Abstract
Gonadotropin-releasing hormone (GnRH) neurons are the final central regulators of reproduction, integrating various inputs that modulate fertility. Stress typically inhibits reproduction but can be stimulatory; stress effects can also be modulated by steroid milieu. Corticotropin-releasing hormone (CRH) released during the stress response may suppress reproduction independent of downstream glucocorticoids. We hypothesized CRH suppresses fertility by decreasing GnRH neuron firing activity. To test this, mice were ovariectomized (OVX) and either implanted with an estradiol capsule (OVX+E) or not treated further to examine the influence of estradiol on GnRH neuron response to CRH. Targeted extracellular recordings were used to record firing activity from green fluorescent protein–identified GnRH neurons in brain slices before and during CRH treatment; recordings were done in the afternoon when estradiol has a positive feedback effect to increase GnRH neuron firing. In OVX mice, CRH did not affect the firing rate of GnRH neurons. In contrast, CRH exhibited dose-dependent stimulatory (30 nM) or inhibitory (100 nM) effects on GnRH neuron firing activity in OVX+E mice; both effects were reversible. The dose-dependent effects of CRH appear to result from activation of different receptor populations; a CRH receptor type-1 agonist increased firing activity in GnRH neurons, whereas a CRH receptor type-2 agonist decreased firing activity. CRH and specific agonists also differentially regulated short-term burst frequency and burst properties, including burst duration, spikes/burst, and/or intraburst interval. These results indicate that CRH alters GnRH neuron activity and that estradiol is required for CRH to exert both stimulatory and inhibitory effects on GnRH neurons.
CRH exerts both stimulatory and inhibitory effects on GnRH neuron firing activity via activation of different receptors in an estradiol-dependent manner.
Gonadotropin-releasing hormone (GnRH) neurons are crucial regulators of the reproductive system. GnRH pulses stimulate secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone, which then induce the gonads to activate gametogenesis and steroidogenesis. GnRH release at the median eminence typically requires action potential firing (1). Many factors that alter GnRH neuron action potential firing regulate fertility (2–4). Stress is one of these factors. In some cases, acute stress can stimulate the reproductive system (5–7). The vast majority of studies, however, suggest that stress exposure suppresses reproductive function. Various types of stressors suppress LH pulses in several species, including rats (8, 9), monkeys (10–12), sheep (13–15), and mice (16).
The hypothalamic-pituitary-adrenal (HPA) axis is one pathway that is activated in response to stress. Stress initiates hypothalamic release of corticotropin-releasing hormone (CRH), which stimulates secretion of adrenocorticotropic hormone from the pituitary and thereby increased glucocorticoid production by the adrenal cortex. Activation of the HPA axis as monitored by increased circulating glucocorticoids during the stress response commonly occurs concomitantly with inhibition of the reproductive system (17–19). Some studies, however, suggest that CRH could have a direct effect on reproduction independent of the downstream glucocorticoid pathway. Pretreatment with CRH receptor (CRHR) antagonists prevents stress-induced suppression of LH pulses (20–22). In addition, intracerebroventricular or median eminence administration of CRH inhibits LH pulses (21, 23, 24) and hypothalamic multiunit electrical activity volleys that are associated with LH pulses (23). This inhibitory effect of CRH on LH pulses persists in adrenalectomized monkeys, which lack the main glucocorticoid production site.
Interactions between the HPA axis and the reproductive system are modulated by estradiol. For example, estradiol potentiates inhibitory effects of insulin-induced hypoglycemia (8, 25) and food deprivation (26) on LH pulses in ovariectomized (OVX) animals compared with those without estradiol treatment. OVX rats with estradiol replacement exhibit more severe suppression of LH secretion after CRH treatment than OVX rats (21). Further, estradiol treatment increases CRHR type-2 (CRHR-2) messenger RNA (mRNA) expression in immortalized GnRH neurons (GT1 cells) (27). These data indicate that estradiol typically enhances the effect of stress on reproduction, potentially via a CRH-mediated pathway.
Anatomical observations suggest that CRH-producing neurons can directly affect GnRH neurons. CRH-containing fibers synapse on GnRH neurons (28, 29). Approximately 30% of GnRH neurons in female mice exhibit CRHR immunoreactivity (30). The antibody used did not distinguish between CRHR subtypes; however, in the same paper, single-cell gene expression profiling was positive for CRHR type-1 (CRHR-1) but not CRHR-2 (30). Intracerebroventricular injection of CRH decreases GnRH mRNA expression in ewes (31), and studies in GT1 cells suggest the inhibitory effect of CRH on GnRH mRNA expression could be direct (27). These lines of evidence suggest CRH may alter GnRH neuron function as one mechanism of altering the reproductive system. Here, we examined the effect of CRH on GnRH neuron activity, the type of receptors involved, and the influence of estradiol milieu on this response in female mice.
Material and Methods
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted.
Animals
Female mice expressing green fluorescent protein under the control of the GnRH promoter were used (32). Mice were on a B6CBA/F1 background and aged from 66 to 126 days. Animals were housed on a 14-hour light, 10-hour dark cycle with light on at 0400 am Eastern Standard Time. Animals were provided with water and Teklad 2916 chow (Envigo, Madison, WI) ad libitum. To study effect of estradiol on CRH response, mice were OVX under isoflurane anesthesia with bupivacaine as a local analgesic. Mice were either implanted with a Silastic (Dow Corning, Midland, MI) capsule containing 0.625 μg of 17β-estradiol in sesame oil (OVX+E group) or not treated further (OVX) at the time of surgery (2). Recordings were performed 2 to 3 days after surgery. The Institutional Animal Care and Use Committee of the University of Michigan approved all procedures.
Brain slice preparation
Solutions were bubbled with 95% O2 and 5% CO2 throughout the experiments and for at least 15 minutes before exposure to tissues. The brain was removed rapidly 1.5 to 2 hours before lights off and placed in ice-cold sucrose saline solution containing 250 mM sucrose, 3.5 mM KCl, 26 mM NaHCO3, 10 mM d-glucose, 1.25 mM Na2HPO4, 1.2 mM MgSO4, and 3.8 mM MgCl2. Coronal brain slices (300 μm) were prepared with a Leica VT1200S (Leica Biosystems, Buffalo Grove, IL). Slices were incubated in a 1:1 mixture solution of sucrose-saline and artificial cerebrospinal fluid (ACSF) containing 135 mM NaCl, 3.5 mM KCl, 26 mM NaHCO3, 10 mM d-glucose, 1.25 mM Na2HPO4, 1.2 mM MgSO4, and 2.5 mM CaCl2 for 30 minutes at room temperature and then transferred to 100% ACSF for at least 30 minutes at room temperature before recording. Slices were placed in the chamber continuously perfused with oxygenated ACSF at a rate of 3 mL/min and heated by an in-line heater (Warner Instruments, Hamden, CT) to maintain temperature at 30°C ± 1°C. Green fluorescent protein-labeled GnRH neurons were identified by brief fluorescent illumination at 488 nm on an upright fluorescence microscope Olympus BX51W1 (Opelco, Dulles, VA).
Electrophysiological recordings
Recording pipettes were pulled from borosilicate capillary glass (type 7052, 1.65-mm outer diameter and 1.12-mm inner diameter; World Precision Instruments, Inc., Sarasota, FL) using a Flaming/Brown P-97 (Sutter Instrument, Novato, CA) to obtain pipettes with a resistance of 2 to 3.5 MΩ when filled with HEPES-buffered solution, containing 150 mM NaCl, 3.5 mM KCl, 10 mM HEPES, 10 mM glucose, 1.3 mM MgCl2, and 2.5 mM CaCl2. Targeted single-cell extracellular recordings were performed with an EPC-8 or EPC-10 dual-patch clamp amplifier (HEKA Elektronik, Holliston, MA) and Patchmaster software (HEKA Elektronik) as data acquisition software. This method does not alter the intracellular milieu of the cell and minimizes interaction between the recording pipette and cell membrane (33, 34). Low resistance seals were made between recording pipette and neuron. Seal resistance was checked every 10 minutes during the recording. Data were excluded if the resistance was >25 MΩ. Recordings were made in voltage-clamp mode at 0 mV holding potential and signal filtered at 10 kHz. Data were obtained from 9 to 12 cells per treatment group, with no more than two cells per mouse; range of firing rate within an animal was similar to that between animals within a group. All recorded neurons were mapped to a brain atlas (35) to determine the relation between anatomical location and response to treatment. No correlation between location of cells and response was observed in this study.
Experimental design
GnRH neurons from OVX+E mice show time-of-day-dependent change in firing activity; the firing rate is low in the morning due to estradiol negative feedback and high in the late afternoon due to estradiol positive feedback (2). To study effects of CRH on GnRH neuron firing activity, recordings were made in late afternoon when mice treated with estradiol exhibit positive feedback (2). After establishing the extracellular recording configuration, recordings were stabilized for 5 to 10 minutes and then spontaneous basal (control) activity was recorded for 5 minutes before treatment. CRH at various doses [0 (i.e., vehicle), 10, 30, 100, or 1000 nM; Bachem, Torrance, CA] was then bath-applied for 5 minutes, followed by a wash period lasting up to 20 minutes to determine if effects were reversible. To study the CRHR subtypes that contribute to effects of CRH on GnRH neuron firing activity, specific agonists were used. The CRHR-1 agonist stressin I (10 nM; Tocris) or the CRHR-2 agonist urocortin (Ucn) III (10 nM; Bachem) were bath-applied to separate sets of brain slices from OVX+E mice in place of CRH in the above paradigm. If no firing activity was observed during the wash out period, ACSF with 20 mM K+ was applied to induce firing to verify cell viability and recording integrity. If a cell did not display action currents in response to high K+ treatment, data from that cell were excluded from the analysis. All drug stocks (CRH, stressin I, Ucn III) were reconstituted in water and diluted at least 1:1000 in ACSF for treatments.
Data analysis
Action currents during targeted-extracellular recording reflect action potential firing. Action currents were detected using custom software written in Igor Pro (Wavemetrics, Lake Oswego, OR). Data were binned at 60-second intervals and mean firing rate (number of action potentials/recording duration) was calculated for control (last 3 minutes of control period), treatment (last 2 minutes of treatment and first minute of wash out period; washout period begins when the intake is switched back to ACSF and treatment exposure continues unaltered for at least another minute before the solution change reaches the chamber), and wash out periods. The wash was divided into sequential 3-minute periods (skipping the first 2 minutes to allow solution exchange) to equalize the duration of recording for analysis. The first wash period in which mean frequency returned to baseline levels was used for analysis; the last wash period was used for cells in which firing rate did not completely recover from treatment. Percent change in mean firing frequency during treatment was calculated relative to that during the control period. Cells were defined as responding if firing frequency during treatment changed by ≥30%; both responding and nonresponding cells were included in statistical analyses for each treatment. In addition to mean firing rate, changes in burst firing characteristics of GnRH neuron were also determined. Groups of action currents (bursts) were identified by custom software in Igor Pro. This software adjusted the maximum time between events (burst window) from 0.01 to 5 seconds at 10-ms intervals. Events are included in a burst if the time interval between events is less than or equal to the burst window. For the present analyses, action currents were included together as a burst if the interval between events was ≤320 ms. This burst window was chosen because it provides the consistent maximum number of bursts detected by the software across the groups in this study. Burst frequency (bursts/3 minutes), burst duration, number of spikes/burst, and intraburst interval were analyzed and compared between control, treatment, and wash out periods.
Statistics
Data are reported as mean ± standard error of the mean (SEM). Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA). Normality of data were analyzed using a Shapiro-Wilk normality test. Two-way repeated-measure analysis of variance (ANOVA) was used to determine effects of CRH 30 and 100 nM on firing activity during control, treatment, and wash out periods of GnRH neurons between OVX and OVX+E mice. Student paired t test was used to analyze the effect of 1 μM CRH on GnRH neurons from OVX mice (no wash period was included for these recordings as the lack of effect was obvious). Effects of CRHR-specific agonists (stressin I and Ucn III) or vehicle on firing activity of GnRH neurons were analyzed by one-way repeated-measure ANOVA for control, treatment, and wash out periods. Burst parameters as defined above were analyzed by two-way repeated-measure ANOVA for control, treatment, and wash out periods. Specific statistical tests are indicated in the figure legends. Significance was set at P < 0.05.
Results
CRH does not affect GnRH neuron firing activity in OVX mice
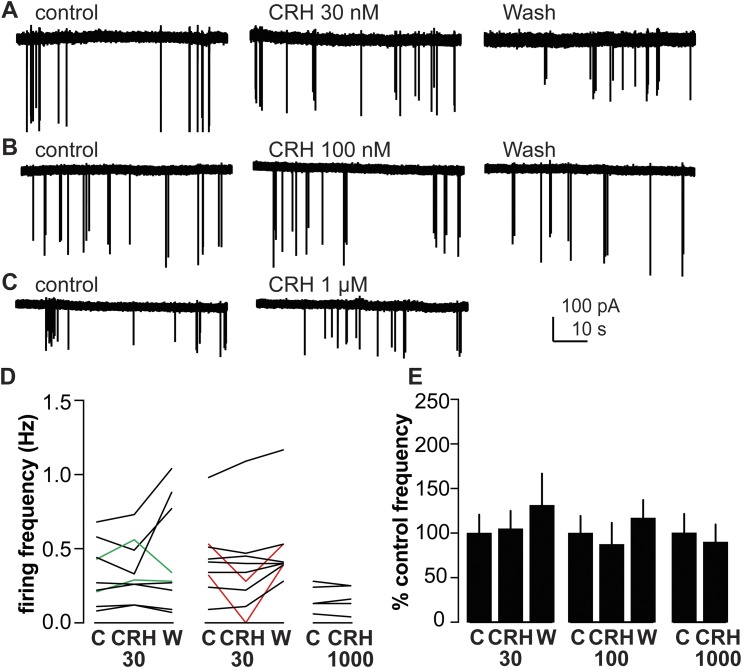
To study the effect of CRH on GnRH neurons in the absence of ovarian estradiol, we tested effects of low (30 nM) and high (100 nM) concentrations of CRH on GnRH neuron firing activity in brain slices from OVX mice. Figures 1A–1C show representative traces from extracellular recording of GnRH neurons from OVX mice before and during treatment with CRH. A minority (two of nine) of GnRH neurons from OVX mice treated with 30 nM CRH exhibited increased firing by the 30% change criteria, but overall this treatment had no effect on firing rate (Fig. 1D and 1E; n = 9; control 0.3 ± 0.1 Hz; CRH 30 nM, 0.4 ± 0.1 Hz; wash 0.4 ± 0.1 Hz; P > 0.9). Similarly, in response to 100 nM CRH, two of nine GnRH neurons from OVX mice exhibited decreased firing, but overall 100 nM CRH had no effect on firing rate (Fig. 1D and 1E; n = 9; control 0.4 ± 0.1 Hz; 100 nM CRH 0.4 ± 0.2 Hz; wash 0.5 ± 0.1 Hz; P > 0.9). To examine if the lack of response in cells from OVX mice was due to insufficient dose, 1000 nM CRH was tested in this group; this dose had no effect on firing frequency of GnRH neurons from OVX mice (Fig. 1D and 1E; n = 5; control 0.2 ± 0.03 Hz; 1 μM CRH 0.2 ± 0.03 Hz; P > 0.8).
Figure 1.
CRH has no effect on the firing rate of GnRH neurons from OVX mice. (A–C) Representative raw data from extracellular recordings of GnRH neurons during each period for (A) 30 nM, (B) 100 nM, and (C) 1000 nM. (D) Firing rate of individual GnRH neurons during control (C), CRH treatment (CRH30, CRH100, or CRH1000), and wash out (W) periods. Green and red lines indicate cells with ≥30% increase or decrease in firing frequency during treatment, respectively. (E) Mean ± SEM percentage of control firing frequency. No differences were detected for either firing frequency [P > 0.5, two-way repeated-measures ANOVA/Tukey (30, 100 nM) or paired Student t test (1000 nM)], or percentage of control frequency (P > 0.1, Friedman test/Dunn post hoc or paired Student t test).
CRH alters firing activity of GnRH neurons from OVX+E mice in a dose-dependent manner
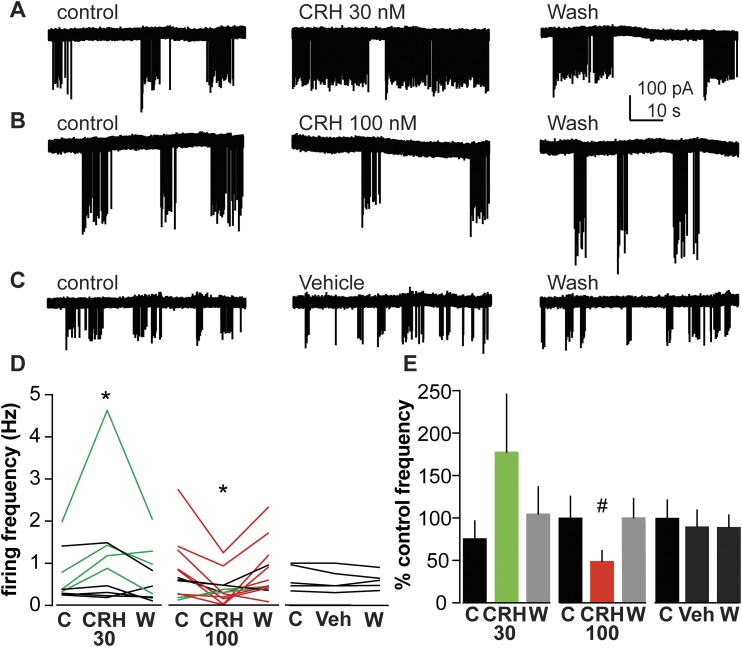
To study if CRH affected GnRH neuron activity in the presence of estradiol, CRH was bath-applied to brain slices from OVX+E mice; no steroids were added during recordings; estradiol was present only in vivo. Representative traces of GnRH neuron firing activity during control, CRH treatment, and wash out periods are shown in Fig. 2A and 2B. In cells from OVX+E mice, 30 nM CRH increased the firing frequency in four of nine cells and increased overall firing rate of the group (Fig. 2D and 2E; n = 9; control 0.7 ± 0.1 Hz; CRH 30 nM 1.2 ± 0.5 Hz; wash 0.7 ± 0.2 Hz; P < 0.05). In marked contrast, 100 nM CRH decreased the firing frequency of 7 of 12 GnRH neurons from OVX+E mice (Fig. 2D and 2E; n = 12; control 0.8 ± 0.2 Hz; CRH 100 nM 0.4 ± 0.1 Hz; wash 0.8 ± 0.2 Hz; P < 0.01). During 100 nM CRH treatment, 2 of 12 cells showed increased in firing frequency, but the overall effect of 100 nM CRH was inhibitory. Because estradiol appeared to induce sensitivity of the GnRH firing response to CRH, we tried a lower CRH dose on slices from OVX+E mice. GnRH neurons did not respond to 10 nM CRH treatment (n = 5; control 0.4 ± 0.2 Hz; CRH 10 nM 0.4 ± 0.2 Hz; P > 0.8). Similarly, vehicle treatment had no effect on GnRH neuron firing frequency (Fig. 2C–2E; n = 5; control 0.6 ± 0.1 Hz; vehicle 0.6 ± 0.1 Hz; P > 0.1). These results suggest that CRH alters GnRH neuron activity in an estradiol-dependent manner with both stimulatory and inhibitory actions observed depending upon dose. Further, the consistent lack of response of cells from OVX mice to CRH and of OVX+E mice to 10 nM CRH or vehicle indicates that the changes attributed to 30 and 100 nM CRH in cells from OVX+E mice are not due to random fluctuations in firing rate of GnRH neurons (2, 36).
Figure 2.
CRH has dose-dependent effects on firing activity of GnRH neurons from OVX+E mice. (A–C) Representative raw data from extracellular recordings of GnRH neurons during each period. (D) Firing rate of individual GnRH neurons during control (C), CRH treatment (CRH30 or CRH100), or vehicle treatment (Veh), and wash out (W) periods. Green and red lines indicate cells with ≥30% increase or decrease in firing frequency during treatment, respectively. *P < 0.05 mean firing frequency compared with control period, two-way repeated-measures ANOVA/Tukey. (E) Mean ± SEM percentage of control firing frequency; gray bars indicate wash after treatment. #P < 0.05 compared with control period, Friedman test/Dunn post hoc.
CRH stimulates GnRH neuron firing activity via CRHR-1
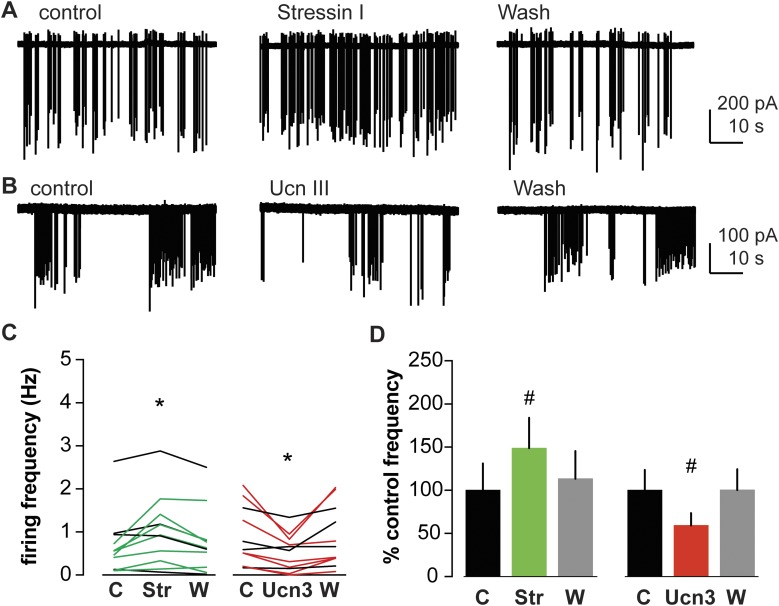
Two types of CRHRs have been identified in mammals: CRHR-1 and CRHR-2 (37, 38). CRHR-1 has a higher affinity for CRH (39). Thus, it is possible that low concentrations of CRH might activate mainly CRHR-1 and lead to stimulation of GnRH neurons, whereas higher concentration of CRH could also bind and activate CRHR-2 and result in a different response. To test if CRH stimulates firing of GnRH neurons via CRHR-1, stressin I (CRHR-1 specific agonist) was bath-applied to brain slices from OVX+E mice and GnRH neuron firing activity recorded. Figure 3A shows representative traces from GnRH neurons in OVX+E before and during stressin I (10 nM) treatment. Stressin I increased firing rate of GnRH neurons in 7 of 11 cells from OVX+E mice and no cells exhibited decreased firing rate (Fig. 3C and 3D; n = 11; control 0.7 ± 0.2 Hz; stressin I 1.0 ± 0.2 Hz; wash 0.8 ± 0.2 Hz; P < 0.05). These data support the postulate that low concentrations of CRH activate GnRH neuron activity via the CRHR-1 pathway.
Figure 3.
Activation of specific CRHRs has different effects on GnRH neuron firing activity. (A and B) Representative raw data from extracellular recordings of GnRH neurons during control (left), CRHR agonist treatment periods (stressin I, top center; Ucn III, bottom center), and wash periods (right). (C) Firing frequency of individual GnRH neurons during control (C), treatment [stressin I (Str) or Ucn3 (Ucn III)], and wash out (W) periods. Green and red lines indicate cells with 30% increase or decrease in firing frequency during treatment, respectively. *P < 0.05 mean firing frequency compared with control period, two-way repeated-measure ANOVA/Tukey. (D) Mean ± SEM percentage of control firing frequency. #P < 0.05 compared with control period, Friedman test/Dunn post hoc.
CRH inhibits GnRH neuron firing activity via CRHR-2
To test if activation of CRHR-2 inhibits GnRH neurons, Ucn III, which has been described as an endogenous ligand specific for CRHR-2 (40), was bath-applied to brain slices from OVX+E mice. Ucn III (10 nM) suppressed firing frequency of GnRH neurons in 7 of 11 cells, and no cells exhibited increased firing (Fig. 3B–3D; n = 11; control 0.9 ± 0.3 Hz; Ucn III 0.5 ± 0.2 Hz; wash 0.9 ± 0.3 Hz; P < 0.05). This suggests that inhibitory effects of CRH on GnRH neuron activity are at least in part mediated through CRHR-2.
Activation of CRHRs alters short-term firing pattern of GnRH neurons
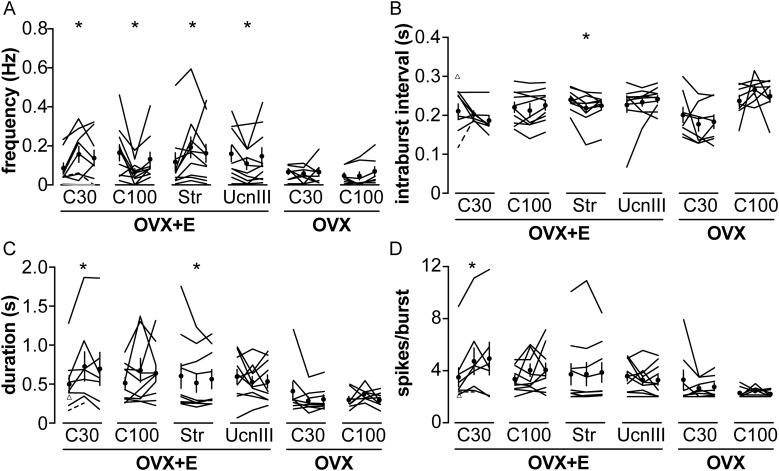
The change in mean firing frequency provides an overview of the effects of CRH on GnRH neuron activity. The short-term pattern of how action currents are grouped together, referred to as burst firing, is also of interest and has been associated with hormone secretion from neuroendocrine cells (41). To further understand the effect of CRH on GnRH neurons, burst firing patterns of the above GnRH neurons from OVX and OVX+E mice were analyzed during control, CRH and CRHR agonist treatments, and wash out periods. All cells were analyzed for burst frequency in all three periods. Of 61 cells studied, 59 cells exhibited bursts during all three periods and were included in the analysis of burst properties. One cell that exhibited bursts only during the control period, and one cell that exhibited bursts during control and treatment periods are shown in Fig. 4 but not included in the repeated-measures analysis for burst properties as they had no bursts to analyze; these cells both fired single spikes throughout the recording. Of note, two-group analysis of control vs treatment and three-group analysis of control, treatment, and washout revealed statistical differences in the same parameters. In GnRH neurons from OVX mice, there was no change in any burst parameter analyzed in response to any treatment. In GnRH neurons from OVX+E mice, however, CRH (30 nM) and stressin I increased burst frequency (Fig. 4A). In contrast, 100 nM CRH and Ucn III decreased burst frequency (Fig. 4A). There was no change in burst duration, spikes/burst, or intraburst interval after 100 nM CRH and Ucn III treatment (Fig. 4B–4D). CRH 30 nM increased both burst duration and spikes/burst (Fig. 4C and 4D). Stressin I also decreased intraburst interval and mean burst duration in GnRH neurons (Fig. 4B and 4C). Although the difference in intraburst interval caused by stressin I treatment achieved a P value indicating statistical significance, the absolute magnitude of the change is small and may have minimal biological effect.
Figure 4.
CRH alters GnRH neuron burst firing pattern in OVX+E mice. (A) Burst frequency of individual GnRH neurons during the control, treatment, and wash periods analyzed in Figs. 1–3. (B) Intraburst interval, (C) burst duration, and (D) spikes/burst of individual GnRH neurons. Values from the same cell are connected by lines. Mean ± SEM are shown as a circle with error bar in (A–D). Triangles in (B–D) indicate control burst parameter values of a cell in the C30 group that had bursts during the control period but not during CRH treatment [gray line in (A)]. Dashed lines in (A–D) connect control and treatment burst parameters of a cell in the C30 group that exhibited bursts during the control and treatment periods but only single spikes during the wash period. *P < 0.05 control vs treatment, two-way repeated-measure ANOVA/Tukey for CRH treatments and Friedman test/Dunn for stressin I and Ucn III. C30, 30 nM CRH; C100, 100 nM CRH; Str, stressin I.
Discussion
GnRH neurons integrate a variety of upstream inputs to produce a secretory output pattern that regulates the downstream reproductive system. Stress exposure can increase or decrease reproductive neuroendocrine output, and response to stress is also modulated by steroid milieu. Here we show that both stimulatory and inhibitory effects of stress can be induced by central interactions among neuroendocrine systems. Specifically, CRH can increase or decrease GnRH neuron firing rate; these effects are estradiol dependent, and the differential response is mediated through activation of specific CRHR.
CRH had a dose-dependent effect on firing rate of GnRH neurons from estradiol-treated OVX mice, with higher concentrations suppressing activity and lower concentrations stimulating it. The effects of CRH on GnRH neurons are mitigated in OVX mice, suggesting estradiol enables the mechanisms that mediate response to CRH. These findings support previous work demonstrating an effect of estradiol on the stress response and extend those studies to effects at the GnRH neuron. At the whole animal level, the inhibitory effect of stress or CRH treatment on LH pulse frequency is stronger in animals with elevated estradiol levels in several species, whether these are due to estradiol treatment or studies in the follicular vs luteal phase of the cycle (8, 11, 13, 21, 25, 26, 42). The mechanisms engaged by estradiol to facilitate the GnRH neuron response to stress are not known but several possibilities exist. First, estradiol may modulate CRHR expression. CRHR is higher during proestrus, when estradiol peaks during the cycle, than in diestrus (43). The CRHR-2 promoter contains a classical estrogen receptor response element (44), which could be a target for regulation of expression. Although GnRH neurons lack estrogen receptor-α, both estrogen receptor-β and membrane estrogen receptors have been reported in GnRH neurons (45, 46). It is thus possible that estradiol acts directly on GnRH neurons to alter CRHR expression in these cells. Second, estradiol alters ionic conductances in GnRH neurons, including voltage-gated potassium and calcium currents, transient receptor potential channels, and hyperpolarization-activated channels (47–52). In other neurons, CRH adjusts excitability by modulating multiple currents (53, 54). Interactions between CRH actions and estradiol-induced changes in intrinsic properties of GnRH neurons could poise these cells to change firing activity. Third, estradiol may facilitate GnRH neuron response by altering synaptic connectivity from afferent populations that are CRH-sensitive. Estradiol alters synaptic interactions in several brain regions, including the hypothalamus (55). Of interest in this regard, kisspeptin stimulation of GnRH neuron firing activity is greater in OVX+E than OVX mice because in the former kisspeptin increases excitatory neurotransmission to GnRH neurons, in addition to direct effects on GnRH neurons observed in both animal models (56, 57). Fourth, estradiol increases CRH mRNA expression in the paraventricular nucleus (PVN) of the hypothalamus in rats (8). This does not apply to the design of the current study because CRH was applied as a treatment, but estradiol upregulation of CRH levels could enhance the effect of stress on the reproductive system in vivo.
The effects of CRH on GnRH neurons observed in this study could be direct and/or indirect. CRH-containing fibers synapse on GnRH neurons, which express CRHR-1, enabling direct action (28–30). Fibers from CRH-producing neurons in the PVN appose GnRH neurons (28). The source of CRH that acts on GnRH neurons remains controversial, however, as retrograde tracing with cholera toxin from the preoptic area revealed only sparse overlap with CRH neurons in the PVN (58). This very sparse PVN CRH input to GnRH neurons may be due to the relative underinnervation of GnRH neurons (59), because direct innervation of GnRH neurons by CRH arises from a different source, and/or because PVN CRH neurons act on GnRH neurons via intermediate cells. Indirectly, GnRH neurons receive numerous upstream inputs and integrate those signals to regulate the downstream reproductive system. Some of these inputs may be CRH-sensitive. Gamma-aminobutyric acid (GABA) and glutamate fast synaptic transmission are the main forms of communication in the brain (60, 61) and GnRH neurons receive both types of fast synaptic inputs (62, 63). Blocking GABAA receptors in the preoptic area in vivo eliminates the ability of CRH to reduce LH pulse frequency (9, 64); because the action of GABA on GnRH neurons is excitatory (65), this suggests receptors on other cells exhibiting the more typical inhibitory response to GABA mediate this response. Stress modifies glutamatergic synapses in other brain regions (66). In addition to fast synaptic transmission, stress may affect neuromodulation of GnRH neurons. In this regard, both the stimulatory kisspeptin system (56, 67, 68), and inhibitory gonadotropin-inhibitory hormone (GnIH) system (69–72) are affected by stress. Kisspeptin neurons both in the arcuate and anteroventral periventricular nucleus regions are reported to express CRHRs (73). Stress exposure or CRH treatment decrease Kiss1 and Kiss1r expression in the hypothalamus (74), possibly reducing excitatory neuromodulation of GnRH neurons via kisspeptin. Although only a small percent (13%) of GnIH neurons express CRH-R1 receptors (75), over half of GnIH neurons have increased Fos expression in response to stress exposure (76); stress also increased the number of appositions from GnIH-immunoreactive fibers on GnRH neurons (76). These changes would potentially enhance effects of this inhibitory system. Action of CRH via GnRH neuron afferents might explain why some GnRH neurons in this study did not respond to CRH treatment; specifically, in some brain slices, upstream cells that are CRH-responsive might be removed during slice preparation.
The dose dependency of the response to CRH suggested the possibility that different CRHRs may mediate these responses. We used receptor-specific agonists to test this and found that activation of CRHR-2 inhibits GnRH neurons, whereas CRHR-1 stimulates GnRH neurons. The involvement of two receptors mediating opposite effects may explain some of the variation observed when CRH itself was used as a treatment. The primary effect of 100 nM CRH was inhibitory, but a couple of cells increased firing in response to this treatment; in contrast, none of GnRH neurons treated with the CRHR-2 specific agonist Ucn III, exhibited stimulation. CRH may activate both receptor subtypes and, in most cases, the inhibitory effects of CRH overpowered the stimulatory effect resulting in suppression of GnRH neuron activity. Similarly, when the specific CRHR-1 agonist was used, a higher percent of GnRH neurons responded than did to 30 nM CRH and all responding cells increased firing rate.
The receptor-specific effects of CRH on GnRH neuron firing may be attributable to signaling pathways engaged upon CRH agonist binding. CRHRs are class-B G-protein coupled receptors that can interact with several Gα subunits (77, 78). CRHR-1 appears to primarily couple to Gαs and Gαq (79, 80). CRHR-2, however, couples with multiple types of G-proteins, including Gαq (80, 81), Gαs (82), and Gαi (80). The type of Gα subunit coupled to CRHR-2, and to some extent CRHR-1, varies among cell types (79). The simplest explanation for the opposite effects of activating CRHR-1 vs CRHR-2 would be direct action on GnRH neurons via Gαs and Gαi subunits. Beyond the current lack of evidence for CRHR-2 expression in GnRH neurons, this explanation does not take into account possible indirect action via afferents that excite or inhibit GnRH neurons. For example, activation of GnRH neuron firing by CRHR-1 agonists may be due to stimulation (via Gαs or Gαq) of an excitatory input. Suppression of GnRH neurons by CRHR-2 agonists may be due to suppression (via Gαi) of an excitatory input or activation (via Gαs and Gαq) of an inhibitory input. Future work to determine the cell signaling pathways in GnRH neurons and their upstream regulators upon activation of CRHRs are needed to test these postulates.
The ability of CRH to both activate and inhibit GnRH neurons could contribute to the different response of the reproductive system to stress. The importance of CRHR-2 in inhibitory effects of stress on fertility has been noted in several studies (20, 27, 83). Pretreatment with a CRHR-2 antagonist prevents stress-induced suppression of LH pulses by metabolic (insulin-induced hypoglycemia), immunological (lipopolysaccharide), and psychological (restraint) stressors (20). Ucn II, which exhibits high affinity for CRHR-2 and low affinity for CRHR-1 similar to Ucn III (40, 84), suppresses LH pulses in female rats in a dose-dependent manner, and blocking of CRHR-2 abolishes the effect of Ucn II on LH pulses (83). Interestingly, expression of mRNA encoding Ucn II in the PVN is increased following restraint stress (85). A role for CRHR-1 in the stimulatory effect of stress on the reproductive system in vivo has also been reported. Exposure to restraint stress on the morning of proestrus induces LH and follicle-stimulating hormone secretion in female proestrous rats (5). This stimulatory effect of restraint stress on LH secretion is blocked by pretreatment of CRHR-1, but not CRHR-2, antagonist suggesting CRHR-1 mediates the stimulatory effect of stress. The present results support and extend these previous findings by demonstrating that activation of CRHR-1 vs CRHR-2 can induce or suppress firing of GnRH neurons, respectively.
Burst firing in neuroendocrine cells is related to the secretion of hormones (41, 86, 87). GnRH neurons exhibit burst firing patterns, although the burst firing in GnRH neurons is slower (88, 89) compared with other neurons (90–92). Treatments that inhibited mean firing rate of GnRH neurons (100 nM CRH or Ucn III) did so by reducing the number of bursts; once initiated, burst duration, number of spikes per burst, and intraburst interval were not different. This may suggest CRHR-2 agonists act indirectly to reduce excitatory drive to GnRH neurons. This would decrease initiation of action potentials and hence number of bursts, but once the burst is initiated, burst characteristics would remain similar because intrinsic properties of GnRH neurons are unaffected. In contrast to CRHR-2, activation of CRHR-1 by 30 nM CRH or stressin I increased burst firing in GnRH neurons. Further, these treatments altered other burst parameters. This suggests a possible direct action via CRHR-1 on GnRH neurons to change intrinsic properties and burst characteristics. Consistent with these postulates and as mentioned above, CRHR-1 mRNA, but not type 2, appears to be expressed in GnRH neurons from female mice (30). Although CRH did not alter mean firing frequency of GnRH neurons from OVX mice, it is possible to alter burst firing without affecting the overall frequency, but we did not observe any change in burst parameters in GnRH neurons from OVX mice during either 30 nM or 100 nM CRH treatments. This emphasizes the importance of estradiol in the response of GnRH neurons to CRH.
The relationship between stress and reproduction varies among studies. The present findings that CRH exerts either stimulatory or inhibitory effects on GnRH neuron activity depending on the CRHR activated provide mechanistic insight into how stress can have different effects. The finding that estradiol potentiates both effects of CRH on GnRH neurons supports and extends previous work indicating steroid milieu alters the response to stress. Together, these observations indicate interactions among the stress and reproductive axes are bidirectional. These findings can help shape future studies of mechanisms that underlie the different responses resulting from stress exposure in animals.
Acknowledgments
We thank Laura L. Burger and Elizabeth R. Wagenmaker for expert technical assistance, and Fred J. Karsch and Elizabeth R. Wagenmaker for comments on the manuscript.
Financial Support: This work was supported in part by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD41469. C.P. was supported by the Anandamahidol Foundation, Thailand.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- CRH
- corticotropin-releasing hormone
- CRHR
- corticotropin-releasing hormone receptor
- CRHR-1
- corticotropin-releasing hormone receptor type-1
- CRHR-2
- corticotropin-releasing hormone receptor type-2
- GABA
- gamma-aminobutyric acid
- GnIH
- gonadotropin-inhibitory hormone
- GnRH
- gonadotropin-releasing hormone
- HPA
- hypothalamic-pituitary-adrenal
- LH
- luteinizing hormone
- mRNA
- messenger RNA
- OVX
- ovariectomized
- OVX+E
- ovariectomized and implanted with an estradiol capsule
- PVN
- paraventricular nucleus
- SEM
- standard error of the mean
- Ucn
- urocortin.
References
- 1.Glanowska KM, Moenter SM. Differential regulation of GnRH secretion in the preoptic area (POA) and the median eminence (ME) in male mice. Endocrinology. 2015;156(1):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102(43):15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roland AV, Moenter SM. Regulation of gonadotropin-releasing hormone neurons by glucose. Trends Endocrinol Metab. 2011;22(11):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology. 2008;149(6):3130–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traslaviña GA, Franci CR. Divergent roles of the CRH receptors in the control of gonadotropin secretion induced by acute restraint stress at proestrus. Endocrinology. 2012;153(10):4838–4848. [DOI] [PubMed] [Google Scholar]
- 6.Tarín JJ, Hamatani T, Cano A. Acute stress may induce ovulation in women. Reprod Biol Endocrinol. 2010;8(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciechanowska M, Łapot M, Antkowiak B, Mateusiak K, Paruszewska E, Malewski T, Paluch M, Przekop F. Effect of short-term and prolonged stress on the biosynthesis of gonadotropin-releasing hormone (GnRH) and GnRH receptor (GnRHR) in the hypothalamus and GnRHR in the pituitary of ewes during various physiological states. Anim Reprod Sci. 2016;174:65–72. [DOI] [PubMed] [Google Scholar]
- 8.Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O’Byrne KT. The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol. 2003;15(5):468–476. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Shao B, Lin C, O’Byrne KT, Lin Y. Stress-induced inhibition of LH pulses in female rats: role of GABA in arcuate nucleus. J Mol Endocrinol. 2015;55(1):9–19. [DOI] [PubMed] [Google Scholar]
- 10.Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56(5):666–673. [DOI] [PubMed] [Google Scholar]
- 11.Norman RL, McGlone J, Smith CJ. Restraint inhibits luteinizing hormone secretion in the follicular phase of the menstrual cycle in rhesus macaques. Biol Reprod. 1994;50(1):16–26. [DOI] [PubMed] [Google Scholar]
- 12.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293(1):E270–E276. [DOI] [PubMed] [Google Scholar]
- 13.Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ. Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav. 2008;54(3):424–434. [DOI] [PubMed] [Google Scholar]
- 14.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150(2):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology. 2007;148(4):1882–1890. [DOI] [PubMed] [Google Scholar]
- 16.Yang JA, Song CI, Hughes J, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS, Breen KM. Acute psychosocial stress inhibits LH pulsatility and Kiss1 neuronal activation in female mice. Endocrinology. 2017. In press. [DOI] [PMC free article] [PubMed]
- 17.Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J Clin Endocrinol Metab. 1988;66(4):733–739. [DOI] [PubMed] [Google Scholar]
- 18.Wagenmaker ER, Moenter SM. Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology. 2017;158(8):2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battaglia DF, Brown ME, Krasa HB, Thrun LA, Viguié C, Karsch FJ. Systemic challenge with endotoxin stimulates corticotropin-releasing hormone and arginine vasopressin secretion into hypophyseal portal blood: coincidence with gonadotropin-releasing hormone suppression. Endocrinology. 1998;139(10):4175–4181. [DOI] [PubMed] [Google Scholar]
- 20.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18(8):602–610. [DOI] [PubMed] [Google Scholar]
- 21.Cates PS, Li XF, O’Byrne KT. The influence of 17beta-oestradiol on corticotrophin-releasing hormone induced suppression of luteinising hormone pulses and the role of CRH in hypoglycaemic stress-induced suppression of pulsatile LH secretion in the female rat. Stress. 2004;7(2):113–118. [DOI] [PubMed] [Google Scholar]
- 22.Chen MD, Ordög T, O’Byrne KT, Goldsmith JR, Connaughton MA, Knobil E. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology. 1996;137(5):2012–2021. [DOI] [PubMed] [Google Scholar]
- 23.Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology. 1990;52(2):133–137. [DOI] [PubMed] [Google Scholar]
- 24.Xiao E, Luckhaus J, Niemann W, Ferin M. Acute inhibition of gonadotropin secretion by corticotropin-releasing hormone in the primate: are the adrenal glands involved? Endocrinology. 1989;124(4):1632–1637. [DOI] [PubMed] [Google Scholar]
- 25.Cagampang FR, Cates PS, Sandhu S, Strutton PH, McGarvey C, Coen CW, O’Byrne KT. Hypoglycaemia-induced inhibition of pulsatile luteinizing hormone secretion in female rats: role of oestradiol, endogenous opioids and the adrenal medulla. J Neuroendocrinol. 1997;9(11):867–872. [DOI] [PubMed] [Google Scholar]
- 26.Cagampang FR, Maeda K, Yokoyama A, Ota K. Effect of food deprivation on the pulsatile LH release in the cycling and ovariectomized female rat. Horm Metab Res. 1990;22(5):269–272. [DOI] [PubMed] [Google Scholar]
- 27.Kinsey-Jones JS, Li XF, Bowe JE, Lightman SL, O’Byrne KT. Corticotrophin-releasing factor type 2 receptor-mediated suppression of gonadotrophin-releasing hormone mRNA expression in GT1-7 cells. Stress. 2006;9(4):215–222. [DOI] [PubMed] [Google Scholar]
- 28.Dudás B, Merchenthaler I. Close juxtapositions between luteinizing hormone-releasing hormone-immunoreactive neurons and corticotropin-releasing factor-immunoreactive axons in the human diencephalon. J Clin Endocrinol Metab. 2002;87(12):5778–5784. [DOI] [PubMed] [Google Scholar]
- 29.MacLusky NJ, Naftolin F, Leranth C. Immunocytochemical evidence for direct synaptic connections between corticotrophin-releasing factor (CRF) and gonadotrophin-releasing hormone (GnRH)-containing neurons in the preoptic area of the rat. Brain Res. 1988;439(1-2):391–395. [DOI] [PubMed] [Google Scholar]
- 30.Jasoni CL, Todman MG, Han SK, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology. 2005;82(5-6):320–328. [DOI] [PubMed] [Google Scholar]
- 31.Ciechanowska M, Łapot M, Malewski T, Mateusiak K, Misztal T, Przekop F. Effects of corticotropin-releasing hormone and its antagonist on the gene expression of gonadotrophin-releasing hormone (GnRH) and GnRH receptor in the hypothalamus and anterior pituitary gland of follicular phase ewes. Reprod Fertil Dev. 2011;23(6):780–787. [DOI] [PubMed] [Google Scholar]
- 32.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–419. [DOI] [PubMed] [Google Scholar]
- 33.Alcami P, Franconville R, Llano I, Marty A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32(9):3118–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G, Franklin KBJ, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 36.Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144(3):823–831. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci USA. 1993;90(19):8967–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA. 1995;92(3):836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrin MH, Sutton SW, Cervini LA, Rivier JE, Vale WW. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther. 1999;288(2):729–734. [PubMed] [Google Scholar]
- 40.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98(13):7570–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290(2):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilbrook AJ, Canny BJ, Serapiglia MD, Ambrose TJ, Clarke IJ. Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. J Endocrinol. 1999;160(3):469–481. [DOI] [PubMed] [Google Scholar]
- 43.Nappi RE, Rivest S. Ovulatory cycle influences the stimulatory effect of stress on the expression of corticotropin-releasing factor receptor messenger ribonucleic acid in the paraventricular nucleus of the female rat hypothalamus. Endocrinology. 1995;136(9):4073–4083. [DOI] [PubMed] [Google Scholar]
- 44.Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW. Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol. 2003;17(3):395–410. [DOI] [PubMed] [Google Scholar]
- 45.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141(9):3506–3509. [DOI] [PubMed] [Google Scholar]
- 46.Terasawa E, Noel SD, Keen KL. Rapid action of oestrogen in luteinising hormone-releasing hormone neurones: the role of GPR30. J Neuroendocrinol. 2009;21(4):316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(10):2255–2265. [DOI] [PubMed] [Google Scholar]
- 48.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30(40):13373–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30(11):3912–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pielecka-Fortuna J, DeFazio RA, Moenter SM. Voltage-gated potassium currents are targets of diurnal changes in estradiol feedback regulation and kisspeptin action on gonadotropin-releasing hormone neurons in mice. Biol Reprod. 2011;85(5):987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29(34):10552–10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C, Kelly MJ, Rønnekleiv OK. 17β-Estradiol rapidly increases K ATP activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151(9):4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kratzer S, Mattusch C, Metzger MW, Dedic N, Noll-Hussong M, Kafitz KW, Eder M, Deussing JM, Holsboer F, Kochs E, Rammes G. Activation of CRH receptor type 1 expressed on glutamatergic neurons increases excitability of CA1 pyramidal neurons by the modulation of voltage-gated ion channels. Front Cell Neurosci. 2013;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Libster AM, Title B, Yarom Y. Corticotropin-releasing factor increases Purkinje neuron excitability by modulating sodium, potassium, and Ih currents. J Neurophysiol. 2015;114(6):3339–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwarz JM, Liang SL, Thompson SM, McCarthy MM. Estradiol induces hypothalamic dendritic spines by enhancing glutamate release: a mechanism for organizational sex differences. Neuron. 2008;58(4):584–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pielecka-Fortuna J, Moenter SM. Kisspeptin increases gamma-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology. 2010;151(1):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hahn JD, Kalamatianos T, Coen CW. Studies on the neuroanatomical basis for stress-induced oestrogen-potentiated suppression of reproductive function: evidence against direct corticotropin-releasing hormone projections to the vicinity of luteinizing hormone-releasing hormone cell bodies in female rats. J Neuroendocrinol. 2003;15(8):732–742. [DOI] [PubMed] [Google Scholar]
- 59.Silverman AJ, Livne I, Witkin JW. The gonadotropin releasing hormone (GnRH) neuronal systems: immunocytochemistry and in situ hybridization In: Knobil E, Neill JD, eds. The Physiology of Reproduction. New York, NY: Raven Press; 1994:1683–1710. [Google Scholar]
- 60.Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302(4):1019–1037. [DOI] [PubMed] [Google Scholar]
- 61.van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250(4985):1276–1278. [DOI] [PubMed] [Google Scholar]
- 62.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27(8):1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod. 2009;80(6):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin YS, Li XF, Shao B, Hu MH, Goundry AL, Jeyaram A, Lightman SL, O’Byrne KT. The role of GABAergic signalling in stress-induced suppression of gonadotrophin-releasing hormone pulse generator frequency in female rats. J Neuroendocrinol. 2012;24(3):477–488. [DOI] [PubMed] [Google Scholar]
- 65.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(12):2872–2891. [DOI] [PubMed] [Google Scholar]
- 66.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28(17):4423–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149(1):268–278. [DOI] [PubMed] [Google Scholar]
- 70.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–667. [DOI] [PubMed] [Google Scholar]
- 71.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–2804. [DOI] [PubMed] [Google Scholar]
- 72.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531(1):40–45. [DOI] [PubMed] [Google Scholar]
- 74.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O’Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21(1):20–29. [DOI] [PubMed] [Google Scholar]
- 75.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA. 2009;106(27):11324–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clarke IJ, Bartolini D, Conductier G, Henry BA. Stress increases gonadotropin inhibitory hormone cell activity and input to GnRH cells in ewes. Endocrinology. 2016;157(11):4339–4350. [DOI] [PubMed] [Google Scholar]
- 77.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10. [DOI] [PubMed] [Google Scholar]
- 78.Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptors signal transduction. Br J Pharmacol. 2012;166:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. [DOI] [PubMed] [Google Scholar]
- 80.Gao L, Tao Y, Hu T, Liu W, Xu C, Liu J, You X, Gu H, Ni X. Regulation of estradiol and progesterone production by CRH-R1 and -R2 is through divergent signaling pathways in cultured human placental trophoblasts. Endocrinology. 2012;153:4918–4928. [DOI] [PubMed] [Google Scholar]
- 81.Kiang JG, Ding XZ, Gist ID, Jones RR, Tsokos GC. Corticotropin-releasing factor induces phosphorylation of phospholipase C-gamma at tyrosine residues via its receptor 2beta in human epidermoid A-431 cells. Eur J Pharmacol. 1998;363:203–210. [DOI] [PubMed] [Google Scholar]
- 82.Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. [DOI] [PubMed] [Google Scholar]
- 83.Li XF, Bowe JE, Lightman SL, O’Byrne KT. Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology. 2005;146(1):318–322. [DOI] [PubMed] [Google Scholar]
- 84.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA. 2001;98(5):2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka Y, Makino S, Noguchi T, Tamura K, Kaneda T, Hashimoto K. Effect of stress and adrenalectomy on urocortin II mRNA expression in the hypothalamic paraventricular nucleus of the rat. Neuroendocrinology. 2003;78(1):1–11. [DOI] [PubMed] [Google Scholar]
- 86.Romanò N, Yip SH, Hodson DJ, Guillou A, Parnaudeau S, Kirk S, Tronche F, Bonnefont X, Le Tissier P, Bunn SJ, Grattan DR, Mollard P, Martin AO. Plasticity of hypothalamic dopamine neurons during lactation results in dissociation of electrical activity and release. J Neurosci. 2013;33(10):4424–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369(1):45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaskins GT, Moenter SM. Orexin a suppresses gonadotropin-releasing hormone (GnRH) neuron activity in the mouse. Endocrinology. 2012;153(8):3850–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu Z, Tomaiuolo M, Bertram R, Moenter SM. Two types of burst firing in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2012;24(7):1065–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith AC, Gerrard JL, Barnes CA, McNaughton BL. Effect of age on burst firing characteristics of rat hippocampal pyramidal cells. Neuroreport. 2000;11(17):3865–3871. [DOI] [PubMed] [Google Scholar]
- 91.Llinás R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297(5865):406–408. [DOI] [PubMed] [Google Scholar]
- 92.Chen L, Deng Y, Luo W, Wang Z, Zeng S. Detection of bursts in neuronal spike trains by the mean inter-spike interval method. Prog Nat Sci. 2009;19(2):229–235. [Google Scholar]