Abstract
Background
Glioma-associated macrophages and microglia (GAMs) are components of the glioblastoma (GBM) microenvironment that express MerTK, a receptor tyrosine kinase that triggers efferocytosis and can suppress innate immune responses. The aim of the study was to define MerTK as a therapeutic target using an orally bioavailable inhibitor, UNC2025.
Methods
We examined MerTK expression in tumor cells and macrophages in matched patient GBM samples by double-label immunohistochemistry. UNC2025-induced MerTK inhibition was studied in vitro and in vivo.
Results
MerTK/CD68+ macrophages increased in recurrent tumors while MerTK/glial fibrillary acidic protein–positive tumor cells did not. Pharmacokinetic studies showed high tumor exposures of UNC2025 in a syngeneic orthotopic allograft mouse GBM model. The same model mice were randomized to receive vehicle, daily UNC2025, fractionated external beam radiotherapy (XRT), or UNC2025/XRT. Although median survival (21, 22, 35, and 35 days, respectively) was equivalent with or without UNC2025, bioluminescence imaging (BLI) showed significant growth delay with XRT/UNC2025 treatment and complete responses in 19%. The responders remained alive for 60 days and showed regression to 1%–10% of pretreatment BLI tumor burden; 5 of 6 were tumor free by histology. In contrast, only 2% of 98 GBM mice of the same model treated with XRT survived 50 days and none survived 60 days. UNC2025 also reduced CD206+ macrophages in mouse tumor samples.
Conclusions
These results suggest that MerTK inhibition combined with XRT has a therapeutic effect in a subset of GBM. Further mechanistic studies are warranted.
Keywords: glioblastoma, macrophage, MerTK, microenvironment
Importance of the study
Our study is a preclinical investigation of an orally bioavailable MerTK inhibitor, UNC2025, as a potential treatment in GBM. We confirmed that the expression of MerTK was increased in the GAM population at tumor progression compared with the initial diagnosis in patients’ GBM samples, suggesting the potential correlation between MerTK activation and tumor progression. Our pharmacokinetic assessment in a syngeneic mouse brain tumor model showed that UNC2025 exhibited excellent brain penetration and, combined with tumor irradiation, provided clinical benefit in a subgroup of GBM-bearing mice. In addition to inhibiting tumor growth, UNC2025 was found to modulate GAM properties within the glioma microenvironment. Our study has suggested that MerTK inhibition, which modulates the GBM microenvironment, is a promising alternative approach for GBM treatment, rather than targeting tumor cells only. It provided the rationale for further development of MerTK inhibitors as GBM therapy in the clinical trial setting.
Glioblastoma (GBM) is the most common malignant primary brain tumor and has a dismal prognosis. The 5-year survival rate remains 9.8% even with aggressive treatments including surgery, concurrent chemoradiation therapy with temozolomide (TMZ), and adjuvant TMZ.1 Despite vigorous research, this disease remains incurable. One focus of advanced cancer therapy is the development of therapies targeted to specific mutations or vulnerabilities in tumor cells. Despite some transient responses, tumors almost invariably develop resistance and eventually recur.2 More recently, knowledge of the adaptive and innate immune systems has produced successful therapeutic approaches in a small but definitive group of metastatic cancer patients.3–5 Therefore, strategically targeting the cells in the immunosuppressive glioma microenvironment, such as glioma-associated macrophages and microglia (GAMs), presents an attractive alternative therapeutic approach.
GAMs comprise a considerable fraction of cells within the glioma microenvironment.6 These myeloid lineage cells include yolk sac–derived microglia, innate immune cells intrinsic to the CNS, as well as bone marrow–derived macrophages that infiltrate as circulating monocytes and mature within the CNS. Studies have shown that GAMs promote glioma progression.7 Unlike microglia and macrophages in nonneoplastic diseases, the inflammatory defensive function of GAMs can be suppressed in the glioma microenvironment, where GAMs shift from an M1, pro-inflammatory phenotype to a wound-healing M2, anti-inflammatory phenotype.8 The numbers of GAMs with M2 phenotype are correlated with higher histological grade of gliomas.9 These findings suggest that modulating the M1/M2 phenotype of GAMs might be an alternative therapeutic strategy that deletes tumor promoting factors and enhances the probability of an immune response. In fact, inhibition of colony stimulating factor 1 receptor (a myeloid lineage growth factor) has been shown to increase survival in platelet derived growth factor–driven glioma mouse models, although tumor resistance eventually develops.10–12
Mer tyrosine kinase (MerTK) is a member of the tumor-associated macrophage (TAM) family of receptor tyrosine kinases (RTKs), which also includes Tyro3 and Axl. These 3 RTKs are overexpressed across a wide range of human tumors; in neoplastic cells, each can signal via canonical survival, motility, and invasion pathways.13,14 Their physiologic function has been studied in innate immune cells, where each can be activated by a complex ligand consisting of phosphatidylserine (PtdSer) linked to the RTK by a vitamin K–dependent protein ligand, Gas6, or Protein S.14,15 Gas6 binds all 3 TAMs, while Protein S binds only to MerTK and Tyro3.15,16 The PtdSer component is provided by apoptotic cells, exosomes, and in some cases, patches of exposed PtdSer on living cells (including T cells).14,15,17 In innate immune cells, TAM family signaling generally produces an anti-inflammatory, homeostatic response that diminishes excessive inflammation and autoimmune responses to the ingestion of “self.”14,18,19 Both the protein ligand and the sources of PtdSer are abundant in the tumor microenvironment, redirecting and thus subverting the homeostatic anti-inflammatory, wound healing, M2-like responses. This action is to the detriment of the host by providing an immunosuppressive milieu and secretion of invasion-promoting cytokines.14,18 Previous studies have shown that MerTK is expressed in all macrophage and microglia subsets and is the predominant TAM family member triggering efferocytosis in macrophages. Axl is more prominent in dendritic cell subsets, although recently studied tolerating dendritic cells are particularly MerTK rich.18,20 MerTK is also expressed in the brain, where it performs several known microglia/macrophage functions, including the pruning of nonproductive synapses.21,22 Overexpression of MerTK has also been found in many human cancers, including myeloid and lymphoblastic leukemia, melanoma, and gliomas.23–25 Prior to this work, short hairpin (sh)RNA–mediated MerTK inhibition has been shown to decrease levels of both pAkt and phosphorylated extracellular signal-regulated kinase (pERK).26
The above evidence suggests that MerTK might be a dual therapeutic target in gliomas. Blocking MerTK signaling will hinder GBM survival and create a pro-inflammatory microenvironment by decreasing M2 polarization in macrophages, so tumor can be eliminated. The development of selective, potent, orally bioavailable, first-in-class MerTK inhibitors including UNC2025 provides us with an opportunity to test the effect of MerTK inhibition on tumor growth as well as in modulating GAM properties within the glioma microenvironment.27 In this study, we investigated the biological functions and pharmacokinetic properties of UNC2025 in both tumor cells and GAMs using a syngeneic, immune competent mouse GBM model.
Materials and Methods
Details of this section can be found in the Supplementary material.
Cell Lines
U87, U251, and EOC 2 were purchased from American Type Culture Collection. Glioma stemlike cell (GSC) lines were provided by Dr E. Sulman. Luciferase-expressing tyrosinase related protein (TRP) astrocytes (TRP–murine stem cell virus [MSCV]–luc cells) were generated as described.28
Animal Studies
Mice were injected with 105 TRP-MSCV-luc cells as described.28 Tumors were established for 7 days and mice were randomized to one of the following 4 treatment groups: vehicle treatment, UNC2025 (65 mg/kg via oral gavage daily starting day 7), external beam radiotherapy (XRT) (5 Gy q.o.d. fractions on days 7, 9, and 11), or concurrent UNC2025 plus fractionated XRT. Bioluminescence imaging (BLI) was performed twice a week using the Xenogen IVIS Kinetic Imaging System. Mice surviving longer than 62 days were censored.
Western Blotting
Western blot analysis was performed using 30 µg of protein separated on 10% Bis-Tris gels. Following transfer to polyvinylidene difluoride membranes, Mer, pMer, Axl, pAxl, Akt, pAkt, ERK, pERK, cleaved caspase-3, and glyceraldehyde 3-phosphate dehydrogenase were probed using primary and horseradish peroxidase–conjugated secondary antibodies (listed in the Supplementary material). Blots were developed using enhanced chemiluminesence and visualized on a GE LAS-4000 Imaging System.
Colony Formation
Colony formation assays were performed on TRP-MSCV-luc cells treated with UNC2025 six hours before irradiation. Fourteen days after irradiation, plates were washed, fixed, stained with 0.05% crystal violet, and visualized on a Typhoon Trio Imager (GE Healthcare).
Proliferation
TRP-MSCV-luc cells were plated in 96-well plates and treated with drug for 5 days, and proliferation was evaluated using MTS ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Promega) as described.28 Relative growth was calculated as absorbance of drug-treated versus dimethyl sulfoxide–treated cells.
Immunofluorescence and Image Analysis
Cluster of differentiation (CD)68/MerTK and glial fibrillary acidic protein (GFAP)/MerTK dual immunofluorescence (IF) staining was performed using CD68 or GFAP antibodies, followed by Dako EnVision Mouse and tyramide Cy3 amplification (Perkin Elmer). MerTK was detected using MerTK antibody followed by Leica Bond polymer and tyramide Cy5 amplification. For dual staining with F4-80/CD206, F4-80 was detected using ImmPRESS anti-rat polymer (Vector Laboratories) and tyramide Cy3 amplification. CD206 was detected using Bond polymer and tyramide Cy5 amplification. Slides were counterstained with Hoechst 33258 (Invitrogen), mounted, and scanned on a Leica Aperio ScanScope FL. Tumor regions were manually identified and annotated using Aperio ImageScope. The Nuclei and Simulated Cells algorithm in Tissue Studio (Definiens) was used to detect and enumerate cells that coexpressed target biomarkers in annotated regions.
UNC2025 Pharmacokinetics
Pharmacokinetic studies were performed in orthotopic TRP allograft models. Tumor growth was confirmed by BLI on day 10 following tumor implantation. Mice were sacrificed prior to administration and at 0.083, 0.5, 1, 3, 6, and 24 hours after single dose administration of UNC2025 at 65 mg/kg by oral gavage (N = 3 per time point). Plasma, brain tumor, peritumor brain, and normal (contralateral, nontumor) brain tissues were collected from each mouse. Drug was detected using liquid chromatography–tandem mass spectrometry where UNC2025 served as a spiked reference standard. Analytes were detected using a TSQ Ultra triple quadrupole mass spectrometer (Thermo Fisher Scientific).
Statistics
Statistics were analyzed using GraphPad Prism. Survival was evaluated by the Kaplan–Meier method, and effects of treatment were compared by log-rank tests. Proliferation data (mean ± SEM) were pooled from 3 independent experiments and fit to a nonlinear, log [inhibitor] versus response curve and half-maximal inhibitory concentration (IC50) values calculated. IC50 values were compared using the extra-sum-of-squares F test. Images from colony formation assays were analyzed with ImageJ (National Institutes of Health). The surviving fraction of initially plated cells was calculated; data (mean ± SEM) were fit to a linear-quadratic equation and compared with an extra-sum-of-squares F test. For dual IF of human GBM, marker expressions in initial and recurrent tumors were compared using a 2-sided paired signed rank test. One-way ANOVA followed by a Newman–Keuls multiple comparison test was used to compare expression by western blots. For dual IF analysis of TRP allografts, one-way ANOVA followed by Dunn’s multiple comparison test was used.
Study Approval
This study was approved by the institutional review board (15–0923) and the Institutional Animal Care and Use Committee (16–112) of the University of North Carolina at Chapel Hill.
Results
MerTK Expression Is Enhanced in CD68+ Cells in Human GBMs
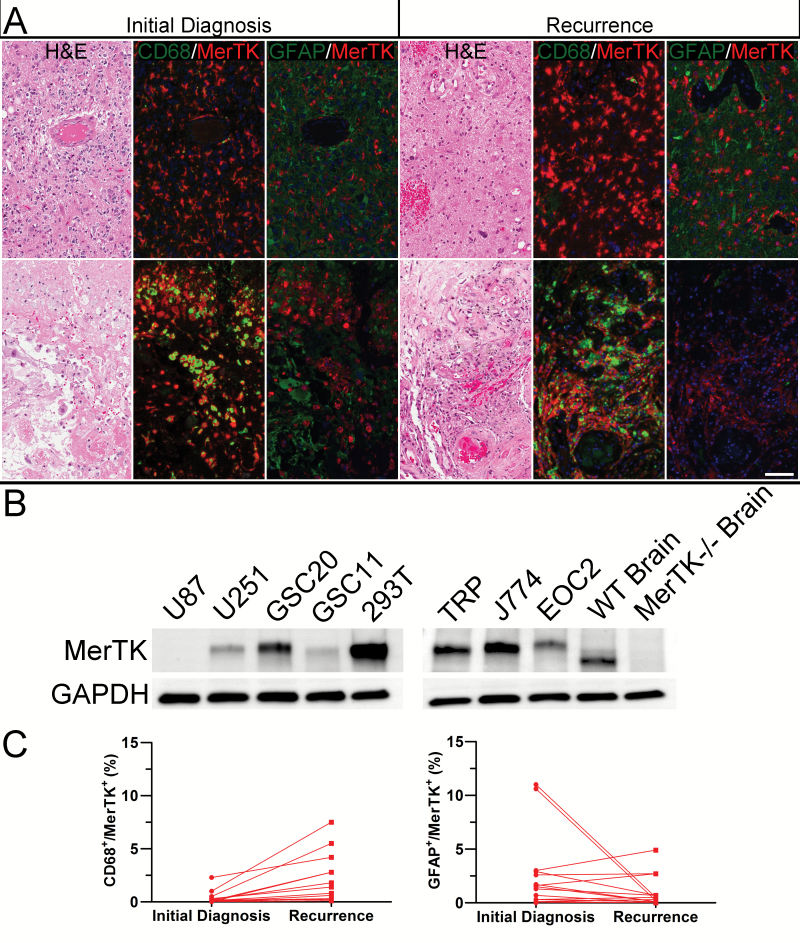
MerTK is expressed in human GBMs.24 To determine which cell populations expressed MerTK and whether expression correlated with disease progression, we performed double-label IF in 40 human GBM samples. GFAP and CD68 were used as markers for GBM tumor cells and GAMs, respectively. MerTK was expressed in both GFAP+ tumor cells and CD68+ GAM cells (Fig. 1A). MerTK was predominantly expressed in perivascular GFAP+ tumor cells (upper panel), as well as CD68+ GAMs in perinecrotic space (lower panel). Thus, MerTK is found in both tumor cells and GAMs in human GBMs.
Fig. 1.
MerTK expression in GBM. (A) MerTK is expressed in both GFAP+ tumor cells and CD68+ macrophages in a representative, matched pair of GBMs taken at initial diagnosis (left panel) and recurrence (right panel). Scale bar = 100 microns. (B) Western blot showed MerTK expression in human GBM (U87, U251, GSC20, GSC11) and mouse cells, including TRP cells, J774 macrophage, and EOC 2 microglia. Human 293T cells served as positive controls. Brain tissues from mertkWT and mertk−/− mice were used as positive and negative controls, respectively. (C) Dual staining for MerTK in CD68+ and GFAP+ cells at initial diagnosis and recurrence was quantified in 15 pairs of GBM. Percentage of MerTK+/CD68+ cells had a 6-fold increase of mean at disease recurrence (N = 15, P < 0.001) (a), while no consistent change of MerTK+/GFAP+ tumor cells was observed (N = 15, P = 0.14) (b).
MerTK was detected by immunoblot in relevant human and mouse cell lines, including high expression in the human GSC lines GSC20 and GSC11. MerTK was found at lower levels in non-GSCs, such as U87. These data are consistent with the finding that MerTK is highly expressed in GBM-derived sphere cultures.24 In mouse cells, MerTK was expressed in TRP cells, as well as J774 macrophage and EOC 2 microglia. MerTK was expressed in the brains of wild-type but not MerTK knockout mice, which have been used to establish physiologic functions for MerTK in nondiseased microglia (Fig. 1B).22
To determine whether MerTK expression changed when GBMs progress, we selected all the cases that had tissue available from both initial diagnosis and recurrent disease from those 40 GBM cases to quantify its expression in human GFAP+ tumor cells and CD68+ GAMs. Quantification in those 15 matched pairs of GBM obtained at initial diagnosis and recurrence showed a 6-fold increase in mean percentage of MerTK-expressing CD68+ macrophages among all nucleated cells (P < 0.001), but no consistent changes in MerTK expressing GFAP+ tumor cells upon recurrence (Fig. 1C). These data suggest that MerTK expression in CD68+ GAMs is correlated with disease recurrence.
UNC2025 Is a MerTK Inhibitor in GBM
Ectopic or aberrant expression of MerTK in pediatric leukemias, lung cancers, and gliomas led to experiments showing that MerTK activation leads to downstream signaling through ERK, Akt, and signal transducers and activators of transcription and that MerTK knockdown by shRNA decreased signaling through these pathways.26 These studies suggest that pharmacological MerTK inhibition in GBM cells might provide an impetus for future human clinical trials.
Our team developed a series of potent and selective MerTK compounds, including UNC2025, a novel small-molecule inhibitor with excellent oral bioavailability.27 UNC2025 inhibits MerTK tyrosine phosphorylation for up to 24 hours when delivered by oral gavage.27 MerTK inhibitory actions could be therapeutic if sufficient levels were obtained in gliomas in vivo. To initiate studies in malignant glioma, we tested the effects of UNC2025 on proliferation in a panel of human and mouse cells in vitro.
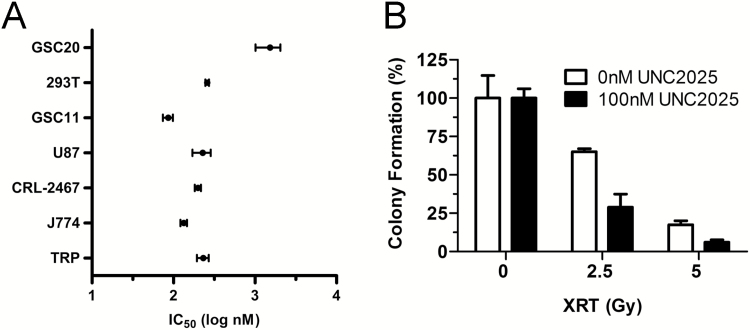
The range of UNC2025 concentration causing 50% of cell growth inhibition was examined in a group of cell lines, including human GSC lines (GSC11 and GSC20), the human GBM cell line U87, the mouse GBM cell line TRP, and the mouse microglial/macrophage cell lines EOC 2 and J774 (Fig. 2A). Among tested tumor cell lines, GSC11 was the most sensitive, with an IC50 of 86 nM. In contrast, GSC20 was 17-fold more resistant (IC50 = 1.5 µM). Sensitivity of EOC 2 microglia and J774 macrophage cells had similar IC50 of 199 and 134 nM, respectively; TRP (IC50 = 231.6 nM) was less sensitive to UNC2025 compared with mouse microglial and macrophage cell lines tested.
Fig. 2.
UNC2025 exerts antiproliferative effects in vitro. (A) UNC2025 inhibited growth of a group of human and mouse cell lines by MTS assay. (B) UNC2025 synergized with radiation in TRP cells by colony formation assay.
Anchorage-independent cell growth is another hallmark of cancer aggressiveness, and the soft agar colony formation assay is a well-established method to characterize this capability in vitro.29 Effects of UNC2025 alone and in combination with radiation were tested on TRP cells using this assay. Both radiation (P < 0.0001) and UNC2025 (P = 0.02) caused significant decrease in TRP colony formation, and a trend toward interaction was revealed by 2-way ANOVA (P = 0.08) (Fig. 2B).
UNC2025 Inhibits MerTK Signaling
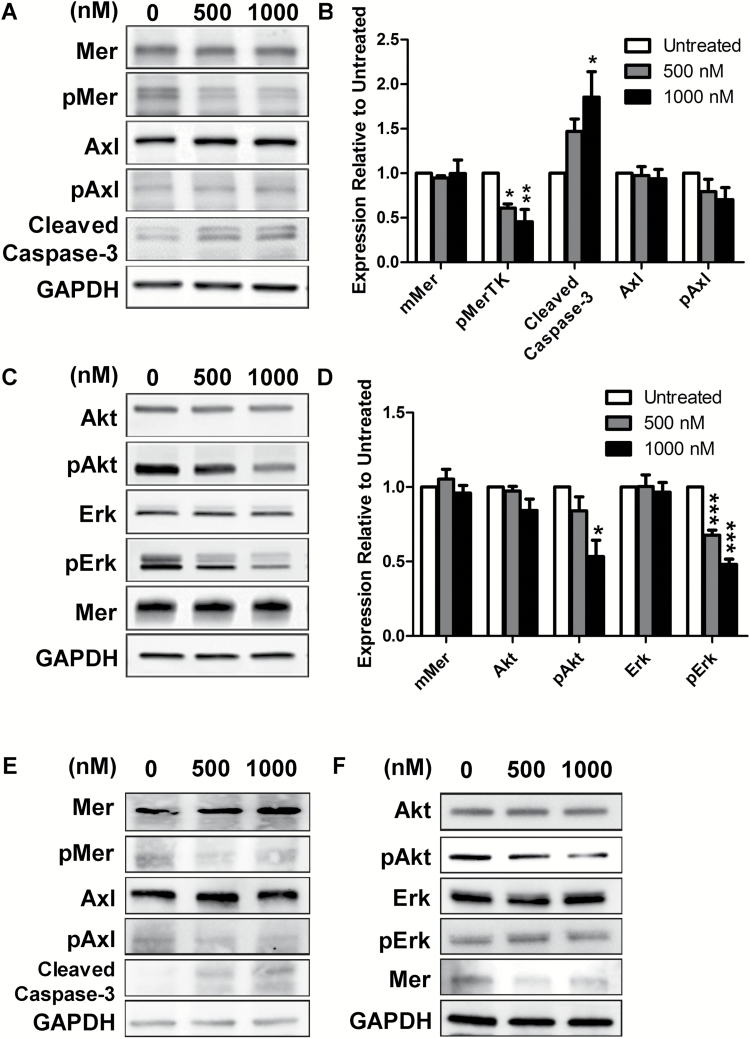
MerTK is linked to pathway activation of phosphatidylinositol-3 kinase and mitogen-activated protein kinase. To assess downstream signaling, we examined phosphorylation of Akt and ERK by immunoblot in TRP and GSC20 cells treated with UNC2025. UNC2025 inhibited MerTK phosphorylation in a dose-dependent manner; total MerTK protein did not significantly change. Activity of Axl, another TAM family member, was not affected significantly, consistent with the relative selectivity of UNC2025 for MerTK within the TAM family (Fig. 3A, B, E).27 Moreover, Akt and ERK phosphorylation decreased in response to UNC2025, while their total levels did not significantly change (Fig. 3C, D, F). UNC2025 induced a dose-dependent increase in cleaved caspase-3 levels, suggesting that MerTK inhibition blocks survival signals and stimulates apoptosis.26
Fig. 3.
UNC2025 regulates MerTK and downstream signaling. (A) Representative western blots of UNC2025-treated TRP cells (3 h) showed decreased MerTK signaling (pMer) and increased apoptosis (cleaved caspase-3), but no change in Axl signaling. (B) Quantification of expression relative to untreated control (mean ± SEM) from 3 independent experiments is shown. (C) Representative western blots of UNC2025-treated TRP cells (1 h) showed decreased signaling of mitogen-activated protein kinase (pERK) and phosphatidylinositol-3 kinase (pAkt). (D) Quantification of expression relative to untreated control (mean ± SEM) from 3 independent experiments is shown. Representative western blots of MerTK signaling in UNC2025-treated GSC20 at 3 h (E) and 1 h (F). *P < 0.05, **P < 0.01, ***P < 0.0001.
UNC2025 Has Desirable Pharmacokinetic Properties for Glioma Treatment
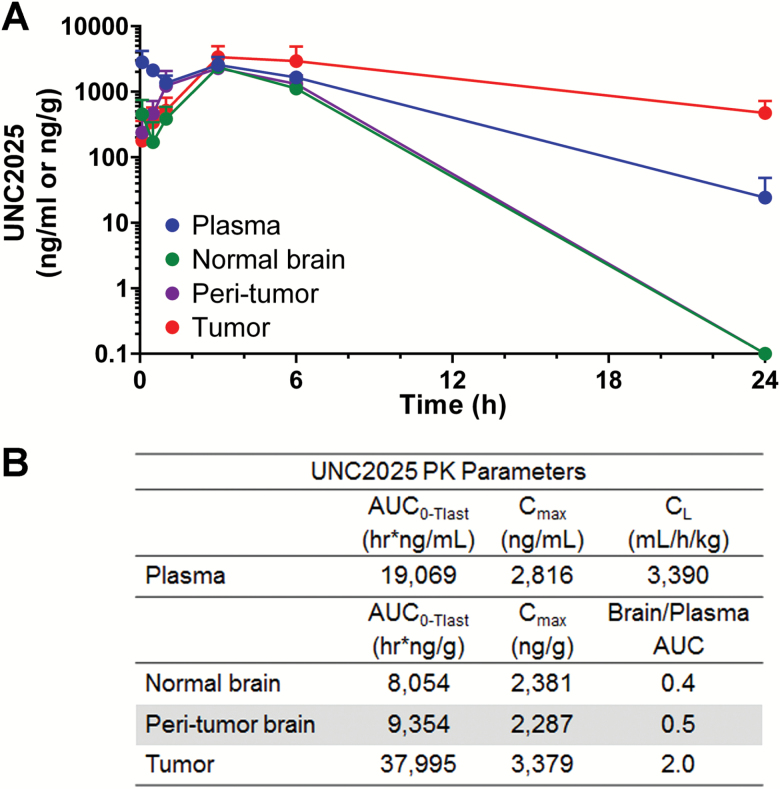
To test the potential of orally administered UNC2025 as an antiglioma drug, pharmacokinetic disposition was studied in the syngeneic, orthotopic TRP allograft model. UNC2025 exposures were evaluated in plasma, brain tumor, peritumoral, and normal (nontumor) tissues from 0–24 hours after one administration of UNC2025 at 65 mg/kg orally (Fig. 4). The highest drug exposure, represented by area under the curve (AUC) from 0 to 24 h, was observed in brain tumor, which resulted in a ratio versus plasma of 2.1 (Fig. 4B). Brain/plasma AUC in contralateral brain and peritumoral tissues was 4- to 10-fold lower. These data suggest that UNC2025 can reach high concentrations in brain tumors relative to plasma, which may portend an excellent profile for brain tumors with diminished systemic toxicity. The lower distribution in normal and peritumoral brain regions may be desirable, as it would lower the potential to disrupt nontumor microglial function.22
Fig. 4.
UNC2025 pharmacokinetics. (A) Mean concentrations of UNC2025 in plasma, normal brain, peritumoral brain, and brain tumor in TRP allograft mice. The lower limits of quantitation (LLOQ) were 58.4 ng/mL (plasma) and 88.0 ng/g for brain tumor and tissues. (B) Summary of pharmacokinetic parameters.
UNC2025 Improves Survival in a Subgroup of Mice with GBM
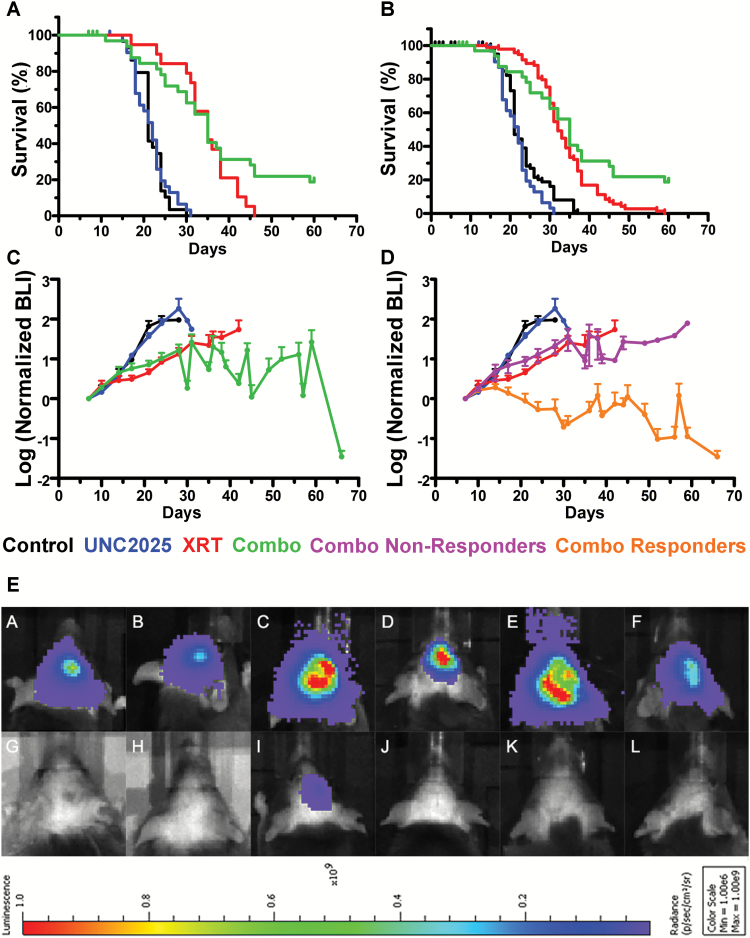
MerTK signaling has an established role in immune modulation, and the full potential antitumor effects of MerTK inhibitors can only be assessed in immune-competent preclinical models.14,18 We have previously described one such model, TRP allografts, which consistently produce high-grade gliomas mimicking human disease. These tumor cells are sensitive to radiation but resistant to TMZ due to their expression of O6-DNA methylguanine-methyltransferase.28 Orthotopic TRP allografts were established for 7 days and mice were randomized into 4 different treatment cohorts based on the BLI tumor values to minimize selection bias: (i) sham water oral gavage, (ii) oral UNC2025, (iii) XRT, or (iv) combination of UNC2025 plus XRT. BLI was utilized to assess the starting values and subsequent growth of individual tumors. Cumulative data from 3 separate experiments showed a median overall survival (OS) of 21, 22, 35, and 35 days, respectively (Fig. 5A). Thus, UNC2025 alone did not produce a significant survival benefit. XRT prolonged survival, consistent with our previous results.28 Adding UNC2025 to XRT did not improve median OS compared with mice receiving XRT alone. However, the combination slowed growth and 7 out of 36 mice treated with combination therapy were long-term survivors (≥60 days). Six of these 7 animals showed minimal neurological signs of disease.
Fig. 5.
UNC2025 induces long-term survival in a subset of TRP allograft mice treated with concomitant radiation. UNC2025 plus radiotherapy results in complete pathological response in a subset of TRP allograft mice. (A) Pooled data from 3 independent experiments showed median survivals of 21, 22, 35, and 35 days for control (N = 29), UNC2025 (N = 32), XRT (N = 19), and UNC2025 plus XRT (N = 36) treatments, respectively. (B) Kaplan–Meier analysis of data in (A) plus historical data from 6 previously published TRP control and XRT-treated allografts showed similar median survivals of 21 (N = 98) and 32 days (N = 98) in control and XRT-treated TRP allografts.28 Only 2 of 98 mice (2%) that received XRT survived beyond 50 days. (C) Longitudinal BLI of mean tumor growth relative to time of treatment randomization and allocation (day 7) shows growth inhibition by XRT with or without UNC2025. (D) However, a significant reduction in mean BLI was evident in mice that responded to XRT plus UNC2025 (N = 6) compared with nonresponders (N = 26). (E) BLI of 6 nonresponders (A to F) and 6 responders (G to L) to XRT plus UNC2025 (see Supplementary Table S1).
To monitor treatment longitudinally in vivo, tumor volume was assessed by BLI. In contrast to XRT alone (doubling time 7 days), UNC2025 alone (4.6 days) produced no significant delay in tumor growth compared with control treated animals (3.8 days). However, tumor doubling time in animals receiving a combination treatment was significantly delayed (34.8 days) (Fig. 5C). Moreover, 19.4% of mice from the combination treatment group remained alive after 60 days. Six responders showed stable tumor growth over the first 30 days of treatment and a remarkable 10–100-fold tumor regression thereafter (Fig. 5D, yellow line). Postmortem histology showed minimal evidence of tumors in these long-term survivors, with 5 of 6 being completely tumor free (Supplementary Table S1). Infiltrative tumors were consistently present in nonresponders (Fig. 5D, E and Supplementary Table S1). As shown in Fig. 5D, the XRT/UNC2025 responders had BLI-positive initial tumors, which grew early in the treatment course. For comparison, 98 TRP-bearing mice have been treated with XRT or XRT plus TMZ by our group; only 2 of 98 mice survived past 50 days and both died from tumors by day 60 (Fig. 5B). Comparison of benefit for the XRT response pattern with the 6 of 32 pathologically confirmed responders to XRT and UNC2025 treatment was statistically significant (Fig. 5B, P < 0.0001) as well as clinically meaningful for this model.
UNC2025 Decreased M2 Glioma-Associated Macrophages
We demonstrated that MerTK was expressed on CD68+ GAMs in human GBMs. Given the complete response in a subgroup of tumor-bearing mice in vivo, but the lack of synergistic effects of UNC2025 and XRT on tumor cells in vitro, we hypothesized that UNC2025 may affect tumor growth by regulating the tumor microenvironment. Specifically, we hypothesized that UNC2025 affected the balance of M2 versus M1 GAMs.
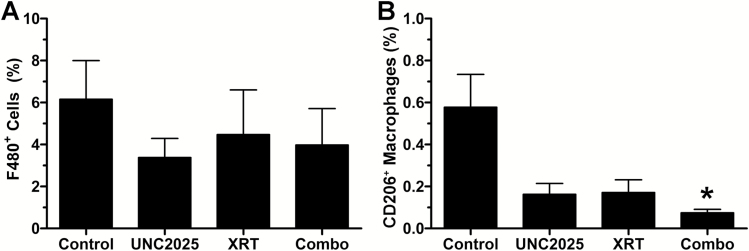
To explore this hypothesis, we performed immunohistochemistry for CD206, a marker of anti-inflammatory M2 macrophages, on tumor tissues from the 4-arm mouse in vivo efficacy experiments. We also examined total tumor macrophage burden using immunohistochemistry for F4-80, a common mouse macrophage marker. None of the treatments, including UNC2025, XRT, or combination of UNC2025 plus XRT had significant impact on the percentage of F4-80+ cells compared with control treated animals (Fig. 6A). The percentage of CD206+ macrophages, however, showed a significant difference by one-way ANOVA followed by Dunn’s multiple comparison test. A significant decrease in CD206+ macrophages was evident in the tumors treated with both UNC2025 and XRT relative to controls (P = 0.02) (Fig. 6B). These data suggest that MerTK inhibition by UNC2025 alters the innate immune repertoire, decreasing a surface marker of M2 GAMs. This effect was also seen with XRT, and seeing that neither alone produced complete or near complete responses, it was the combination that presumably altered the microenvironment and presumptive effective immune response.
Fig. 6.
UNC2025 modulates macrophage phenotype. (A) Percentage (mean ± SEM) of F4-80+ GAM was decreased by UNC2025 (N = 12) and XRT (N = 11) in TRP allografts, but these effects did not reach statistical significance. (B) UNC2025 plus XRT (N = 13) significantly (P = 0.02) decreased percentage (mean ± SEM) of GAMs with M2 phenotype (CD206+). *P < 0.05.
Discussion
Consistent with prior reports, we showed that MerTK is expressed in cultured GBM cells, particularly GSC populations and macrophage/microglial cell lines. We also evaluated the percentage of MerTK+GFAP+ tumor cells and MerTK+CD68+ GAMs among all nucleated cells in patients’ GBM samples and demonstrated an increase in MerTK+ GAMs, but not in the GFAP+ tumor cells compared with the paired initial and recurrent tumors. The correlation of increased MerTK+ GAMs in GBM from patients’ tumors in recurrent disease suggests a possible correlation between MerTK activation in GAMs and progressive disease status.
Although inhibition of MerTK signaling has been studied in several cancer models, the antitumor effects have primarily been demonstrated by genetic, shRNA-mediated MerTK knockdown or deletion of MerTK innate immune function using MerTK−/− mice.25,26,30 In this study and another recent study of leukemia, we used a potent, orally bioavailable, small-molecule inhibitor of MerTK to investigate the effects of MerTK inhibition in glioma cells and a syngeneic, immunocompetent GBM model.31 Importantly, pharmacokinetic data demonstrated significant UNC2025 concentrations in brain tumor tissue following a single oral dose, which suggests that UNC2025 could be a promising agent capable of penetrating the blood–brain barrier and providing a novel therapeutic action. The 4-fold higher ratio of drug exposure in brain tumor relative to nontumor brain tissues demonstrates improved delivery and prolonged exposure to the tumor, which could also be attributed to the tumor microenvironment. Lemke and coworkers have recently used MerTK and Axl knockout mice to show that both RTKs have a role in maintaining the integrity of the blood–brain barrier.32 Therefore, while the chemical properties of UNC2025 may allow passage through the blood–brain barrier, the biologic target of the drug, MerTK, may also be involved in allowing drug to concentrate in brain and GBM.
In the in vivo experiment using a syngeneic mouse GBM model, the treatment with UNC2025 alone did not lead to a survival benefit compared with the control group (Fig. 5A), even though it altered the expression of M2 macrophage markers (Fig. 6B). This suggests that modulation of the tumor microenvironment by MerTK inhibition by itself may be insufficient to block GBM growth. While combination treatment with UNC2025 plus XRT did not prolong median OS compared with XRT alone, the combination significantly prolonged survival in a subgroup of animals (~20%) in each of 3 separate experiments. These long-term survivors showed minimal neurological symptoms and were largely free of disease by necropsy, even though each of these surviving mice was shown by BLI to harbor established tumors at the beginning of the study. Each responder’s individual tumors exhibited stable growth over the first month of combination treatment and then regressed 10–100-fold over the next month in each long-term survivor. Complete response was confirmed in 5 of the 6 mice by postmortem histology. This obliteration of tumor was likely due to an immune-mediated response triggered by XRT and a MerTK initiated innate response.
The numbers of GAMs were not affected by UNC2025, either alone or in combination with XRT. However, the percentage of GAMs with an M2 phenotypic marker decreased significantly after combination treatment. This finding suggests that MerTK inhibition of M2 phenotypic markers combined with XRT produces a response not previously observed in this model. In the 19 mice treated with XRT in this study and the 98 TRP-bearing GBM mice tested over time with either XRT or XRT plus TMZ, none showed complete response or survival to 60 days. Response rates of ~15%–20% to immune checkpoint therapies are seen in studies of non–small cell lung cancer and bladder patients. However, the mice in our study are genetically identical. Thus, to implicate an immune-mediated regression mechanism, we postulate that the combination of XRT-induced necrosis in the setting of a more M1 polarized innate immune system leads stochastically to an adaptive response and tumor regression. The regression was both clear and dramatic, with complete resolution of 5 of 6 GBMs in a model in which we have not observed full tumor resolution with any standard therapies. Thus, further experiments and analysis of posttreatment tumor tissues from long-term responders may lead to the identification of biomarkers that correlate with MerTK drug response.
Macrophages have plasticity to adapt to different microenvironments. They are activated by a group of stimuli to acquire specialized functional phenotypes.33 Two overall polarization states have been defined, including classically activated (M1) macrophages and alternatively activated (M2) macrophages. Most recognize that this is a useful but oversimplified nomenclature.34,35 M1 macrophages are capable of phagocytosis and antigen presentation and promote inflammatory responses. In contrast, alternatively activated macrophages promote wound healing and decrease inflammatory responses.36,37 Studies with experimental murine tumors provided evidence that TAMs share similarity with M2 macrophages, where they promote angiogenesis and tumor growth.38,39 The number of GAMs with M2 phenotype was shown to correlate with histological grade in gliomas, and macrophage colony stimulating factor has been shown to induce a shift of GAMs toward M2.8 A more recent study has shown that inhibition of colony stimulating factor 1 receptor alters macrophage polarization by decreasing the number of M2-like tumor-associated macrophage/microglia and blocks glioma progression.10 In our study, we demonstrated that MerTK inhibition resulted in decreased number of GAMs with the M2 marker CD206, whose activation correlates M2 phenotype and has been used as an M2 marker in other studies.10,40 Our results and those of others suggest that MerTK signaling may be involved in promoting an M2 phenotype shift and thus contributes to the immune-suppressed glioma microenvironment.30,40
Glioma targeted therapy or cytotoxic agents have been investigated for decades but have yet to lead to significantly improved clinical outcomes. Because of cross-talk between glioma cells and other parenchymal cells, including GAMs, a pro-tumorigenic microenvironment is usually created. Modulating the glioma microenvironment while simultaneously targeting glioma cells represents a logical therapeutic approach. Our study has suggested the role of MerTK inhibition in regulating GAMs in glioma microenvironment, which brings promising therapeutic options for better glioma management. Based on the potential antiglioma effect of UNC2025, we would postulate that the optimal timing of MerTK inhibition in GBM would be along with concurrent chemoradiation therapy in the newly diagnosed disease setting. With the availability of pharmacologic agents that gain access to the CNS and the GBM microenvironment, such as UNC2025, potential clinical studies can be considered in the future.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This research was supported by the UNC University Cancer Research Fund and the NCI Intramural Research Program.
Conflict of interest statement
H.S.E. has filed patents on targeting MerTK in cancer therapy. X.W. and S.V.F have filed patents on UNC2025. X.W., S.V.F., and H.S.E. hold stock in Meryx Inc.
Supplementary Material
Acknowledgments
We thank Dr Glenn Matsushima at UNC Neuroscience Center for mer−/− mice.
References
- 1. Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Kamiya-Matsuoka C, Gilbert MR. Treating recurrent glioblastoma: an update. CNS Oncol. 2015;4(2):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C, Schachter J, Long GV et al. ; KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg JE, Hoffman-Censits J, Powles T et al. . Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferris RL, Blumenschein G Jr, Fayette J et al. . Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh A, Chaudhuri S. Microglial action in glioma: a boon turns bane. Immunol Lett. 2010;131(1):3–9. [DOI] [PubMed] [Google Scholar]
- 7. Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59(3):472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. [DOI] [PubMed] [Google Scholar]
- 9. Prosniak M, Harshyne LA, Andrews DW et al. . Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19(14):3776–3786. [DOI] [PubMed] [Google Scholar]
- 10. Pyonteck SM, Akkari L, Schuhmacher AJ et al. . CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quail DF, Bowman RL, Akkari L et al. . The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016; 352(6288):aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butowski N, Colman H, De Groot JF et al. . Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18(4):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer. 2014;14(12):769–785. [DOI] [PubMed] [Google Scholar]
- 15. Birge RB, Boeltz S, Kumar S et al. . Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23(6):962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsou WI, Nguyen KQ, Calarese DA et al. . Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J Biol Chem. 2014;289(37):25750–25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carrera Silva EA, Chan PY, Joannas L et al. . T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013;39(1):160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan PY, Carrera Silva EA, De Kouchkovsky D et al. . The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science. 2016;352(6281):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cabezón R, Carrera-Silva EA, Flórez-Grau G et al. . MerTK as negative regulator of human T cell activation. J Leukoc Biol. 2015;97(4):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung WS, Clarke LE, Wang GX et al. . Astrocytes mediate synapse elimination through MEGF10 and MerTK pathways. Nature. 2013;504(7480):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fourgeaud L, Través PG, Tufail Y et al. . TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532(7598):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graham DK, Salzberg DB, Kurtzberg J et al. . Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2006;12(9):2662–2669. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Moncayo G, Morin P Jr et al. . Mer receptor tyrosine kinase promotes invasion and survival in glioblastoma multiforme. Oncogene. 2013;32(7):872–882. [DOI] [PubMed] [Google Scholar]
- 25. Schlegel J, Sambade MJ, Sather S et al. . MerTK receptor tyrosine kinase is a therapeutic target in melanoma. J Clin Invest. 2013;123(5):2257–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keating AK, Kim GK, Jones AE et al. . Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang W, DeRyckere D, Hunter D et al. . UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor. J Med Chem. 2014;57(16):7031–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmid RS, Simon JM, Vitucci M et al. . Core pathway mutations induce de-differentiation of murine astrocytes into glioblastoma stem cells that are sensitive to radiation but resistant to temozolomide. Neuro Oncol. 2016;18(7):962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borowicz S, Van Scoyk M, Avasarala S et al. . The soft agar colony formation assay. J Vis Exp. 2014;(92):e51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cook RS, Jacobsen KM, Wofford AM et al. . MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest. 2013;123(8):3231–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minson KA, Smith CC, DeRyckere D et al. . The MerTK/FLT3 inhibitor MRX-2843 overcomes resistance-conferring FLT3 mutations in acute myeloid leukemia. JCI Insight. 2016;1(3):e85630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miner JJ, Daniels BP, Shrestha B et al. . The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21(12):1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. [DOI] [PubMed] [Google Scholar]
- 35. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 37. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 38. Gocheva V, Wang HW, Gadea BB et al. . IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24(3):241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189(7):3508–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.