Abstract
Endometriosis is a gynecological disease that negatively affects the health of 1 in 10 women. Although more information is known about late stage disease, the early initiation of endometriosis and lesion development is poorly understood. Herein, we use a uterine tissue transfer mouse model of endometriosis to examine early disease development and its dependence on estradiol (E2) and estrogen receptor (ER) α within 72 hours of disease initiation. Using wild-type and ERα knockout mice as hosts or donors, we find substantial infiltration of neutrophils and macrophages into the peritoneal cavity. Examining cell infiltration, lesion gene expression, and peritoneal fluid, we find that E2/ERα plays a minor role in early lesion development. Immune-mediated signaling predominates E2-mediated signaling, but 48 hours after the initiation of disease, a blunted interleukin (IL)-6-mediated response is found in developing lesions lacking ERα. Our data provide evidence that the early initiation of endometriosis is predominantly dependent on the immune system, whereas E2/ERα/IL-6-mediated cross-talk plays a partial role. These findings suggest there are two phases of endometriosis—an immune-dependent phase and a hormone-dependent phase, and that targeting the innate immune system could prevent lesion attachment in this susceptible population of women.
A mouse model of endometriosis, used to examine the early initiation of disease, revealed that two phases of disease exist—an immune-predominant phase and hormone-predominant phase.
Endometriosis, the presence of proliferating uterine endometrial tissue outside the uterine cavity, affects the quality of life and reproductive health of roughly 7.4 million American women (1). Symptoms of disease include dysmenorrhea, chronic pain, dyspareunia, and infertility (2). Treatment of endometriosis is purely palliative because no cure exists, and diagnosis is made only through invasive laparoscopic surgery (3). Endometrial lesions are often found attached to sites in the peritoneal cavity, such as the rectouterine cul-de-sac, fallopian tubes, ovarian fossa, peritoneal wall, and bowel (2). A current view regarding pathogenesis is that disease arises via retrograde menstruation, in which viable endometrial tissue flows back through the fallopian tubes and into the peritoneal cavity (4). Although greater than 90% of women have retrograde menstruation, only 10% develop endometriosis (4). The nature of individual predisposing factors in the endometrium and/or peritoneum remains unclear (2, 5).
The major functions of the endometrium are growth, implantation, menstruation, and repair. The endometrium, whether it is within the uterine cavity (eutopic) or found in endometriotic lesions (ectopic) is hormone regulated (6). In the uterus, hormones mediate their endometrial effects through the activity of steroid hormones and steroid hormone receptors. Estradiol (E2) is a ligand for estrogen receptor (ER) α and ERβ and is both pro- and anti-inflammatory (7). Endometriosis is an E2-dependent disease (2). Endometriotic lesions have altered ratios of ERα and ERβ expression compared with eutopic endometrium (8), and both ERs are present in uterine and multiple immune cell types (i.e., neutrophils, macrophages, T cells). Using a uterine tissue transfer mouse model of endometriosis, in which donor minced uterine tissue is injected into the peritoneal cavity of a host, we previously found that ERα-deficient (αERKO) uterine tissue transferred to wild-type (WT) mice (αERKO to WT) does not develop lesions, WT tissue transferred into ERα-deficient mice (WT to αERKO) does not exhibit the classic progesterone receptor switch from the epithelial cells to the stromal cells in response to E2, and inflammation scores (pathology and gene expression) increased with ERα knocked out in the host and donor (9). These data suggest that both uterine tissue (host) and peritoneal environment (donor) are important in disease-mediated signaling with ERα dramatically participating in paracrine signaling (9). ERβ is expressed at a low level in the uterus; however, when ERβ knockout (βERKO) uterus is transferred into a WT host (βERKO to WT), disease development is comparable to WT uterine tissue into a WT host (WT to WT) (9). Recently, Zhao et al. (10) demonstrated that ligands selective for ERα or ERβ and optimized for anti-inflammatory activity were able to suppress ER-mediated effects and inflammation. These ligands lead to a decrease in endometriosis lesion size in a mouse model of endometriosis where uterine tissue is sutured into the peritoneal wall (10). The peritoneal fluid of women with endometriosis contains elevated numbers of activated macrophages, which express increased ERα (7, 8, 11–14). Most current therapies for endometriosis aim to decrease E2 production or counteract E2 effects (2). Together the aforementioned findings indicate a role for E2 and ERα-mediated signaling in endometriosis; however, adverse side effects limit long-term use of these therapies and upon cessation endometriosis symptoms often return (2, 15, 16).
Women with endometriosis demonstrate increased expression of angiogenic factors and higher incidences of autoimmune disorders (17–19). E2 is mitogenic and can be both pro- and anti-inflammatory, which helps regulate angiogenesis and immune protective responses, functions that are normal to the uterus (20–23). Cytokines produced from cells of the innate immune system are critical for mediating cellular recruitment, neoangiogenesis, and resolution of inflammation (24–29). Among these cells, neutrophils are major effectors of acute and chronic inflammatory conditions, whereas macrophages cooperate to coordinate repair processes (25, 30). Although cytokine regulation within the endometrium is a normal part of menstruation, implantation, and for the defense of the mucosal epithelium (21), women with endometriosis often exhibit increased activation of peritoneal macrophages and associated inflammatory cytokines (31–33). These findings suggest altered immune surveillance may contribute to disease development (32). The dependence of chronic endometriosis disease on E2 is well established (2, 9, 13), but little is known regarding the molecular mechanisms underlying the early initiation of endometriosis and lesion formation. Menstruation is an inflammatory event for the uterus; consequently, the menstrual fluid/tissue leaving the body or flowing retrograde into the peritoneal cavity where lesions are formed is ∼40% composed of neutrophils, macrophages, and uterine natural killer (uNK) cells (22, 34, 35).
Based upon our previous studies, we hypothesized ERα/E2 is necessary for immune modulation and angiogenesis in the early initiation of endometriosis. To test our hypothesis, we used an endometriosis mouse model in which syngeneic donor uterine tissue is minced and injected into the peritoneal cavity of a host mouse to address the role of ERα and E2 in the early initiation of endometriosis. Contrary to our hypothesis, we find a predominant role for the signaling of the innate immune system, <72 hours after the initiation of disease and during the early development of endometriosis lesions. At this stage of disease, signaling by the immune system predominates E2 mediated signaling in disease. Notably, 48 hours after the initiation of disease, a blunted interleukin (IL)-6-mediated response is found in developing lesions lacking ERα. Our data provide evidence that the early initiation of endometriosis is predominantly dependent on the immune system with cross-talk through an E2/ERα/IL-6-mediated signaling axis.
Materials and Methods
Animal care and treatment
All animal studies were conducted in accordance with the National Institutes of Health Guidelines for Humane Use and Care of Animals and with approved National Institute of Environmental Health Sciences (NIEHS) animal protocol. Mice (αERKO) containing a deletion of exon 3 of the Esr1 (ERα) gene were generated as described previously (36). Adult female C57/BL6 mice were purchased from Charles River Laboratories (Raleigh, NC), adult female IL-6KO mice (B6.129S2-IL6tm1/Kopf/J) were purchased from The Jackson Laboratory (Bar Harbor, ME), αERKO mice were generated from the NIEHS αERKO colonies at Charles River Laboratories (Wilmington, MA) or were generated by in-house breeding at NIEHS. Mice used were female and aged between 2 and 6 months. Mice were in a controlled temperature range (22°C to 23°C) on a 12-hour light, 12-hour dark cycle. Mice were given food and water ad libitum.
Recipient mice of various genotypes depending on the experimental design were ovariectomized through two 0.5-cm dorsolateral skin incisions, and endogenous hormones were allowed to clear for 7 to 10 days. Mice were then randomly divided into two treatment groups, E2 valerate (2.5 µg/mouse/wk; Sigma-Aldrich, St. Louis, MO) in corn oil or corn oil vehicle (n = total of 6 to 12 mice per group with experimental replicates). Mice were dosed subcutaneously once prior to experimental endometriosis induction and then once weekly for the duration of the study for long-term studies. Donor mice were primed 41 hours prior to uterus removal with pregnant mare serum gonadotropin 5 IU intraperitoneal (9). The donor uterus was removed en bloc after euthanasia, cleaned of excess tissue, and the outer myometrium was peeled away. The tissue was then washed thrice in sterile phosphate-buffered saline (PBS). In a glass 60-mm dish, the uterus was slit with a linear incision longitudinally and minced (≤1.5 mm). Recipient mice were anesthetized using isoflurane/oxygen and given buprenorphine (0.1 mg/kg) for pain management. A 0.5-cm right dorsolateral incision was made in the recipient abdomen while the donor uterus was minced. The minced donor uterine tissue was suspended in 500 µL of PBS, was injected into the peritoneal cavity of the recipient using a p1000 tip, and the peritoneal wall was overlapped to close the cavity, the outer skin was closed with 9-mm clips, and a gentle massage was given to disperse the tissue throughout the peritoneal cavity. An equivalent amount (∼100 mg) of minced tissue was transferred into all recipients (WT and IL-6KO donors were used at a one donor uterus: one host ratio, whereas the αERKO donors, with hypoplastic uteri, were used at a five donor uteri: one host ratio). Sham mice received the same operations as experimental mice but were injected with PBS alone. Mice were treated up to an additional 3 weeks with E2 valerate (International Union of Pure and Applied Chemistry: [(8R,9S,13S,14S,17S)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] pentanoate) or vehicle (Fig. 1A). Groups were designated in the following manner: donor to host: WT to WT, WT to αERKO, and αERKO to WT. For anti-IL-6 experiments, mice were injected subcutaneously with 200 µg/mouse anti-IL-6 (Bio X Cell, West Lebanon, NH; clone MP5-20F3) every third day starting 3 days prior to tissue injection. Mice per group are based on sample size calculations done using preliminary data to compare sham vs endometriosis or treated vs control and were conducted using SAS Proc Power (SAS Institute, Cary, NC; 2008).
Figure 1.
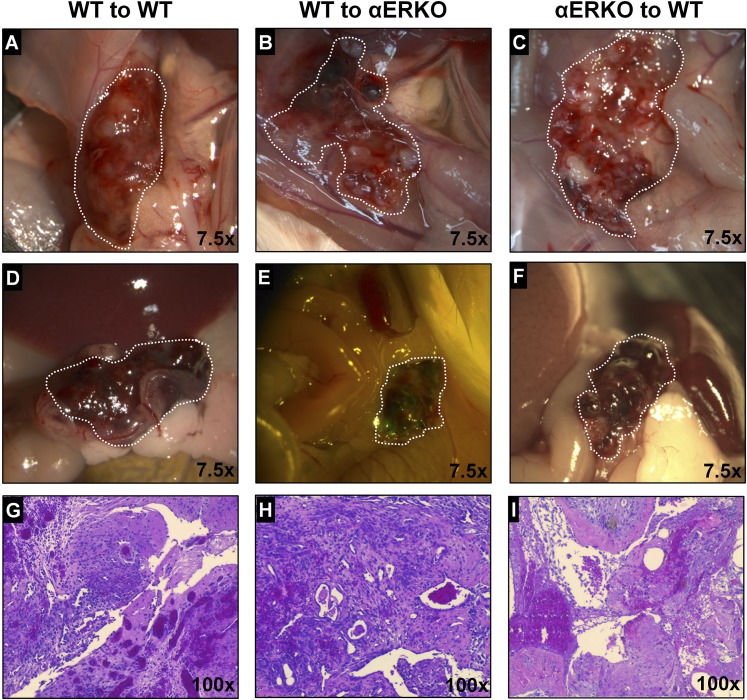
Endometriosis lesion macroscopic and microscopic appear similar 72 hours after disease initiation. (A–F) Gross appearance of lesions (×7.5) in WT to WT (left column), WT to αERKO (middle column), and αERKO to WT (right column). Lesions in A–C are localized to the injection site. Lesions in D–F are localized throughout the peritoneal cavity. Lesions are outlined by dotted white line. (G–I) Histological evaluation (hematoxylin and eosin) of lesion tissue (×100). Representative examples are E2 treated.
After 24, 48, or 72 hours or 3 weeks (Supplemental Fig. 1 (334.6KB, pdf) ), mice were euthanized with CO2. Peritoneal wash was performed by injecting 1 mL of PBS + 0.5% bovine serum albumin (BSA) + 2 mM EDTA into the peritoneal cavity. The cavity was gently massaged, a small incision was made into the inner skin lining the peritoneal cavity, and the fluid was gently removed not drawing organs into the syringe. The peritoneal wash was immediately spun at 800g for 5 minutes, the supernatant was snap-frozen on dry ice and stored at −80°C until use, and the cell pellet was resuspended in PBS + 0.5% BSA + 2 mM EDTA and kept cold on ice until antibody staining. To assess the effects of genotype on ectopic uterine tissue, ectopic lesions were photographed to document in situ images of endometriosis-like lesions (Leica dissecting microscope MZ16FA and Leica camera DFC490, Germany). Endometriosis-like lesions were visualized, dissected, measured, weighed, and then removed and either fixed in 10% formalin or snap frozen on dry ice and stored at −80°C until use. Resuspended cell pellets containing red blood cells (RBCs) were lysed one to two times with RBC lysis buffer for 15 to 30 seconds and 10 times volume of PBS was immediately added. Cells were resuspended for cell counting, cytospin, and flow cytometry analysis. A hemocytometer was used for cell counting, and 150,000 cells were used for differentials and the remaining for fluorescence-activated cell sorting (FACS; see later).
Cytology
The fixed tissues were routinely processed for paraffin embedding. Five micron sections were cut and the slides were used for hematoxylin and eosin (Sigma-Aldrich) staining. All the slides were deparaffinized and hydrated through descending grades of alcohol, stained, dehydrated, and cover slipped.
Differentials were stained with modified Giemsa (Hema 3 according to manufacturer’s protocol).
RNA isolation and real-time polymerase chain reaction
Frozen endometriosis-like lesions from the mice were pulverized under liquid nitrogen and RNA was isolated using TRIzol as per manufacturer’s instructions (Invitrogen, Carlsbad, CA). Using a previously described method, complementary DNA was synthesized and analyzed by real-time polymerase chain reaction (RT-PCR) using Fast SYBR (37). Relative transcript levels were quantified in comparison with the WT to WT vehicle group and normalized to Rpl7 using the model described by Pfaffl (38). Primer sequences (Supplemental Table 1 (334.6KB, pdf) ) purchased from Sigma-Aldrich were selected using Primer Express (Applied Biosystems, Foster City, CA), Harvard Primer Bank (Harvard University, Cambridge, MA), or PrimerBot! (McDonnell Laboratory, Duke University, Durham, NC).
Analysis of cytokine production
Peritoneal cavity lavage fluid was used neat according to the manufacturer’s protocol for multiplex analysis (BioRad, Hercules, CA). Bio-Plex Pro™ Cytokine 23-plex Assay (M60009RDPD) and Bio-Plex Custom Assays were used for the detection of vascular endothelial growth factor (VEGF), IL-6, granulocyte colony-stimulating factor (G-CSF), monocyte chemotactic protein 1 (MCP1), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-10, and IL-17.
Flow cytometric analysis
Peritoneal fluid cells were spun and resuspended in 450 µL of FACS buffer (0.5% BSA, 0.1% NaN3, 2 mM EDTA in PBS). Two antibody panels were run for each sample and 200 µL of cells (1–2 × 106 cells) were placed into a round bottom 96-well plate. Cells were spun and resuspended and blocked for 30 minutes in nonspecific binding blocking reagent cocktail made in FACS buffer with 5% normal mouse serum (#015-000-120; Jackson ImmunoResearch, West Grove, PA), 5% normal rat serum (#012-000-120; Jackson ImmunoResearch), and 5 µg/mL anti-CD16/32 (2.4G2 hybridoma). Antibody cocktails (Table 1) in FACS buffer were added to the samples for an additional 30 minutes. For staining, cells were incubated with fluorochrome Allophycocyanin (APC), APC-Alexa Fluor-647, APC-Cy7, eFluor-450, eFluor-605, eFluor-780, phycoerythrin, Pacific Blue, PerCP-Cy5.5, or biotin-conjugated antibodies against mouse B220/CD45r (RA3-6B2), CD4 (L3T4), NK1.1 (PK136), CD3e (145-2C11), EpCAM/CD326 (G8.8), Ly-6G (1A8), MHC class II/I-Ab (AF6-120.1), CD11b (M1/70), CD11c (N418), F4/80 (BM8), CD115 (AFS98), and Ly-6C (AL-21) from BD Biosciences (San Jose, CA), Thermo Fisher Scientific (formerly eBiosciences; Waltham, MA), and BioLegend (San Diego, CA). Stained cells were analyzed on a FACS LSRII flow cytometer (BD Biosciences). Data from these studies were analyzed using FlowJo software (Treestar, Ashland, OR). Only single cells were analyzed.
Table 1.
Antibodies Used
| Antibody | RRID | Vendor, Catalog No. | Host Organism; Antibody Type | Clone | Dilution |
|---|---|---|---|---|---|
| Ly-6C, APC-Cy7 conjugated | AB_1727555 | BD Biosciences, 560596 | Rat; monoclonal | AL-21 | 1:200 |
| CD115 (c-fms), APC conjugated | AB_1210789 | Thermo Fisher Scientific, 17-1152-82 | Rat; monoclonal | AFS98 | 1:200 |
| F4/80, phycoerythrin conjugated | AB_465922 | Thermo Fisher Scientific, 12-4801-80 | Rat; monoclonal | BM8 | 1:200 |
| CD11c, PerCP-Cyanine5.5 conjugated | AB_925727 | Thermo Fisher Scientific, 45-0114-82 | Armenian hamster; monoclonal | N418 | 1:400 |
| CD11b, eFluor 605NC conjugated | AB_1944342 | Thermo Fisher Scientific, 93-0112-42 | Rat; monoclonal | M1/70 | 1:200 |
| MHC Class II I-Ab, eFluor 450 conjugated | AB_10669941 | Thermo Fisher Scientific, 48-5320-82 | Mouse; monoclonal | AF6-120.1 | 1:200 |
| CD326 (Ep-CAM), Alexa Fluor® 647 conjugated | AB_1134101 | BioLegend, 118212 | Rat; monoclonal | G8.8 | 1:200 |
| CD3e, phycoerythrin conjugated | AB_465497 | Thermo Fisher Scientific, 12-0031-83 | Armenian hamster; monoclonal | 145-2C11 | 1:96 |
| NK-1.1, PerCP-Cy5.5 conjugated | AB_914361 | Thermo Fisher Scientific, 45-5941-82 | Mouse; monoclonal | PK136 | 1:100 |
| CD4, eFluor 605NC conjugated | AB_1834368 | Thermo Fisher Scientific, 93-0041-42 | Rat; monoclonal | GK1.5 | 1:200 |
| CD45R/B220, Pacific Blue conjugated | AB_397031 | BD Biosciences, 558108 | Rat; monoclonal | RA3-6B2 | 1:100 |
| Ly-6G, Biotin conjugated | AB_1036096 | Miltenyi Biotec, 130-093-141 | Rat; monoclonal | 1A8 | 1:500 |
Abbreviation: RRID, Research Resource Identifier.
Statistical analysis
One-way analysis of variance (ANOVA) with Tukey posttest, two-way ANOVA with Bonferroni posttest, and one-way ANOVA with Bonferroni Multicomparison posttest P < 0.05 were performed using GraphPad Prism version 7.01 (GraphPad Software, San Diego, CA). Means not sharing a letter are significantly different from each other (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from each other (P > 0.05).
Results
Endometriosis lesions are found regardless of ERα or E2 in early endometriosis
Our previous findings demonstrated the importance of ERα and E2 in chronic endometriosis (9). In the current study, we evaluated the role of ERα and E2 in the early development of endometriosis with the hypothesis that ERα-mediated signaling is critical for lesion development. A syngeneic mouse model of endometriosis was used to assess early endometriosis lesion formation (Supplemental Fig. 1 (334.6KB, pdf) ). We first chose to examine lesion development 72 hours post disease initiation to capture early lesion development. At necropsy, lesions and peritoneal fluid/peritoneal cells were collected. Representative macroscopic and microscopic images of lesions collected from WT to WT, WT to αERKO, αERKO to WT 72 hours after disease initiation are shown (Fig. 1). Regardless of ERα genotype, lesions are found throughout the peritoneal cavity. At the injection site, these lesions are visually often hemorrhagic, vascularized, and not securely attached to the peritoneal wall (Fig. 1A–1C), whereas lesions distal to the injection site are also often hemorrhagic, vascularized, and beginning to attach by 72 hours (Fig. 1D–1F). Lesions were similar across groups (Supplemental Fig. 1 (334.6KB, pdf) ), which is in contrast to what is observed at 3 weeks (9). At 3 weeks, αERKO to WT do not develop lesions, and WT to WT or WT to αERKO lesions are not hemorrhagic, but cystic with clear fluid, which demonstrates the disease has progressed and established (9). At 72 hours postinjection, the transferred uterine tissue is localized to the same sites of attachment found 3 weeks post disease initiation. Lesions are often attached to the peritoneal wall, intestinal mesentery, fat pads, behind the stomach, in the rectouterine cul-de-sac area, and to the uterine blood supply. Lesions are not found attached to the spleen, liver, or kidneys. Histological evaluation of 72-hour lesion tissue (Fig. 1G–1I) shows lesion tissue is highly disorganized and infiltrated with white and RBCs. The disorganization at 72 hours is in contrast to what was observed 3 weeks after disease initiation where lesions are organized with distinct epithelial and stromal cell layers (9). These data show similar lesion number, weight, and histopathological characteristics between all experimental groups.
Inflammatory factors and angiogenic factors are increased following disease initiation but are independent of E2 treatment
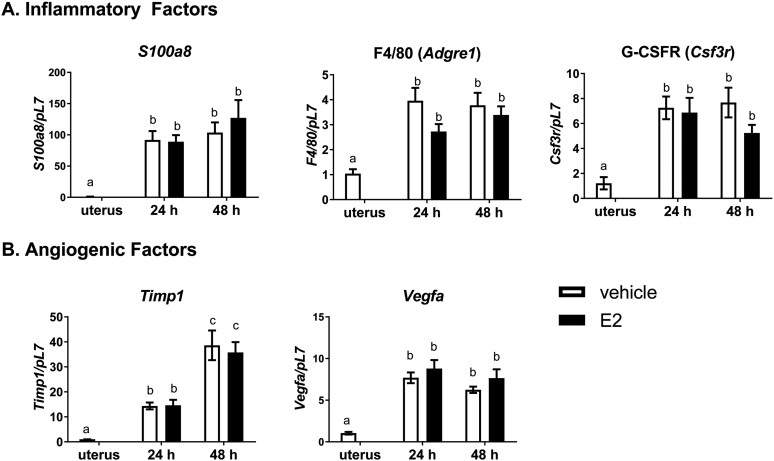
Our laboratory’s previous work with ERα chromatin immunoprecipitation sequencing of whole uterine chromatin uncovered a host of ERα bound genes associated with angiogenesis and inflammatory factors (39). To investigate how these ERα bound genes were altered by early endometriosis (72 hours), ERα status, and E2 treatment, endometriosis was initiated in WT to WT, αERKO to WT, and WT to αERKO with and without E2 treatment. When examining lesion gene expression of a multitude of these factors, with a focus on immune and angiogenic factors 72 hours post disease initiation, no statistical differences were found between the groups or with E2 treatment (data not shown). Therefore, to more closely examine the regulation surrounding the development of a blood supply and the seemingly E2-independent effect, we isolated lesions 24 and 48 hours after disease initiation in WT to WT lesions. Genes expressed in lesions were compared with noninjected minced uterine tissue by RT-PCR analysis. Lesions removed 24 and 48 hours after disease initiation have increased gene expression of inflammatory factors (Fig. 2A: S100a8, F4/80/Adgre1, G-CSFR/Csf3r) and angiogenic factors known to be regulated in endometriosis (Fig. 2B: Timp1, Vegfa) compared with minced uterine tissue (set to 1) at both time points. Again, no further increase in gene expression was observed with E2 treatment. These data suggest increased activity of angiogenic and immune factors in early lesion development are independent of E2 treatment.
Figure 2.
Estrogen does not further increase lesion marker gene expression 24 or 48 hours after endometriosis disease initiation. (A) Gene expression of immune cell markers for neutrophils (S100A8), macrophages (F4/80), and granulocytes (G-CSFR) from WT to WT lesions at 24 and 48 hours after endometriosis-like disease initiation. Lesions are compared with minced uterine tissue (set to 1). (B) Gene expression from angiogenic factors (Timp1 and Vegfa) from WT to WT lesions at 24 and 48 hours after endometriosis-like disease initiation. Lesions were removed, RNA was isolated, and gene expression was determined by RT-PCR. Means not sharing a letter are significantly different from each other (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from each other (P > 0.05; one-way ANOVA). Error bars represent standard error of the mean; n = 8 to 11.
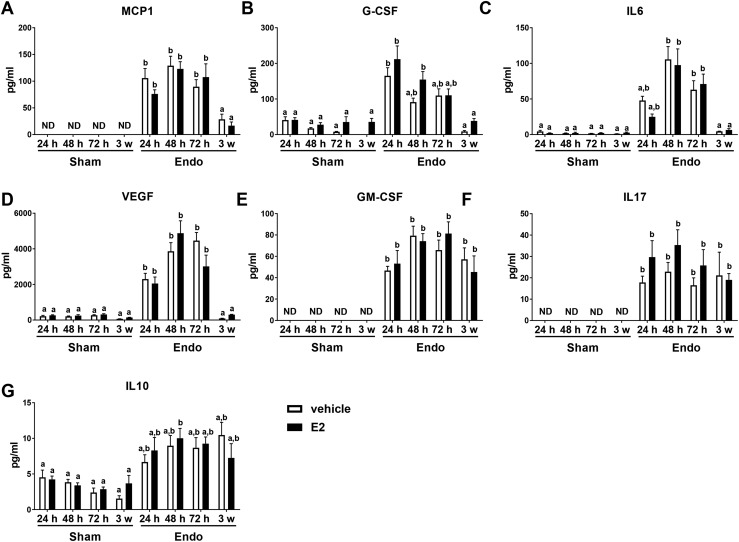
As women with endometriosis often have an aberrant peritoneal fluid cytokine milieu (40), we next evaluated peritoneal fluid cytokines from mice induced with endometriosis. At necropsy, peritoneal fluid was collected from WT to WT at 24, 48, and 72 hours after disease initiation and compared with sham peritoneal fluid (24, 48, and 72 hours) or 3-week peritoneal fluid to determine the role E2 plays in early disease initiation. All animals received vehicle or E2 and surgery, but sham animals were injected with PBS alone. MCP1/CCL2, G-CSF, IL-6, and VEGF increase in endometriosis peritoneal fluid independent of E2 treatment at 24, 48, and 72 hours after disease initiation and return to sham levels by 3 weeks after disease initiation (Fig. 3A–3D). Although other cytokines, such as GM-CSF, IL-10, and IL-17, remain chronically elevated at 3 weeks, these pro- and anti-inflammatory cytokines show no E2-mediated differences (Fig. 3E–3G). These data again suggest that during early disease development the immune-mediated responses outweigh potential E2-mediated effects.
Figure 3.
Peritoneal lavage fluid from WT to WT endometriosis mice treated with and without E2 have increased cytokine and chemokine production 24, 48, and 72 hours after disease initiation that is disease dependent using enzyme-linked immunosorbent assay (ELISA). (A–D) Transient increase is seen with MCP1, G-CSF, IL-6, and VEGF. (E–G) Chronic increase is seen with GM-CSF, IL-17, and IL-10. Endometriosis (Endo) was induced with injection of minced uterine tissue and compared with sham operated animals. At necropsy, 1 mL of saline was injected into the peritoneal cavity, mice were gently massaged, and fluid was removed for cytokine/chemokine analysis by ELISA. Means not sharing a letter are significantly different from one another (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from one another (P > 0.05; two-way ANOVA). Error bars represent standard error of the mean. Three weeks: n = 5; 24 to 72 hours: n = 8 to 12. ND, not detected.
White blood cells are recruited to the peritoneal cavity in a disease-dependent manner during the early initiation of endometriosis
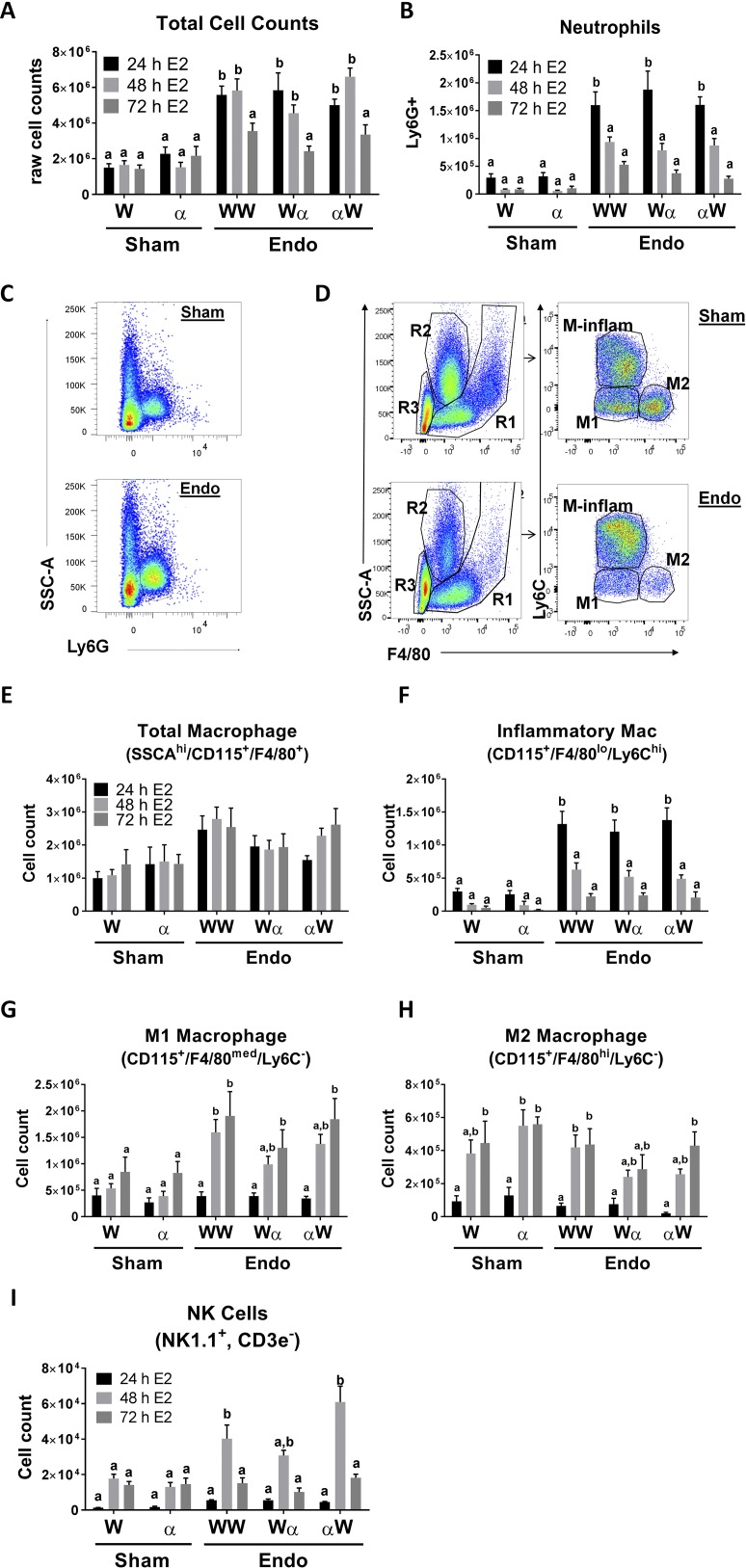
As mentioned, menstruation is an inflammatory process with 40% of menstrual tissue being composed of neutrophils, macrophages, and uNK cells (22, 34, 35). Leukocytes secrete cytokines into the peritoneal cavity, and because our data display no differences between vehicle and E2 treatment, we next examined the cell population(s) infiltrating into the peritoneal cavity after the initiation of endometriosis in mice only treated with E2. A representative differential from each group is shown in Supplemental Fig. 2 (334.6KB, pdf) . The cell differentials from the sham groups are visually different from the endometriosis groups. Leukocytes are present in the peritoneal cavity of endometriosis animals and sham animals (Supplemental Fig. 2 (334.6KB, pdf) ). In the endometriosis samples, increased numbers of recruited neutrophils and activated macrophages are seen. Total cell counts in the peritoneal fluid determined from WT to WT, WT to αERKO, and αERKO to WT 24, 48, and 72 hours after disease initiation were compared with WT and αERKO sham animals. Total cell counts demonstrated immune cells are infiltrating into the peritoneal cavity and are increased transiently regardless of ERα genotype 24 and 48 hours after disease initiation (Fig. 4A). By 72 hours, cell numbers decreased and were statistically unchanged from sham levels.
Figure 4.
Innate immune cells infiltrate into the peritoneal cavity after the initiation of endometriosis in a disease-dependent manner. (A) Total cell counts in sham WT (W), sham αERKO (α), endometriosis (endo) WT to WT (WW), endo WT to αERKO (Wα), and endo αERKO to WT (αW) 24, 48, and 72 hours after the initiation of endometriosis. A representative experiment is shown (n = 5). (B) Quantitation of peritoneal neutrophil counts. A representative experiment is shown (n = 5). (C) Neutrophils were gated for Ly6G+. (D) Macrophage gating strategy. Regions (R1, R2, and R3) were gated. R1 was then gated for F4/80+ and Ly6C+ to determine M1, M2, M-inflammatory (M-inflam) macrophages. (E) Quantitation of total macrophages (SSCAhi/CD115+,F4/80+). (F) Quantitation of inflammatory macrophages (SSCAhi/CD115+,F4/80lo/Ly6Chi) (n = 8 to 12). (G) Quantitation of M1 macrophages (SSCAhi/CD115+,F4/80med/Ly6C−) (n = 8 to 12). (H) Quantitation of M2 macrophages (SSCAhi/CD115+,F4/80+/Ly6C−) (n = 8 to 12). (I) Quantitation of NK cells (NK1.1+,CD3e−). A representative experiment is shown (n = 5). Means not sharing a letter are significantly different from each other (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from each other (P > 0.05; two-way ANOVA). Error bars represent standard error of the mean.
Cells infiltrating into the peritoneal cavity were immunophenotyped using flow cytometric analysis. The antibody panels used to stain and immunophenotype neutrophils, macrophages, epithelial cells, B cells, T cells, NK cells, and dendritic cells (DCs) are listed in the materials and methods. Cells were first gated for single cell populations and then gated based on the specific cell type antibody marker(s). Neutrophils were gated based on side scatter (SSChi), exclusion of B cells (B220−)/T cells (CD3e−), and Ly6G+ expression (Fig. 4B and 4C). Neutrophil infiltration increases 24 hours after the initiation of disease relative to sham animals. The numbers of neutrophils are reduced at 48 hours, but return to sham levels by 72 hours. No differences were observed regardless of the presence of ERα in the donor or host peritoneal cavity.
Total macrophages were gated following the peritoneal fluid gating strategy of Xia et al. (41). In brief, total macrophages were gated for SSC-Ahi, CD115+, and F4/80+ (Fig. 4D). No differences in total macrophages are seen between endometriosis and sham groups at 24, 48, or 72 hours after the initiation of endometriosis (Fig. 4E). The total macrophage sample was further immunophenotyped (Fig. 4F–4H) by gating for inflammatory macrophages (CD115+, F4/80low, Ly6Chi), M1 macrophage/proinflammatory macrophages (CD115+, F4/80med, Ly6C−), and M2 macrophage/resident anti-inflammatory macrophages (CD115+, F4/80hi, Ly6C−). No ERα genotype–dependent changes in macrophages were observed. Inflammatory macrophages increased 24 hours after disease initiation and by 72 hours return to sham levels. M1 macrophages increased 48 and 72 hours after disease initiation in endometriosis animals compared with sham animals. M2 macrophages show no striking differences between sham animals or in any of the experimental combinations at 24, 48, or 72 hours. Neutrophil and macrophage populations are the predominant cell types recruited to the peritoneal cavity after disease initiation.
Because uNK cells are a known component of menstrual effluent and can assist in vascular remodeling (42), uNK cells were immunophenotyped by excluding any CD3e+ cells (excludes NK T cells) and then gated for NK cell marker NK1.1 (Fig. 4I). NK cells increased 48 hours after disease initiation in endometriosis animals when compared with sham animals at 24, 48, and 72 hours. To ensure robust characterization of the peritoneal cavity immune cell population after the initiation of endometriosis, we also examined DCs, B cells, and T cells. Additionally, endometrial glandular cells secrete chemokines (43); therefore, recruitment of epithelial cells was examined. DCs were characterized (Ly6C+, IA-b+, CD11chi) and, as previously published by Stanic et al. (44), we observed a twofold increase in DCs compared with sham animals (Supplemental Fig. 3 (334.6KB, pdf) ). In contrast, B cells (B220+), T cells (CD3e+, CD4+ vs CD4−), and epithelial cells (EpCAM+) exhibit no statistical changes relative to sham operated or ERα status (Supplemental Fig. 3 (334.6KB, pdf) ). Additionally, naïve mice were staged based on estrus cycle (proestrus, estrus, metestrus, and diestrus) and the peritoneal cavity cell populations remained static throughout the cycle stages with our immunophenotyping parameters (data not shown). These data demonstrate innate immune cell populations are involved in the early initiation of endometriosis. Macrophages and neutrophils predominate in the early initiation of endometriosis with neutrophils peaking and inflammatory macrophages at 24 hours, and M1 macrophages at 48 hours.
Cross-talk occurs between ERα and IL-6 pathways during the early initiation of endometriosis lesion development
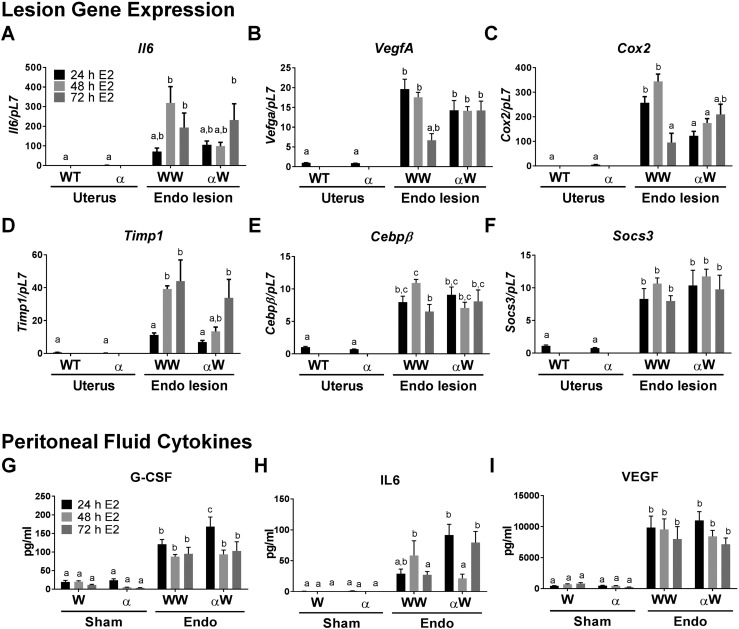
Examining the presence of factors known to be expressed in human endometriosis, we examined relative messenger RNA expression in the two main groups, WT to WT and αERKO to WT with E2 treatment compared with WT and αERKO minced uterine tissue. Our focus was on IL-6 signaling as IL-6 was one of the highest modulated cytokines in our analyses (Fig. 5A–5F). IL-6 message mirrors the secretory IL-6 levels and is not elevated at 48 hours after the initiation of endometriosis in the αERKO to WT group (Fig. 5A). ERα is known to modulate IL-6 via CEBPβ and nuclear factor κB (NF-κB) pathways (45–48); therefore, gene targets in these pathways were examined. Prostaglandin-endoperoxide synthase 2 (Cox2/Ptgs2), Timp1, Cxcl2, and Cebpβ are significantly decreased in the αERKO to WT lesions at 48 hours and suggests this pathway is involved in lesion development. Vegfa and Socs3, also associated with these pathways, were increased in αERKO to WT lesions similarly to WT to WT lesions 48 hours after the initiation of endometriosis. These data at 48 hours suggest an ERα-IL-6 axis may contribute to early lesion development and a potential pathway for therapeutic targeting.
Figure 5.
Endometriosis lesions exhibit changes in the IL-6 pathway that are blunted in the absence of ERα in donor tissue. (A–F) Gene expression in endometriosis (Endo) lesions when compared with WT and αERKO minced uterine tissue. Lesions were removed, RNA was isolated, and gene expression was determined by RT-PCR (n = 5 from a representative experiment). (G–I) Gene expression for peritoneal fluid cytokines. Peritoneal fluid from sham and endo were removed and fluid was analyzed by enzyme-linked immunosorbent assay (n = 5 to 10 from a representative experiment). For gene expression data, means not sharing a letter are significantly different from one another (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from one another (P > 0.05; one-way ANOVA). For cytokine analysis, means not sharing a letter are significantly different from one another (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from one another (P > 0.05; two-way ANOVA).
To further examine an ERα-mediated effect on early disease, peritoneal fluid from WT to αERKO and αERKO to WT at 24, 48, and 72 hours was examined and compared with WT to WT fluid for a specific subset of cytokines (Fig. 5G–5I). The proangiogenic factor VEGF is strongly increased in the peritoneal fluid of all endometriosis groups at 24, 48, and 72 hours after disease initiation when compared with sham. G-CSF shows increased levels in all endometriosis groups and times after disease initiation. IL-6 secretion decreased 48 hours after disease initiation in αERKO to WT compared with WT to WT. These findings further indicate a role for the immune system in early disease, but also implicate a specific role for ERα-IL-6-mediated cross-talk in lesion development. It is notable to see an increase in both angiogenic and early inflammatory cytokines, regardless of ERα genotype, in factors known to be important for angiogenesis and disease progression. On the other hand, IL-6 and targets downstream of IL-6 (Cox2 and Timp1) are decreased at 48 hours when ERα is knocked out of the donor uterine tissue, suggesting ERα-IL-6 cross-talk is important in lesion development.
Disruption of IL-6 signaling increases endometriosis lesion numbers
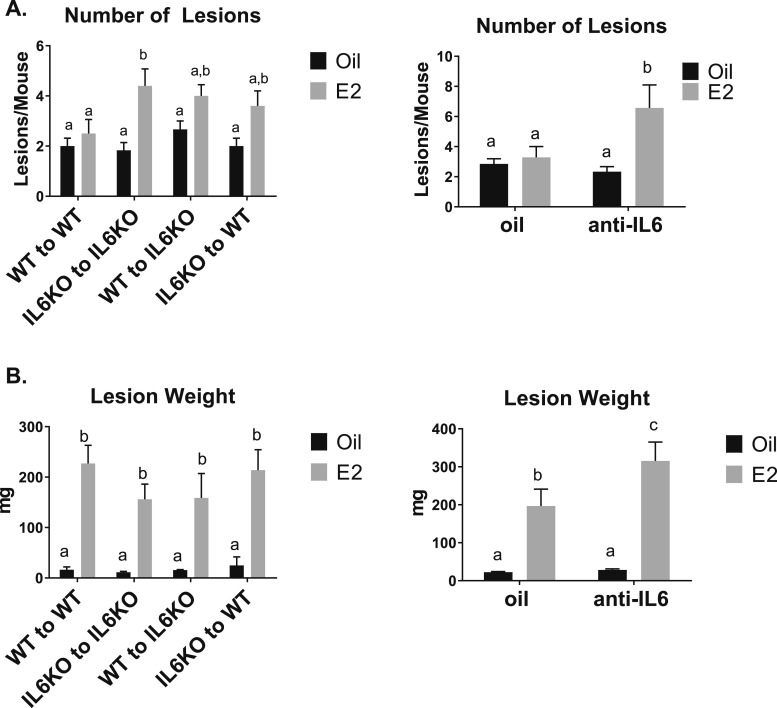
With the decreased response of IL-6 48 hours after the initiation of endometriosis, we examined the ability of endometriosis lesions to attach and grow using IL-6KO mice or in the presence of anti-IL-6 treatments at 3 weeks. IL-6KO or anti-IL-6 treatment did not alter the uterine weight increase with E2 (data not shown). After 3 weeks, as expected, no increased number of lesions is observed in WT to WT with E2 treatment; however, using IL-6KO animals increased the number of lesions in IL-6KO to IL-6KO with E2 treatment (Fig. 6A). Additionally, anti-IL-6 treatment + E2 increased the number of lesions (Fig. 6A). WT to IL-6KO or IL-6KO to WT with E2 treatment tended toward, but did not reach significance and demonstrate that both host and donor IL-6 plays a role in lesion development. Lesion weight was not altered based on IL-6 genotype, but lesion weight increased with anti-IL-6 + E2 cotreatment relative to E2 treatment alone (Fig. 6B). These findings suggest IL-6 plays a larger role in the number of lesions formed and E2 plays a role in lesion growth. These data further support cross-talk between ERα and IL-6 in the development of endometriosis.
Figure 6.
KO of IL-6 or anti-IL-6 treatment increases endometriosis lesion numbers. Lesion number and lesion weight were examined in the absence of IL-6 using IL-6KO or anti-IL-6 treatment with or without E2 treatment (n = 6). Means not sharing a letter are significantly different from one another (P < 0.05). Means sharing a same single letter or a letter in combination with other letters are not significantly different from one another (P > 0.05; two-way ANOVA).
Discussion
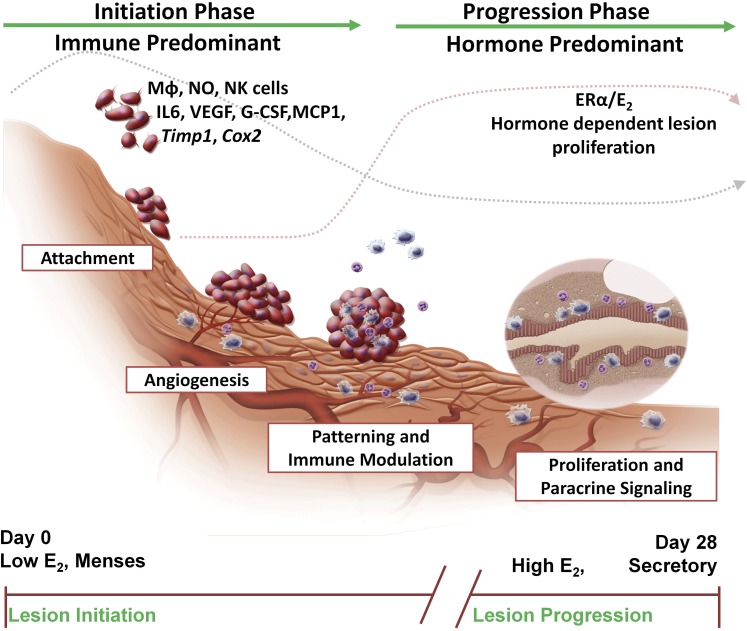
Using a mouse model of endometriosis to examine the early initiation of endometriosis disease, we find that two phases contribute to the development and maintenance of the disease—an immune predominant phase and a hormone/ERα/E2 predominant phase (Fig. 7). Based on our own and other’s findings, endometriosis develops in interconnected stages shown in Fig. 7. Herein, we find the early initiation phase of endometriosis (<72 hours) is largely modulated by the innate immune system. Many changes in gene expression, the infiltration of immune cells, and altered cytokine secretions are disease-mediated and irrespective of E2 or ERα status; however, a role for ERα-IL-6 cross-talk has emerged. Further, IL-6KO to IL-6KO and anti-IL-6 treatment revealed increased lesion numbers in the absence of IL-6 in both the host and the donor with E2 treatment. Treatment with anti-IL-6 and E2 additionally demonstrated an increase in lesion weight, suggesting even further a role for an ERα–IL-6 axis in the development and subsequent proliferation of lesions. Our findings consistently align with the hormonal changes that occur cyclically each month in women at menstruation, hormone levels are low (i.e., immune-predominant phase) then during the follicular phase, hormone levels rise (i.e., hormone/ER/E2-predominant phase). Each menstrual cycle has the potential to establish new endometriotic lesions, as greater than 90% of women have retrograde menstruation (4); whereas, concomitantly, already established endometriotic lesions continue to respond to hormonal and paracrine signals during the menstrual cycle. Our findings support the clinical observations about endometriosis; hormone alterations do not cure disease, but render the disease in a suspended state.
Figure 7.
Schematic representation of proposed development of an endometriosis lesion. The initiation phase of disease that is immune predominant includes the stages of attachment, angiogenesis, patterning, and immune modulation (<72 hours after disease initiation—dotted gray line). The progression phase of disease is hormone predominant and includes the proliferation and paracrine signaling stages of disease (dotted pink line). From our studies, these two different phases have emerged to increase understanding of the development of endometriosis lesions, with ERα and IL-6 both having roles in disease development. Correlated with menses, during the initiation of endometriosis, hormone levels are low, but when lesions are established and proliferating, they respond to hormonal regulation.
Endometriosis naturally occurs in humans and nonhuman primates because these species have an open reproductive system (49). Mice, on the other hand, have a closed reproductive system and thus, do not develop endometriosis naturally. To circumvent this issue, we use a mouse model of disease that recapitulates endometriosis by injecting syngeneic minced uterine tissue into the peritoneal cavity of a host mouse (9). In our model, endometriotic lesion development mimics human disease by forming lesions attached to the uterine blood supply, cul-de-sac region, fat pads, peritoneal wall, bladder, and bowel. Also similar to human disease, we rarely find lesions that are attached to the liver, kidney, or spleen. As seen in human disease and presented in this study, mice display increased peritoneal cavity neutrophils, macrophages, NK cells, and increased levels of IL-6, VEGF, G-CSF, MCP1, and other chemokines/cytokines. The mouse lesions respond to hormonal stimulation and have altered gene expression similar to what is observed with human lesions (40). A limitation to the mouse model is that in humans, the tissue shed from the eutopic uterus gives rise to the ectopic lesions; therefore, inherent defect in the eutopic uterus will not be reflected in the mouse model. However, suspected human eutopic uterine defects can be examined in the mouse model through the utilization of genetically modified mice to study lesion development. As we are unsure of how endometriosis develops in humans, our model, where tissue attaches naturally to sites within the peritoneal cavity, gives us the unique perspective into the early initiation of disease. Studying the early initiation of disease in humans would require extensive efforts to follow a susceptible population of adolescents, perform surgery for endometriosis diagnosis, and acquire peritoneal fluid, menstrual tissue, and serum at the time of menses to begin analyses; consequently, the use of a mouse model with controlled variables sheds invaluable light into the orchestration of the initiation of endometriosis.
Neutrophils, macrophages, and uNK cells aid in orchestrating the simultaneous breakdown and repair of the eutopic endometrium during menstruation (22, 35, 50, 51). These leukocytes secrete chemokines and cytokines, which then amplify inflammation and further leukocyte recruitment. Women with endometriosis have increased numbers of immune cells in their peritoneal cavity (52). Although our endometriosis model is a mouse model of disease and can be viewed as a limitation, we find similar increases in total cell recruitment into the peritoneal cavity of WT to WT, WT to αERKO, and αERKO to WT groups supporting the findings that ERα activity is not required in either the donor or the recipient in early endometriosis. In patients with endometriosis, peritoneal cavity neutrophil counts are approximately threefold to fivefold higher than healthy women (52). In our experimental model, neutrophils are dominant in the initial leukocyte influx into the peritoneal cavity. Similar recruitment of neutrophils, dependent on disease state and not ERα status, is observed in WT to WT, WT to αERKO, and αERKO to WT. Neutrophils, when activated, can release IL-6 that allows endothelial cells to express adhesion molecules (53, 54). Although it seems most likely this orchestration is from neutrophils, uterine epithelial cells can also secrete IL-6 (55). Additionally, IL-17A (52, 56) and IL-6 (57, 58) are among the known chemokines/cytokines increased in the peritoneal fluid of women with endometriosis, and these are also elevated in the peritoneal fluid of our mouse model. Neutrophils are capable of a vast array of specialized functions, which will be the focus of future studies, ranging from neutrophil activation to promotion of adhesion of endometrium (59, 60).
Following neutrophil activation monocytes are recruited and differentiate into macrophages (54, 61). Women with endometriosis have a fourfold to sixfold increase in peritoneal fluid macrophages (12, 52, 62), have higher peritoneal fluid volumes, higher protein concentrations, and, determined visually, increased activated macrophages compared with healthy women (63). Macrophage activation is reflected, often, in a continuous spectrum of phenotypes that rapidly change in response to the local environment (64). Similarly to women with endometriosis, in our model, we observe different macrophage types have abundantly infiltrated into the peritoneal cavity. This phenotype demonstrates complex macrophage plasticity that is dependent on the initiation of endometriosis and occurs irrespective of ERα status in the host or donor mouse.
Endometriosis is called a sterile inflammatory environment and a disease of the macrophage (63, 65, 66). Although macrophage type was not determined, peritoneal fluid of women with endometriosis, analyzed using a consensus cytokine signature enrichment analysis, found a macrophage-directed inflammatory phenotype (67). In our study, the total macrophage population did not alter, but M1 and inflammatory macrophages play a predominant role in the initiation of endometriosis 48 and 72 hours after disease initiation. Importantly, as described in women with endometriosis (63), we visualize activated macrophages by cell differential. Additionally, in support of our findings that macrophages are important to lesion establishment, 12 days after the initiation of endometriosis, Tie2+ macrophages contribute to lesion tissue organization and are required for blood vessels to reach lesion inner layers (68, 69). Macrophage chemokines and cytokines, such as MCP-1, GM-CSF, and G-CSF, are secreted regardless of host or donor ERα status, which further suggests the initial phase of endometriosis is not dependent on ERα. Supported by other endometriosis studies, Cao et al. (70), without examining lesions or lesion development, find the presence of endometrial cells in the peritoneal cavity initiate recruitment of monocytes, and Zhao et al. (10) demonstrated that chemicals suppressing both estrogenic and inflammatory activities are potential therapeutic options for endometriosis. In contrast to our studies, a suture model of endometriosis that placed peritoneal cavity cells in ex vivo culture suggests neutrophils and macrophages are important in early endometriosis; the results showed changes at 4 days (71). In our studies, by 4 days immune cell infiltration and dynamic signaling had already allowed uterine tissue to develop a blood supply to form endometriotic lesions, which, to us, demonstrates the critical nature of using a dispersal mouse model to more accurately study the early initiation of endometriosis, as it parallels more closely human disease.
IL-6 has both pro- and anti-inflammatory properties (72). Invading neutrophils drive IL-6 trans-signaling that is important to recruit monocytes, stimulate the induction of integrins, cell adhesion, actin polymerization, chemotaxis, transmigration, and proliferation (72, 73). Once recruited, monocytes can differentiate into macrophages that express ERα, where migration and adherence have been associated with E2 because ERα/E2 can regulate the IL-6 promoter through NF-κB and CEBPβ (45–48, 74–77). Interestingly, these same responses do not occur via progesterone- or ERβ-mediated signaling (45–48, 74–77). Our data and others suggest IL-6 has feed forward and feedback regulation with itself (78, 79), which can then signal via STAT3 (80, 81) to NF-κB targets (Cox2, Cebpβ, IL-6). In 60% of peritoneal endometriosis cases, NF-κB is constitutively active (82). Additionally, COX2 in combination with SRC1/SRC1-isoform, known to play a role in endometriosis (83, 84), can signal to increase mediators responsible for vascular permeability and cell sprouting (85, 86), suggesting this dynamic pathway is important for endometriosis lesion vascularization. Our data fully support the current paradigm for endometriosis, but uniquely suggest the early initiation of endometriosis is immune predominated to initiate chemotaxis and immune cell infiltration into the peritoneal cavity, which then signals via an ERα/IL-6-mediated axis when ectopic uterine cells are developing a blood supply. E2 through ERα is required for the repressive activity of IL-6, which leads to increased lesion number. Additionally, anti-IL-6 treatment increased lesion size. We find with IL-6, as we found previously for ERα (9), that both host and donor contribute to lesion properties because only when IL-6 is knocked out in the host and the donor, lesion number is affected.
In conclusion, we have uncovered a dynamic role for the innate immune system in the early initiation of endometriosis that uniquely parallels human disease. Our findings support that the early initiation of endometriosis is predominated by the innate immune system less than 3 days after the initiation of disease. Further, our studies demonstrate that an ERα/IL-6-mediated cross-talk is important for disease development. Our findings strongly support the need for detailed studies that focus on the innate immune system to further identify and characterize underlying causes of endometriosis and the potential for appropriate therapeutic development. This area of research is paramount, not only to treat disease, but to prevent endometriosis in the millions of women around the world afflicted with this disease.
Acknowledgments
We thank Dr. Yin Li and Sylvia Hewitt for critical reading of the manuscript, the National Institute of Environmental Health Sciences (NIEHS) Flow Cytometry Core and Dr. Hideki Nakano for guidance on flow cytometry, Page Myers and Sandy Hackney for surgical assistance, and the NIEHS Histology Core for histology assistance.
Financial Support: This research was supported by National Institutes of Health Grants Z01ES70065 (to K.S.K.) and R00 ES021737 (to K.A.B.).
Current Affiliation: K.A. Burns’ current affiliation is the Division of Environmental Genetics and Molecular Toxicology, Department of Environmental Health, University of Cincinnati College of Medicine, Cincinnati, Ohio 45267.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- APC
- Allophycocyanin
- BSA
- bovine serum albumin
- DC
- dendritic cell
- E2
- estradiol
- ER
- estrogen receptor
- FACS
- fluorescence-activated cell sorting
- G-CSF
- granulocyte colony-stimulating factor
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- IL
- interleukin
- KO
- knockout
- MCP1
- monocyte chemotactic protein 1
- NF-κB
- nuclear factor κB
- NIEHS
- National Institute of Environmental Health Sciences
- NK
- natural killer
- PBS
- phosphate-buffered saline
- RBC
- red blood cell
- RT-PCR
- real-time polymerase chain reaction
- uNK
- uterine natural killer
- VEGF
- vascular endothelial growth factor
- WT
- wild-type.
References
- 1.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D’Hooghe T. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27(5):1292–1299. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. [DOI] [PubMed] [Google Scholar]
- 3.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010;16(6):651–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. 1927;3(2):93–110. [PMC free article] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 7.Capellino S, Montagna P, Villaggio B, Sulli A, Soldano S, Ferrero S, Remorgida V, Cutolo M. Role of estrogens in inflammatory response: expression of estrogen receptors in peritoneal fluid macrophages from endometriosis. Ann N Y Acad Sci. 2006;1069:263–267. [DOI] [PubMed] [Google Scholar]
- 8.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149(3):1190–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology. 2012;153(8):3960–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Gong P, Chen Y, Nwachukwu JC, Srinivasan S, Ko C, Bagchi MK, Taylor RN, Korach KS, Nettles KW, Katzenellenbogen JA, Katzenellenbogen BS. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7(271):271ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: possible role in pathogenesis of endometriosis. Am J Obstet Gynecol. 1987;156(4):783–789. [DOI] [PubMed] [Google Scholar]
- 12.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril. 2004;81(3):652–661. [DOI] [PubMed] [Google Scholar]
- 13.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S. Estrogen production and metabolism in endometriosis. Ann N Y Acad Sci. 2002;955:75–85; discussion 86–78, 396–406. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001;943:131–147. [DOI] [PubMed] [Google Scholar]
- 15.Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–461. [DOI] [PubMed] [Google Scholar]
- 16.Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag. 2008;4(5):905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87(10):4782–4791. [DOI] [PubMed] [Google Scholar]
- 18.Ricci AG, Olivares CN, Bilotas MA, Meresman GF, Barañao RI. Effect of vascular endothelial growth factor inhibition on endometrial implant development in a murine model of endometriosis. Reprod Sci. 2011;18(7):614–622. [DOI] [PubMed] [Google Scholar]
- 19.Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM, Peeraer K, Tomassetti C, Meuleman C, D’Hooghe TM. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front Biosci (Elite Ed). 2009;1:444–454. [DOI] [PubMed] [Google Scholar]
- 20.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RW, King AE, Critchley HO. Cytokine control in human endometrium. Reproduction. 2001;121(1):3–19. [DOI] [PubMed] [Google Scholar]
- 22.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6(1):16–27. [DOI] [PubMed] [Google Scholar]
- 23.Kaitu’u-Lino TJ, Morison NB, Salamonsen LA. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007;328(1):197–206. [DOI] [PubMed] [Google Scholar]
- 24.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 26.Christoffersson G, Vågesjö E, Vandooren J, Lidén M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G, Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. [DOI] [PubMed] [Google Scholar]
- 28.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17(11):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol. 1996;174(5):1522–1526. [DOI] [PubMed] [Google Scholar]
- 32.Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149(2):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60(5):383–404. [DOI] [PubMed] [Google Scholar]
- 34.Battersby S, Critchley HO, de Brum-Fernandes AJ, Jabbour HN. Temporal expression and signalling of prostacyclin receptor in the human endometrium across the menstrual cycle. Reproduction. 2004;127(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salamonsen LA, Woolley DE. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol. 1999;44(1-2):1–27. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB J. 2010;24(12):4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winuthayanon W, Piyachaturawat P, Suksamrarn A, Ponglikitmongkol M, Arao Y, Hewitt SC, Korach KS. Diarylheptanoid phytoestrogens isolated from the medicinal plant Curcuma comosa: biologic actions in vitro and in vivo indicate estrogen receptor-dependent mechanisms. Environ Health Perspect. 2009;117(7):1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, DeMayo FJ, Schütz G, Korach KS. Novel DNA motif binding activity observed in vivo with an estrogen receptor α mutant mouse. Mol Endocrinol. 2014;28(6):899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152(3):R63–R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113(2):438–446. [DOI] [PubMed] [Google Scholar]
- 42.Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S, Mandelboim O. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181(3):1869–1876. [DOI] [PubMed] [Google Scholar]
- 43.Hannan NJ, Paiva P, Meehan KL, Rombauts LJ, Gardner DK, Salamonsen LA. Analysis of fertility-related soluble mediators in human uterine fluid identifies VEGF as a key regulator of embryo implantation. Endocrinology. 2011;152(12):4948–4956. [DOI] [PubMed] [Google Scholar]
- 44.Stanic AK, Kim M, Styer AK, Rueda BR. Dendritic cells attenuate the early establishment of endometriosis-like lesions in a murine model. Reprod Sci. 2014;21(10):1228–1236. [DOI] [PubMed] [Google Scholar]
- 45.Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, Gjyshi O, Cavett V, Nowak J, Garcia-Ordonez RD, Houtman R, Griffin PR, Kojetin DJ, Katzenellenbogen JA, Conkright MD, Nettles KW. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. eLife. 2014;3:e02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15(9):4971–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pottratz ST, Bellido T, Mocharla H, Crabb D, Manolagas SC. 17 beta-Estradiol inhibits expression of human interleukin-6 promoter-reporter constructs by a receptor-dependent mechanism. J Clin Invest. 1994;93(3):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Liu K, Bodenner DL. Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor kappaB transactivation. Cytokine. 2005;31(4):251–257. [DOI] [PubMed] [Google Scholar]
- 49.D’Hooghe TM, Bambra CS, De Jonge I, Lauweryns JM, Koninckx PR. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75(2):98–101. [DOI] [PubMed] [Google Scholar]
- 50.Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Rev Endocr Metab Disord. 2012;13(4):277–288. [DOI] [PubMed] [Google Scholar]
- 51.Maybin JA, Hirani N, Brown P, Jabbour HN, Critchley HO. The regulation of vascular endothelial growth factor by hypoxia and prostaglandin F2α during human endometrial repair. J Clin Endocrinol Metab. 2011;96(8):2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milewski Ł, Dziunycz P, Barcz E, Radomski D, Roszkowski PI, Korczak-Kowalska G, Kamiński P, Malejczyk J. Increased levels of human neutrophil peptides 1, 2, and 3 in peritoneal fluid of patients with endometriosis: association with neutrophils, T cells and IL-8. J Reprod Immunol. 2011;91(1-2):64–70. [DOI] [PubMed] [Google Scholar]
- 53.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–439. [DOI] [PubMed] [Google Scholar]
- 54.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112(4):1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piva M, Horowitz GM, Sharpe-Timms KL. Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotic cells in vitro: a model for endometrial-peritoneal interaction in endometriosis. J Clin Endocrinol Metab. 2001;86(6):2553–2561. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Xu H, Lin J, Qian Y, Deng L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG. 2005;112(8):1153–1155. [DOI] [PubMed] [Google Scholar]
- 57.Akoum A, Lemay A, Paradis I, Rheault N, Maheux R. Secretion of interleukin-6 by human endometriotic cells and regulation by proinflammatory cytokines and sex steroids. Hum Reprod. 1996;11(10):2269–2275. [DOI] [PubMed] [Google Scholar]
- 58.Martínez S, Garrido N, Coperias JL, Pardo F, Desco J, García-Velasco JA, Simón C, Pellicer A. Serum interleukin-6 levels are elevated in women with minimal-mild endometriosis. Hum Reprod. 2007;22(3):836–842. [DOI] [PubMed] [Google Scholar]
- 59.Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, Rot A. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32(10):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114(21):4613–4623. [DOI] [PubMed] [Google Scholar]
- 62.Olive DL, Weinberg JB, Haney AF. Peritoneal macrophages and infertility: the association between cell number and pelvic pathology. Fertil Steril. 1985;44(6):772–777. [DOI] [PubMed] [Google Scholar]
- 63.Haney AF. Endometriosis, macrophages, and adhesions. Prog Clin Biol Res. 1993;381:19–44. [PubMed] [Google Scholar]
- 64.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, Yoshida S, Sado T, Oi H, Kobayashi H. New insights into the pathophysiology of endometriosis: from chronic inflammation to danger signal. Gynecol Endocrinol. 2011;27(2):73–79. [DOI] [PubMed] [Google Scholar]
- 66.Capobianco A, Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beste MT, Pfäffle-Doyle N, Prentice EA, Morris SN, Lauffenburger DA, Isaacson KB, Griffith LG. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci Transl Med. 2014;6(222):222ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175(2):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Capobianco A, Monno A, Cottone L, Venneri MA, Biziato D, Di Puppo F, Ferrari S, De Palma M, Manfredi AA, Rovere-Querini P. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. 2011;179(5):2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cao X, Yang D, Song M, Murphy A, Parthasarathy S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: implications for endometriosis. Fertil Steril. 2004;82(Suppl 3):999–1007. [DOI] [PubMed] [Google Scholar]
- 71.Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147(3):1278–1286. [DOI] [PubMed] [Google Scholar]
- 72.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. [DOI] [PubMed] [Google Scholar]
- 73.Clahsen T, Schaper F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J Leukoc Biol. 2008;84(6):1521–1529. [DOI] [PubMed] [Google Scholar]
- 74.Ray A, Prefontaine KE, Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269(17):12940–12946. [PubMed] [Google Scholar]
- 75.Cutolo M, Villaggio B, Bisso A, Sulli A, Coviello D, Dayer JM. Presence of estrogen receptors in human myeloid monocytic cells (THP-1 cell line). Eur Cytokine Netw. 2001;12(2):368–372. [PubMed] [Google Scholar]
- 76.Okada M, Suzuki A, Mizuno K, Asada Y, Ino Y, Kuwayama T, Tamakoshi K, Mizutani S, Tomoda Y. Effects of 17 beta-estradiol and progesterone on migration of human monocytic THP-1 cells stimulated by minimally oxidized low-density lipoprotein in vitro. Cardiovasc Res. 1997;34(3):529–535. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki A, Mizuno K, Ino Y, Okada M, Kikkawa F, Mizutani S, Tomoda Y. Effects of 17 beta-estradiol and progesterone on growth-factor-induced proliferation and migration in human female aortic smooth muscle cells in vitro. Cardiovasc Res. 1996;32(3):516–523. [PubMed] [Google Scholar]
- 78.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21(13):3763–3770. [DOI] [PubMed] [Google Scholar]
- 79.Baumgarten SC, Frasor J. Minireview: Inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26(3):360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, Fazleabas AT, Young SL, Lessey BA, Jeong JW. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30(5):1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoo JY, Jeong JW, Fazleabas AT, Tayade C, Young SL, Lessey BA. Protein inhibitor of activated STAT3 (PIAS3) is down-regulated in eutopic endometrium of women with endometriosis. Biol Reprod. 2016;95(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.González-Ramos R, Donnez J, Defrère S, Leclercq I, Squifflet J, Lousse JC, Van Langendonckt A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. 2007;13(7):503–509. [DOI] [PubMed] [Google Scholar]
- 83.Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O’Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18(7):1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O’Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31(2, Suppl 7):2–11. [DOI] [PubMed] [Google Scholar]
- 86.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA. 2005;102(41):14641–14646. [DOI] [PMC free article] [PubMed] [Google Scholar]