (n = 35)
| Gender, n (%) | |
| Male | 21 (60) |
| Female | 14 (40) |
| Age (years) | |
| Mean ± SD | 44.7 ± 17.4 |
| Range | 7–76 |
| Type of sting, n (%) | |
| Wasp | 32 (91.4) |
| Bee | 3 (8.6) |
| Month of the year, n (%) | |
| April | 1 (2.9) |
| May | 2 (5.7) |
| June | 1 (2.9) |
| July | 2 (5.7) |
| August | 4 (11.4) |
| September | 8 (22.9) |
| October | 11 (31.4) |
| November | 5 (14.3) |
| December | 1 (2.9) |
| Number of stings | |
| Mean ± SD | 57 ± 49 |
| Median | 40 |
| Range | 15-200 |
| Duration at arrival (days) | |
| Mean ± SD | 5.1 ± 4.8 |
| Median | 4 |
| Range | 0.2–16 |
| Oligura, n (%) | 29 (82.9) |
| Haematuria, n (%) | 20 (57.1) |
| Dialysis therapy, n (%) | 29 (82.9) |
| Haemodialysis | |
| Mean ± SD | 4.4 ± 3.1 |
| Range | 1–14 |
| Outcome, n (%) | |
| Survived | 25 (71.4) |
| Died | 10 (28.6) |
| Time duration to normal kidney function (weeks) | |
| Mean ± SD | 5.5 ± 2.6 |
| Median | 6 |
| Range | 1–8 |
Laboratory results
The Hb was 9 ± 2.4 g/dL and white blood cell (WBC) count was 13.9 ± 7.3 (×103/mm3). Peak values of serum urea and creatinine were 238 ± 117 and 10.2 ± 6 mg/dL, respectively. The mean values of bilirubin were 1.5 ± 1.1 mg/dL, AST 627 ± 1068 IU/L and ALT 393 ± 481 IU/L. Serum CK was 2593 ± 4093 U/L and LDH was 1756 ± 1862 IU/L (Table 2). The hematological and biochemical laboratory abnormalities included anaemia (97.1%), leucocytosis (54.3%), hyperkalaemia (68.6%), metabolic acidosis (51.4%), hepatic dysfunction (74.3%), haemolysis (91.4%) and rhabdomyolysis (62.9%).
Table 2.
Laboratory results of the study patients (n = 35)
| Mean ± SD | Range | |
|---|---|---|
| Haemoglobin (g/dL) | 9 ± 2.4 | 2.5–12.9 |
| WBC count (×103/mm3) | 13.9 ± 7.3 | 4–35.5 |
| Urea (mg/dL) | 238 ± 117 | 65–516 |
| Creatinine (mg/dL) | 10.2 ± 6 | 1.2–22.2 |
| Bilirubin (mg/dL) | 1.5 ± 1.1 | 0.3–5.2 |
| AST (U/L) | 627 ± 1068 | 17–4870 |
| ALT (U/L) | 393 ± 481 | 27–2370 |
| CK (U/L) | 2593 ± 4093 | 44–22 000 |
| LDH (U/L) | 1756 ± 1862 | 120–9971 |
Complications
The main complications were ARDS and encephalopathy in four (11.4%) patients each; GI bleed, hypertension and panniculitis in two (5.7%) patients each and one (2.9%) patient each developed intra-abdominal bleeding, stroke and polyserositis.
Clinical outcomes
Of the 35 patients, 10 died and 25 survived (case fatality rate 28.6%). Mortality was due to ARDS [4], massive bleeding [3], metabolic causes [2] and stroke [1]. Three patients with bee stings and two patients with wasp stings were treated with alkaline diuresis, as they arrived early (a few to <24 h) and recovered without dialysis support. Twenty-nine (82.9%) patients required dialysis. Each patient received 4.4 ± 3.1 sessions of HD. The kidney functions normalized at 5.5 ± 2.6 weeks in patients who survived (Table 1).
Comparison between the survival (n = 25) and non-survival group (n = 10)
The WBC count was significantly higher in patients who died (P = 0.05). The complications of ARDS and encephalopathy were only seen in patients who died (P = 0.004). Dialysis was required in 9 (90%) patients who died as compared with 20 (80%) of the patients who survived (P = 0.649). There were no significant differences in the type of bite, the severity of haematological and biochemical abnormalities, other complications and the need for dialysis therapy in the non-survival group as compared with the survival group (Tables 3 and 4).
Table 3.
Comparison of demographic and laboratory results between the survival and non-survival group (n = 35)
| Survived | Died | ||
|---|---|---|---|
| (n = 25) | (n = 10) | P-value | |
| Age (years) | 46.3 ± 16.5 | 40.5 ± 19.8 | 0.380a |
| Sex, n (%) | |||
| Male | 15 (60) | 6 (60) | 1.000c |
| Female | 10 (40) | 4 (40) | |
| Type of sting, n (%) | |||
| Wasp | 22 (88) | 10 (100) | 0.542c |
| Bee | 3 (22) | 0 (0) | |
| Number of stings, mean ± SD | 65 ± 56.3 | 38.5 ± 13.5 | 0.160a |
| Duration at arrival (days) | 5.4 ± 5.2 | 4.1 ± 3.5 | 0.486a |
| Haemoglobin (g/dL) | 9.2 ± 2.2 | 8.4 ± 2.8 | 0.404a |
| WBC count (×103/mm3) | 12.5 ± 6.7 | 17.3 ± 7.8 | 0.054b |
| Urea (mg/dL) | 226 ± 114 | 270 ± 102 | 0.460b |
| Creatinine (mg/dL) | 10.8 ± 6.5 | 8.8 ± 4.7 | 0.358 b |
| Bilirubin (mg/dL) | 1.6 ± 1.3 | 1.1 ± 0.7 | 0.397b |
| AST (U/L) | 542 ± 888 | 841 ± 1461 | 0.460b |
| ALT (U/L) | 324 ± 358 | 567 ± 696 | 0.131b |
| CK (U/L) | 1675 ± 2273 | 4940 ± 6486 | 0.078b |
| LDH (U/L) | 1459 ± 1161 | 2573 ± 3045 | 0.256b |
Independent samples t-test, significance level (two-tailed).
Mann–Whitney U test, asymptotic significance (two-tailed), non-parametric independent samples test.
Chi-square with Fisher’s exact test.
Table 4.
Comparison of laboratory abnormalities and complications between the survival and non-survival group (n = 35)
| Survived | Died | ||
|---|---|---|---|
| (n = 25) | (n = 10) | P-value | |
| Anaemia, n (%) | 24 (96) | 10 (100) | 1.000a |
| Leucocytosis, n (%) | 11 (60.2) | 8 (80) | 0.071a |
| Hyperkalaemia, n (%) | 16 (64) | 8 (80) | 0.447a |
| Metabolic acidosis, n (%) | 9 (37.5) | 9 (90) | 0.458a |
| Haemolysis, n (%) | 23 (92) | 9 (90) | 1.000a |
| Rhabdomyolysis, n (%) | 15 (60) | 7 (70) | 0.709a |
| Hepatic dysfunction, n (%) | 18 (72) | 8 (80) | 1.000a |
| ARDS, n (%) | 0 (0) | 4 (40) | 0.004a |
| Encephalopathy, n (%) | 0 (0) | 4 (40) | 0.004a |
| Dialysis, n (%) | 20 (80) | 9 (90) | 0.649a |
Chi-square with Fisher’s exact test.
Comparison between AKI due to bee (n = 3) and wasp stings (n = 32)
The mean urea (P = 0.021) and creatinine (P = 0.011) values were significantly higher in patients with wasp sting–induced AKI. The age (P = 0.024) and the number of stings (P = 0.001) were significantly higher in bee sting–induced AKI. The complications of ARDS (P = 0.004), encephalopathy (P = 0.004), dialysis treatment (P = 0.003) and mortality (P = 0.001) were seen only in patients with wasp sting–induced AKI. There were no significant differences in the clinical characteristics, other laboratory abnormalities and complications between the two groups. In patients with bee sting–induced AKI, none required dialysis and none died.
Renal histology
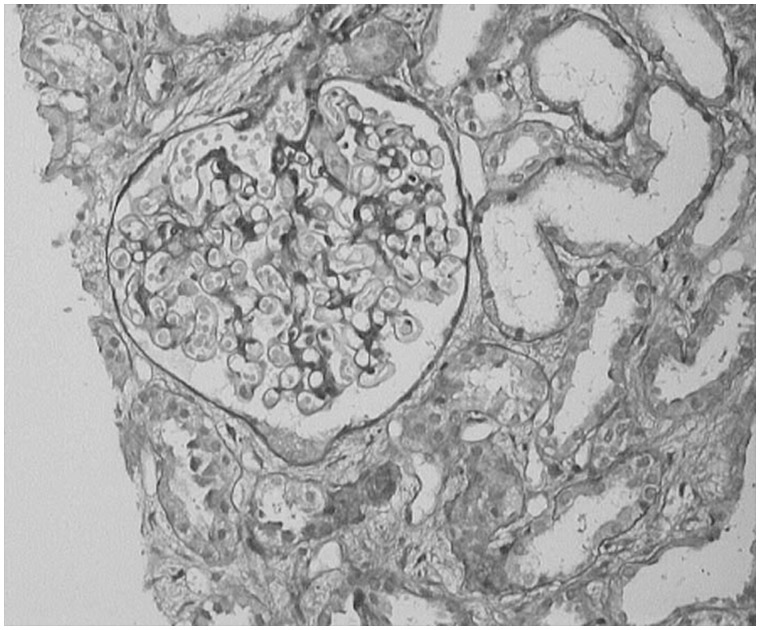
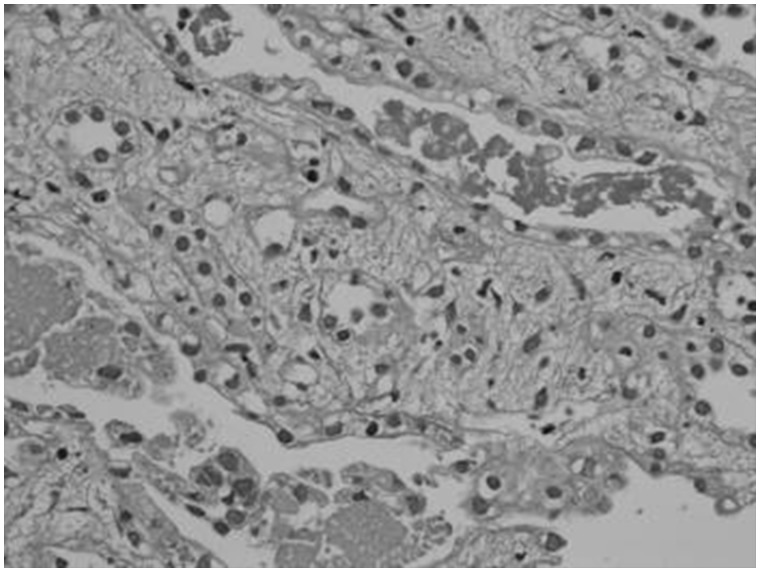
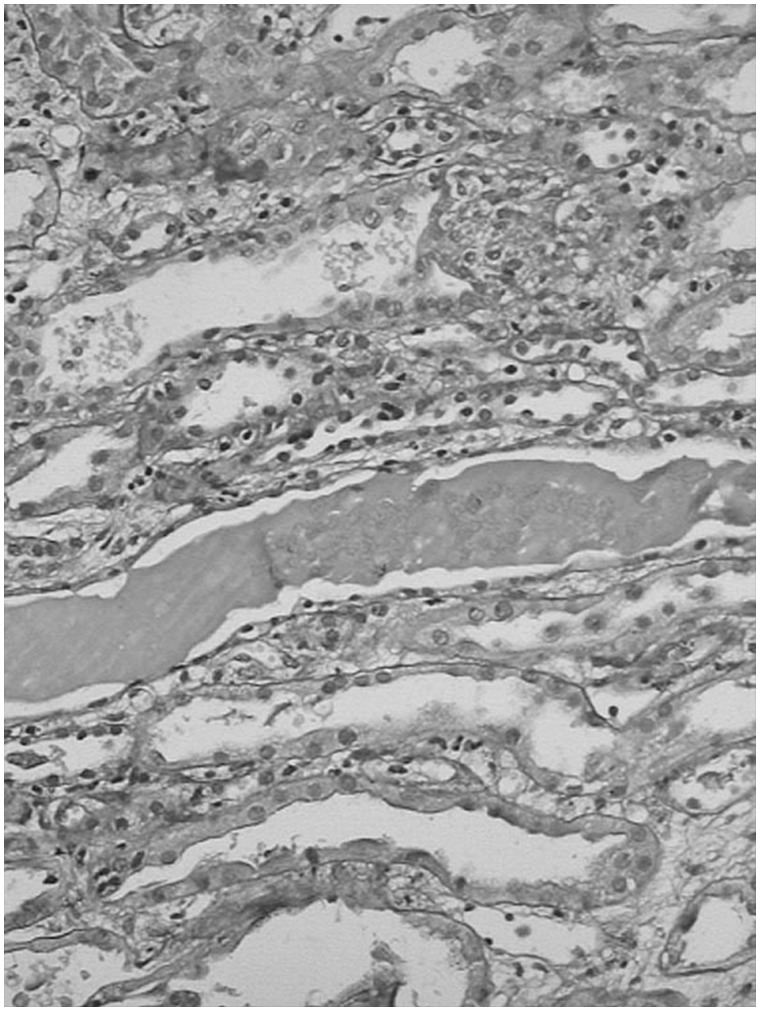
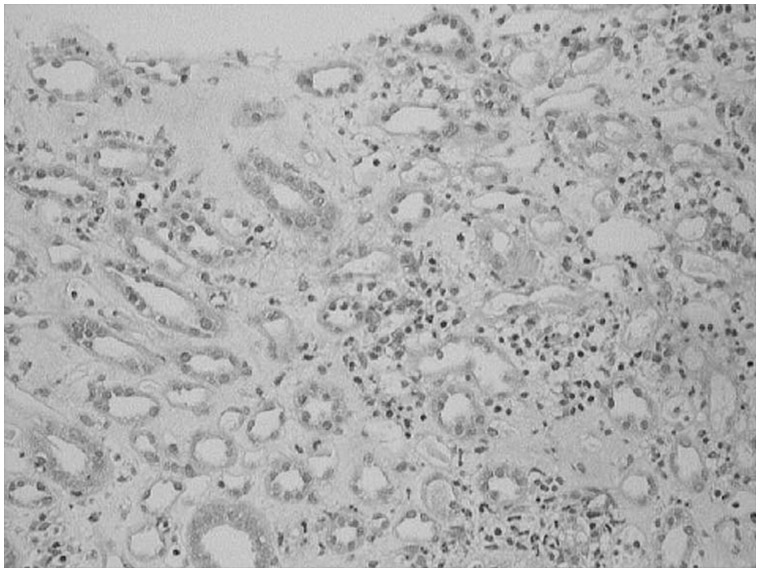
Kidney biopsy was done in 13 patients. The predominant lesion was ATN with or without pigmented granular cast in 10 (77%) patients (Figure 1). In four (30.8%) patients the kidney biopsy showed severe ATN and in the other six (46.2%) patients the kidney biopsy showed features of ATN associated with mild to moderate AIN (Figure 2). In three (23%) patients the histopathological examination revealed only AIN (Figures 3 and 4), and these patients were treated with a short course of steroids. Overall, AIN was seen in nine (69.2%) kidney biopsies.
Fig. 1.
Kidney biopsy image showing severe ATN.
Fig. 2.
Kidney biopsy image shows ATN associated with moderate interstitial nephritis.
Fig. 3.
Kidney biopsy image shows severe acute interstitial nephritis.
Fig. 4.
Kidney biopsy image shows moderate acute interstitial nephritis.
Discussion
AKI after multiple Hymenoptera stings is well known but is still a rare phenomenon. Thiruventhiran et al. [6] reviewed 24 cases of AKI following multiple Hymenoptera stings published over a quarter-century. Many large series have been published since then (Table 5) [6, 8, 9, 15–21]. The current study of 35 cases is the largest series encompassing clinicopathological aspects, course, treatment and outcomes.
Table 5.
Studies of AKI due to multiple Hymenoptera stings
| Author | No. of cases | Types of insects | Haemolysis (%) | Rhabdomyolysis (%) | Hypotension (%) | Hepatitis (%) | Renal biopsy (%) | RRT (%) | Mortality (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Thiruventdiran et al. [6] | 24 | Wasp | 16 | 88 | 85 | 38 | 75 | ATN 9 | 77.3 | 25 |
| Bee | 7 | |||||||||
| Yellow | ||||||||||
| jacket | 1 | |||||||||
| Wiwanitkit [15] | 16 | Wasp | NA | NA | NA | NA | ATN 8 | 68.8 | 50 | |
| Vachvanichsanong | ||||||||||
| et al. [16] | 7 | Wasp | Yes | Yes | NA | Yes | NI | Yes | 14.2 | |
| Paudel and Paudel [9] | 9 | Wasp | 89 | 89 | NA | 78 | NI | 89 | 22.2 | |
| Xuan et al. [8] | 38 | Hornet | NI | Yes | 18.4 | Yes | NI | 55.3 | 18.4 | |
| Mejía Vélez [17] | 43 | Bee | Yes | Yes | Yes (61) | NA | NI | 86 | 16.3 | |
| Zhang et al. [18] | 75 | Wasp | Yes | Yes | NA | Yes | NI | Yes | 9.3 | |
| Sigdel and Raut [19] | 18 | Wasp | NA | NA | NA | NA | NI | Yes | 5.6 | |
| Si et al.* [20] | 19 | Wasp | Yes | Yes | Yes | Yes | NI | Yes | 31.6 | |
| Dhanapriya et al. [21] | 11 | Wasp | 27.3 | 100 | 18.2 | NA | ATN 2 | 91 | 9.1 | |
| AIN 1 | ||||||||||
| ATN+ | ||||||||||
| AIN 1 | ||||||||||
| Current study | 35 | Wasp 32 | 91.4 | 63 | No | 74 | ATN 4 | 83 | 28.6 | |
| Bee 3 | AIN 3 | |||||||||
| ATN+ | ||||||||||
| AIN 6 |
NA, not available; NI, not investigated.
The main clinical features at presentation included oliguria (83%) and haematuria (57%). These were also the main symptoms reported in other studies [19, 21].
Hymenoptera venoms are complex mixtures of biologically active components primarily composed of peptides, enzymes and amines. Bee venom contains melittin, phospholipase A2, mast cell degranulating peptide (peptide 401), hyaluronidase and apamin, among other constituents [4]. These components have direct and indirect cytotoxic (hepatic, renal and myocyte membrane), haemolytic, neurotoxic and vasoactive properties that can cause intravascular haemolysis and rhabdomyolysis. Our study found incidences of intravascular haemolysis (91.4%), rhabdomyolysis (63%) and hepatic dysfunction (74.3%) in AKI patients. Similar to our study, other studies have reported intravascular haemolysis [6, 9, 16–18, 20, 21] to be 27.3–89% [6, 9, 21], rhabdomyolysis [6–9, 16–18, 20, 21] to be 85–100% [6, 9, 21] and hepatic dysfunction [6–9, 16, 18, 20] to be 75–78%. Studies have reported hypotension [6, 8, 17, 20, 21] to be 18.2–61% [6, 8, 17, 21]. Our study patients did not have hypotension/anaphylaxis nor were these documented by the referring hospital.
Hymenoptera venom can cause AKI by several mechanisms, including ATN, AIN and pigment nephropathy resulting from rhabdomyolysis (myoglobinuria) or intravascular haemolysis (Hb) and hypotension caused by anaphylactic reaction and direct nephrotoxicity of the venom [6]. ATN is the primary cause of AKI following Hymenoptera envenomation. AKI, however, is likely to be secondary to intravascular haemolysis and/or rhabdomyolysis. Indeed, there was evidence of intravascular haemolysis and rhabdomyolysis in reported studies where this effect was sought (Table 5). Pigment casts in renal tubules have been documented in addition to ATN, supporting the view that renal complications are likely to be a secondary rather than primary effect of venomous toxins [6].
ATN was the most common (>90%) histopathological finding on renal biopsies in patients with AKI following multiple Hymenoptera stings [6, 15, 21]. Some patients in our study had neither evidence of intravascular haemolysis or rhabdomyolysis nor a history of hypotension or anaphylaxis. Although kidney biopsy was not done, it is possible that direct nephrotoxicity or AIN might have been responsible for AKI in these patients. Hypersensitivity reaction to wasp venom leading to AIN has been reported as a cause of AKI alone [22, 23] or in combination with pigment nephropathy [7, 21]. Our study also found ATN with or without pigment nephropathy to be the most common (77%) lesion on kidney biopsy that was associated with AIN in a significant number (46.2%). AIN alone was seen in three (23%) patients who completely recovered after a course of steroids. Overall, AIN was seen in 69.2% of kidney biopsies.
Since there is no antivenom, treatment in all such cases is essentially supportive. Following multiple Hymenoptera stings, patients are likely to develop the complications of intravascular haemolysis, rhabdomylysis and AKI. To prevent systemic and renal complications, patients should be hospitalized early and treated with aggressive hydration, loop diuretic and urine alkalization. Indeed, patients who are treated early may not require dialysis, as was seen in five study patients who were treated with alkaline diuresis since they arrived early (a few to <24 h) and recovered without dialysis. However, once the overt kidney injury has been established, the only reliable therapeutic intervention is extracorporeal blood purification such as IHD, continuous renal replacement therapy (CRRT), PD or plasmapheresis (whenever indicated) [6, 8, 16–21]. Better renal outcomes have been reported in patients treated with CRRT alone or in combination with plasmapheresis as compared with IHD [18]. Thus the treatment of established AKI is largely supportive in nature, RRT being the cornerstone [24]. RRT is required in a high proportion (55–91%) of patients [6, 8, 15, 17, 21].
AKI or death may result from 20 to 200 wasp stings or 150 to 1000 bee stings [3]. In our study, the number of stings ranged from 15 to 100 for wasps and 200 for bees. Mortality of 28.6%, all due to wasp stings, was observed in our study. A higher WBC count (P = 0.05) and the complications of ARDS (P = 0.004) and encephalopathy (P = 0.004) were associated with mortality. None of the patients who experienced bee stings required RRT or died, however, these patients experienced fewer stings compared with ∼ 900 per patient reported in the largest case series of AKI due to bee stings [17].
The duration of AKI varies from 1 week to several weeks and is prolonged in elderly patients. Recovery of renal function is usual, but there may be residual renal damage, and elderly patients and children are at high risk of a fatal outcome [2]. Of the study patients, 71.4% survived and had a complete recovery of renal function over 1–8 weeks.
A few patients have been reported to develop chronic kidney disease on follow-up [18, 21]. Long-term outcome could not be studied in our patients due to a lack of such follow-up.
Our study is a large series on the clinicopathological spectrum of AKI following multiple Hymenoptera stings. There are certain disadvantages in this study. First, it was a retrospective study carried out at a tertiary care renal unit where patients were referred from far-flung rural areas. Second, many clinical details might have been missed due to failure on the part of peripheral hospitals to observe or document them. The special investigations, such as complete coagulation profile as evidence for disseminated intravascular coagulation, which were not done in all patients, are the other deficiency. Finally, we also could not assess long-term residual injury in these cases of Hymenoptera stings.
In conclusion, AKI is an important complication of multiple Hymenoptera stings that occurs in association with intravascular haemolysis, rhabdomyolysis and hepatic dysfunction. The pathogenesis of AKI is likely to be mediated by pigmented nephropathy caused by precipitation of Hb and myoglobin casts in renal tubules. However, in some patients a direct nephrotoxic effect or AIN from a hypersensitivity to insect venom may result in AKI. The most common findings on renal histological examination are pigment-induced ATN. AIN alone or in combination with ATN is an important contributor to AKI. Early recognition and prompt and aggressive treatment, in particular, forced alkaline diuresis, may help avert the need for dialysis and reduce morbidity. Mortality remains significant, but in the majority who survive the prognosis is good, with satisfactory recovery of renal function.
Conflict of interest statement
None declared.
References
- 1. WHO Weekly Epidemiological Record (February 2, 2002) Poisonous animal bites and stings. http://www.who.int/iris/handle/10665/229584 (25 November 2016, date last accessed)
- 2. Sitprija V. Animal toxins and the kidney. Nat Clin Pract Nephrol 2008; 4: 616–627 [DOI] [PubMed] [Google Scholar]
- 3. Vetter RS, Visscher PK, Camazine S.. Mass envenomations by honey bees and wasps. West J Med 1999; 170: 223–227 [PMC free article] [PubMed] [Google Scholar]
- 4. Fitzgerald KT, Flood AA.. Hymenoptera stings. Clin Tech Small Anim Pract 2006; 21: 194–204 [DOI] [PubMed] [Google Scholar]
- 5. Reisman RE. Unusual reactions to insect stings. Rev Curr Opin Allergy Clin Immunol 2005; 5: 355–358 [DOI] [PubMed] [Google Scholar]
- 6. Thiruventhiran T, Goh BL, Leong CL. et al. Acute renal failure following multiple wasp stings. Nephrol Dial Transplant 1999; 14: 214–217 [DOI] [PubMed] [Google Scholar]
- 7. Chao YW, Yang AH, Ng YY. et al. Acute interstitial nephritis and pigmented tubulopathy in a patient after wasp stings. Am J Kidney Dis 2004; 43: e15–e19 [DOI] [PubMed] [Google Scholar]
- 8. Xuan BH, Mai HL, Thi TX. et al. Swarming hornet attacks: shock and acute kidney injury—a large case series from Vietnam. Nephrol Dial Transplant 2010; 25: 1146–1150 [DOI] [PubMed] [Google Scholar]
- 9. Paudel B, Paudel K.. A study of wasp bites in a tertiary hospital of western Nepal. Nepal Med Coll J 2009; 11: 52–56 [PubMed] [Google Scholar]
- 10. Bresolin NL, Carvalho LC, Goes EC. et al. Acute renal failure following massive attack by Africanized bee stings. Pediatr Nephrol 2002; 17: 625–627 [DOI] [PubMed] [Google Scholar]
- 11. Bridi RA, Balbi AL, Neves PM. et al. Acute kidney injury after massive attack of Africanised bees. BMJ Case Rep 2014; 2014: pii: bcr2013201381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wikipedia. Himachal Pradesh. BMJ Case Rep 2014; pii: bcr2013201381; doi: 10.1136/bcr-2013-201381
- 13. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vanholder R, Sever MS, Erek E. et al. Rhabdomyolysis. J Am Soc Nephrol 2000; 11: 1553–1561 [DOI] [PubMed] [Google Scholar]
- 15. Wiwanitkit V. Renal failure from wasp stings: an appraisal on the previous reported Thai cases. Nephrology 2005; 10: 537. [DOI] [PubMed] [Google Scholar]
- 16. Vachvanichsanong P, Dissaneewate P.. Acute renal failure following wasp sting in children. Eur J Pediatr 2009; 168: 991–994 [DOI] [PubMed] [Google Scholar]
- 17. Mejía Vélez G. Acute renal failure due to multiple stings by Africanized bees. Report on 43 cases. Nefrologia 2010; 30: 531–538 [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Yang Y, Tang Y. et al. Recovery from AKI following multiple wasp stings: a case series. Clin J Am Soc Nephrol 2013; 8: 1850–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigdel MR, Raut KB.. Wasp bite in a referral hospital in Nepal. J Nepal Health Res Counc 2013; 11: 244–250 [PubMed] [Google Scholar]
- 20. Si X, Li J, Bi X. et al. Clinical evaluation of high-volume hemofiltration with hemoperfusion followed by intermittent haemodialysis in the treatment of acute wasp stings complicated by multiple organ dysfunction syndrome. PLoS One 2015; 10: e0132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dhanapriya J, Dineshkumar T, Sakthirajan R. et al. Wasp sting-induced acute kidney injury. Clin Kidney J 2016; 9: 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang R, Meleg-Smith S, Batuman V.. Acute tubulointerstitial nephritis after wasp stings. Am J Kidney Dis 2001; 38: E33. [DOI] [PubMed] [Google Scholar]
- 23. Li XD, Liu Z, Zhai Y. et al. Acute interstitial nephritis following multiple Asian giant hornet stings. Am J Case Rep 2015; 16: 371–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fieghen H, Wald R, Jaber BL.. Renal replacement therapy for acute kidney injury. Rev Nephron Clin Pract 2009; 112: c222–c229 [DOI] [PubMed] [Google Scholar]