Abstract
Lack of standard response criteria in clinical trials for medulloblastoma and other seeding tumors complicates assessment of therapeutic efficacy and comparisons across studies. An international working group was established to develop consensus recommendations for response assessment. The aim is that these recommendations be prospectively evaluated in clinical trials, with the goal of achieving more reliable risk stratification and uniformity across clinical trials. Current practices and literature review were performed to identify major confounding issues and justify subsequently developed recommendations; in areas lacking scientific investigations, recommendations were based on experience of committee members and consensus was reached after discussion. Recommendations apply to both adult and pediatric patients with medulloblastoma and other seeding tumors. Response should be assessed using MR imaging (brain and spine), CSF cytology, and neurologic examination. Clinical imaging standards with minimum mandatory sequence acquisition that optimizes detection of leptomeningeal metastases are defined. We recommend central review prior to inclusion in treatment cohorts to ensure appropriate risk stratification and cohort inclusion. Consensus recommendations and response definitions for patients with medulloblastomas and other seeding tumors have been established; as with other Response Assessment in Neuro-Oncology recommendations, these need to now be prospectively validated in clinical trials.
Keywords: brain, medulloblastoma, RANO, response, tumor
Medulloblastomas (MBL) are malignant embryonal tumors of the cerebellum with a propensity to invade and disseminate in the cerebrospinal fluid (CSF). They are among the most common CNS tumors of childhood, accounting for 10%–15% of pediatric CNS tumors.1 Although relatively rare, MBL can also affect adults. Over the past 5 years, considerable advances have been made in understanding MBL biology; distinct molecular subgroups have been identified,2 and the recent World Health Organization update has incorporated histological and molecular schemes into the classification of MBL.3 Clinical trials incorporating biologic and molecular features and risk stratification are being developed, making accurate disease assessment and determination of response to therapy critical.
Determination of disease assessment and response to therapy has relied heavily on MRI, although clinical examination and CSF analysis are also considered. MBL have a variable appearance on MRI (Fig. 1). They are typically hypointense or isointense on T1- and T2-weighted imaging. The tumors may be homogeneous, although some display heterogeneous enhancement, intratumoral cysts, and necrotic foci.4 Because most tumors comprise compact tumor cells, diffusion is restricted, resulting in decreased apparent diffusion coefficient values.5 Leptomeningeal dissemination may be noted at diagnosis or throughout the course of the disease.
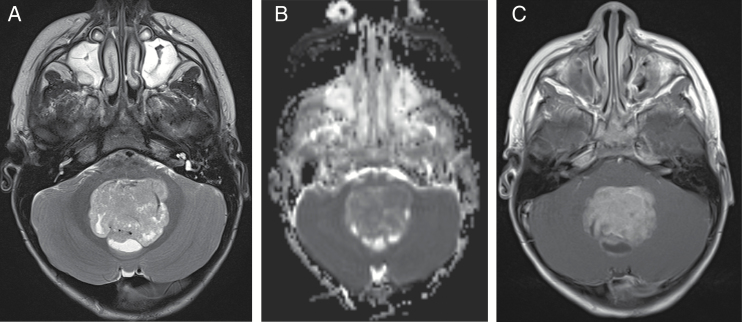
Fig. 1.
A 4-year-old boy with medulloblastoma. (A) Axial T2 image demonstrates T2 hypointense mass with posterior cysts in the fourth ventricle. (B) Axial apparent diffusion coefficient map image demonstrates restricted diffusion within the mass. (C) Axial T1 postcontrast image demonstrates enhancement in the mass, posterior cysts, and leptomeningeal seeding in bilateral internal auditory canals.
Patients with MBL are generally treated with surgical resection of the primary mass, followed by craniospinal radiation therapy and chemotherapy. Attempts have been made to reduce dose intensity, particularly for average- or low-risk pediatric patients, due to significant treatment-related adverse effects. Known risk factors, such as significant postresection residual tumor and metastatic/leptomeningeal disease, are critically important to characterize in order to stratify patients enrolled on clinical trials appropriately. Uniform criteria to assess disease burden and response evaluation are also essential to compare results across trials. However, there is currently no single standardized imaging protocol, no recommendations for timing to obtain scans, no radiographic standards to evaluate leptomeningeal disease, and no clinical standards to investigate CSF cytology.
The need for meticulous imaging techniques in patients with MBL was well demonstrated in the Children’s Oncology Group ACNS9961 study.6 In this study of average-risk MBL, patients who were determined at central neuroradiology review to have no dissemination or significant postoperative residual disease and who had good quality staging imaging studies (ie, “fully assessable” cases) had an 83% 5-year event-free survival. In contrast, patients with metastatic deposits at diagnosis who were overlooked fared much worse, with a 5-year event-free survival of 36%; patients with excess residual tumor after surgery had a 5-year event-free survival of 75%; patients with inadequate staging imaging studies had a 5-year event-free survival of 73%—that is, all inferior to the fully assessable group (Fig. 2).
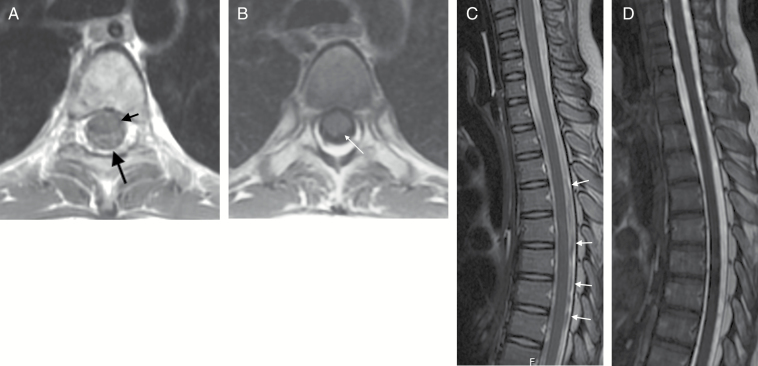
Fig. 2.
(A) Axial T1-weighted image at the level of the carina. Image was obtained using an interleaved slice acquisition order. Prominent CSF pulsation artifacts (long black arrow) are present around the spinal cord (short black arrow). These pulsation artifacts can obscure subarachnoid metastatic deposits. (B) Axial T1-weighted image obtained a few days later, without use of interleaved image acquisition. The spinal cord is well demarcated from the surrounding T1 hypointense CSF. (C) Sagittal 2D FSE T2 of the upper spine of the same patient. Many hypointense artifacts (arrows) are evident within the CSF surrounding the spinal cord. These artifacts are produced by physiologic CSF pulsation and could obscure subarachnoid metastatic deposits. (D) Sagittal 3D FIESTA T2-weighted image obtained a few days later. CSF has a homogeneous T2 hyperintense (myelographic) appearance, which increases sensitivity to the presence of lesions within the thecal sac.
Optimizing the conduct of clinical trials involves use of consistent, objective disease assessments and standardized response criteria. The Response Assessment in Pediatric Neuro-Oncology (RAPNO) committee, consisting of an international panel of pediatric and adult neuro-oncologists, clinicians, radiologists, radiation oncologists, and neurosurgeons, was established to address issues and unique challenges in assessing response in children with CNS tumors.7 A subcommittee of RAPNO was formed to specifically address response assessment in children and adults with MBL and other CSF seeding tumors and to develop a consensus on recommendations for response assessment that can then be prospectively evaluated in clinical trials. The committee first identified major confounding issues, reviewed the literature and current practices, and subsequently developed recommendations.
Issues with Response Assessment in Medulloblastoma
In addition to general issues with assessing response in patients with CNS tumors, patients with MBL present distinct challenges, described below.
Different Patient Populations
While MBL is considered one of the most common pediatric malignant CNS tumors, it also occurs in adults, accounting for 2% of CNS tumors in adults age 20–34 years, and an overall incidence in adults of 0.5–1 per million.8,9 Diagnostic evaluations, treatment, and follow-up assessments may differ between adult and pediatric patients with similar disease processes.
Disease Classification and Subclassification
In efforts to identify prognostic factors and patients with high- or low-risk disease, several methods of classification and subclassification for MBL have been developed. Historically, patients have been classified as average or high-risk based on disease staging using the Chang classification, which incorporates age, postresection tumor size, CSF cytology, and CNS and extra-CNS metastases.10,11 MBL are also subclassified histologically as classic; nodular or desmoplastic; with extensive nodularity; or as anaplastic/large cell variants. Most recently, MBL have been subcategorized based upon genomic findings into 4 groups, including WNT, sonic hedgehog, Group 3 (classic), and Group 4.2 Interestingly, preliminary studies suggest imaging may differ by subgroup, although analysis of larger cohorts is needed.12 Current treatment strategies differ for average- versus high-risk groups based on Chang or Chang-modified criteria, and prospective clinical trials incorporating the recent genomic subgrouping are being developed. At this time, no specific imaging criteria are used to delineate prognostic subgroups or disease cohorts.
Tendency to Metastasize Throughout CNS
MBL frequently metastasize throughout the CNS. Multiple lesions, both nodular and laminar, may be present in the brain and spine (Fig. 3). The presence of metastases influences prognosis and therapeutic decisions. Patients are generally assessed for metastatic dissemination by postcontrast spine MRI as well as CSF cytology, as the sensitivity of each alone is thought to be relatively low, although somewhat improved when both are used.13 However, absolute quantification of tumor cells in the CSF is complex and rarely performed and its interpretation is unclear. CSF cytology is generally a qualitative study, with tumor cells assessed as present or absent. No standards exist for timing, volume, or location of CSF acquisition.
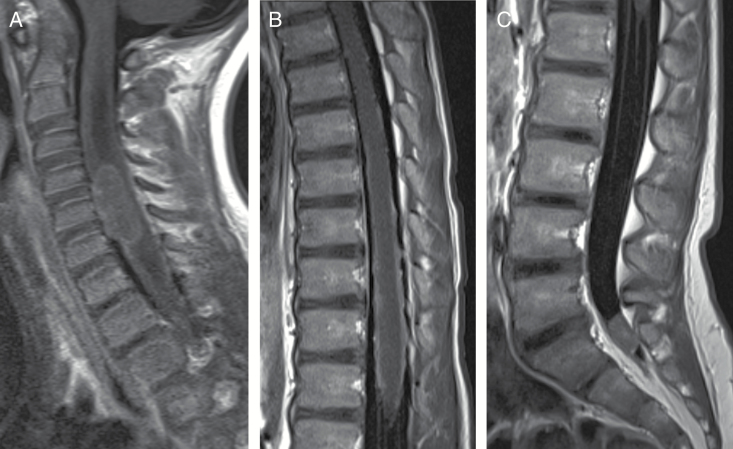
Fig. 3.
A 4-year-old girl with medulloblastoma. (A) Sagittal T1 postcontrast image of cervical spine demonstrates large nodule on ventral surface of cervical spinal cord and leptomeningeal laminar seeding. (B) Sagittal T1 postcontrast image of thoracic spine demonstrates linear leptomeningeal seeding along surface of thoracic spinal cord. (C) Sagittal T1 postcontrast image of lumbar spine demonstrates nodule in distal thecal sac.
Defining Baseline Scans
Patients with posterior fossa tumors frequently present emergently with signs and symptoms of increased intra cranial pressure due to obstructive hydrocephalus. Rapid preoperative imaging includes CT and/or MRI to detect and characterize the lesion. Appearance on MRI can vary, with mild to diffuse enhancement, cystic components, and calcifications.14 Maximum safe surgical resection is generally recommended. Several questions arise, then, in defining and measuring tumor size to be used as baseline tumor measurements in clinical trials. Although preoperative scans are used for radiation planning, they do not reflect tumor burden at the time of study entry, yet postoperative scans may have surgical-related artifact.
Neurocognitive Impact
Significant neurocognitive issues emerge in nearly all survivors. Treatment regimens associated with less neurocognitive toxicity would be viewed favorably compared with alternate regimens with otherwise similar response rates or survival outcomes. Assessment of neurocognitive outcome is complicated due to the potential impact of confounding factors such as the presence of increased intracranial pressure or hydrocephalus, surgery, postsurgical complications such as cerebellar mutism, and different sensitivities to radiation therapy and related neurotoxicities.
The RAPNO medulloblastoma subcommittee addressed these issues, reviewed literature and current practices, and developed recommendations with scientific justification where possible. Recommendations for issues not yet scientifically tested were developed based upon experience, current practices, and discussion within the committee. It is important to emphasize that these are recommendations that need to be prospectively tested in appropriate populations as objectives in the next generation of clinical trials.
Recommendations
While there has been an exponential increase in the understanding of the biology of MBL, there is no evidence to date that separate response criteria should apply to different subgroups. There is also no evidence that separate criteria should be developed for adults versus children. Additionally, there is no evidence that separate radiographic or CSF cytology criteria are necessary for other CSF seeding CNS tumors. The common seeding tumors, including germ cell subtypes, choroid plexus tumors, and embryonal tumors such as pineoblastoma, embryonal tumors (previously defined as primitive neuroectodermal tumor [PNET]), and atypical teratoid rhabdoid tumor, can, like medulloblastoma, have variable MRI characteristics and metastasize throughout the brain and spine with diffuse infiltrative, nodular, and/or leptomeningeal spread. Serum or CSF biomarkers have application for some seeding tumors (eg, germ cell tumors) and are considered in response criteria when obtained for these tumors. Otherwise, these recommendations apply to all patients with MBL and other CSF seeding tumors enrolling on clinical trials, and may include newly diagnosed or progressive/recurrent disease.
The following methods should be used to assess response: MR imaging (brain and spine), CSF cytology, and neurologic examination. Specific recommendations are presented below.
Imaging Standards for Clinical Trials for MBL and CSF Seeding Tumors
Clinical trials assessing therapeutic efficacy for a specific disease should have uniform criteria for image acquisition and response assessments in order to compare results across studies. Recently, the Brain Tumor Imaging Standardization Steering Committee recommended a standardized brain MRI protocol to be used for endpoints primarily in clinical trials of adult patients with glioblastoma.15 These recommendations were based on the principle of balancing compliance, acquisition feasibility, and quality of data acquired from imaging centers ranging from small community medical or imaging centers to large university research hospitals. While uniform acquisition criteria are ideal, implementing these recommendations for patients with MBL and other seeding tumors may prove difficult. Given the tendency for leptomeningeal dissemination, imaging of the spine is performed at relatively frequent intervals in this population compared with adults with glioma. Children frequently need sedation to obtain adequate MR images free of significant motion artifacts. In efforts to avoid multiple imaging sessions and reduce anesthesia risk, brain and spine images are frequently acquired during the same imaging session. How images obtained in a single session compare with images of brain and spine from patients who have been imaged in different sessions is unknown.
As recommended by the Brain Tumor Imaging Standardization Steering Committee, clinical trials should have prespecified imaging parameters, and patients should be assessed with the same method and magnet strength throughout the trial.15 In keeping with the principles of maximizing compliance and imaging quality across imaging centers with varied capacity, and standardizing imaging-based risk and response assessment in patients with leptomeningeal seeding tumors, the RAPNO committee recommends image acquisition utilizing common sequences readily available at most centers to address the primary study endpoints. Additional imaging sequences may be added at sites with capability in efforts to address specific additional research objectives. The following imaging techniques and sequences are recommended.
Brain Imaging
Basic characterization of the tumor is achieved with T1, T2, fluid attenuated inversion recovery (FLAIR), diffusion, and postcontrast T1-weighted images (Table 1). Pre- and postcontrast T1-weighted images should be obtained. T1-weighted image acquisition utilizing isotropic volume (3D) MRI sequences allows for improved resolution and, hence, better detection of small lesions, as well as image reconstruction in any plane, allowing better characterization of lesions, volumetric assessments, and coregistration. T1-weighted images should be free of artifacts originating from CSF pulsation and from vascular structures, as these artifacts can obscure or mimic leptomeningeal metastatic lesions. For postcontrast 3D T1-weighted sequences, fast (turbo) spin echo (FSE [TSE]) sequences are recommended (ie, sampling perfection with application optimized contrasts using different flip angle evolution [SPACE]/Cube/volumetric isotropic T2-weighted acquisition [VISTA]), as vascular signal is much less on these than on contrast-enhanced 3D radiofrequency spoiled gradient recalled acquisitions (spoiled gradient [SPGR]/fast low angle shot (FLASH)/T1 contrast-enhanced fast field echo [CE-FFE]). Alternately, postcontrast 2D T1-weighted images can be acquired in at least 2 different planes. In the brain, most 2D T1 techniques (ie, SE, TSE/FSE, FLAIR) produce good quality images with few vascular or CSF pulsation artifacts. Images acquired in an interleaved order (ie, when images are acquired in 2 or more packets, such as when order acquisition is 1, 3, 5, 7 . . . followed by 2, 4, 6, 8 . . .) can suffer from greater vascular signal and CSF pulsation; in this situation, image acquisition in a consecutive fashion (ie, as 1, 2, 3, 4 . . .) may be necessary. Maximum 2D slice thickness acquisition should be 4 mm; a small interslice gap (of 10% of the slice thickness) can be introduced to minimize any cross-talk artifacts if a consecutive image acquisition order is used. Vascular flow compensation and fat saturation pulses are strongly discouraged.
Table 1.
Sample brain and spine MRI protocol for children with leptomeningeal seeding tumors
| Seq. # | Sequence | Slice Thickness (mm) | Gap % | In-Plane Resolution | Comment |
|---|---|---|---|---|---|
| Brain | |||||
| 1 | Axial T2 TSE/FSE | ≤4 | 0 | ≤1.0 × 1.0 mm | |
| 2 | Axial DWI (b = 0,1000) with ADC | ≤4 | 0 | 2.0 × 2.0 mm | Or axial DTI |
| 3 | 3D T1 MPRAGE/SPGR/FFE/TFE | 1–1.5 | 0 | 1.0 × 1.0 mm | Sagittal, coronal, or axial plane |
| OR Axial T1 SE/TSE/FSE | ≤4 | 0–10% | 1.0 × 1.0 mm | ||
| Gadolinium contrast administration | |||||
| 4 | Axial T1 SE/TSE/FSE +C | ≤4 | 0–10% | ≤1.0 × 1.0 mm | Avoid flow compensation and fat saturation |
| 5 | 3D T1 SPACE/Cube/VISTA +C | 1 | 0 | 1.0 × 1.0 mm | Sagittal or coronal plane |
|
OR, if 3D has motion or is not possible/available:
Coronal T1 SE +C |
≤4 | 0–10% | ≤1.0 × 1.0 mm | Avoid flow compensation. Acquire images in consecutive order |
|
| 6 | Axial T2 FLAIR +C | ≤4 | 0 | ≤1.0 × 1.0 mm | Can acquire as first postcontrast sequence |
| Spine | |||||
| 1&2 | Sagittal T1 SE upper/lower +C | 3 | 0–10% | <1.0 × 1.0 mm | Use anterior saturation band |
| 3&4 | Axial T1 VIBE/FAME/LAVA/THRIVE +C upper/lower | 3 | 0 | <1.0 × 1.0 mm | |
| OR: Axial SE T1 upper/lower +C | 4–5 | 10% | <1.0 × 1.0 mm | Acquire images in consecutive order | |
| OR: Axial T1 FLAIR (propeller) upper/lower +C | 4–5 | 10% | <1.0 × 1.0 mm | ||
| 5&6 | Sagittal CISS/FIESTA upper/lower | 1 | 0 | ≤1.0 × 1.0 mm | Can replace with sag Cube/SPACE/VISTA T2 |
Abbreviations: ADC, apparent diffusion coefficient; +C, with contrast; CISS, constructive interference in steady state; DTI, diffusion tensor imaging; DWI, diffusion-weighted imaging; FAME, fast acquisition with multiphase enhanced fast gradient echo; MPRAGE, magnetization-prepared rapid acquisition with gradient echo.
Diffusion-weighted sequences should be routinely acquired, as hypercellular tumors (such as medulloblastoma) present with hyperintense signal on diffusion images. Both the primary tumor site and metastatic deposits can be identified as high signal intensity lesions on the diffusion images.16–18 T2-weighted FLAIR images are preferably obtained after contrast administration, as contrast-enhanced T2 FLAIR images are very sensitive to the presence of leptomeningeal disease, including metastatic lesions.19,20
Timing of Postoperative Brain MRI
MRI evaluation for residual tumor is best performed within 72 hours postoperatively. Intraoperative MRI should not be considered a substitute for the baseline postsurgical brain MRI. If there are extensive parenchymal postsurgical changes that may obscure residual tumor, a second brain MRI approximately 2–3 weeks after surgery is recommended. Follow-up brain MRI for assessment of response should be performed every 2 cycles, with possible exceptions depending on timing of anticipated effects of therapy (eg, immunotherapy), but no less frequently than every 3 months.
Spine Imaging
There is little information in the literature regarding standards for spinal leptomeningeal tumor screening. It was recognized by the committee that practice and experience of individual institutions differ, studies optimizing and comparing these techniques are currently limited, and further evaluations as secondary objectives within a clinical trial are warranted. However, standards for uniform acquisition and quality control should be incorporated into clinical trials, and the following recommendations are made:
For detection of small spinal leptomeningeal deposits, postcontrast T1-weighted images, free of pulsation and physiologic motion artifacts within the CSF-containing spaces, are essential. In addition, 3D myelographic T2-weighted images can highlight small metastatic deposits that are not well visualized on the T1-weighted images. High quality T1 images (with low intensity, homogeneous CSF signal) and myelographic T2 images can be difficult to produce in children, especially younger ones, as the pulsatility of CSF is much greater in children than in adults.
Imaging of the spine can be performed immediately following brain MRI, with T1-weighted series acquired first, followed by the T2-weighted images. An additional injection of intravenous contrast is not required (or recommended), as all T1-weighted spine imaging can usually be acquired within 45 minutes of initial contrast administration using the recommended sequences. For postcontrast sagittal T1-weighted imaging, slice thickness ≤3 mm and minimal or no gap are optimal. To minimize motion artifacts from the chest and abdomen, anterior saturation pulses should be placed close to the anterior margin of the spinal column.21
Postcontrast axial T1-weighted images should be free of CSF pulsation artifacts. Axial 3D volume-interpolated gradient recalled echo sequences (ie, volume interpolated breathhold examination [VIBE]/ liver acquisition with volume acceleration [LAVA]/T1 high resolution isotropic volume excitation [THRIVE]) provide excellent resolution with minimal CSF pulsation artifacts.22 For 2D SE techniques, the image order acquisition should be consecutive, not interleaved (as explained above in the “Brain Imaging” section); interleaved acquisitions are sometimes degraded by prominent CSF pulsation artifacts and therefore their routine use is not recommended. Maximum image thickness should be 4 mm (5 mm for larger patients). As pulsation artifacts are frequently observed in the spine, suspicious signal abnormalities in the subarachnoid spaces seen only in one plane may not represent metastatic dissemination; this can be confirmed on myelographic T2 images.
For myelographic T2 images, thin (1 mm) 3D sagittal acquisitions are preferred, as they produce more reliable myelographic effects than 2D FSE/TSE techniques. Fast imaging employing steady state acquisition (FIESTA)/true fast imaging with steady-state precession (FISP)/balanced FFE usually produce excellent myelographic effects but can be sensitive to susceptibility effects. Three-dimensional T2-weighted FSE/TSE sequences (SPACE/Cube/VISTA) generally produce good myelographic images with less susceptibility artifacts.
Timing of Spine MRI
Several studies support preoperative screening for leptomeningeal dissemination due to the possibility of obscuring metastases by postoperative sequelae such as subdural collections.23,24 We recommend that preoperative spine MRI be used as the baseline spine evaluation in clinical trials when feasible. If not clinically feasible, or if significant motion artifact is present, the baseline spine MRI for clinical trials should be obtained within 72 hours postoperatively. In cases where extensive postoperative enhancing subdural effusions are present, a repeat spine MRI approximately 2–3 weeks postsurgery is recommended.
Currently, there is no standard regarding the timing for follow-up spinal surveillance imaging. Some centers perform spine screening concurrently with brain imaging at defined time points during therapy for MBL, with decreased frequency of screening after completion of therapy; other centers perform spine surveillance less frequently. The frequency is sometimes risk stratified, with higher-risk MBL patients receiving more frequent surveillance. The typical European practice is to perform spine screening at diagnosis, with follow-up only in cases where metastasis is subsequently detected by spine MRI or CSF, or if dictated by symptoms. This practice more closely reflects typical adult spine surveillance imaging practice in the United States. A 2006 study of 73 children with MBL/PNET found that of 19 subjects with leptomeningeal recurrence, none had isolated spinal disease on imaging and only 7 were symptomatic.25 In a more recent study of 89 children with MBL, 51 had relapsed in the brain, while 5/89 (7%) had isolated spinal leptomeningeal metastasis.26 Neither study described imaging parameters such as slice thickness or gap; thus, the influence of imaging quality on detection rate cannot be assessed. In a study of 297 children with MBL on the German Hirntumoren (HIT) trial, up to 20% had isolated spinal metastasis (personal communication, Dr Monika Warmuth-Metz).
The development of recommendations for spine surveillance may be confounded by differences in patterns and timing of metastases between MBL subtypes. For example, a 2013 multicenter study found that the sonic hedgehog group had a higher propensity for local recurrence, while Groups 3 and 4 subgroups had more frequent leptomeningeal recurrences. Nonetheless, isolated spinal metastases were noted in all 3 subgroups, and some recurred relatively late. Although the current literature is sparse, a relatively high incidence of isolated spine metastasis, coupled with a low incidence of associated symptoms in children with MBL, support risk-stratified spinal surveillance MRI, particularly in high-risk groups during the first 3 to 4 years after diagnosis.26,27 However, there is no consistent data from large cohorts to recommend timing for MBL subtype-specific spine surveillance imaging at this time. Therefore, at present, the committee recommends surveillance spine imaging to be done concurrently with brain imaging in patients with initially positive radiographic findings or positive CSF cytology at baseline, and repeat spine imaging in patients with new spine symptoms.
Quality Control for Imaging
Retrospective evaluations of MRI of patients with MBL entered on clinical trials have shown a significant rate of discordance between local centers and central reviewers regarding residual disease and presence of metastatic disease. In the previously discussed ACNS9961 trial for average-risk patients, 7% of enrolled patients were found to have either excess residual disease (n = 15 of 405 evaluated) or dissemination (n = 15 of 405 evaluated); this was critical, as these patients had decreased event-free survival and therefore affected study results.6 In a French study, 4% of patients were retrospectively diagnosed as having local residual tumor.28 In both trials, the number of patients with nonconclusive MRI series due to incomplete or poor quality imaging was high. There were errors in both interpretation (especially for non-enhancing primary lesions) and quality of images (inadequate sequences, movement artifact, lack of imaging in 2 planes, and skip regions). Despite education, there was an essentially identical incidence and pattern of deficiencies noted in review of the first 400+ patients in the Children’s Oncology Group successor study, ACNS0331.
Attempts have been made to address the issue of discordant MRI interpretation to avoid enrolling ineligible patients. In the PNET4 trial, a pre-inclusion imaging review was recommended. Of note, patients lacking this central review had a lower event-free survival compared with the cohort of patients for whom absence of metastases and absence of a residual tumor >1.5 cm2 was centrally confirmed.29 In the German HIT group, pre-inclusion central MRI review has been established since the 1990s. Data on the HIT-2000 trial show a high rate of divergent results on central neuroradiological upfront review compared with the local radiology reports, with relevant discrepancies described in 171 of 697 (25%) reviewed patients.30 As the central MRI review is performed upfront, repeat MRI examination is initiated in case of relevant uncertainty regarding clinical staging.
These data demonstrate that reliance on local MRI results is not adequate for clinical trials assessing efficacy of new therapies. Discordant results have an impact on interpretation of clinical trial results and are of relevance for determining optimal treatment for an individual patient. The following are therefore recommended for determining acceptable imaging for use in clinical trials:
Central review for eligibility by neuroradiologists with expertise in pediatric brain tumor imaging should be mandatory for appropriate risk stratification. We recommend more than one central reviewer, particularly for cases where there is disagreement between the local center and central review. Central review should be performed prior to inclusion in treatment cohorts, ideally within a week after images are submitted for central review.
Absence of movement artifacts
Available preoperative contrast-enhanced MRI, to allow sufficient delineation of primary tumor and assessment of leptomeningeal metastases
Spinal MRI with full visualization of the dural sac, performed in 2 planes without skip regions and with adequate slice thickness and resolution
Tumor measurements should be made using the sequence that best demonstrates the extent of tumor; non-enhancing and enhancing primary lesions should be measured similarly.
Assessing Leptomeningeal Disease
Patients diagnosed with leptomeningeal metastasis require aggressive therapy to mitigate their poor prognosis.10,31,32 It is crucial to obtain the data to accurately diagnose leptomeningeal disease while avoiding false positive and false negative results. Although leptomeningeal disease is most often diagnosed by MRI or with a CSF sample obtained by either lumbar or an intraventricular collection,33 diagnosis can be complicated due to variable findings. Studies pertaining to the importance and variability of the CSF sampling site for CSF cytology to rule out leptomeningeal metastasis differ in their conclusions.34–37 Although gadolinium-enhanced spinal MRI has been a vital tool in diagnosing leptomeningeal disease for decades, some studies have shown discordance between CSF sampling and MR spinal imaging.38,39 Full assessment of leptomeningeal disease should therefore include both adequate MR imaging and CSF cytology. A positive finding in either one is diagnostic of CSF dissemination.40
The committee recommends the following for assessment of leptomeningeal disease on clinical trials:
Baseline evaluation: neurologic examination (using a standardized method, if available), CSF cytology/flow cytometry, and pre- and postcontrast MRI. Radioisotope CSF flow studies should be added for trials with intra-CSF chemotherapy administration.
During clinical trial: neurologic examination, CSF cytology/flow cytometry concurrently with brain MRI
Staging assessment should be binary, that is, tumor cells present or absent on cytology; present or absent leptomeningeal disease on MRI.
Assessment of CSF Cytology
CSF collection for cytology or flow cytometry differs among studies in regard to timing, collection site, and volume of CSF collected. The committee recommends the following for standardization and optimization of leptomeningeal metastases assessment:
Timing
CSF sampling should be obtained within 14 days postsurgery. Intraoperative CSF sampling should be avoided, as it may lead to false positives from floating cells that may disseminate during surgery.38,41 Conventionally, CSF cytology is generally repeated for confirmation in patients who have a positive CSF after surgery. However, given the variability of CSF sampling, the relative significance of a negative CSF assessment after an initial positive assessment is of unclear prognostic or therapeutic significance. Given these caveats, the committee is recommending that if the CSF obtained within 14 days of surgery is positive for tumor cells, a repeat CSF sample should be obtained at least 15 days postsurgery and prior to initiation of therapy for confirmation.41 If confirmed positive, in patients with an initial positive CSF cytology, 2 negative samples at least 2 weeks apart are considered a response.42
Site
There is some evidence in the literature that directing the site of sampling based on symptoms or radiographic leptomeningeal disease may reduce false negative cytology,42 although these studies primarily included patients without MBL and results have been mixed. Others propose that, when possible, CSF should be sampled both intraventricularly (eg, Ommaya reservoir) as well as lumbar to reduce false negatives.37,42 However, the risks and benefits of these approaches must be weighed, particularly in the pediatric population. For this reason, we recommend lumbar sampling of CSF be performed unless contraindicated.
Volume and Processing
In general, higher CSF sample volumes reduce false negative rates. Again, though, there must be a balance between patient safety and consistent, optimal sampling. For infants and children under 5 years of age, the committee recommends obtaining a minimum CSF volume of 2.5 mL. Higher volumes may be considered in older children and adults.43 Samples should be cytospun immediately; if not feasible, they should be refrigerated without alcohol and processed as soon as possible and no longer than 24 hours to minimize cell loss.43 A delay in processing samples makes the sample less viable and can result in false negatives.43
Assessing Extra-CNS Metastases
While MBL can metastasize outside the CNS, particularly to bone marrow, the incidence is estimated to be low. Measurement of the degree of bone marrow involvement is unreliable, and the clinical significance of a decline or increase in bone marrow disease beyond present or absent has not been determined. Systemic metastases may be present at diagnosis or may be identified many years later, therefore the optimal timing and frequency of extraneural assessment is unclear. The low incidence, unclear natural history, and unreliable measurements make recommendations for assessment and surveillance of extra-CNS metastases difficult. Therefore, specific recommendations for routine quantitative assessment of extra-CNS metastasis for response are not being made. However, if systemic metastases are suspected or known, they must be reassessed after therapy and be negative to meet complete response criteria.
Quality of Life and Neurocognitive Outcomes
The committee agreed that quality of life (QoL) and cognitive outcomes are critically important in assessing treatment effects, particularly in this population and with the known neurocognitive consequences of craniospinal radiation. However, there are no standard, validated, multilanguage pediatric QoL tests or standardized patient reported outcome measures in pediatrics, hence there may be issues when applying different measures across age groups, and language issues exist particularly for international studies. Effects on QoL and cognition may occur late and although they are considered important outcome measures, they are not useful for response evaluation within individual clinical trials. The committee is therefore not making specific recommendations at this time but suggests that future studies prospectively include a select QoL test for validation in multiple languages.
Definitions of Response
Based on the literature, existing practice, and experience, the committee has developed criteria for defining response or progression for patients with MBL and other seeding tumors enrolled on clinical trials (Table 2). Importantly, all criteria must be met in order to determine objective response and stable disease, while progression is defined when any of the listed criteria are met. If any criterion has not been adequately assessed, such as CSF cytology, objective response is considered indeterminate, although progression may still be determined. If any criterion is not clearly met for disease progression (eg, unclear worsening neurologic examination, increase in tumor measurements but suspected pseudoprogression), discretion may be used to retain a patient on study until disease progression is definitive, but the date of progression should be backdated to the initial questionable time point if ultimately confirmed on subsequent assessments.
Table 2.
Response definitions: patients must meet ALL criteria in each response/stable disease category, or ANY criteria in the progressive disease category
| Complete Response (CR) | Partial Response (PR) | Stable Disease | Progressive Disease (PD) | |
|---|---|---|---|---|
| Criteria | Must meet ALL criteria | Must meet ALL criteria | Must meet ALL criteria | Must meet ANY criteria |
| MRI-brain | Complete disappearance of all disease (enhancing and non-enhancing, measurable and nonmeasurable) for a minimum of 4 weeks; no new lesions | ≥50% decrease (compared with baseline) in the sum of the products of perpendicular diameters of all (up to 4) measurable lesions sustained for at least 4 weeks; no progression of nonmeasurable disease | Does not meet criteria for CR, PR, or PD | ≥25% increase (compared with smallest measurement at any time point) in the sum of the products of perpendicular diameters of all measurable lesions; significant progression of nonmeasurable disease not attributed to prior therapy; any new tumor (any new lesions suspected to be treatment related should be confirmed by biopsy) |
| MRI-spine | Complete disappearance of all disease (enhancing and non-enhancing, measurable and nonmeasurable) for a minimum of 4 weeks; no new lesions | ≥50% decrease (compared with baseline) in the sum of the products of perpendicular diameters of all measurable lesions sustained for at least 4 weeks; no progression of nonmeasurable disease. If negative at baseline, must remain negative | Does not meet criteria for CR, PR, or PD | ≥25% increase (compared with smallest tumor measurement at any time point) in the sum of the products of perpendicular diameters of all (up to 4) measurable lesions; significant progression of nonmeasurable disease not attributed to prior therapy; any new tumor (any new lesions suspected to be treatment related should be confirmed by biopsy) |
| CSF cytology | If tumor cells are present at baseline, must be negative × 2 (sampling at least 2 weeks apart) | If absent (negative) at baseline, must remain absent. If present at baseline, can be present or absent | If absent at baseline, must remain absent. If present at baseline, can be present or absent | Previously absent tumor cells in CSF now present (positive) |
| *Neurologic exam | Stable or improving | Stable or improving | Stable or improving | Clinical deterioration not attributable to other causes |
| Steroid use | Off steroids or physiologic replacement doses only | Stable or less than baseline dose | Stable or less than baseline dose | |
| Extra-CNS disease | If positive at any time point, must be reevaluated and have no evidence of disease | No new sites of disease | No new sites of disease | New sites of disease |
| Serum or CSF AFP, βhCG (if obtained, eg, germ cell tumors) | Must be within normal range for age | A previously negative (normal) assessment becomes positive |
Abbreviations: AFP, alpha-fetoprotein; βhCG, beta human chorionic gonadotropin.
*If it is unclear that the patient has disease progression, it may be a reasonable option to keep the patient on study until subsequent assessments (eg, MRI, CSF cytology) confirm progression. If subsequent testing confirms progression, the date of progression should be backdated to the onset of neurologic deterioration.
For imaging studies, the size of a measurable lesion at baseline should be at least 2 times the thickness of the slices showing the tumor (adding the interslice gap). Patients should be assessed using the same method and magnet strength throughout the study. If multiple measurable lesions are present, up to 4 target lesions should be selected to follow for response assessment. Standard 2-dimensional measurements (ie, largest tumor diameter and its largest perpendicular) should be used unless otherwise defined in a specific study protocol.
Conclusions
MBL and other CSF seeding tumors embody distinct issues for tumor staging and response assessment. Accurately and adequately determining efficacy of new agents, particularly in the current climate of decreasing therapeutic intensity, is critical. It is important that all clinical trials assessing efficacy do so in a manner in which results can be compared across trials. The recommendations presented here represent an initial effort to uniformly collect and evaluate response assessment criteria; these recommendations can now be incorporated into clinical trials to assess feasibility and corroboration with patient outcomes.
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Acknowledgment
The committee would like to acknowledge Julie Harreld for her thoughtful discussion and criticisms.
Conflict of interest statement. The authors report no conflict of interest.
References
- 1. Ostrom QT, de Blank PM, Kruchko C et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16 Suppl 10:x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Northcott PA, Korshunov A, Witt H et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pierce T, Kranz PG, Roth C, Leong D, Wei P, Provenzale JM. Use of apparent diffusion coefficient values for diagnosis of pediatric posterior fossa tumors. Neuroradiol J. 2014;27(2):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Packer RJ, Gajjar A, Vezina G et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 7. Warren KE, Poussaint TY, Vezina G et al. Challenges with defining response to antitumor agents in pediatric neuro-oncology: a report from the response assessment in pediatric neuro-oncology (RAPNO) working group. Pediatr Blood Cancer. 2013;60(9):1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fellay CN, Frappaz D, Sunyach MP, Franceschi E, Brandes AA, Stupp R. Medulloblastomas in adults: prognostic factors and lessons from paediatrics. Curr Opin Neurol. 2011;24(6):626–632. [DOI] [PubMed] [Google Scholar]
- 9. Verma S, Tavaré CJ, Gilles FH. Histologic features and prognosis in pediatric medulloblastoma. Pediatr Dev Pathol. 2008;11(5):337–343. [DOI] [PubMed] [Google Scholar]
- 10. Bailey CC, Gnekow A, Wellek S et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol. 1995;25(3):166–178. [DOI] [PubMed] [Google Scholar]
- 11. Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 12. Perreault S, Ramaswamy V, Achrol AS et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35(7):1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gajjar A, Hernan R, Kocak M et al. Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22(6):984–993. [DOI] [PubMed] [Google Scholar]
- 14. Barkovich A. Intracranial, Orbital and Neck Tumors of Childhood. 3rd ed Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 15. Ellingson BM, Bendszus M, Boxerman J et al. ; Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pierce TT, Provenzale JM. Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol J. 2014;27(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamashita Y, Kumabe T, Higano S, Watanabe M, Tominaga T. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol Res. 2009;31(9):940–946. [DOI] [PubMed] [Google Scholar]
- 18. Koral K, Alford R, Choudhury N et al. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology. 2014;56(9):781–788. [DOI] [PubMed] [Google Scholar]
- 19. Kremer S, Abu Eid M, Bierry G et al. Accuracy of delayed post-contrast FLAIR MR imaging for the diagnosis of leptomeningeal infectious or tumoral diseases. J Neuroradiol. 2006;33(5):285–291. [DOI] [PubMed] [Google Scholar]
- 20. Griffiths PD, Coley SC, Romanowski CA, Hodgson T, Wilkinson ID. Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am J Neuroradiol. 2003;24(4):719–723. [PMC free article] [PubMed] [Google Scholar]
- 21. Shapiro MD. MR imaging of the spine at 3T. Magn Reson Imaging Clin N Am. 2006;14(1):97–108. [DOI] [PubMed] [Google Scholar]
- 22. Cho HH, Choi YH, Cheon JE et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient-echo sequence for contrast-enhanced pediatric spinal imaging: comparison with T1-weighted turbo spin-echo sequence. AJR Am J Roentgenol. 2016;207(1):177–182. [DOI] [PubMed] [Google Scholar]
- 23. Warmuth-Metz M, Kühl J, Krauss J, Solymosi L. Subdural enhancement on postoperative spinal MRI after resection of posterior cranial fossa tumours. Neuroradiology. 2004;46(3):219–223. [DOI] [PubMed] [Google Scholar]
- 24. Harreld JH, Mohammed N, Goldsberry G et al. Postoperative intraspinal subdural collections after pediatric posterior fossa tumor resection: incidence, imaging, and clinical features. AJNR Am J Neuroradiol. 2015;36(5):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartels U, Shroff M, Sung L et al. Role of spinal MRI in the follow-up of children treated for medulloblastoma. Cancer. 2006;107(6):1340–1347. [DOI] [PubMed] [Google Scholar]
- 26. Perreault S, Lober RM, Carret AS et al. Surveillance imaging in children with malignant CNS tumors: low yield of spine MRI. J Neurooncol. 2014;116(3):617–623. [DOI] [PubMed] [Google Scholar]
- 27. Ramaswamy V, Remke M, Bouffet E et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oyharcabal-Bourden V, Kalifa C, Gentet JC et al. Standard-risk medulloblastoma treated by adjuvant chemotherapy followed by reduced-dose craniospinal radiation therapy: a French Society of Pediatric Oncology Study. J Clin Oncol. 2005;23(21):4726–4734. [DOI] [PubMed] [Google Scholar]
- 29. Lannering B, Rutkowski S, Doz F et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187–3193. [DOI] [PubMed] [Google Scholar]
- 30. Warmuth-Metz M, Bison B, Leykamm S. Neuroradiologic review in pediatric brain tumor studies. Klin Neuroradiol. 2009;19(4):263–273. [DOI] [PubMed] [Google Scholar]
- 31. Boyett J, Zeltzer P, Finlay J et al. Progression free survival and risk factors for primitive neuroectodermal tumors (PNET) of the posterior fossa (PF)[medulloblastoma] in children: report of the Children’s Cancer Study Group (CCG) randomized trial, CCG 921. Proc Am Soc Clin Oncol. 1995;14:147–153. [Google Scholar]
- 32. Kuhl J, Berthold F, Bode U et al. Preradiation chemotherapy of children with poor prognosis medulloblastoma: response rate and toxicity of the ifosfamide-containing multi-drug regimen HIT’88/’89. Am J Pediatr Hematol Oncol. 1993;15:67–71. [Google Scholar]
- 33. Chamberlain MC. New approaches to and current treatment of leptomeningeal metastases. Curr Opin Neurol. 1994;7(6):492–500. [DOI] [PubMed] [Google Scholar]
- 34. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. [DOI] [PubMed] [Google Scholar]
- 35. Murray JJ, Greco FA, Wolff SN, Hainsworth JD. Neoplastic meningitis. Marked variations of cerebrospinal fluid composition in the absence of extradural block. Am J Med. 1983;75(2):289–294. [DOI] [PubMed] [Google Scholar]
- 36. Rogers LR, Duchesneau PM, Nunez C et al. Comparison of cisternal and lumbar CSF examination in leptomeningeal metastasis. Neurology. 1992;42(6):1239–1241. [DOI] [PubMed] [Google Scholar]
- 37. Gajjar A, Fouladi M, Walter AW et al. Comparison of lumbar and shunt cerebrospinal fluid specimens for cytologic detection of leptomeningeal disease in pediatric patients with brain tumors. J Clin Oncol. 1999;17(6):1825–1828. [DOI] [PubMed] [Google Scholar]
- 38. Fouladi M, Gajjar A, Boyett JM et al. Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol. 1999;17(10):3234–3237. [DOI] [PubMed] [Google Scholar]
- 39. Terterov S, Krieger MD, Bowen I, McComb JG. Evaluation of intracranial cerebrospinal fluid cytology in staging pediatric medulloblastomas, supratentorial primitive neuroectodermal tumors, and ependymomas. J Neurosurg Pediatr. 2010;6(2):131–136. [DOI] [PubMed] [Google Scholar]
- 40. Chamberlain MC. Leptomeningeal metastases: a review of evaluation and treatment. J Neurooncol. 1998;37(3):271–284. [DOI] [PubMed] [Google Scholar]
- 41. Packer RJ, Sutton LN, Elterman R et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81(5):690–698. [DOI] [PubMed] [Google Scholar]
- 42. Chamberlain MC, Kormanik PA, Glantz MJ. A comparison between ventricular and lumbar cerebrospinal fluid cytology in adult patients with leptomeningeal metastases. Neuro Oncol. 2001;3(1):42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Glantz MJ, Cole BF, Glantz LK et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. [DOI] [PubMed] [Google Scholar]