Abstract
Background
Cancer immunotherapy represents a promising treatment approach for malignant gliomas but is hampered by the limited number of ubiquitously expressed tumor antigens and the profoundly immunosuppressive tumor microenvironment. We identified cluster of differentiation (CD)70 as a novel immunosuppressive ligand and glioma target.
Methods
Normal tissues derived from 52 different organs and primary and recurrent low-grade gliomas (LGGs) and glioblastomas (GBMs) were thoroughly evaluated for CD70 gene and protein expression. The association between CD70 and patients’ overall survival and its impact on T-cell death was also evaluated. Human and mouse CD70-specific chimeric antigen receptors (CARs) were tested respectively against human primary GBMs and murine glioma lines. The antitumor efficacies of these CARs were also examined in orthotopic xenograft and syngeneic models.
Results
CD70 was not detected in peripheral and brain normal tissues but was constitutively overexpressed by isocitrate dehydrogenase (IDH) wild-type primary LGGs and GBMs in the mesenchymal subgroup and recurrent tumors. CD70 was also associated with poor survival in these subgroups, which may link to its direct involvement in glioma chemokine productions and selective induction of CD8+ T-cell death. To explore the potential for therapeutic targeting of this newly identified immunosuppressive axis in GBM tumors, we demonstrate that both human and mouse CD70-specific CAR T cells recognize primary CD70+ GBM tumors in vitro and mediate the regression of established GBM in xenograft and syngeneic models without illicit effect.
Conclusion
These studies identify a previously uncharacterized and ubiquitously expressed immunosuppressive ligand CD70 in GBMs that also holds potential for serving as a novel CAR target for cancer immunotherapy in gliomas.
Keywords: CD70, chimeric antigen receptors (CARs), gliomas, immunosuppressive ligand, immunotherapy
Importance of the study
This study provides evidence that glioma-associated CD70 expression was not detected in normal tissues from different organs but overexpressed predominantly in subgroups of patients with gliomas (ie, IDH wild-type LGGs and mesenchymal GBMs). The overexpressed CD70 gene was associated with poor survival in patients with LGGs (69.8 mo short, P = 0.0005), IDH wild-type LGGs (13.8 mo short), and mesenchymal GBMs (5.5 mo short). We found that CD70 is directly involved in immunosuppression by mediating production of protumor chemokines (ie, interleukin-8, chemokine C-C ligand 2, and C-X-C motif chemokine ligand 1) and selectively inducing CD8+ T-cell death. Importantly, our preclinical studies show that adoptively transferring CD70 CAR T cells can induce potent antitumor response in xenograft and syngeneic models without adverse effects, suggesting that CD70 may be employed to improve outcomes in patients with refractory brain tumors.
Despite highly toxic multimodal treatments, the 5-year survival rate is less than 10% for patients with primary glioblastomas (GBMs), necessitating development of new agents that promote survival without illicit effects.1,2 Cancer immunotherapy represents a promising new treatment paradigm, and patients with malignant gliomas have been shown to develop immunotherapeutic response against autologous specimens.3–5 Modification of patient-derived T cells with chimeric antigen receptors (CARs) is a uniquely specific and promising approach for ex vivo activation of tumor-specific T cells, but remains limited by the lack of ideal or appropriate targets in solid tumors. Important features of an ideal tumor immunotherapeutic target include (i) distinct expression patterns over normal cells to avoid off-target effects, (ii) involvement in malignant propagation, and (iii) ubiquitous expression within tumor cells to limit immune escape.
Cluster of differentiation (CD)70, a member of the tumor necrosis factor receptor family, may meet these characteristics in gliomas. CD70 is a type II transmembrane protein that represents the only ligand for CD27, a glycosylated transmembrane protein of the tumor necrosis factor receptor family.6,7 CD70/CD27 interactions play an important role in providing co-stimulation during the development of functional lymphocytes; strict control of CD70 expression is required for optimal signaling for immune cell activation.8,9 While CD70 expression is restricted to highly activated T/B lymphocytes and a small subset of mature dendritic cells; distinct hematologic and solid tumor malignancies, including gliomas, can constitutively overexpress CD70.9–13 Although CD70 has been demonstrated to play an important role in dendritic cell–induced enhanced T-cell antitumor immunity,14,15 without adequate T-cell receptor signals provided by tumor cells the CD27–CD70 interaction may not induce a strong immunological response, and inversely, promote apoptosis16–18 and dysregulate T cells’ function. The relevance and physiological consequence of ectopic CD70 expression on these tumors have yet to be thoroughly investigated.
In this study, our efforts have focused on CD70’s mechanism of action in glioma immunosuppression. By investigating the impact of tumor CD70 expression on CD8+ T cells and evaluating CD70-specific CAR against this molecule, we found that CD70 expression in malignant gliomas correlates with poor patient survival and directly facilitates immune suppression by selectively inducing CD8+ T-cell death. Importantly, we provide preclinical proof-of-principle evidence that targeting CD70-positive tumors with CAR T cells induces a potent antitumor response. These data suggest that CD70 is an optimal tumor immunotherapeutic target in glioma and may be employed to improve outcomes in patients with refractory brain tumors.
Materials and Methods
Patient Samples
A summary of tumor samples and clinicopathological details of the patients is shown in Supplementary Table S1. Healthy donors’ and cancer patients’ peripheral blood mononuclear cell (PBMC) samples were from HMU or LifeSouth. Institutional review board and Institutional Animal Care and Use Committee rules were followed.
Immunohistochemistry and FACS Analysis
CD70 was assessed using the mouse monoclonal anti-CD70 antibody (Santa Cruz). Other immunohistochemical staining was performed for CD3, CD4, CD8 (Zhongshan), and CD27 (Abcam). Three independent pathologists counted the positive cells in 8–10 randomized high-power fields of each sample. For the fluorescence activated cell sorting (FACS) analysis, anti-human and mouse CD8, CD4, CD3, and CD70 (BD Biosciences) were used.
CD70 GBM Lines
Lentiviral gene constructs encoding human CD70 and controls (pD2109-EF1a-CD70, pD2109-EF1a vector) or CD70 short hairpin (sh)RNA and controls (pLKO.1-puro-CD70-shRNA, pLKO.1-puro-nt-shRNA)14 were transduced into GBM lines. pLenti-CMV-Puro-Luc (gift from Eric Campeau, Addgene plasmid #17477) was used in the transduction of U87 (U87.Luc).
Murine glioma lines GL261 and KR158B were transduced with lentiviral constructs containing mouse CD70 gene or vector control (pUltra-EGFP-CD70-Luc or pUltra-EGFP-Luc), designated as GL70 or KR70. The CD70+ clone was achieved by single cell sorting using a flow cytometer, and the bulk CD70+ cells were from nonselected cells. The backbone pUltra was a gift from Malcolm Moore (Addgene plasmid #24129). All lentiviruses were produced with packaging plasmid psPAX2 and envelope plasmid pMD2.G (gifts from Didier Trono, Addgene plasmid #12260 and #12259).
RNA Sequencing and Data Analysis
Triplicated RNA products of 5 CD70 manipulated conditions of the pGBM#3 line were sent for RNA sequencing (RNA-seq) (Novogene). For the data analysis, clean data were provided by the company; paired-end reads were mapped to the human genome (version GRCh37/hg19) and assembled by TopHat Alignment and Cufflinks Pipeline from Illumina BaseSpace as a default setup. Data from The Cancer Genome Atlas (TCGA) used in this study were downloaded from https://cancergenome.nih.gov/. Gene expression by RNA-seq (IlluminaHiSeq version 2) was used.
CAR Transduction of Human and Mouse T Cells
The pMSGV8-hCAR and pMSGV8-mCAR generations and T-cell transductions were described previously.19 We added a tdTomato as a CAR tracker following the CD3-zeta by employing self-cleaving 2A peptide. Nontransduced (NT) T cells were activated under the same conditions. Mouse T-cell transduction was performed using the same methodology as the human T-cell transduction. T-cell transduction efficiency was determined by tdTomato-positive cells.
Real-Time PCR
Real-time PCR was performed using the SsoAdvanced Universal Probes Supermix (Bio-Rad Laboratories) and run on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The primers and probes were purchased from Thermo-Fisher Scientific. RPL13A was used for normalization.
CAR T-Cell Tumor Recognition
Human or mouse CAR T cells were cocultured with human or mouse glioma cell lines, respectively. Cell culture supernatants were harvested and assayed for interferon (IFN)-γ 18 h later. For the specific lysis assays, luciferase-expressing tumor targets were cocultured with varying amounts of mCD70 CAR T cells or NT T cells, respectively.20 Luminescence was measured in a Cytation 3 Cell Imaging Multi-Mode Reader (BioTek).
Syngeneic and Xenograft Mouse Models and Bioluminescence Imaging
For the syngeneic model, KR70 tumor cells were inoculated into the brains of C57BL/6J mice (Jackson Laboratory), and mCAR or NT T cells were delivered systemically via tail-vein injection. All mice were preconditioned with 5 Gy total body irradiation to induce lymphodepletion. In the human xenograft model, U87.Luc cells were implanted into the brain of NSG-B2m mice (NOD.Cg-B2mtm1UncPrkdcscid Il2rgtm1Wjl/SzJ) (Jackson Laboratory), and hCAR or NT T cells were delivered systemically via tail-vein injection. To monitor tumor growth, animals were imaged using the IVIS system (Xenogen). The bioluminescence image was analyzed using Living Image software (Caliper Life Sciences).
Statistical Methods
We considered CD70 as a continuous predictor of survival. Because of the observational nature of our study survival data, we also included variables in each model to account for possible confounding, and we included sex and age in all patient models. We used restricted cubic splines to assess possible nonlinearity in the association of continuous predictors with survival. We transformed all continuous predictors to a log base 2 scale before inclusion in our models. We used our fitted Cox models to estimate adjusted hazard ratio (HR) for CD70 and other included predictors along with 95% confidence intervals and Wald tests for the null hypothesis HR = 1. We estimated median survival in a similar manner for selected levels over the range of observed CD70 levels. We used these estimates to generate a median survival trend curve for CD70. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Overexpressed CD70 Is Presented on Primary and Recurrent Gliomas, but Not on Tumor Infiltrating T Cells
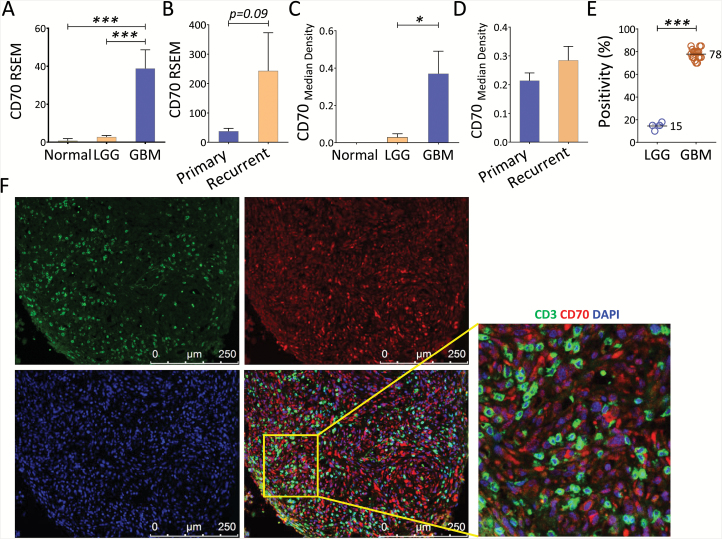
We and others have shown that T-cell senescence, characterized by CD27 loss and telomere shortening, is a signature of tumor-associated immune erosion.21 We sought to determine the expression profile of CD70 in patients with primary low-grade gliomas (LGGs) and GBMs. Using RNA-seq data culled from TCGA, we detected no CD70 in normal brains, but a significant CD70 upregulation was observed in both primary LGGs and GBMs; notably, primary and recurrent GBMs expressed the highest levels of CD70 (Fig. 1A–B). For protein expression, regular or fluorescent immunohistochemistry staining was carried out using surgical resected primary and recurrent LGGs and GBMs. A significantly elevated expression in patients with GBM primary and recurrent tumors was observed (Fig. 1C–D). Heterogeneous expression was observed on the surface and/or within the cytoplasm of the positive tumor cells, and no CD70 expression was observed on endothelial cells of the microvascular proliferation. Many GBMs demonstrated diffuse strong membranous and cytoplasmic staining and an average of 78% positivity in each CD70-expressing primary GBM; expression in LGGs was limited to about 15% (Fig. 1E). Since activated T cells potentially express CD70, we wanted to determine if these T cells also contribute to CD70 positivity. Dual-colored staining of CD3 and CD70 was performed in CD70+ patients’ samples. Overlaid images in these samples demonstrated that CD70 expression was limited to tumor cells and was not on infiltrating T cells (Fig. 1F). While 35% of primary GBMs were CD70+, only 10% of the primary LGGs tested were CD70 expressing. Compared with recurrent LGGs, which displayed 17% CD70 expression, 69% of recurrent GBMs expressed CD70 (Table 1). To confirm that CD70 was a tumor-specific target, we evaluated CD70 gene expression in all 52 normal tissue types (total 8555 samples from different organs, including 1259 brain tissues) and Epstein–Barr virus (EBV)–transformed B cells using an RNA-seq database culled from GTEx Portal. Based on these analyses, only the EBV-transformed B cells showed significant expression of CD70; CD70 levels from normal samples were nondetectable (Supplementary Figure S1). These results indicate that CD70 is a potential glioma target for the development of novel therapeutic strategies.
Fig. 1.
Overexpression of CD70 on primary and recurrent gliomas, but not on tumor infiltrating T cells. (A–B) Differential CD70 gene expression in normal brain, primary and recurrent LGG, and GBM. The gene expression level was defined as RSEM (RNA-seq by expectation-maximization), and patient’s informations were culled from TCGA RNA-seq datasets. (C) Protein level of CD70 was evaluated by immunohistochemistry using tumors from primary LGGs (n = 41), GBMs (n = 44), and normal brains (n = 7). (D) CD70 protein expression between primary and recurrent GBMs. Tumors from 7 paired GBMs before and after recurrence were evaluated. (E) The CD70 positivity within each CD70-expressing primary GBM. (F) Determination of CD70 expression on tumor and T cells. Seven CD70+ GBMs were co-stained with fluorescent conjugated CD3 (green) and CD70 (red) antibodies; 4′,6′-diamidino-2-phenylindole was used for nuclear staining. Representative pictures (with an enlarged overlaid area) from one tumor are shown. Mann–Whitney U test was used.
Table 1.
CD70 positivity and CD3+ infiltration in resected tumor from primary and recurrent gliomasa
| WHO Grade | Total Patients |
CD70 Expression by Glioma | CD3 Tumor Infiltration | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive % | Positive | Negative | Positive% | ||
| Primary LGG | 41 | 4 | 37 | 10 | 25 | 16 | 61 |
| Primary GBM | 46 | 16 | 30 | 35 | 40 | 6 | 90 |
| Recurrent LGG | 6 | 1 | 5 | 17 | N/A | N/A | N/A |
| Recurrent GBM | 16 | 11 | 5 | 69 | N/A | N/A | N/A |
aThe demographic and clinical characteristics for most of the patients are shown in Supplementary Table S1.
CD70 Expression Correlates with Poor Survival for Patients with LGGs and Mesenchymal GBMs
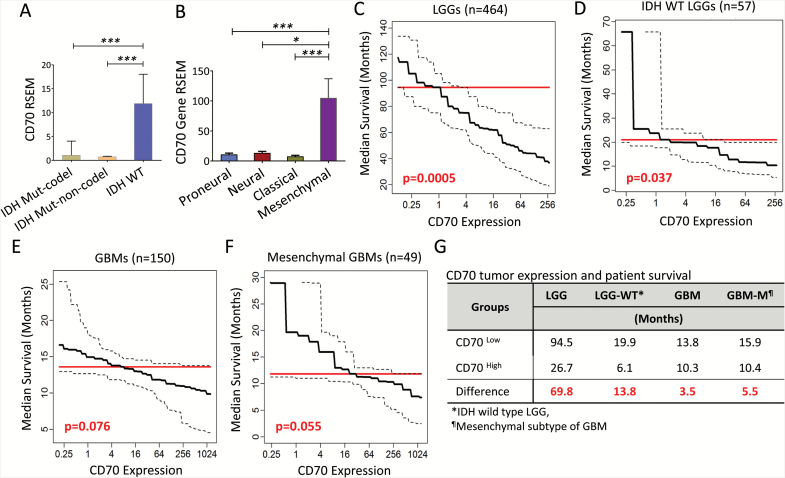
To understand the effects of CD70 expression in gliomas on clinical outcomes, we utilized existing large RNA-seq datasets and determined the association between CD70 gene expression and survival based on molecular classifications.22,23 In primary LGGs, CD70 was predominantly expressed by isocitrate dehydrogenase (IDH) wild-type tumors compared with IDH mutated tumors, including both chromosome 1p/19q codeletion and non-codeletion (Fig. 2A). In GBM, CD70 was overexpressed in tumors with mesenchymal gene signatures (Fig. 2B). Since CD70 has been shown to assist T-cell function,14 we assessed whether CD70 could be a favorable prognostic factor in gliomas. CD70 expression levels were culled from TCGA and correlated with survival outcomes. In LGGs, elevated CD70 tumor expression was significantly associated with decreased overall survival. The median overall survival for patients with elevated CD70 expression (above the median expression level) was only 26.7 months, compared with 94.5 months for patients in the CD70-low group (below median) (69.8 mo difference, P = 0.0005; Fig. 2C). Because wild-type IDH status is associated with poor survival, we evaluated the correlation between CD70 expression levels and survival outcomes in this population. Indeed, CD70 was found to be inversely associated with patient survival (13.8 mo difference, P = 0.037; Fig. 2D). The same trends were revealed for patients with GBM (3.5 mo difference, P = 0.076), specifically mesenchymal subtypes23 (5.5 mo difference, P = 0.055; Fig. 2E–F). In summary (Fig. 2G), these data suggest that CD70 expression on tumor cells is negatively associated with patients’ survival in LGGs and GBMs.
Fig. 2.
CD70 gene expression is inversely correlated with overall survival in patients with primary gliomas. (A–B) CD70 expression among different subgroups of LGGs and GBMs. (C–F) Predicted median overall survival decreases with increasing CD70 expression in primary LGGs, IDH wild-type LGGs, GBMs, and GBMs in the mesenchymal subgroup (CD70 RNA-seq expression was considered as a continuous predictor of survival, adjusted for gender and age in all subgroup models using Cox proportional hazards (PH) regression; further adjustment was made for tumor subtype in the GBM subgroup model). (G) Summary of differential median overall survival between CD70 high and low groups defined by mean values of CD70 RSEM in LGGs and GBMs (subgroup median survival estimates adjusted for age, gender and GBM tumor subtype via Cox PH regression).
CD70 Is Directly Involved in Chemokine/Cytokine Signaling Pathway in Primary GBM
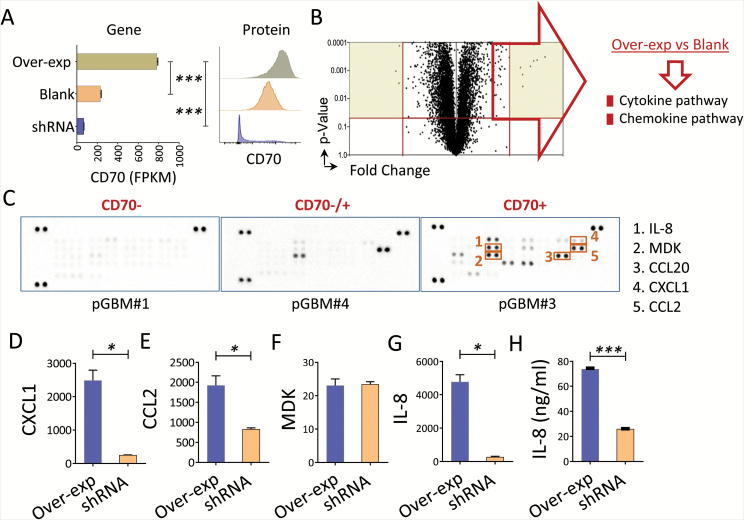
To better understand the mechanism of action for CD70 in gliomas, we did pathway enrichment analysis using a TCGA RNA-seq dataset, which indicated the involvement of CD70 in chemokine/cytokine signaling pathways.24 Since the analysis from TCGA samples illustrated entire tumor tissues that contained immune infiltrates, we next wanted to determine if CD70 directly affects the chemokine/cytokine pathway in pure tumors. We investigated chemokine/cytokine pathway changes in a primary CD70+ line (pGBM#3) after manipulating the CD70 gene (Fig. 3A and Supplementary Table S2). We confirmed that CD70 overexpression enhanced these genes in the chemokine/cytokine pathways (Fig. 3B). Additionally, CD70+ primary GBM lines elicited greater amounts of chemokines in cell culture supernatants, including interleukin (IL)-8, midkine (MDK), chemokine C-C ligand (CCL)20, C-X-C motif chemokine ligand (CXCL)1, and CCL2 (Fig. 3C). The gene levels of the chemokines (IL-8, CXCL1, CCL2, MDK) were abrogated by CD70 silencing (Fig. 3D–G). Additionally, the protein levels of IL-8 were significantly reduced when the CD70 gene was silenced (Fig. 3H). These data suggest that CD70 is directly involved in glioma tumor chemokine productions.
Fig. 3.
CD70 involved in cytokine/chemokine productions in GBM. (A–B) CD70 overexpression influences the cytokine/chemokine pathway in a primary GBM line. Triplicated RNA samples from pGBM#3 (CD70+) were transduced with CD70 (Over-exp) or lentiviral vector control (blank), confirmed by gene (fragments per kilobase of transcript per million mapped reads, FPKM) and protein expression (flow cytometry). Gene enrichment of the upregulated gene list (Over-exp vs blank) was performed by PANTHER-Pathway; a volcano plot and the top-ranked pathways are shown. (C) Primary GBM lines secrete various chemokines. Culture supernatants from the primary lines were collected 8 days after the tumors were seeded, and chemokine array was performed. (D–G) Gene expression changes between overexpression and silence of CD70 in pGBM#3 were evaluated by real-time quantitative PCR. (H) IL-8 secretion was measured by enzyme-linked immunosorbent assay. Unpaired t-test was used.
CD70 Associates with Enhanced Tumor T-Cell Infiltration and Induction of Selective CD8+ T-Cell Death
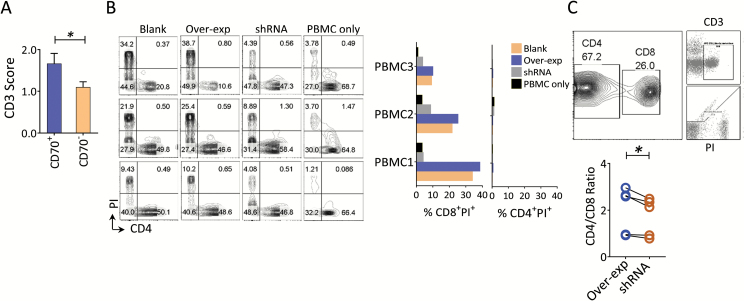
Tumor-derived chemokines have been shown to recruit macrophages and T cells into the tumors.25,26 Having demonstrated a direct connection between CD70 and tumor-associated chemokine production, we sought to assess the association of CD70 with T-cell infiltration. We found that CD70 was moderately associated with tumor infiltrating CD3+ T cells in primary GBMs (Fig. 4A). To more broadly interrogate T-cell infiltration in primary GBMs, a large dataset culled from TCGA was analyzed, and a predilection for CD4 over CD8 transcripts (~29-fold higher) was revealed in primary gliomas; no such phenomenon has been described in other cancer types (Mu L et al, manuscript submitted). These data suggest that diminished CD8 transcripts in GBMs may represent tumor immune erosion. To further determine the impact of CD70-expressing tumor cells on T cells, allogeneic PBMCs were cocultured with GBM lines, which expressed various levels of CD70 using forced overexpression or shRNA knockdown (described in Fig. 3A). Interestingly, no major cell death was found in the CD4+ T-cell population, but CD8+ T cells displayed a significant level of cell death as measured by propidium iodide (PI) staining. Silencing CD70 significantly reduced CD8+ T-cell death. Although there were large variations in PI+ CD8+ T cells across PBMC samples, the trend was greater for CD8+ versus CD4+ T-cell death, and an increased CD4+ to CD8+ T-cell ratio was consistently observed (Fig. 4B–C). Together, these data suggest that CD70 on gliomas may help recruit T cells into the tumor but also induce CD8+ specific cell death in part through direct cell contact.
Fig. 4.
CD70 is associated with T-cell infiltration and CD8+ T-cell death in GBM. (A) Relative higher numbers of infiltrating CD3+ T cells in CD70+ compared with CD70− tumors. Paraffin-embedded GBM tumor samples described in Fig. 1 were analyzed by immunohistochemistry for CD70 and CD3, respectively, using serial slides. (B) CD70 predominantly induces CD8+ T-cell death after engaging tumor cells. CD70-manipulated pGBM#3 cells were cocultured with 3 allogeneic PBMCs obtained from healthy donors. Fluorescence activated cell sorting (FACS) analysis was performed 14 days after the coculture (left). The cell death of T-cell subsets was quantitated by propidium iodide (PI) staining for both CD4+ and CD8+ T cells (right). These results were representative of 4 individual experiments. Mann–Whitney test was used to assess statistical significance. (C) The ratio of CD4+ to CD8+ T cells among the cocultured T cells. FACS analysis from (B) was also evaluated by CD4+ to CD8+ ratio, and the gating strategy is shown in the upper panel. Paired t-test was used to assess statistical significance.
Glioma Recognitions of Human and Mouse CD70 CAR T Cells In Vitro
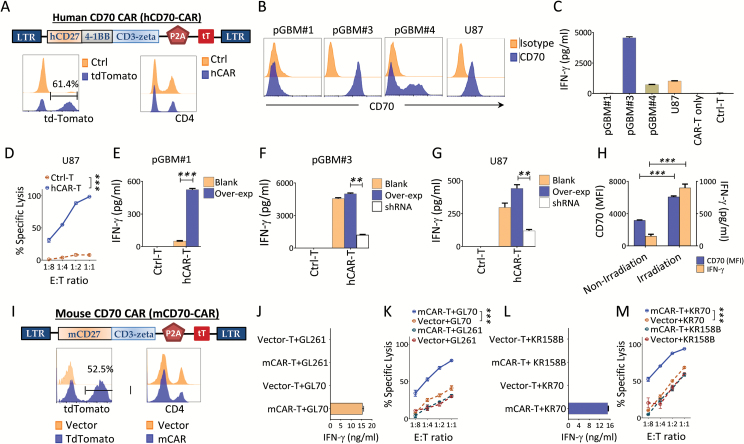
To explore whether the restricted expression of an immunosuppressive ligand on GBM tumor cells could serve as a novel target for immunotherapy, CD70-specific CAR constructs of human and mouse were made. The human CD70 CAR (hCD70-CAR, MSGV8 [(MSGV1-(Bam-)]-human CD27-4-1BB-CD3-zeta) is a retroviral-based vector that encodes CD27 for antigen recognition and transmembrane domains followed by intracellular portions of the 4-1BB and the CD3-zeta chain.19 We added tdTomato sequences following the CD3-zeta linked by P2A (the MSGV8 vector was used as a control). Based on tdTomato expression, we obtained >50% of T-cell transduction efficiency, and the majority of the transduced T cells were CD8+. CAR transduction did not significantly alter the ratio of CD4+/CD8+ T cells when the transduced and NT T cells were compared (a representative case is shown in Fig. 5A). Using both primary and commercial GBM lines that do or do not naturally express CD70 (Fig. 5B) as hCD70-CAR T cell targets, we showed that hCD70-CAR T cells recognize only CD70+ lines, but not CD70-negative lines; no recognition was found by NT or vector-transduced control T cells, as measured by IFN-γ release and cytotoxic killing assay (Fig. 5C–D). Overexpression or knockdown of CD70 enhanced or diminished, respectively, recognition by the CD70 CAR T cells (Fig. 5E–G). Irradiation of CD70+ tumors enhanced their CD70 expression and recognition by CAR T cells (Fig. 5H). A similar approach was also carried out for the murine GBM lines. The murine-derived CD70 CAR (mCD70-CAR), MSGV8 [(MSGV1-(Bam-)]-mouse CD27-CD3-zeta was the same retroviral vector used for the hCAR, but it contains a mouse’s full-length CD27 and CD3-zeta chain19 (tdTomato was added after the CD3-zeta by 2A peptide). The transduction efficiencies were generally >50% in our experiments (a representative analysis is shown in Fig. 5I). We next determined the tumor-specific recognition against CD70 transduced murine glioma lines GL70 and KR70 by INF-γ production and cytotoxic killing assay, respectively. We demonstrated that mCD70-CAR T cells were able to kill CD70+ targets in vitro (Fig. 5J–M). These results demonstrated that both human and mouse CD70 CAR T cells recognize CD70+ glioma targets specifically.
Fig. 5.
Tumor recognitions of gliomas by human and mouse CD70 CAR T cells. (A) Human CAR construct and its transduction efficiency in human T cells. The transduction efficiency was measured by tdTomato positive cells and the frequency of CD4+/CD8+ T cells 4 days posttransduction are shown. (B) GBM targets for hCAR T cells. GBM lines naturally expressing various levels of CD70 were tested for the CD70 surface expression by FACS. (C–D) Recognition of these lines by hCAR T cells. The recognitions were measured by IFN-γ release and cytotoxic killing assay. (E–G) Manipulation of tumor CD70 expression alters the CAR T cells recognition. The tumor lines were cocultured with manipulated GBM lines by either overexpressing or silencing CD70. (H) Irradiation enhances tumor CD70 expression and CAR T-cell recognition. The U87 line was irradiated with 6 Gy, CD70 expression was determined by FACS on day 3 and then cocultured with CAR T cells. (I) Transduction of mCD70 CAR to mouse T cells. Construct of mouse CD70-specific CAR (mCD70-CAR), transduction efficiency determined by tdTomato and frequency of CD4+ /CD8+ T cells in the mCAR T cells 4 days posttransduction are shown. (J–M) CD70-overexpressed murine glioma lines were recognized by mCD70-CAR T cells. The tumors (104) were cocultured with (104) mCD70-CAR T cells or vector/NT control T cells, respectively. For the killing assay, various ratios of effector to target (E:T) were respectively cocultured. Unpaired t-test was used.
Antitumor Response of CD70 CAR T Cells Against CD70+ Gliomas In Vivo
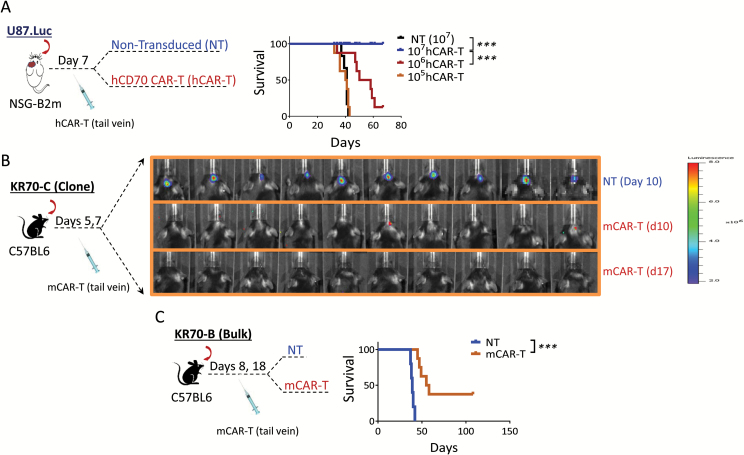
Next, we want to test the human and mouse CARs in human xenograft and syngeneic orthotopic glioma models. For hCAR, various doses (105–107/mouse) of the CAR T cells were adoptively transferred into tumor-bearing NSG-B2m mice 7 days after tumor inoculation. The results demonstrated a T-cell dose-dependent manner: a complete regression was seen in 107/mouse, an intermediate effect in 106/mouse, and no effect in the 105/mouse group in survival (Fig. 6A). Since the NSG-B2m mice lacked major immune cell components, we also made a mouse version of CD70 CAR and tested it in C57BL/6J syngeneic mice. Due to the heterogeneous nature of gliomas, we employed 2 types of CD70-overexpressing glioma cells: (i) tumors derived from a single clone of a CD70+ glioma cell line, KR70-C; and (ii) inoculated nonselective bulk CD70-expressing tumors, KR70-B, which expressed various levels of CD70 (~70% positivity for CD70). Complete tumor regressions were observed in KR70-C tumor-bearing mice 17 days after tumor implantation (Fig. 6B). Significantly prolonged survival was observed in the CAR T treated group of KR70-B tumor-bearing mice compared with the NT T cell treated group, and 38% of the mice were cured by CAR T cells (P < 0.001, HR = 3.5, Fig. 6C). No adverse effect was observed in these models, confirming CAR safety in vivo.19 In summary, CD70-specific CAR T cells generate potent antitumor response against CD70+ gliomas in xenograft and syngeneic animal models.
Fig. 6.
Antitumor response of CD70 CAR T cells against CD70+ gliomas in vivo. (A) Antitumor response of hCAR T cells in human xenograft glioma model. CD70+ (U87.Luc, 5 × 104/mouse) tumor-bearing (confirmed by imaging 6 days after tumor inoculation) mice were adoptively transferred through tail-vein injection with NT or various doses (105–107/mouse, 8/group) of hCAR T cells 7 days after the tumor implantation. (B–C) A complete response and prolonged survival induced by mCAR T cells in syngeneic glioma models. Two models were used: groups of 6–8 weeks C57BL/6J mice (10/group) were intracranially inoculated with 1 × 105 KR70 tumor cells derived from a tumor clone, designated as KR70-C, and then adoptively transferred (1 × 107) NT or mCAR T cells on days 5 and 7 post tumor implantation. Luciferase imaging was carried out 10 and 17 days after the tumor implantation. The same experiment described in (B) was performed, except the inoculated tumor cells were derived from bulk tumors, KR70-B. All experiments were repeated at least 2 times. Mann–Whitney test and the log-rank test were used.
Discussion
Identifying molecular targets that contribute to tumor pathogenesis with restricted specificity to tumor expression is crucial for effective and sustained therapy. The tumor microenvironment in GBM is notoriously immunosuppressive and appears co-opted in part by CD70 tumor overexpression. We found in a recent study that CD70 is involved in promoting tumor migration and M2 macrophage infiltration in GBM.24 In this report, we showed that CD70 protein was overexpressed by subgroups of primary LGG (10%) and GBM (35%). No CD70 was detected on tumor infiltrating CD3+ T cells and CD163+ macrophages in these tumors.24 Importantly, little to no expression was detected in all 52 types of normal tissue, including peripheral blood. These results indicate that CD70 expression is mostly restricted to tumors. The gene expression of CD70 was predominantly found in IDH wild-type LGG patients, a subgroup with strikingly similar molecular characteristics and overall survival to GBMs.27 CD70 was associated with poor survival outcomes in IDH wild-type LGGs. CD70-expressing GBMs were mainly observed in the mesenchymal subgroups and were negatively associated with survival, suggesting that CD70 may have a vital role in glioma progression.
Since CD70 is the only known ligand for CD27 (a T-cell co-stimulatory receptor that plays a critical role in activation, effector function, and survival of T cells), we initially thought that glioma-CD70 expression would impact tumor infiltrating T-cell function via CD27 signaling. Unexpectedly, we found no downregulation of CD27 on tumor infiltrating lymphocytes (data not shown), suggesting that a CD27-independent mechanism has taken place. Although CD70 expression on primary GBMs positively correlates with tumor infiltrating lymphocytes, several obstacles may potentially prevent these T cells from mounting a strong antitumor response. These obstacles include: (i) overwhelming presentation of M2 macrophages that may stymie T-cell function; (ii) the possibility that promoting regulatory T cells, which we have shown can act as a predictor of tumor progression (Mu L et al, manuscript submitted), will utilize the CD27/CD70 axis; and (iii) inducing CD8+ specific T-cell death through a novel mechanism involving the epiregulin–epidermal growth factor receptor pathway (unpublished data). Our studies provide scientific evidence that targeting CD70-expressing gliomas may offset the immunosuppressive effect and promote strong and sustained antitumor responses.
Several immunotherapeutic approaches targeting CD70 in other malignancies have been reported, such as antibody blockade, antibody-dependent cell-mediated cytotoxicity, and CAR T cells for cancers such as lymphoma and renal carcinoma.28,29 Due to the existence of the blood–brain barrier in gliomas, CAR T therapy is a superior strategy to other methods, as activated T cells are not only able to pass through the barrier, but are also capable of inducing a potential antitumor response.30–32
Our results illustrate that CD70 is highly expressed not only by primary tumors, but also by recurrent tumors, which presents a consistent therapeutic target for primary and recurrent gliomas. In addition, irradiation enhances CD70 expression on tumors, providing good opportunity to enhance antitumor efficacy to combine standard care with CD70 CAR therapy in glioma patients. Our previous study showed that the impact of CD27 on T-cell activation depends (in part) on the extracellular domain of membrane-bound CD27 (soluble [s]CD27), such that addition of sCD27 into T-cell cultures promotes strong T-cell proliferation.33 Thus, introducing the extracellular motif could potentially enhance the CAR T-cell function. Nevertheless, we demonstrated that CD70-specific human and murine CAR T cells induce complete regression/improved survival in CD70+ gliomas in both xenograft and syngeneic models without toxicity.
Toxicity is one of the great obstacles for CAR T therapy. Since inherited deficiency of CD70 (ie, homozygous mutations in CD70) can cause impaired function in immune response to viral infections,34,35 there is concern that targeting CD70 may induce immunosuppression. However, adverse effects from CD70 deficiency may be distinct from adverse effects mediated by CD70 CAR T-cell targeting. While the effects of CD70 CAR T-cell targeting on progenitor cells remain to be determined, based on previously published data, CD70 CAR T-cell targeting does not appear to affect dendritic or T cells. CD70 expression is limited only to the most activated immune cells, which are relatively sparse in cancer patients.14,36 Moreover, a recent report demonstrated that CD70 CAR T cells “did not appear to block adaptive host immune responses,”19 and we showed in this report that CD70 CAR T cells potently induce antitumor reactivity against CD70+ gliomas, both in vitro and in vivo. Taken together, our data suggest that CD70 may be harnessed as an immunotherapeutic target to improve outcomes in patients with gliomas.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the Wells Endowment [grant number 00107592]; Heilongjiang Province Program for Application Technology Research and Development [grant number GA15C108]; and Ministry of Science and Technology of the People’s Republic of China [grant number 2014DFA31630].
Conflict of interest statement
All authors have declared there are no financial conflicts of interest in regard to this work.
Supplementary Material
References
- 1. Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan RA, Johnson LA, Davis JL et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23(10):1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowman MR, Crimmins MA, Yetz-Aldape J, Kriz R, Kelleher K, Herrmann S. The cloning of CD70 and its identification as the ligand for CD27. J Immunol. 1994;152(4):1756–1761. [PubMed] [Google Scholar]
- 7. Hintzen RQ, Lens SM, Beckmann MP, Goodwin RG, Lynch D, van Lier RA. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol. 1994;152(4):1762–1773. [PubMed] [Google Scholar]
- 8. Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154(6):2612–2623. [PubMed] [Google Scholar]
- 9. Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229(1):216–231. [DOI] [PubMed] [Google Scholar]
- 10. Held-Feindt J, Mentlein R. CD70/CD27 ligand, a member of the TNF family, is expressed in human brain tumors. Int J Cancer. 2002;98(3):352–356. [DOI] [PubMed] [Google Scholar]
- 11. Ryan MC, Kostner H, Gordon KA et al. Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75. Br J Cancer. 2010;103(5):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wischhusen J, Jung G, Radovanovic I et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62(9):2592–2599. [PubMed] [Google Scholar]
- 13. Adam PJ, Terrett JA, Steers G et al. CD70 (TNFSF7) is expressed at high prevalence in renal cell carcinomas and is rapidly internalised on antibody binding. Br J Cancer. 2006;95(3):298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang J, Kerstann KW, Ahmadzadeh M et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176(12):7726–7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keller AM, Xiao Y, Peperzak V, Naik SH, Borst J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood. 2009;113(21):5167–5175. [DOI] [PubMed] [Google Scholar]
- 16. Chahlavi A, Rayman P, Richmond AL et al. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005;65(12):5428–5438. [DOI] [PubMed] [Google Scholar]
- 17. Diegmann J, Junker K, Loncarevic IF, Michel S, Schimmel B, von Eggeling F. Immune escape for renal cell carcinoma: CD70 mediates apoptosis in lymphocytes. Neoplasia. 2006;8(11):933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruf M, Mittmann C, Nowicka AM et al. pVHL/HIF-regulated CD70 expression is associated with infiltration of CD27+ lymphocytes and increased serum levels of soluble CD27 in clear cell renal cell carcinoma. Clin Cancer Res. 2015;21(4):889–898. [DOI] [PubMed] [Google Scholar]
- 19. Wang QJ, Yu Z, Hanada K-i et al. Pre-clinical evaluation of chimeric antigen receptors targeting CD70-expressing cancers. Clinical Cancer Research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson LA, Scholler J, Ohkuri T et al. Rational development and characterization of humanized anti–EGFR variant III chimeric antigen receptor T cells for glioblastoma. Science Translational Medicine. 2015; 7(275):275ra222–275ra222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175(10):7046–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Che JW, Kraft AR, Selin LK, Welsh RM. Regulatory T cells resist virus infection-induced apoptosis. J Virol. 2015;89(4):2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verhaak RG, Hoadley KA, Purdom E et al. ; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ge H, Mu L, Jin L, . et al. Tumor associated CD70 expression is involved in promoting tumor migration and macrophage infiltration in GBM. Int J Cancer. 2017. doi:10.1002/ijc.30830 [DOI] [PubMed] [Google Scholar]
- 25. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oelkrug C, Ramage JM. Enhancement of T cell recruitment and infiltration into tumours. Clin Exp Immunol. 2014;178(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Network TCGAR. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015; 372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silence K, Dreier T, Moshir M et al. ARGX-110, a highly potent antibody targeting CD70, eliminates tumors via both enhanced ADCC and immune checkpoint blockade. Paper presented at: MAbs2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaffer DR, Savoldo B, Yi Z et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117(16):4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm (Vienna). 2006;113(4):477–485. [DOI] [PubMed] [Google Scholar]
- 31. Miao H, Choi BD, Suryadevara CM et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One. 2014;9(4):e94281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown CE, Alizadeh D, Starr R et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375(26): 2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang J, Jochems C, Anderson AM et al. Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity. J Immunol. 2013;190(12):6250–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abolhassani H, Edwards ES, Ikinciogullari A et al. Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency. J Exp Med. 2017;214(1): 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Izawa K, Martin E, Soudais C et al. Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection. J Exp Med. 2017;214(1):73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang J, Wang QJ, Yang S et al. Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro. J Immunother. 2011;34(4):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.