See the article by Jahan et al on pages 44–54.
Immunotherapies represent a promising treatment option for different types of cancer, as evidenced by the increasing number of approved immunotherapeutic drugs. Despite impressive successes in treating systemic tumors such as malignant melanomas, the early hopes that immunotherapy would similarly act in glioma therapy have been dampened by negative outcomes in clinical trials—most recently the CheckMate 143 trial (NCT02017717).1 Although this randomized phase III clinical trial implementing checkpoint inhibition in the treatment of recurrent glioblastoma did not prolong overall patient survival, other studies have shown in principle that immunotherapy can induce a brain tumor–specific immune response.2 Therefore, rather than be discouraged by negative results, this should serve as a motivation to further understand the unique features of immunotherapy within the brain. Only by understanding these challenges will we be able to eventually realize effective immunotherapies for brain tumors. To this end, preclinical studies will play an essential role in exploiting the molecular mechanisms of response and resistance.
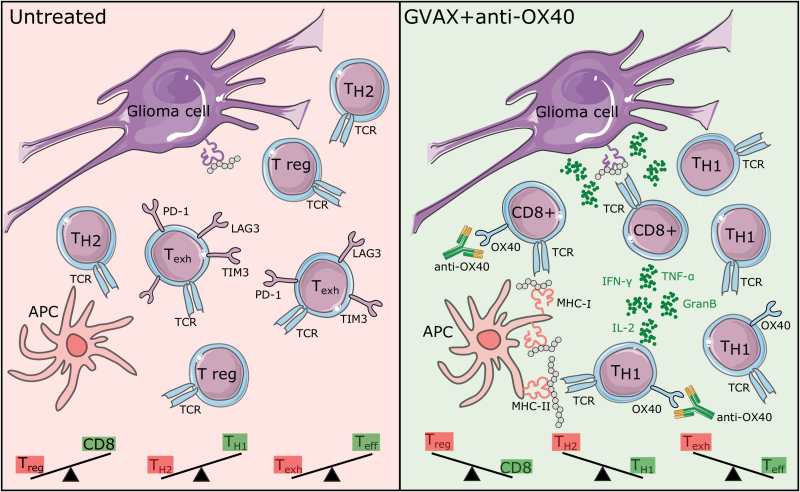
An example of such a study is presented here by Jahan and colleagues.3 The authors show that vaccination with irradiated whole tumor cells engineered to release the immunostimulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF; GVAX) combined with agonistic OX40 therapy acts synergistically against experimental gliomas. The authors observe an induction of a systemic T helper cell 1–driven immune response, an improvement in the ratio of cytotoxic to regulatory glioma-infiltrating lymphocytes, and a decrease in the lymphocyte exhaustion markers programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3 (LAG3), and T-cell immunoglobulin and mucin-domain containing-3 (TIM3) within the glioma microenvironment (Fig. 1).
Fig. 1.
Schematic overview of the glioma immune microenvironment and changes after combined GVAX + anti-OX40 treatment.
Abbreviations: APC, antigen-presenting cell; GranB, granzyme-B; IFN-γ, interferon-γ; IL-2, interleukin-2; TCR, T cell receptor; Teff, effector T cell; Texh, exhausted T cell; TH2, T helper 2 cell; TNF-α, tumor necrosis factor-α; T reg, regulatory T cell. Illustrations modified from Servier, CC BY 3.0.
Combinatory therapy of GVAX and checkpoint antagonists such as anti–cytotoxic T lymphocyte antigen 4 (CTLA-4)4 or agonists such as glucocorticoid-induced tumor necrosis factor receptor and OX40 has already been described to prolong survival in GL261 glioma bearing mice in a study by Murphy et al.5 The present study by Jahan and coworkers now provides comprehensive insights into the immunological mechanism of combinatory therapy. These findings may represent a general effector mechanism of combinatorial immunotherapeutic approaches and may therefore offer an opportunity to monitor treatment response.
While blocking antibodies against inhibitory immune checkpoints such as PD-1 and CTLA-4 prevent negative T-cell signaling and T-cell suppression, agonists of activating immune checkpoints such as OX40 increase positive signals and T-cell activation.6 Proliferation of effector cells consequently enhances the ratio between CD8 effector and regulatory T cells and restores antitumor immunity. A possible explanation for the limited effect of an immune checkpoint monotherapy in the context of malignant gliomas is that the low mutational load (and therefore potential immunogenicity) and the tight immune regulation within the CNS result in limited T-cell effector responses.7 As immune checkpoint therapies reactivate exhausted T cells, sufficient numbers of effector T cells must already be present for such an approach to be effective. In contrast, vaccination strategies—which have been shown to effectively delay tumor growth in syngeneic mouse tumor models—mediate their effects by inducing and expanding tumor-specific effector T cells.8 While considerable attention has focused on targeting single tumor-specific antigens to produce highly specific immune responses, vaccination with tumor lysates or whole irradiated tumor cells (as in this study) induces a polyclonal immune response that includes multiple epitopes for both major histocompatibility complex (MHC) class I and II. Although this approach lacks antigen specificity, it may result in the induction of MHC class II–driven CD4-mediated immune responses, which have been shown to represent a majority of tumor-specific adaptive immunity,9 provided the relevant antigens are present at a sufficient concentration in the vaccine. A phase I study previously performed by Curry and colleagues for patients with recurrent glioblastomas not only demonstrated the safety and feasibility of autologous whole tumor cell vaccination with GM-CSF–producing bystander cells,10 but intriguingly found that OX40 expression on CD4 T cells was increased after vaccination. Building on these clinical results, the authors have returned to a preclinical setting to demonstrate the benefits of combining vaccination with OX40 agonists, a promising strategy for future patient trials. By investigating the efficacy and underlying mechanism of a (combinatorial) immunotherapeutic intervention and by identification of potential biomarkers, preclinical studies such as that of Jahan et al provide a basis for translation into a clinical setting.
As a next step, translational research programs should be seen as a core feature of clinical immunotherapy trials. Only once we understand the reasons why immunotherapy does—or even does not—work will we be able to improve future therapies, realizing the hope for an effective immunotherapy for malignant glioma.
References
- 1. Reardon DA, Omuro A, Brandes AA, Rieger J. Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017; 19 (suppl_3): iii21. [Google Scholar]
- 2. Schumacher T, Bunse L, Pusch S et al. . A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014;512(7514):324–327. [DOI] [PubMed] [Google Scholar]
- 3. Jahan N, Talat H, Curry WT Jr. Agonist OX40 Immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF–expressing tumor cells. Neuro Oncol. 2018;20(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT Jr. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35(5):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murphy KA, Lechner MG, Popescu FE et al. . An in vivo immunotherapy screen of costimulatory molecules identifies Fc-OX40L as a potent reagent for the treatment of established murine gliomas. Clin Cancer Res. 2012;18(17):4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindy MS, Yu J, Zhu H, Smith MT, Gattoni-Celli S. A therapeutic cancer vaccine against GL261 murine glioma. J Transl Med. 2016;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kreiter S, Vormehr M, van de Roemer N et al. . Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curry WT Jr, Gorrepati R, Piesche M et al. . Vaccination with irradiated autologous tumor cells mixed with irradiated GM-K562 cells stimulates antitumor immunity and T lymphocyte activation in patients with recurrent malignant glioma. Clin Cancer Res. 2016;22(12):2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]