Abstract
Critical windows of development are often more sensitive to endocrine disruption. The murine pituitary gland has two critical windows of development: embryonic gland establishment and neonatal hormone cell expansion. During embryonic development, one environmentally ubiquitous endocrine-disrupting chemical, bisphenol A (BPA), has been shown to alter pituitary development by increasing proliferation and gonadotrope number in females but not males. However, the effects of exposure during the neonatal period have not been examined. Therefore, we dosed pups from postnatal day (PND)0 to PND7 with 0.05, 0.5, and 50 μg/kg/d BPA, environmentally relevant doses, or 50 μg/kg/d estradiol (E2). Mice were collected after dosing at PND7 and at 5 weeks. Dosing mice neonatally with BPA caused sex-specific gene expression changes distinct from those observed with embryonic exposure. At PND7, pituitary Pit1 messenger RNA (mRNA) expression was decreased with BPA 0.05 and 0.5 μg/kg/d in males only. Expression of Pomc mRNA was decreased at 0.5 μg/kg/d BPA in males and at 0.5 and 50 μg/kg/d BPA in females. Similarly, E2 decreased Pomc mRNA in both males and females. However, no noticeable corresponding changes were found in protein expression. Both E2 and BPA suppressed Pomc mRNA in pituitary organ cultures; this repression appeared to be mediated by estrogen receptor-α and estrogen receptor-β in females and G protein–coupled estrogen receptor in males, as determined by estrogen receptor subtype-selective agonists. These data demonstrated that BPA exposure during neonatal pituitary development has unique sex-specific effects on gene expression and that Pomc repression in males and females can occur through different mechanisms.
Neonatal BPA exposure alters pituitary Pit1 and Pomc mRNA levels sex specifically. Furthermore, using estrogen receptor–selective agonists, we showed how Pomc can be regulated at the pituitary level.
The pituitary is the master gland of the endocrine system, releasing hormones that control reproduction, lactation, growth, metabolism, and stress. To release these hormones, the anterior pituitary gland must develop five hormone-secreting cell types: gonadotropes, lactotropes, somatotropes, thyrotropes, and corticotropes. Development of the hormone-secreting cell types occurs during two key periods in mouse development: embryonic and neonatal. During embryonic development, cell specification is regulated by intrinsic and paracrine signals from the pituitary and surrounding tissue (1). However, during the neonatal period, the capability for communication between the hypothalamus, pituitary gland, and target organs develops (2, 3), suggesting a potential role for axis hormones in this window of development. Additionally, at birth, the testosterone surge establishes sex differences in the hypothalamic–pituitary–gonadal (HPG) and the hypothalamic–pituitary–adrenal (HPA) axes through local aromatization of testosterone to estradiol in the male brain (4, 5). Thus, the endocrine milieu postnatally can alter pituitary sensitivity to exposure to endocrine-disrupting chemicals (EDCs).
Bisphenol A (BPA) is an EDC found in several items such as polycarbonate plastics, epoxy resins, and thermal paper, leading to ubiquitous exposure (6). Studies have suggested that the developing organs of fetuses and neonates have heightened sensitivity to EDCs such as diethylstilbestrol and BPA in both rodents and humans (7–9). BPA is readily detectible in fetal cord serum, maternal serum, and fetal amniotic fluid in humans (10), demonstrating exposure to BPA during critical developmental windows. A possible mechanism by which BPA is considered to act is by disrupting the actions of estrogens. The classic estrogen receptors estrogen receptor-α (ESR1) and estrogen receptor-β (ESR2) and the membrane G protein–coupled estrogen receptor (GPER) are found in the hypothalamus and pituitary gland (11–15). However, the roles of estrogen signaling and the potential effects of BPA during neonatal pituitary development are unexplored.
Developmental exposure to environmentally relevant doses of BPA at or less than the no observed adverse effect level can affect every axis of the endocrine system regulated by the pituitary gland, including the reproductive axis (16–20) in rats and mice and the prolactin levels (21), growth axis (22), thyroid axis (23), and stress axis (24) in rats. BPA also alters sex differences of the HPG and HPA axes established by sex steroids (24–27). Despite the relatively quick metabolism of BPA (28), changes can sometimes occur well after exposure has ended. For example, embryonic exposure to 25 to 250 ng/kg/d BPA led to reproductive tissue weight changes in adult female mice and changes in estrogen and progesterone receptor expression (9). Also, neonatal exposure to 50 μg/kg/d to 50 mg/kg/d BPA had different outcomes on the rat hypothalamus at 2 days after exposure than at 8 days after exposure (27), indicating the importance of examinations at multiple points after exposure. Therefore, it is clear that developmental exposure to BPA can influence sex differences and the endocrine axes, although regulation could be time and/or context specific. Despite this evidence, few studies have examined closely the effects of neonatal BPA exposure on the development and expression of genes in the pituitary gland itself.
Previously, our laboratory showed that embryonic exposure of pregnant mice to 0.5 or 50 μg/kg/d BPA increased pituitary proliferation and gonadotrope cell numbers in female offspring (29), a response similar to that of estradiol (E2) (30). However, the effects of neonatal exposure on the second wave of pituitary development are unknown. We hypothesized that BPA exposure would have different effects on the pituitary gland, depending on sex, the developmental period of exposure, and the time of examination after exposure. Additionally, we hypothesized that some of these effects might be similar to that of E2. To test this hypothesis, two experiments were performed. First, we examined pituitary glands at two time points after exposure neonatally to three environmentally relevant doses of BPA and E2 and compared the effects in males and females. Second, we treated isolated pituitary glands in culture with E2, BPA, and estrogen receptor subtype-specific agonists to determine the direct effects on the pituitary gland and an estrogen receptor pathway important in gene regulation.
Materials and Methods
Mice
CD-1 mice, obtained from Charles River, were bred in-house, kept in polysulfone cages containing corn cob bedding material, and fed Teklad 8664 rodent chow (Envigo) and water ad libitum through glass bottles. Sex was confirmed by visual inspection, SRY genotyping as previously described (31), or gonadal removal. The University of Illinois Urbana-Champaign institutional animal care and use committee approved all procedures.
Experiment 1 design: effects of neonatal dosing
In the first experiment, we examined the effects of dosing male and female mice during the critical neonatal period of pituitary development. CD-1 litters were culled to 8 to 12 pups on the day of birth, and the neonatal pups were dosed orally through consumption from a pipet tip, once daily, from postnatal day (PND)0 to PND7 with control (0.1% ethyl alcohol), 0.05, 0.5, or 50 μg/kg/d BPA [BPA0.05, BPA0.5, and BPA50, respectively; Sigma-Aldrich; purity, ≥99%; Chemical Abstracts Service (CAS) no. 80-05-7], or 50 μg/kg/d E2 (17β-estradiol; Tocris; purity, >99%; CAS no. 50-28-2) dissolved in tocopherol-stripped corn oil (MP Biomedicals). The litters were randomly assigned to a treatment group. The 0.05- and 0.5-μg/kg/d doses of BPA were chosen because they are within the range of human exposure levels (32). The 50-μg/kg/d dose of BPA was chosen because it is the oral reference dose for BPA (33). The 50-μg/kg/d dose of E2 was chosen because it was a dose sufficient to induce expression of estrogen-regulated genes in our system (31). All pups in a litter were dosed with a single compound, and six separate litters were dosed with each compound. One male and one female were taken from each litter at each measurement point for each assay between 10 and 11 am.
Part A: on neonatal pituitary development
Part A of the experiment examined the immediate effects of exposure to BPA and E2 during the neonatal period. The mice were euthanized on PND7, 1 hour after the final dosing. For western blot analysis, an additional dosing of each compound was performed, and all pups were collected at PND7 for western blot analysis.
Part B: at 5 weeks
Part B of the experiment examined whether neonatal dosing would influence the pituitary outside the first postnatal rapid growth phase, which ends at 3 weeks of age (34). The 5-week-old siblings of the mice examined at PND7 were collected for RNA analysis. One male and female were analyzed from each litter, if possible. Because of the sex distribution, it was not always possible to have the number equal six. The estrous cycles were monitored beginning at 5 weeks by vaginal smear (35), and the mice were euthanized when in estrus. Female mice were euthanized in estrus because neonatal E2 treatment caused persistent estrus.
Measurements, weights, and puberty assessment
At PND7 and 5 weeks, the weights and measurements were recorded to determine whether any gross systemic effects of BPA or E2 exposure had occurred. The wet weight for the testes, ovaries, uteri, and liver were all compared with the body weight. The anogenital distance was measured using calipers and compared with the body weight. Puberty measurements were observed, stratified by the day of vaginal opening for females and preputial separation for males.
Experiment 2 design: pituitary cultures
PND1 pituitary glands were cultured overnight on Millicell CM membranes (Millipore), as previously described (31). The next day, the media were replaced with media containing the following compounds alone or in combination: 10−8 M pyrazole triol (PPT; Tocris; purity, >99%; CAS no. 263717-53-9), 10−8 M diarylpropionitrile (DPN; Tocris; purity, >99%; CAS no. 1428-67-7), 10−8 M E2 (Tocris; purity, >99%; CAS no. 50-28-2), 4.4 × 10−5 M BPA (Sigma-Aldrich; purity, ≥99%; CAS no. 80-05-7), 10−6 M G1 (Cayman; purity, >98%; CAS no. 881639-98-1), or vehicle control consisting of 0.1% ethanol for treatment with a single compound, 0.2% ethanol for cotreatment, or 0.1% dimethyl sulfoxide for G1 treatment. The pituitary glands were also treated with an estrogen dendrimer conjugate (10−9 M) or empty dendrimer conjugate (dendrimer control; 10−9 M) (36, 37). For the cotreatment experiments with PPT and DPN, 0.2% ethanol vehicle was added to the dendrimer control. The pituitary glands were treated for 48 hours before collection (n = 6 to 13).
Quantitative reverse transcription polymerase chain reaction
For RNA analysis, males and females were analyzed separately (n = 3 to 6). RNA was processed, as previously described (38). The expression levels of the genes of interest were normalized to Actb messenger RNA (mRNA) levels, with the primer sequences of forward 5′ to 3′: GACATGGAGAAGATCTGGCA and reverse 5′ to 3′: GGTCTCAAACATGATCTGGGT (Life Technologies). Gene expression for Mki67, Lhb, Nr5a1, Tshb, Gh, and Prl was analyzed using primer sequences previously reported (29) and Pit1 and Tpit previously reported (38). Finally, the Pomc sequences used were forward 5′ to 3′: GATAGCGGGAGAGAAAGCCG and reverse 5′ to 3′: GGGACCCCGTCCTGTCCTAT. The data were analyzed using the standard comparative change in threshold cycle(ΔCt) value method, as previously described (39). For in vivo analysis, individual points were graphed, with the sex indicated and averages shown as a line. For pituitary cultures, bar graphs representing the data were prepared.
Immunohistochemistry
Pituitary glands were collected at PND7 from the exposed mice and fixed in 3.7% paraformaldehyde for 1 hour, cryoprotected in 30% sucrose/phosphate-buffered saline (PBS) solution, and frozen in optimal cutting temperature compound (Electron Microscopy Sciences). The pituitary glands were sectioned into 10-μm slices before being mounted onto Superfrost plus slides (Fisher Scientific).
Before antibody treatments, frozen sections were thawed for 10 minutes, fixed in 4% paraformaldehyde diluted in PBS, and blocked using 5% normal donkey serum diluted in an immunohistochemical (IHC) block, which had 3% bovine serum albumin and 0.5% TritonX-100 diluted in PBS. The slides were then treated with the following primary antibodies overnight at 4°C: LHβ (luteinizing hormone-β; National Hormone and Peptide Program), phosphorylated histone H3 (Millipore), PIT1 (gift from Simon Rhodes), and POMC (Dako). After a series of PBS washes, the slides were treated with biotin-conjugated rabbit secondary antibody (Jackson ImmunoResearch) diluted 1:200 in an IHC block for 60 minutes at room temperature. After another series of PBS washes, the slides were treated with streptavidin-conjugated cy3 tertiary antibody (Jackson ImmunoResearch) diluted 1:200 using an IHC block. Control slides were prepared that did not include the primary antibody.
All slides were counterstained with 4′,6-diamidino-2-phenylindole (1:1000; Sigma-Aldrich) and visualized under a Leica DM2560 microscope. Photographs were taken using a Retiga 2000R camera (Q-Imaging) and acquired using Q-Capture Pro software (Q-Imaging). Images were processed using Adobe Photoshop CS2.
Cell counting
POMC cell counts were performed using National Institutes of Health ImageJ. For each dose (0.05, 0.5, and 50 µg/kg/d BPA, and control) four to five pituitary glands were examined, with three to four slides per pituitary. The percentage of POMC-positive cell numbers was determined by comparing the number of POMC immunoreactive cells per 4′,6-diamidino-2-phenylindole –stained nuclei in a defined area. The sections on each slide, and all the slides per animal (3), were averaged together for the mean percentage of positive POMC cells for each mouse.
Western blot
Three pituitary glands were frozen on dry ice and lysed using radioimmunoprecipitation assay buffer. Protein fractions were obtained by centrifugation for 5 minutes at 4°C at 20,817 relative centrifugal force. The total protein concentration was measured via a BCA protein assay kit (Thermo Fisher) according to the manufacturer’s instructions. Protein samples (20 μg) were mixed with radioimmunoprecipitation assay buffer and 3× Laemmli loading dye and then heated for 12 minutes at 57°C. The samples were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane (BioRad). The membrane was blocked for 2 hours in 5% nonfat dried milk in Tris-buffered saline and incubated with either PIT1 antibody (rabbit; 1:1000; gift from Dr. Simon Rhodes), POMC antibody (rabbit; 1:250; Dako), β-actin antibody (rabbit; 1:1000; Cell Signaling), or α-tubulin antibody (mouse; 1:5000; Sigma-Aldrich) in 1% milk with 0.2% Tween, overnight at 4°C. Secondary goat anti-rabbit (1:500) or goat anti-mouse (1:5000) IgG conjugated to IRdye 800CW (LiCor) was added at room temperature for 1 hour. The membrane was then imaged with the Odyssey IR imaging system (LiCor). The relative protein levels were analyzed using National Institutes of Health ImageJ.
Statistical analysis
Statistical significance among the controls, three BPA treatment groups, and E2 was determined using one-way analysis of variance within sex. Within sex was used because sex differences were expected in some gene expression levels between the control males and females (31). Only one pituitary gland of each sex from each litter was used in the quantitative polymerase chain reaction (PCR) analysis; thus, litter was not considered as a covariate. If significance was found, the mean fold change between groups was compared using the Tukey honest significant difference test in Microsoft Excel for both quantitative reverse transcription PCR and cell counting. For quantitative reverse transcription PCR of pituitary explant culture experiments, significance was determined using a two-tailed t test. P values of ≤ 0.05 were considered statistically significant.
Results
Experiment 1: neonatal dosing caused few changes in gross appearance of mice
Previous studies have demonstrated that the embryonic pituitary gland is sensitive to BPA exposure. Therefore, we sought to examine the effects of BPA exposure specifically during neonatal development. Organ weights and measurements were taken to determine whether any gross effects were present. The testes, ovaries, uteri, and liver weights are provided as a percentage of body weight (Table 1). At PND7, the lowest dose of BPA decreased the ovary weight compared with the control, and E2 had increased the uterine weight compared with the control. At 5 weeks, the same weights and measurements were gathered. No statistically significant differences were found in the body weights or measurements between any of the treatments at the 5-week point (Table 2).
Table 1.
PND7 Wet Weights and Measurements
| Variable |
Weight, g |
Testes, %BW | Ovaries, %BW | Uteri, %BW | Liver, %BW |
AGD, mm/gBW |
||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Control | 4.725 | 4.417 | 0.175 | 0.049 | 0.093 | 3.118 | 1.14 | 0.91 |
| BPA0.05 | 4.217 | 3.995 | 0.177 | 0.038a | 0.094 | 3.012 | 1.16 | 0.82 |
| BPA0.5 | 4.611 | 4.250 | 0.170 | 0.043 | 0.096 | 3.137 | 1.10 | 0.83 |
| BPA50 | 4.606 | 4.483 | 0.179 | 0.044 | 0.104 | 3.201 | 1.20 | 0.82 |
| E2 | 4.683 | 4.555 | 0.183 | 0.057 | 0.195a | 3.163 | 1.15 | 0.82 |
Abbreviations: AGD, anogenital distance; gBW, grams of body weight; %BW, percentage of body weight.
Table 2.
Five-Week Wet Weights and Measurements
| Variable |
Weight, g |
Testes, %BW | Ovaries, %BW | Uteri, %BW | Liver, %BW |
AGD, mm/gBW |
||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||
| Control | 27.446 | 23.517 | 0.499 | 0.092 | 0.792 | 6.470 | 0.47 | 0.28 |
| BPA0.05 | 26.950 | 22.350 | 0.506 | 0.112 | 0.937 | 6.570 | 0.50 | 0.29 |
| BPA0.5 | 27.813 | 23.083 | 0.509 | 0.095 | 0.689 | 5.373 | 0.49 | 0.30 |
| BPA50 | 29.743 | 23.136 | 0.505 | 0.110 | 0.760 | 6.309 | 0.51 | 0.31 |
| E2 | 27.111 | 24.675 | 0.573 | 0.080 | 0.598 | 6.842 | 0.48 | 0.28 |
Abbreviations: AGD, anogenital distance; gBW, grams of body weight; %BW, percentage of body weight.
Experiment 1: neonatal exposure to BPA did not affect pituitary proliferation
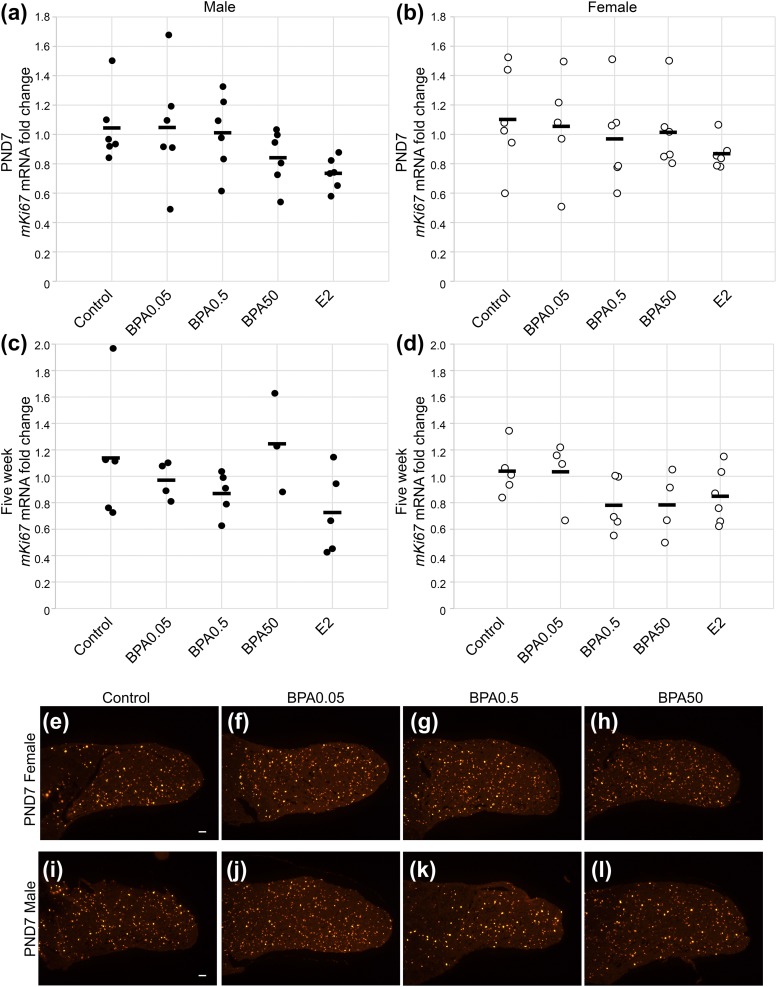
Exposure to BPA or E2 during the neonatal period had no statistically significant effect on pituitary proliferation at PND7, as measured by Mki67 mRNA levels, in males [Fig. 1(a); F(4, 25) = 1.82; P = 0.16) and females [Fig. 1(b); F(4, 25) = 0.74; P = 0.57]. Immunohistochemistry for the mitosis marker phosphorylated histone H3 in the female [Fig. 1(e)–1(h)] and male [Fig. 1(i)–1(l)] pituitary glands at PND7 revealed no obvious change in the distribution of cells stained in response to exposure to BPA. We next examined the proliferation at 5 weeks to determine whether an effect of neonatal BPA exposure would be present at a later time point. The Mki67 mRNA levels were not significantly altered statistically by BPA or E2 in the males [Fig. 1(c); F(4, 17) = 1.66; P = 0.21] or females [Fig. 1(d); F(4, 19) = 1.61; P = 0.21] at 5 weeks.
Figure 1.
Proliferation was not changed by neonatal exposure to BPA or E2. Proliferation was measured by Mki67 mRNA levels in (a) males and (b) females at PND7, demonstrating no statistically significant change in proliferation. Also, at 5 weeks, no statistically significant change was observed in proliferation in (c) males or (d) females (n = 3 to 6 for each sex). Circles represent individual mice, and average is represented by a line. Proliferation, as assessed by phosphorylated histone H3 expression in sections of PND7 females, was similar among (e) control-, (f) BPA0.05-, (g) BPA0.5-, and (h) BPA50-treated pituitary glands. Proliferation in male pituitary glands was also similar among (i) control, (j) BPA0.05, (k) BPA0.5, and (l) BPA50 exposure (n = 3 to 4 for each sex and each condition).
Experiment 1: neonatal BPA exposure did not affect gonadotrope lineage gene expression
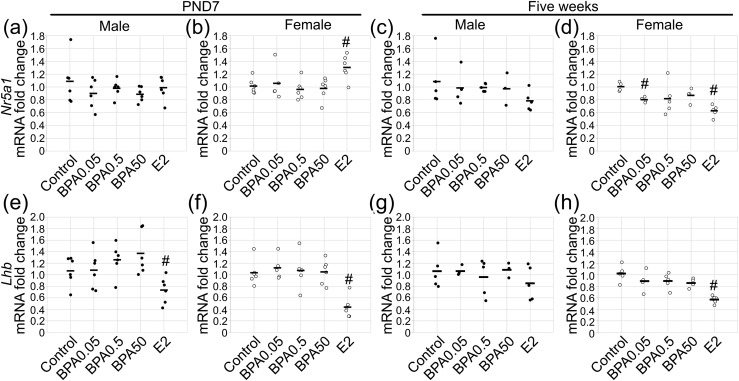
To examine whether BPA exposure during the neonatal period affected the gonadotrope lineage, the mRNA expression levels of the gonadotrope lineage transcription factor Nr5a1 were examined. No statistically significant effect of exposure on Nr5a1 mRNA levels was found in males at PND7 [Fig. 2(a); F(4, 25) = 0.81; P = 0.53). In females, a statistically significant effect of exposure was found on Nr5a1 mRNA levels [Fig. 2(b); F(4, 25) = 2.85; P ≤ 0.04]. An increase in Nr5a1 with E2 treatment was found (P ≤ 0.01). To explore whether a difference in Nr5a1 mRNA levels would exist later, we examined the pituitary glands at 5 weeks. In these mice, the males showed no statistically significant main effect of treatment on Nr5a1 mRNA levels [Fig. 2(c); F(4, 17) = 0.94; P = 0.47]. However, in females, an effect of treatment was seen [F(4, 18) = 4.92; P ≤ 0.007], and the Nr5a1 mRNA levels were decreased with the BPA0.05 and E2 exposures [Fig. 2(d); P ≤ 0.002 and P ≤ 7 × 10−5].
Figure 2.
Gonadotrope markers were unchanged by neonatal BPA exposure, despite regulation by E2. Gonadotrope marker Nr5a1 mRNA was measured in (a) males and (b) females at PND7, with no response to BPA. At 5 weeks, no effect of BPA or E2 was found in (c) males. (d) However, suppression of Nr5a1 mRNA expression was found in females with exposure to BPA0.05 and E2. Lhb mRNA was decreased by E2 in (e) males and (f) females at PND7 but (e) was unchanged by BPA. (g) At 5 weeks, Lhb was unaltered by any treatment group in males. (h) In females at 5 weeks, E2 caused substantial repression of Lhb mRNA. For PND7, n = 5 to 6; for 5 weeks, n = 3 to 6. Circles represent individual mice, and average is represented by a line. #P ≤ 0.05.
To further explore the gonadotrope lineage during neonatal exposure to E2 or BPA, Lhb mRNA expression was analyzed. With neonatal dosing, a statistically significant effect of exposure on Lhb mRNA levels was found in males [F(4, 25) = 4.04; P ≤ 0.01] and females [F(4, 25) = 9.70; P ≤ 7 × 10−5]. BPA exposure did not statistically significantly alter Lhb mRNA levels. However, E2 significantly decreased the levels of Lhb mRNA in both males [Fig. 2(e); P ≤ 0.04] and females [Fig. 2(f); P ≤ 0.0005] at PND7. Immunohistochemistry for LHβ did not reveal any obvious differences in the BPA treatment groups in females (Supplemental Fig. 1 (4MB, tif) ). Additionally, mRNA levels for the common α-subunit Cga were examined at PND7 and were not statistically significantly affected by exposure in males [F(4, 24) = 0.78; P = 0.55] or females [F(4, 24) = 0.40; P = 0.81]. We next examined the 5-week-old mice. For the Lhb mRNA levels, no statistically significant effect of BPA or E2 exposure was found in males [Fig. 2(g); F(4, 17) = 0.78; P = 0.55]. A statistically significant effect of exposure was found for females [Fig. 2(h); F(4, 19) = 11.61; P ≤ 6 × 10−5]. No statistically significant difference was found in Lhb mRNA with BPA exposure; however, suppression of Lhb mRNA occurred with E2 exposure [Fig. 2(h); P ≤ 6 × 10−5]. Despite finding effects on the gonadotrope lineage with E2 treatment, no statistically significant effects were found on the timing of puberty, as assessed by preputial separation or vaginal opening with E2 or BPA treatment (Table 3).
Table 3.
Puberty Measurements
| Variable | Preputial Separation (d) | Vaginal Opening (d) |
|---|---|---|
| Control | 23.695 | 22.514 |
| BPA0.05 | 23.700 | 23.533 |
| BPA0.5 | 24.222 | 23.167 |
| BPA50 | 24.104 | 23.422 |
| E2 | 25.780 | 21.667 |
Day (d) of preputial separation and vaginal opening were monitored and averaged for males (n = 3 to 5) and females (n = 4 to 6).
Experiment 1: low levels of BPA exposure decreased Pit1 mRNA in males only, without altering hormone transcript levels
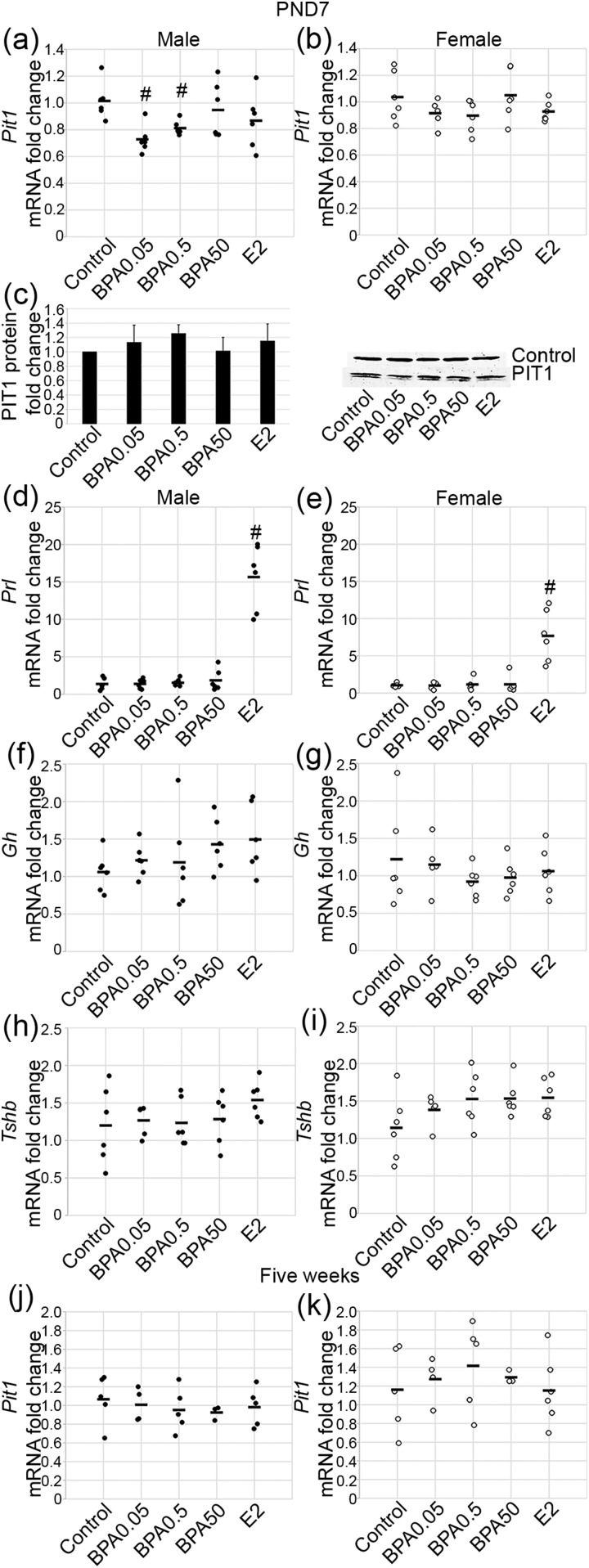
PIT1 is a transcription factor found exclusively in the pituitary gland and is important in the development of lactotropes, somatotropes, and thyrotropes and expression of their respective hormones. A statistically significant effect of exposure on Pit1 mRNA levels was found in males [Fig. 3(a); F(4, 25) = 3.23; P ≤ 0.03]. The lowest doses of BPA, BPA0.05 and BPA0.5, decreased the levels of Pit1 mRNA (P ≤ 0.002 and P ≤ 0.007, respectively); however, BPA50 and E2 had no statistically significant effect on Pit1 mRNA during this period. In females, exposure did not have a statistically significant effect on Pit1 mRNA [Fig. 3(b); F(4, 25) = 1.84; P = 0.15]. To determine whether the decrease in Pit1 mRNA in males would correspond with a decrease in protein, western blot analysis was performed. No statistically significant change in PIT1 protein was seen with any treatment group in the males at PND7 [Fig. 3(c)]. Additionally, immunohistochemistry for PIT1 did not reveal any obvious differences in the treatment groups in the males (Supplemental Fig. 1 (4MB, tif) ). PIT1 is a transcriptional regulator of Gh, Prl, and Tshb. Consequently, we examined mRNA expression of these hormones to determine whether BPA-mediated repression of Pit1 mRNA resulted in downstream effects on hormone mRNA expression. A statistically significant effect of exposure was found for Prl mRNA levels in males [F(4, 25) = 53.86; P ≤ 6 × 10−12] and females [F(4, 25) = 17.81; P ≤ 1 × 10−6]. BPA had no substantial effect on Prl mRNA; however, the increase in Prl mRNA with E2 was statistically significant in males [Fig. 3(d); P ≤ 1 × 10−5] and females [Fig. 3(e); P ≤ 0.0002]. Neither BPA nor E2 had any statistically significant effect on Gh mRNA levels in males [Fig. 3(f); F(4, 25) = 1.17; P = 0.35] or females [Fig. 3(g); F(4, 25) = 0.68; P = 0.61]. BPA and E2 also had no statistically significant effects on Tshb mRNA levels in males [Fig. 3(h); F(4, 24) = 0.92; P = 0.47] or females [Fig. 3(i); F(4, 24) = 1.76; P = 0.17]. Finally, to determine whether the decrease in Pit1 mRNA seen in males at PND7 would be present after removal of BPA, we examined the 5-week-old mice. No statistically significant effect of exposure was found at 5 weeks in the males [Fig. 3(j); F(4, 17) = 0.28; P = 0.89] or females [Fig. 3(k); F(4, 18) = 0.42; P = 0.79].
Figure 3.
BPA suppressed Pit1 mRNA at low doses in males but did not alter transcription of PIT1 lineage hormones. Pit1 mRNA was decreased at PND7 with 0.05 and 0.5 μg/kg/d BPA in (a) males but not (b) females. (c) However, PIT1 protein was not changed by BPA or E2 in males by western blot analysis. Prl mRNA was not altered by BPA but was increased with E2 in (d) males and (e) females. No difference was seen in Gh mRNA with any treatment in (f) males or (g) females. Also, no difference in Tshb mRNA with BPA or E2 exposure in (h) males or (i) females was detected. Pit1 mRNA at 5 weeks was not changed in (j) males or (k) females. For quantitative PCR, n = 3 to 6; for PND7 western blot, n = 3. Circles represent individual mice, and average is represented by a line. #P ≤ 0.05.
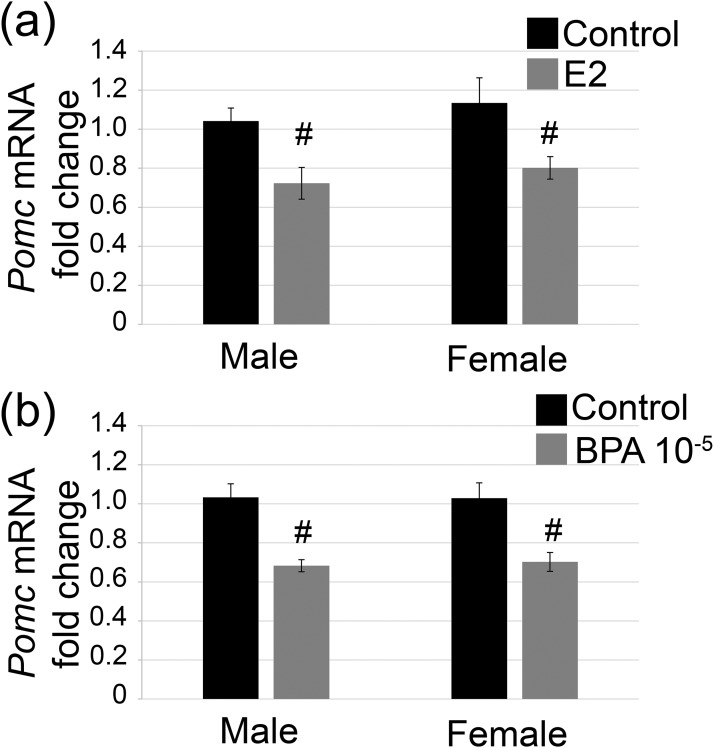
Experiment 1: BPA decreased Pomc mRNA in a dose- and sex-specific manner
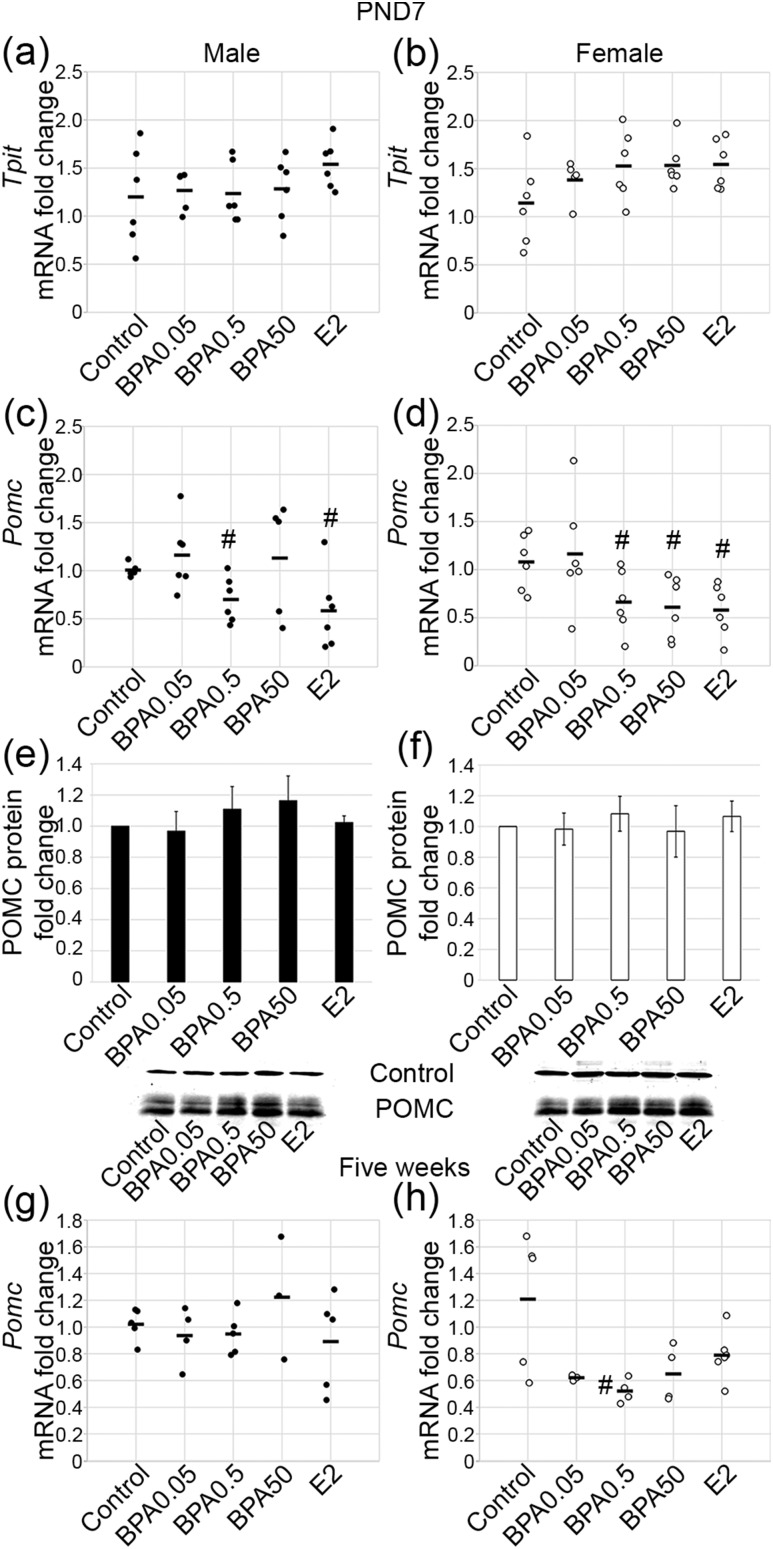
The last lineage examined was the TPIT lineage, which includes the corticotrope and melanotrope cells. At PND7, no statistically significant effect of BPA or E2 exposure was found on Tpit mRNA levels in males [Fig. 4(a); F(4, 25) = 0.29; P = 0.88] or females [Fig. 4(b); F(4, 25) = 0.74; P = 0.57]. A statistically significant effect of exposure on Pomc mRNA levels was observed in males [F(4, 24) = 4.01, P ≤ 0.01] and females [F(4, 25) = 3.31, P ≤ 0.03]. The middle dose of BPA, BPA0.5, and E2 both decreased Pomc mRNA in males [Fig. 4(c); P ≤ 0.02; P ≤ 0.01]. In contrast, exposure to the lowest dose of BPA, BPA0.05, and the highest dose, BPA50, showed no statistically significant change in Pomc mRNA [Fig. 4(c)]. In females, Pomc mRNA was reduced with exposure to BPA0.5, BPA50, and E2 [Fig. 4(d); P ≤ 0.04, P ≤ 0.02, and P ≤ 0.01, respectively]. No statistically significant change in Pomc mRNA was observed with the BPA0.05 dose. However, POMC immunostaining and corticotrope number appeared unaffected by exposure in females (Supplemental Fig. 1 (4MB, tif) ). Next, intrapituitary POMC protein levels were examined by western blot analysis in both males [Fig. 4(e)] and females [Fig. 4(f)], and no statistically significant changes in protein levels were detected in either sex. Next, Pomc mRNA levels were assessed at 5 weeks. No statistically significant changes in Pomc mRNA were observed in males [Fig. 4(g); F(4, 17) = 0.81; P = 0.53]. In females, a statistically significant effect of treatment was seen [F(4, 17) = 4.18; P ≤ 0.02]. BPA0.5-treated females had lower Pomc mRNA levels compared with the levels in the control females [Fig. 4(h); P ≤ 0.03]. Those treated with BPA0.05 (P = 0.10), BPA50 (P = 0.08), and E2 (P = 0.09) had levels approaching statistical significance.
Figure 4.
Pomc mRNA was suppressed by BPA and E2 neonatal exposure. Tpit mRNA was unchanged by BPA or E2 at PND7 in (a) males and (b) females. Pomc mRNA was (c) decreased with 0.5 μg/kg/d BPA and E2 at PND7 in males and (d) decreased with 0.5 μg/kg/d BPA, 50 μg/kg/d BPA, and E2 in females. POMC protein was unchanged by BPA in (e) males and (f) females. (g) Pomc mRNA was not different with treatment at 5 weeks in males. (h) However, a persistent decrease was seen in females treated with 0.5 μg/kg/d BPA. For quantitative PCR, n = 3 to 6; for PND7 western blot, n = 3. Circles represent individual mice, and average of combined sexes is represented by a line. #P ≤ 0.05.
Experiment 2: E2 and BPA decrease Pomc mRNA directly at the pituitary
Owing to the striking suppression of Pomc mRNA in vivo with both E2 and BPA, we sought to determine whether these changes resulted from direct effects at the level of the pituitary. PND1 pituitary glands were placed in culture and treated with either E2 (10−8 M) or BPA (4.4 × 10−5 M). In both males and females, E2 decreased Pomc mRNA levels directly at the pituitary [Fig. 5(a)]. Next, pituitary glands were treated with BPA, which substantially reduced Pomc mRNA in both sexes, similar to the effects with E2 [Fig. 5(b)], suggesting these compounds can act directly at the pituitary to exact changes in Pomc expression similar to that found in vivo.
Figure 5.
Pomc mRNA was suppressed by BPA and E2 in pituitary cultures. Pomc mRNA was suppressed by (a) 10−8 M estradiol and (b) 4.4 × 10−5 M BPA in pituitary explant cultures in both males and females (E2 males, n = 6 to 10; E2 females, n = 10 to 15; BPA males, n = 9 to 15, BPA females, n = 7 to 14). #P ≤ 0.05.
Experiment 2: Pomc mRNA is decreased by ESR1 and ESR2 in females and by GPER in males
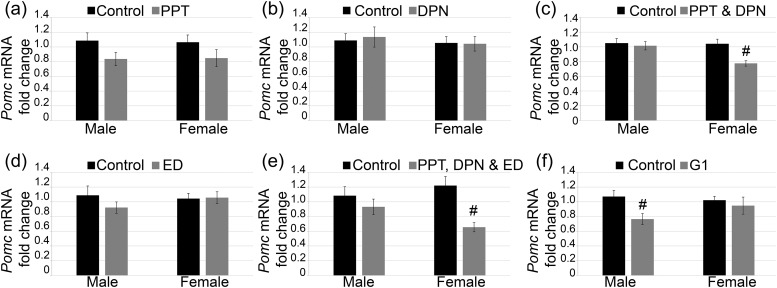
Because Pomc was suppressed by E2 and BPA directly at the pituitary, the precise estrogen signaling pathway that might be involved in this process was examined. First, the pituitary glands were treated with an ESR1 selective agonist, PPT. PPT alone was unable to statistically significantly suppress levels of Pomc mRNA [Fig. 6(a); male, P = 0.09; female, P = 0.17]. Next, pituitary glands were treated with the ESR2 selective agonist, DPN. DPN alone was also unable to statistically significantly suppress the levels of Pomc mRNA [Fig. 6(b); male, P = 0.79; female, P = 0.93]. Because PPT or DPN alone did not suppress Pomc sufficiently, the pituitary glands were cotreated with PPT and DPN to determine whether activation of both ESR1 and ESR2 would lead to relevant repression. This cotreatment was able to suppress Pomc mRNA in females but not in males [Fig. 6(c); male, P = 0.71; female, P ≤ 0.002].
Figure 6.
Pomc mRNA was regulated by various estrogen receptors in a sex-specific manner. (a) ESR1 selective agonist PPT and (b) ESR2 selective agonist DPN did not affect Pomc mRNA. (c) PPT and DPN showed decreased Pomc mRNA in females. (d) Membrane estrogen signaling agonist, estrogen dendrimer conjugate (ED), showed no regulation of Pomc mRNA. (e) PPT plus DPN plus ED decreased Pomc mRNA in females. (f) Finally, the GPER agonist, G1, decreased Pomc mRNA in males. PPT males, n = 11; PPT females, n = 9 to 10; DPN males, n = 9 to 10; DPN females, n = 8; PPT plus DPN males, n = 11; PPT plus DPN females, n = 8 to 10; ED males, n = 10; ED females, n = 9; PPT plus DPN plus ED males, n = 7 to 13; PPT plus DPN plus ED females, n = 9 to 12; G1 males, n = 9 to 10; G1 females, n = 6 to 10. #P ≤ 0.05.
To determine whether this regulation of Pomc might occur through membrane estrogen receptors, pituitary glands were treated with an estrogen dendrimer conjugate. This compound is too large to enter the nucleus and thus only activates estrogen receptors in the plasma membrane or cytoplasmic sites. Treatment of pituitary glands with this compound did not lead to any statistically significant changes in Pomc expression [Fig. 6(d); male, P = 0.26; female, P = 0.91]. Next, cotreatment of PPT, DPN, and estrogen dendrimer conjugate, meant to activate both membrane and nuclear signaling pathways, showed suppression of Pomc in females but, again, not in males [Fig. 6(e); male, P = 0.37; female, P ≤ 0.003]. Finally, pituitary glands were treated with the GPER selective agonist G1. G1 was able to decrease the levels of Pomc mRNA in males but not in females [Fig. 6(f); male, P ≤ 0.01; female, P = 0.64]. This showed that suppression of Pomc can occur through multiple pathways and can be different between males and females.
Discussion
We have demonstrated that critical windows of development exist in which the mouse pituitary gland is sensitive to BPA exposure and that differential effects occur based on age and sex. Embryonic exposure to 0.5 or 50 μg/kg/d BPA increased pituitary proliferation and gonadotrope number in a sex-specific manner (29). Exposure to BPA during the neonatal period did not affect proliferation and also had little effect on gonadotropes. However, we did see a decrease in Pit1 mRNA expression with lower doses of BPA in males and sex-specific decreases in Pomc mRNA with exposure to BPA and E2. Pomc mRNA regulation by BPA and E2 can occur at the level of the pituitary in males and females; however, sex-specific downregulation of Pomc mRNA levels occurs with distinct estrogen receptor agonists. Therefore, these findings highlight that the pituitary is dynamically affected by BPA exposure in a sex-specific manner and emphasize the need for examining different periods of exposure and times after exposure.
Previously, our laboratory showed that embryonic exposure to BPA in mice led to an increase in progenitor proliferation and differentiation to the gonadotrope lineage at PND0 in females (29). We found that the effect of neonatal exposure to BPA was not similar to the effects observed with embryonic dosing. Neonatal exposure demonstrated no substantial change in proliferation or gonadotropes in either sex. A higher concentration of 50 mg/kg/d BPA and direct stimulation of the neonatal pituitary also did not regulate Lhb and Fshb mRNA (31), demonstrating effectively that in the neonatal pituitary, gonadotropes are less affected by BPA exposure than during embryogenesis. Many reasons could exist for why the neonatal period did not respond to BPA the same as during the embryonic period. Pituitary cell composition and receptor levels vary during the developmental period (40–42), which could alter the precise cellular dynamics leading to these changes. Additionally, the functionality of the HPG axis during these two periods might play a role. Development of the HPG axis begins before birth (2) and is of critical importance to the pituitary, because both hpg (hypogonadal) mice lacking gonadotropin-releasing hormone (GnRH) and kisspeptin receptor, GPR54, knockout mice have lower serum gonadotropin levels at birth (41, 43). However, after birth, the pituitary does not respond to GnRH with increased luteinizing hormone release until 2 weeks of age, despite a high frequency of GnRH stimulation in the first week of life (44). This suggests that the pituitary is hyporesponsive to stimuli during the neonatal period, in contrast to the embryo or the adult. Perhaps, late during the embryonic period, the HPG axis and regulation of proliferation and Lhb mRNA is active; however, after birth, the responsiveness of the pituitary to GnRH and proliferation signals is decreased, preventing disruption of Lhb mRNA and proliferation by BPA during the neonatal period.
Despite an absence of similar changes in the neonatal and embryonic periods, effects were observed that were specific to neonatal exposure. The lowest doses of BPA, within the range of human exposure, decreased Pit1 mRNA in males. To the best of our knowledge, BPA has not been previously shown to affect Pit1 transcription. This could be a nonestrogenic action because the same effect was not seen with E2. However, E2 has been shown to increase Pit1 mRNA in cell lines and somatotrope-specific estrogen receptor-α knockout mice in adults (45). It is possible that regulation of Pit1 mRNA by E2 was not seen in our experiments because of the neonatal timing. BPA has been shown to bind different receptors, including the androgen receptor, estrogen-related receptor, thyroid hormone receptor, peroxisome proliferator-activated receptor, aryl hydrocarbon receptor, and glucocorticoid receptor (46, 47). Therefore, it is possible that BPA might be acting through one of these other receptors to reduce Pit1 mRNA levels. Despite the decrease in Pit1 mRNA with low doses of BPA in males, we did not see any effect on the transcript levels of Prl, Gh, or Tshb. Furthermore, we did not find any change in the protein levels of PIT1, supporting the lack of changes in the transcription of genes regulated by PIT1. These data suggest the pituitary might use compensatory mechanisms to maintain consistent levels of PIT1 in the presence of BPA exposure.
Neonatal BPA exposure also had striking effects on the TPIT lineage that were not seen after embryonic dosing. We observed a decrease in Pomc mRNA in the E2-, BPA0.5-, and BPA50-exposed females and with BPA0.5 and E2 exposure in males, independent of the Tpit mRNA changes. E2 exposure via an implanted 0.5-mg pellet in the adult rat has been shown to reduce Pomc mRNA in the pituitary, which also leads to decreased plasma adrenocorticotropic hormone (ACTH) and corticosterone levels when stressed (48). Seeing the same suppression of Pomc in the neonate suggests that the mechanism by which this inhibition occurs is established early on. However, the precise mechanism is unclear. BPA50-exposed males did not show a statistically significant decrease in Pomc mRNA. This represents a crucial sex difference in Pomc mRNA regulation with varying amounts of exposure and, potentially, a nonmonotonic dose response in males. Despite this, we did not see a decrease in intrapituitary POMC protein expression or cell number. There could be a variety of explanations, such as increased translation, to compensate for decreased transcription. Alternatively, it could be that hormone release is also affected, and intrapituitary POMC levels are kept consistent. Other studies in rats have demonstrated the ability of 2 or 40 μg/kg/d BPA to regulate Pomc mRNA levels and blood ACTH levels in males compared with females in response to stress (24, 49), emphasizing the importance of sex as a variable in research on the HPA axis.
Of the parameters examined, few statistically significant effects of BPA exposure at 5 weeks after neonatal dosing. A decrease was seen in Nr5a1 in BPA0.05-treated female pituitary glands at 5 weeks; however, no corresponding decrease was seen in Lhb. Additionally, changes in Pit1 at PND7 were not seen at 5 weeks. However, a suppression of Pomc in BPA0.5-treated females was found at 5 weeks, suggesting the possibility of lasting effects on the stress axis. Despite the relative lack of changes due to BPA, E2 exposure affected the reproductive axis of 5-week females, leading to lower Nr5a1 and Lhb levels and persistent estrus. Therefore, neonatal E2 exposure might have permanently altered the HPG axis, which could affect fertility in females. Although the effects of BPA were minimal on the 5-week old pituitary gland, it is possible that changes could be present at different time points. Additionally, these data highlight that it is vital to examine exposure during critical windows of development.
The strongest effect of neonatal dosing was a decrease in Pomc mRNA with both E2 and BPA. Further analysis demonstrated that this decrease might be a direct effect on the pituitary itself. Although it has been reported that E2 can regulate Pomc mRNA, conflicting findings have been reported on the direction and mechanism. In the adult rat pituitary, E2 decreased Pomc mRNA, leading to decreased stress-related ACTH release (48). This effect, however, might not be due to a direct effect on the pituitary gland because, in adult anterior pituitary-cultured cells, E2 treatment did not alter Pomc mRNA expression. However, in the neurointermediate lobe, E2 treatment increased Pomc mRNA (50). In contrast, neonatal testosterone propionate decreased Pomc mRNA in the adult, and treatment of E2 returned the levels of Pomc mRNA to normal (51). Pomc is also expressed in the hypothalamus, and E2 treatment decreases Pomc mRNA in embryonic hypothalamic cultures (52). Alternatively, in adult mice, E2 increased Pomc mRNA in POMC-expressing neurons (53). Although different effects of E2 on Pomc expression have been observed, it is clear that E2 can regulate Pomc in an age-dependent and sex-specific manner. The mechanism by which estradiol regulates Pomc expression is not well understood; however, in the hypothalamus ESR1 colocalizes with POMC-expressing neurons (54), suggesting a role for ESR1. In our studies of the neonatal pituitary gland, ESR1 and ESR2, together, decreased the levels of Pomc mRNA in females, but not in males. In contrast, activation of GPER decreased the levels of Pomc mRNA in males but not in females. This interesting observation can be explained by differing levels of estrogen receptors or isoforms of the receptors. In the adult pituitary gland, estrogen receptor mRNA isoforms are differentially expressed in males and females. In addition, pituitary expression of these isoforms can vary in females depending on the stage of the estrous cycle (55). Therefore, it is possible that differences in receptor expression or activation during this neonatal period could lead to the differing pathways for Pomc mRNA downregulation and should be further explored. However, it seems that despite the possible differences in the roles of the various estrogen receptors, it is clear that estrogen stimulation in the neonatal pituitary leads to decreased Pomc mRNA expression.
Conclusions
Overall, we found contrasting effects of BPA exposure in the embryonic vs the neonatal period. This might be demonstrating that the critical window for proliferation and gonadotrope regulation is embryonic, and the critical window for corticotrope regulation is neonatal. After removal of BPA, minimal statistically significant effects were seen, potentially demonstrating the plasticity of the pituitary in regulating itself to maintain the proper cell number and hormonal signaling. A commonality in both the embryonic and the neonatal periods was the existence of sex-specific effects, which could hint at either baseline differences in male and female pituitary glands or differences in hormone signaling, both worthy of further exploration. Finally, in our studies, we began to explore the mechanism of estrogenic regulation of Pomc mRNA. We observed that receptor-selective agonists have sex-specific effects on Pomc gene expression. This might suggest that BPA can activate different estrogen receptors in males and females and, therefore, could be a potential mechanism by which BPA mediates sex-specific effects.
Acknowledgments
We thank Karen Weis and Liying Gao for technical assistance.
Financial Support: This work was supported by the National Institutes of Health (Grant R01 DK076647 to L.T.R.; Grant P01 ES022848 to J.A.F.; Grant DK015556 to J.A.K.; and Grant T32 ES007326 to K.S.E. and W.W.). This work was also supported by the Environmental Protection Agency (Grant RD-83459301 to J.A.F.) and the Midwest Society of Toxicology (Young Investigator Award to K.S.E.).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| ACTH | Human ACTH AA 1-24 | Polyclonal rabbit anti-human ACTH | DakoCytomation, A0571 | Rabbit; polyclonal | 1:250 | No longer commercially available |
| Mouse β-actin | NA | β-Actin (D6A8) rabbit mAb | Cell Signaling Technology, 8457 | Rabbit; monoclonal | 1:1000 | AB_10950489 |
| Chicken α-tubulin | NA | Mouse anti–α-tubulin monoclonal antibody, unconjugated, clone DM1A | Sigma-Aldrich, T9026 | Mouse; monoclonal | 1:5000 | AB_477593 |
| Rat PIT1 | NA | Anti-PIT1 | Simon Rhodes | Rabbit; polyclonal | 1:1000 | Not commercially available |
| Rat βLH | NA | Anti-rβLH-IC | National Hormone and Peptide Program, Dr. A.F. Parlow | Rabbit; polyclonal | 1:1000 | AB_2665533 |
| Human phosphorylated histone H3 | Histone H3 at serine 10 | Anti-phosphorylated histone H3 (Ser10) antibody | Millipore | Rabbit; polyclonal | 1:1000 | AB_310177 |
Abbreviations: AA, amino acid; AB, antibody; LHβ, luteinizing hormone-β; mAb, monoclonal antibody; NA, not available; rβLH-IC, rat LH β for immunocytochemistry; RRID, Research Resource Identifier.
Footnotes
- ACTH
- adrenocorticotropic hormone
- BPA
- bisphenol A
- BPA0.05
- 0.05 μg/kg/d bisphenol A
- BPA0.5
- 0.5 μg/kg/d bisphenol A
- BPA50
- 50 μg/kg/d bisphenol A
- CAS
- Chemical Abstracts Service
- DPN
- diarylpropionitrile
- E2
- estradiol
- EDC
- endocrine-disrupting chemical
- ESR1
- estrogen receptor-α
- ESR2
- estrogen receptor-β
- GnRH
- gonadotropin-releasing hormone
- GPER
- G protein–coupled estrogen receptor
- HPA
- hypothalamic–pituitary–adrenal
- HPG
- hypothalamic–pituitary–gonadal
- IHC
- immunohistochemical
- PBS
- phosphate-buffered saline
- PND
- postnatal day
- PPT
- pyrazole triol.
References
- 1.Davis SW, Ellsworth BS, Peréz Millan MI, Gergics P, Schade V, Foyouzi N, Brinkmeier ML, Mortensen AH, Camper SA. Pituitary gland development and disease: from stem cell to hormone production. Curr Top Dev Biol. 2013;106:1–47 10.1016/B978-0-12-416021-7.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pointis G, Mahoudeau JA. Release of immuno-reactive and biologically active LH from fetal mouse pituitary in response to synthetic gonadotropin releasing factor (LRF). Experientia. 1976;32(10):1347–1348 10.1007/BF01953132. [DOI] [PubMed] [Google Scholar]
- 3.Milković S, Milković K, Paunović J. The initiation of fetal adrenocorticotrophic activity in the rat. Endocrinology. 1973;92(2):380–384 10.1210/endo-92-2-380. [DOI] [PubMed] [Google Scholar]
- 4.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81(6):1216–1225 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 5.McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have “organizational” effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105(2):295–307. [DOI] [PubMed] [Google Scholar]
- 6.Shelby MD. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR Mon. 2008;7–9(22):1–64 passim. [PubMed] [Google Scholar]
- 7.Herbst AL. The current status of the DES-exposed population. Obstet Gynecol Annu. 1981;10:267–278. [PubMed] [Google Scholar]
- 8.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109(1):55–60 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72(6):1344–1351 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol. 2007;24(2):139–177 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Nishihara E, Nagayama Y, Inoue S, Hiroi H, Muramatsu M, Yamashita S, Koji T. Ontogenetic changes in the expression of estrogen receptor alpha and beta in rat pituitary gland detected by immunohistochemistry. Endocrinology. 2000;141(2):615–620 10.1210/endo.141.2.7330. [DOI] [PubMed] [Google Scholar]
- 12.Ogasawara K, Nogami H, Tsuda MC, Gustafsson JA, Korach KS, Ogawa S, Harigaya T, Hisano S. Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinology. 2009;150(2):1061–1068 10.1210/en.2008-1151. [DOI] [PubMed] [Google Scholar]
- 13.Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202(2):223–236 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 15.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473(2):270–291 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 16.Losa-Ward SM, Todd KL, McCaffrey KA, Tsutsui K, Patisaul HB. Disrupted organization of RFamide pathways in the hypothalamus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol Reprod. 2012;87(2):28. 10.1095/biolreprod.112.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monje L, Varayoud J, Muñoz-de-Toro M, Luque EH, Ramos JG. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reprod Toxicol. 2010;30(4):625–634 10.1016/j.reprotox.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Fernández M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117(5):757–762 10.1289/ehp.0800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276(2):157–164 10.1016/j.taap.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401(6755):763–764 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 21.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141(12):4512–4517 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez MC, Bourguignon NS, Bonaventura MM, Lux-Lantos V, Libertun C, Becu-Villalobos D. Neonatal xenoestrogen exposure alters growth hormone-dependent liver proteins and genes in adult female rats. Toxicol Lett. 2012;213(3):325–331 10.1016/j.toxlet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146(2):607–612 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 24.Chen F, Zhou L, Bai Y, Zhou R, Chen L. Sex differences in the adult HPA axis and affective behaviors are altered by perinatal exposure to a low dose of bisphenol A. Brain Res. 2014;1571:12–24 10.1016/j.brainres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J. 1995;9(5):419–423. [DOI] [PubMed] [Google Scholar]
- 26.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 27.Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117(5):784–789 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod. 2012;87(4):82. 10.1095/biolreprod.112.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YJ, Chen DW, Liu JL, Zhang JH, Luo HS, Cui S. Estradiol promotes pituitary cell proliferation and gonadotroph differentiation at different doses and with different mechanisms in chick embryo. Steroids. 2009;74(4-5):441–448 10.1016/j.steroids.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Eckstrum KS, Weis KE, Baur NG, Yoshihara Y, Raetzman LT. Icam5 expression exhibits sex differences in the neonatal pituitary and is regulated by estradiol and bisphenol A. Endocrinology. 2016;157(4):1408–1420 10.1210/en.2015-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakind JS, Naiman DQ. Bisphenol A (BPA) daily intakes in the United States: estimates from the 2003-2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol. 2008;18(6):608–615 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- 33.US Environmental Protection Agency Health assessment information on bisphenol A (CASRN 80-05-7). Available at:http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickview&substance_nmbr=0356#reforal. First online 1998. Accessed 24 August 2017. [PubMed]
- 34.Gremeaux L, Fu Q, Chen J, Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012;21(5):801–813 10.1089/scd.2011.0496. [DOI] [PubMed] [Google Scholar]
- 35.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Katzenellenbogen JA. Hormone-PAMAM dendrimer conjugates: polymer dynamics and tether structure affect ligand access to receptors. Angew Chem Int Ed Engl. 2006;45(43):7243–7248 10.1002/anie.200601923. [DOI] [PubMed] [Google Scholar]
- 38.Nantie LB, Himes AD, Getz DR, Raetzman LT. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol Endocrinol. 2014;28(5):731–744 10.1210/me.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol. 2011;358(1):23–32 10.1016/j.ydbio.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura F, Harumiya K, Kiyama H. Light and electron microscopic studies of the cytogenesis of anterior pituitary cells in perinatal rats in reference to the development of target organs. Arch Histol Jpn. 1970;31(3):333–369 10.1679/aohc1950.31.333. [DOI] [PubMed] [Google Scholar]
- 41.Carbajo-Pérez E, Watanabe YG. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261(2):333–338 10.1007/BF00318674. [DOI] [PubMed] [Google Scholar]
- 42.Pasqualini C, Guivarc’h D, Boxberg YV, Nothias F, Vincent JD, Vernier P. Stage- and region-specific expression of estrogen receptor α isoforms during ontogeny of the pituitary gland. Endocrinology. 1999;140(6):2781–2789 10.1210/endo.140.6.6752. [DOI] [PubMed] [Google Scholar]
- 43.Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153(2):782–793 10.1210/en.2011-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069 10.1523/JNEUROSCI.2200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avtanski D, Novaira HJ, Wu S, Romero CJ, Kineman R, Luque RM, Wondisford F, Radovick S. Both estrogen receptor α and β stimulate pituitary GH gene expression. Mol Endocrinol. 2014;28(1):40–52 10.1210/me.2013-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acconcia F, Pallottini V, Marino M. Molecular mechanisms of action of BPA. Dose Response. 2015;13(4):1559325815610582. 10.1177/1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasanth GK, Divya LM, Sadasivan C. Bisphenol-A can bind to human glucocorticoid receptor as an agonist: an in silico study. J Appl Toxicol. 2010;30(8):769–774 10.1002/jat.1570. [DOI] [PubMed] [Google Scholar]
- 48.Redei E, Li L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology. 1994;60(2):113–123 10.1159/000126741. [DOI] [PubMed] [Google Scholar]
- 49.Panagiotidou E, Zerva S, Mitsiou DJ, Alexis MN, Kitraki E. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. J Endocrinol. 2014;220(3):207–218 10.1530/JOE-13-0416. [DOI] [PubMed] [Google Scholar]
- 50.Matsumura R, Takeuchi S, Takahashi S. Effect of estrogen on melanocortin-3 receptor mRNA expression in mouse pituitary glands in vivo and in vitro. Neuroendocrinology. 2004;80(3):143–151 10.1159/000082355. [DOI] [PubMed] [Google Scholar]
- 51.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Postnatal masculinization alters the HPA axis phenotype in the adult female rat. J Physiol. 2005;563(Pt 1):265–274 10.1113/jphysiol.2004.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao Y, He JR, Kapcala LP. Estrogen inhibits hypothalamic pro-opiomelanocortin gene expression in hypothalamic neuronal cultures. Brain Res Dev Brain Res. 1997;45(2):340–344. [DOI] [PubMed] [Google Scholar]
- 53.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol. 2007;19(6):426–431 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 54.de Souza FSJ, Nasif S, López-Leal R, Levi DH, Low MJ, Rubinsten M. The estrogen receptor α colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur J Pharmacol. 2011;660(1):181–187 10.1016/j.ejphar.2010.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demay F, Tiffoche C, Thieulant M-L. Sex- and cell-specific expression of an estrogen receptor isoform in the pituitary gland. Neuroendocrinology. 1996;63(6):522–529 10.1159/000127081. [DOI] [PubMed] [Google Scholar]