Abstract
Laminin α4 (LAMA4) is located in the extracellular basement membrane that surrounds each individual adipocyte. Here we show that LAMA4 null (Lama4−/−) mice exhibit significantly higher energy expenditure (EE) relative to wild-type (WT) mice at room temperature and when exposed to a cold challenge, despite similar levels of food intake and locomotor activity. The Lama4−/− mice are resistant to age- and diet-induced obesity. Expression of uncoupling protein 1 is higher in subcutaneous white adipose tissue of Lama4−/− mice relative to WT animals on either a chow diet or a high-fat diet. In contrast, uncoupling protein 1 expression was not increased in brown adipose tissue. Lama4−/− mice exhibit significantly improved insulin sensitivity compared with WT mice, suggesting improved metabolic function. Overall, these data provide critical evidence for a role of the basement membrane in EE, weight gain, and systemic insulin sensitivity.
Despite similar food consumption and activity, mice lacking basement membrane protein laminin α4 were resistant to obesity and had increased beige subcutaneous adipose tissue and energy expenditure.
Adipocytes are lined by a thin network of proteins known as the basement membrane. Basement membranes are submicron-sized extracellular matrix (ECM) structures that are distinct in composition, structure, and location from the stromal ECM. Stromal ECM proteins such as collagen I, III, and VI are present in adipose connective tissue and areas of fibrosis, and their contributions to adipose tissue expansion have been investigated (1–4), but little is known about how basement membrane proteins contribute to adipose tissue function. The basement membrane consists primarily of various laminin isoforms, collagen IV, entactin, and perlecan (5). These components interact in a defined way to form a mesh-like network that interacts with adipocytes and also serves as a link to the stromal ECM. In humans, obese adipose tissue has a disorganized basement membrane with collagen IV clusters frequently associated with adipocytes from obese subjects compared with adipocytes from lean subjects (6). Recently, basement membranes have emerged as a critical regulator of both normal physiological conditions and pathologic events in many tissues and organs. For example, altered composition of kidney basement membranes (7, 8) and increased stiffness of the retinal basement membrane (9) contribute to comorbidities observed in people with diabetes.
Laminin isoforms are fundamental to both the assembly and function of basement membranes. The laminin family of glycoproteins has heterotrimeric structures of α, β, and γ subunit chains (10–13). Laminins interact with other basement membrane proteins, cell membrane receptors, and also the stromal ECM. The specific isoforms present in a particular tissue vary with anatomic location and disease state. The primary isoforms present in adipose tissue are α4, α2, β1, and γ1 (14–17). The composition of the adipose basement membrane changes during adipocyte differentiation, primarily through increased expression of laminin α4 (LAMA4) (18–21). However, the relative importance of specific isoforms in terms of regulating tissue physiology is unclear.
Recently, mice with a null mutation in the laminin α4 gene (Lama4−/−) have been shown to exhibit reduced weight gain and adipose mass accumulation under both standard chow and high-fat diets (HFDs) (17). However, the mechanism underlying this resistance has not been defined. Brown adipose tissue (BAT) is an adipose depot that plays a role in energy expenditure (EE) and heat generation. Interest in BAT has increased in recent years, particularly as a potential treatment of obesity and metabolic disease. However, the small volume of BAT relative to white adipose tissue (WAT) in adults suggests that its therapeutic potential may be limited when targeted directly. Beige or brite (brown-in-white) cells are adipocytes that reside in WAT depots that have been induced to behave in a thermogenic manner similar to BAT (22). Beige adipocytes exhibit characteristics of both white and brown adipocytes and have a thermogenic capacity that can enhance both local adipose function and systemic metabolism (22–24). Recent evidence suggests that laminin α1–based and laminin γ1–based peptides (25) and substrate properties (26) influence adipocyte expression of uncoupling protein 1 (UCP1) in three-dimensional culture but, to our knowledge, there have not been any studies showing that the basement membrane influences beige or brown adipose function in vivo.
In the current studies, we examine the mechanism underlying resistance to weight gain observed in Lama4−/− mice. We found that energy expenditure was increased without any substantial changes in locomotor activity on both chow and HFDs. Enhanced UCP1 expression was observed in subcutaneous WAT (sWAT) and, depending on diet, in BAT as well. In addition, systemic insulin sensitivity was increased when mice were challenged with HFD. These data provide important evidence for a role of the basement membrane in adipocyte metabolic function and beige adipose formation.
Research Design and Methods
Animals, diets, and housing
The number of animals to be used was based on the expected effects size. All animal procedures and animal numbers were approved by the University of Chicago Institutional Animal Care and Use Committee. The generation of Lama4−/− was previously described (27). The mice were backcrossed to C57 BL/6 mice (Charles River) for more than 10 generations. Mice were fed either standard chow diet (Teklad 2918; Harlan Laboratories) or 45% HFD (Teklad custom diet TD.06415; Harlan Laboratories). Both the chow diet and HFD were matched for micronutrient content. The animals were fed ad libitum. Animals were weighed weekly starting at 8 weeks of age. The mouse studies were conducted without randomization to experimental groups, but the investigators were blinded to the genotype for immunostaining analysis, indirect calorimetry measurements, and dual-energy X-ray absorptiometry (DEXA) scan measurements.
Immunostaining
For immunostaining in mouse tissues, animals at 13 to 15 weeks of age were euthanized and tissues harvested. The tissues were fixed in 4% paraformaldehyde, paraffin embedded, and sectioned (5μm thickness) with a microtome. Immunohistochemical staining for UCP1 was performed. Briefly, tissue sections were first incubated with primary antibody (anti-UCP1, abcam 10983, produced in rabbit, 5.2 μg/mL) and 5% goat serum at 4°C. After overnight incubation and rinsing with phosphate-buffered saline, the avidin-biotin complex method (Vectastain Elite ABC kit) was used as secondary antibody. sWAT- and UCP1-positive area percentages were then quantified and compared using bright-field microscope images.
Indirect calorimetry
Four male Lama4−/− and four Lama4+/+ aged 11 to 14 weeks on a chow diet and four male Lama4−/− and three Lama4+/+ on HFD were evaluated for metabolic parameters using the TSE Systems LabMaster—Metabolism Research Platform. Physical activity was monitored in the X and Z planes using an infrared light beam. Measurements for animals’ oxygen consumption (VO2) and carbon dioxide production (VCO2) were used to estimate various metabolic parameters, including the respiratory exchange ratio, EE, and substrate utilization. Mice were provided free access to food and water, in a 12-hour light (6 am to 6 pm) and 12-hour dark (6 pm to 6 am) cycle. The experimental protocol was 5-day acclimation and a 2- to 3-day experimental period. For the initial 5 days, the cage ambient temperature was at room temperature (25°C), followed by 24 hours of thermoneutrality at 30°C and then finally 24 hours in cold challenge at 16°C.
DEXA
DEXA (Lunar PIXImus densitometer system; GE Healthcare) was conducted on 15-week-old mice to measure body composition using PIXImus 2 software. Prior to the initiation of the experiment, the system was calibrated according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction
Freshly collected tissues or cells were flash frozen in liquid nitrogen and stored at −80°C for later processing or immediately processed using an E.Z.N.A Total RNA kit II (Omega) extraction kit following the manufacturer’s instructions. The purity and concentration of the isolated RNA was assessed using a Nanodrop 2000; 260/280 ratios were approximately 2.0. The complementary DNA was synthesized with Quanta Biosciences Qscript. Quantitative real-time polymerase chain reaction was performed using SYBER green on a Bio-Rad MyiQ RT-PCR detection system (Bio-Rad). Primers (Table 1) from Integrated DNA Technologies were selected from literature or designed using Mouse Primer Depot (http://mouseprimerdepot.nci.nih.gov/) and Primer-Blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Melt curve analysis was used to assess primer specificity, and 18S ribosomal RNA was used as the reference gene to control for total messenger RNA recovery. Gene expression levels were evaluated by the ΔΔ threshold cycle (Ct) method.
Table 1.
Quantitative Real-Time Polymerase Chain Reaction Primer Sequences
| Primer | Forward Primer | Reverse Primer |
|---|---|---|
| UCP1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG |
| CIDEA | TGCTCTTCTGTATCGCCCAGT | GCCGTGTTAAGGAATCTGCTG |
| Cox7a1 | GTACTGGGAGGTCATTGTCG | GCTGAGGACGCAAAATGAG |
| 18S | AACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Insulin and glucose tolerance testing
A glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed on 13- and 15-week-old male mice, respectively. The mice were fasted for 6 hours. For GTT, animals were injected 1 g/kg d-glucose intraperitoneally (IP), whereas for the ITT, 0.75 U/kg human insulin was injected IP with glucose levels measured from tail bleeds at 0, 15, 30, 45, 60, 90, and 120 minutes after IP injection using a Freestyle lite blood glucose monitor (Abbott). The area under the curve was calculated using SigmaPlot 11.0 software (Systat Software, Inc., San Jose, CA) for the GTT and ITT.
Primary fibroblast isolation
The method for depositing ECM from fibroblasts has been previously described (28). Primary fibroblast cells were isolated from Lama4−/− and Lama4+/+ mice skin following this previously published procedure (29). Briefly, dermal tissue was explanted from euthanized mice. The tissue was minced and placed in tissue plates and the fibroblast migrated out of the tissue and adhered to the tissue culture plates. Tissue fragments and cells were cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Primary adipose-derived stem cell isolation
Subcutaneous adipose depots were harvested from euthanized Lama4−/− and Lama4+/+ mice and digested in collagenase type I. The adipose-derived stem cells were enzymatically isolated following the published protocol (30). Briefly, digestion was performed in an orbital shaker at 37°C for 60 minutes. The digest was then centrifuged with the floating adipocyte layer discarded. The pelleted stromal vascular fraction was washed two times with complete media and plated on tissue culture plastic. The complete media were DMEM/F12 with 10% FBS and 1% penicillin/streptomycin. Cells at passages 3 to 6 were used for experiments.
Fibroblast ECM generation
The method for depositing ECM from fibroblasts has been previously described (28). Primary Lama4−/− and Lama4+/+ fibroblast cells were isolated from mice skin and lung following a previously published procedure (29). Briefly, fibroblast cells were plated on gelatin-coated well plates at 2.5 × 105 cells/mL and cultured for 8 days using media containing 0.5 mg/mL ascorbic acid to enhance ECM deposition. Next, the ECM was decellularized using 0.5% Triton X-100 and 20 mM NH4OH in phosphate-buffered saline. Then, isolated Lama4−/− and Lama4+/+ adipose-derived stem cells were seeded on the ECM at 2 × 104 cells/mL. These cells were then cultured overnight and then given media to induce differentiation into adipocytes. Induction media were given to the cells for 48 hours to induce adipocyte differentiation. The induction media consisted of DMEM/F12 with 10% FBS, 1% penicillin/streptomycin, 125 μM indomethacin, 2 μg/mL dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 0.5 μM rosiglitazone, 1 nM 3,3′,5-triiodo-l-thyronine, and 5 μg/mL insulin (31). After 2 days of induction media, cells were given maintenance media consisting of F12/DMEM with 10% FBS, 1% penicillin/streptomycin, 1 nM 3,3′,5-triiodo-l-thyronine, and 5 μg/mL insulin. On the first maintenance media change (day 2) to maintenance media, 0.5 μM rosiglitazone was added. On the second maintenance media change (day 4), 1.0 μM rosiglitazone was added. Additional maintenance media changes past day 7 did not receive additional rosiglitazone. Cells were incubated at 37°C and 5% CO2.
Statistics
The repeated-measures analysis of variance (ANOVA) followed with the Tukey test was used for repeated measurements of weight, food consumption, movements, EE, VO2, VCO2, and respiratory exchange ratio over time. The two-way repeated-ANOVA with one-factor repetition was followed by the Holm-Sidak method for individual comparison posttest. The comparison of cells grown on different ECM matrix used Kruskal-Wallis one-way ANOVA on ranks followed by all pairwise multiple comparison procedures (Dunn’s method). The Student t test was used for all other statistical analysis. In all cases, P < 0.05 was considered significant.
Results
Lama4−/− mice consume the same amount of food as wild-type mice
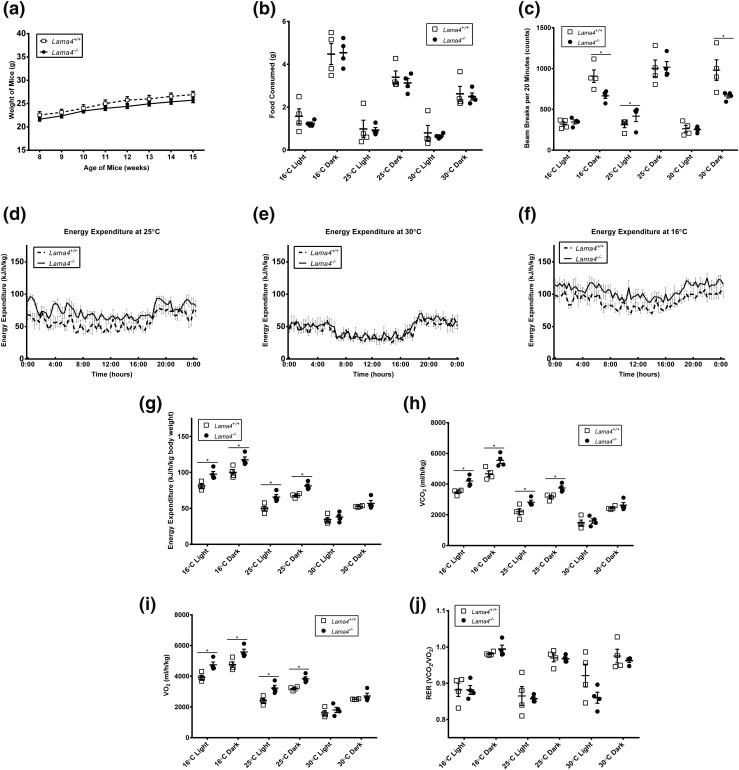
Lama4−/− and Lama4+/+ mice on a chow diet were evaluated at 12 to 15 weeks of age. This age range was selected because we wished to assess metabolic and other responses prior to any significant alterations in weight. Lama4−/− mice have been shown to be resistant to age-induced obesity, with significant differences in weight observed only after 15 weeks of age (17). As expected, Lama4−/− and Lama4+/+ mice weights on a chow diet were the same during this time frame [Fig. 1(a)]. In addition, we found that Lama4−/− mice consume equal amounts of standard chow food as the Lama4+/+ mice at each of three temperatures: cold challenge (16°C), room temperature (25°C), and thermoneutrality (30°C), during both light and dark cycles [Fig. 1(b)]. Food consumption for mice housed in thermoneutral conditions is lower compared with mice housed at room temperature or in cold cages for both Lama4−/− and Lama4+/+ mice. These findings are expected and consistent with other literature on rodent food intake at different housing temperatures (32).
Figure 1.
Lama4−/− mice phenotype on a standard diet. (a) Average body weight of mice fed standard chow diet (Lama4−/− mice, n = 19; Lama4+/+ mice, n = 15). (b) Average food consumption of mice fed standard chow diet (n = 4 mice per group). (c) Average total movements in the x direction by mice fed a standard chow diet (n = 4 mice per group). (d) Average EE of mice housed at 25°C and fed a standard chow diet (n = 4 mice per group). (e) Average EE of mice housed at 30°C and fed a standard chow diet (n = 4 mice per group). (f) Average EE of mice housed at 16°C and fed a standard chow diet (n = 4 mice per group). (g) Average EE over a 12-hour light and dark cycle at each temperature: 16°C, 25°C, and 30°C (n = 4 mice per group). (h) Average VCO2 levels during a 12-hour dark cycle (n = 4 mice per group). (i) Average VO2 levels during a 12-hour dark cycle at each temperature: 16°C, 25°C, and 30°C (n = 4 mice per group). (j) Average respiratory exchange ratio (RER) during a 12-hour dark cycle at each temperature: 16°C, 25°C, and 30°C (n = 4 mice per group). Data are presented as mean ± standard error of the mean with *P < 0.05 as significant from repeated-measures ANOVA.
Lama4−/− mice exhibit comparable physical activity to wild-type mice
Physical activity was evaluated for the mice at all three temperatures. Movement measurements evaluated included ambulatory movement (breaking two beams consecutively), fine movements, and rearing. Lama4−/− mice exhibit the same or decreased movement relative to Lama4+/+ mice in most metrics [Fig. 1(c)]. In particular, during the 16°C cold challenge and at thermoneutrality, Lama4−/− mice moved significantly less in total, fine, and ambulatory movements [Fig. 1(c)] and rearing (data not shown) in comparison with Lama4+/+ mice during dark cycles. In only one condition during the 25°C light cycle did Lama4−/− mice exhibit significantly higher movement (ambulatory movement). Overall, these results suggest that locomotor activity of Lama4−/− mice is not an explanation for the resistance to weight gain.
Energy expenditure is increased in Lama4−/− mice at room temperature (25°C) and during a cold challenge (16°C)
Energy expenditure was assessed based on the guidelines described by Tschöp et al. (33) for lower body weight in comparison with age- and sex-matched wild-type (WT) controls. Energy expenditure was increased in Lama4−/− mice at room temperature [Fig. 1(d) and 1(g)]. However, EE was equal at thermoneutral conditions (30°C) [Fig. 1(e) and 1(g)], consistent with decreased sympathetic activation of BAT and/or beige adipose activation (34). In contrast, cold exposure results in metabolic changes due to an upregulation of BAT and beige cell thermogenesis. Energy expenditure of the Lama4−/− mice was significantly higher at both ambient temperature and when cold challenged [Fig. 1(f) and 1(g)]. This was true during both the light and dark cycles [Fig. 1(d)–1(j)]. VCO2 and VO2 levels were both increased for Lama4−/− mice at 25°C and 16°C [Fig. 1(h) and 1(i)] without a difference in the respiratory exchange ratio [Fig. 1(j)]. Thus, Lama4−/− mice exhibit increased EE without any increase in movement or decrease in food intake, at both room temperature and during cold challenges.
UCP1 expression is significantly increased in Lama4−/− mice sWAT depots
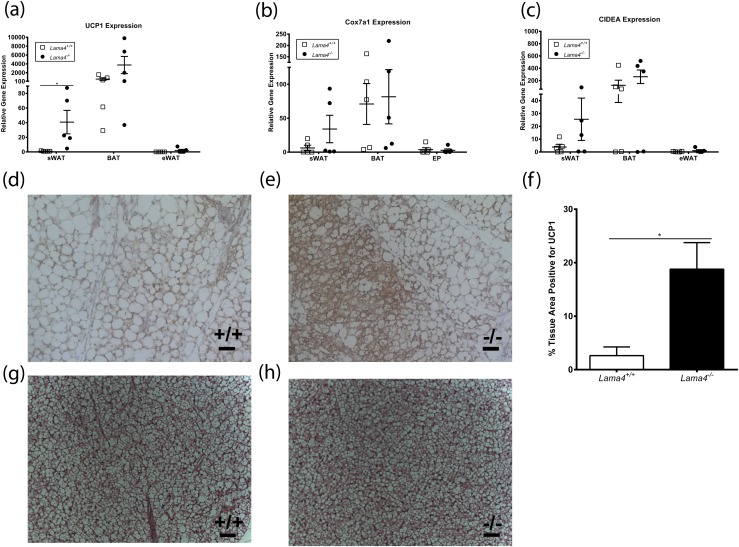
WAT is primarily involved in energy storage and cytokine production, whereas BAT is a major site of thermogenesis. UCP1 is a mitochondrial protein that contributes to heat generation in BAT. To determine if altered UCP1 expression could explain the increase in EE seen in Lama4−/− mice, we first assessed UCP1 messenger RNA expression. UCP1 expression was not significantly different in BAT from chow-fed Lama4−/− mice [Fig. 2(a)] relative to control mice.
Figure 2.
Increased beige markers in Lama4−/− subcutaneous white adipose depot. Gene expression profile of the epididymal, subcutaneous, and brown fat depots from Lama4−/− mice housed at 25°C and fed a standard diet for (a) UCP1 (n = 5 mice per group), (b) Cox7a1 (n = 5 mice per group), and (c) CIDEA (n = 5 mice per group). (d, e) UCP1 immunohistochemistry sWAT for (d) Lama4+/+ and (e) Lama4−/−. Scale bar: 50 µm. (f) Percentage of sWAT area positive for UCP1 (n = 5 mice per group). (g, h) Histology of BAT stained with hematoxylin and eosin from (g) Lama4+/+ and (h) Lama4−/−. Data are presented as mean ± standard error of the mean. Two-tailed Student t test with *P < 0.05 as significant.
WAT depots can develop UCP1-expressing cells based on environmental conditions. These cells have been termed beige or brite adipocytes. To determine if there was an increase in beige adipocytes in Lama4−/− mice, UCP1 expression was assessed in both sWAT and epididymal WAT (eWAT). sWAT from Lama4−/− mice had higher UCP1 expression (40.7 ± 16.1-fold expression, n = 5) than WT mice (1.0 ± 0.2-fold expression, n = 5) [Fig. 2(a)]. In contrast, UCP1 expression in eWAT was not significantly different between Lama4−/− and Lama4+/+ mice. Expression of other genes involved in thermogenesis was also evaluated in sWAT, eWAT, and BAT from chow-fed Lama4−/− and WT mice. sWAT had a trend toward increased expression of Cox7a1 and CIDEA in Lama4−/− mice relative to WT mice [Fig. 2(b) and 2(c)], but levels were not significantly different for eWAT, BAT, or sWAT in Lama4−/− mice relative to controls. Immunostaining was performed on sWAT harvested from Lama4−/− and Lama4+/+ mice. sWAT depots showed significantly higher levels of UCP1-positive tissue [Fig. 2(d) and 2(e)]. When quantifying the area of UCP1 staining, Lama4−/− mice had a mean of 18.79% ± 4.97% of UCP1-positive tissue, whereas Lama4+/+ mice subcutaneous WAT (sWAT) had only 2.62% ± 1.63% [Fig. 2(f), n = 5, P = 0.015]. No differences were observed between hematoxylin and eosin stains of BAT from Lama4+/+ [Fig. 2(g)] and Lama4−/− [Fig. 2(h)] mice. These data suggest that there are increased levels of beige adipocytes in sWAT depot from Lama4−/− mice.
Lama4−/− mice exhibit increased EE and resistance to obesity when challenged with HFD
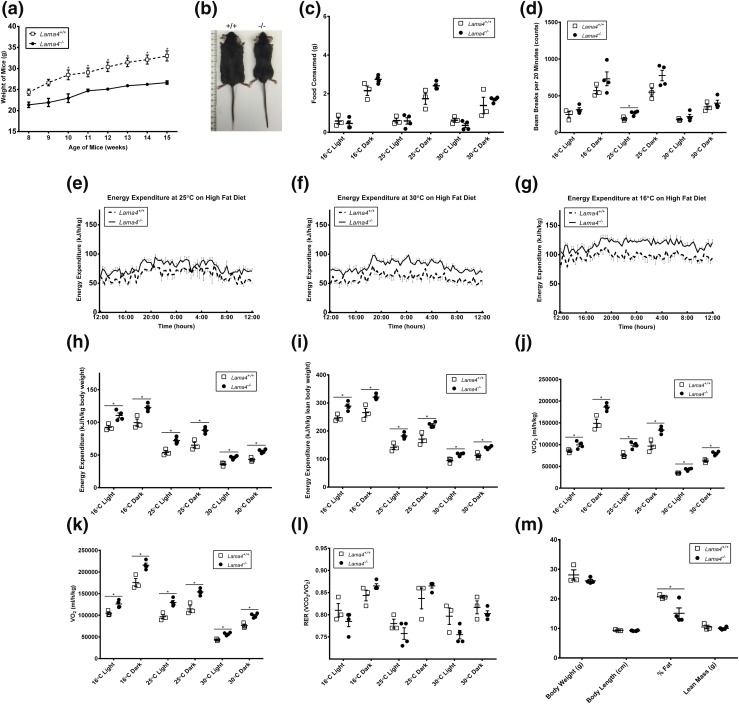
After 2 weeks on HFD, Lama4−/− mice gained significantly less weight compared with control Lama4+/+ mice [Fig. 3(a) and 3(b)]. In fact, Lama4−/− mice on HFD maintained their lean weight as the control mice gained weight (Lama4+/+). Lama4−/− mice consumed the same or slightly more HFD food compared with the Lama4+/+ mice at each of three temperatures: cold challenge (16°C), room temperature (25°C) conditions, and thermoneutrality (30°C), during both the light and dark cycles [Fig. 3(c)]. In addition, the Lama4−/− mice exhibited the same or fewer movements on the HFD than Lama4+/+ mice in most metrics [Fig. 3(d)]. In only one condition (ambulatory movement) during the 25°C light cycle did the Lama4−/− mice move significantly more than the Lama4+/+ mice. As with chow diet, differences in food intake and locomotor activity do not appear to explain the resistance to obesity seen in Lama4−/− mice when challenged with HFD.
Figure 3.
Lama4−/− mice phenotype on HFD. (a) Average body weight of mice fed HFD: Lama4−/−, n = 6 (weeks 8 to 10), n = 9 (weeks 11 to 12), and n = 13 (weeks 13 to 15); Lama4+/+, n = 10 (weeks 8 to 10), n = 14 (weeks 11 to 12), and n = 20 (weeks 13 to 15). Data are presented as mean ± standard error of the mean (SEM) with *P < 0.05 as significant from repeated-measures ANOVA. (b) Lama4−/− mice remained lean compared with control mice when fed HFD at age 15 weeks; ruler is in centimeters. (c) Average food consumption of mice fed HFD (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (d) Average total movements in the x direction by mice fed HFD (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (e) Average EE of mice housed at 25°C and fed HFD (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (f) Average EE of mice housed at 30°C and fed HFD (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (g) Average EE of mice housed at 16°C and fed HFD (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (h) Average EE of mice fed HFD normalized to body weight (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (i) Average EE of mice fed HFD normalized to lean weight (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (j) Average VCO2 levels during a 12-hour dark cycle (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). (k) Average VO2 levels during a 12-hour dark cycle at each temperature: 16°C, 25°C, and 30°C (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (l) Average respiratory exchange ratio (RER) during a 12-hour dark cycle at each temperature: 16°C, 25°C, and 30°C (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from repeated-measures ANOVA. (m) DEXA analysis of percent fat and lean tissue mass In mice (Lama4−/− mice, n = 4; Lama4+/+ mice, n = 3). Data are presented as mean ± SEM with *P < 0.05 as significant from two-tailed Student t test.
Energy expenditure for Lama4−/− mice is increased when fed HFD
Lama4−/− and Lama4+/+ mice were housed in metabolic cages at room temperature (25°C), in thermoneutral (30°C) conditions, and with a cold challenge (16°C). Energy expenditure was significantly increased for Lama4−/− compared with WT control mice at all temperature conditions when mice were challenged with HFD [Fig. 3(e)–3(i)]. The Lama4−/− VCO2 and VO2 were significantly higher compared with WT control mice [Fig. 3(j) and 3(k)]. The respiratory exchange ratio was not significantly higher at any temperature [Fig. 3(l)]. DEXA scanning indicated that the percentage of fat was lower in Lama4−/− mice, whereas body length and lean mass were both similar [Fig. 3(m)].
Beige markers are upregulated in Lama4−/− mice fed HFD
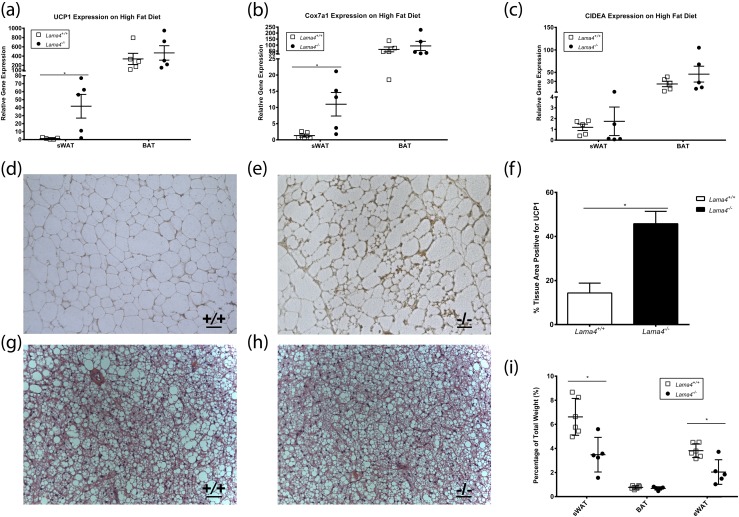
Similar to a chow diet, messenger RNA expression of UCP1 was increased in sWAT of Lama4−/− mice on HFD (41.7 ± 14.8-fold expression, n = 5) compared with Lama4+/+ mice (1.4 ± 0.5-fold expression, n = 5, P = 0.026) [Fig. 4(a)]. Interestingly, UCP1 expression was not increased in BAT from Lama4−/− mice (Lama4−/− mice 448.1 ± 162.5-fold and Lama4+/+ mice 250.3 ± 60.9-fold, n = 5) [Fig. 4(a)]. Cox7a1 expression was also significantly higher in sWAT from Lama4−/− mice on HFD relative to controls. However, no differences in Cox7a1 were observed in BAT [Fig. 4(b)]. Expression of CIDEA was not increased in sWAT or BAT [Fig. 4(c)]. Immunostaining for UCP1 was performed on adipose from Lama4−/− and Lama4+/+ mice aged 15 weeks fed HFD for 7 weeks. Large regions of UCP1-positive stained area could be seen in sWAT adipose from Lama4−/− mice but not in WT mice [Fig. 4(d) and 4(e)]. As observed on a chow diet, HFD-fed Lama4−/− mice exhibited increased protein expression of UCP1 in the sWAT. Lama4−/− mice had a mean of 45.81% ± 12.54% of UCP1-positive tissue, whereas Lama4+/+ mice sWAT had only 14.35% ± 10.03% [Fig. 4(f), n = 5]. In Lama4−/− and Lama4+/+ mice fed HFD, the BAT had a similar appearance [Fig. 4(g) and 4(h)]. The sWAT depot and epididymal WAT (eWAT) depots were significantly increased in weight in Lama4+/+ mice fed HFD; notably, the BAT depots were similar in size [Fig. 4(i)].
Figure 4.
Increased beige markers in HFD-fed Lama4−/−. Gene expression profile of the epididymal, subcutaneous, and brown fat depots from Lama4−/− mice housed at 25°C and fed HFD: (a) UCP1 (n = 5 mice per group), (b) Cox7a1 (n = 5 mice per group), and (c) CIDEA (n = 5 mice per group). (d, e) UCP1 immunohistochemistry sWAT for (d) Lama4+/+ and (e) Lama4−/−. Scale bar: 50 µm. (f) Percentage of sWAT area positive for UCP1 (n = 5 mice per group). (g, h) Histology of BAT stained with hematoxylin and eosin from (g) Lama4+/+ and (h) Lama4−/−. (i) Fat pad weights as a percentage of total weights (Lama4+/+, n = 5; Lama4−/−, n = 6). Data are presented as mean ± standard error of the mean with *P < 0.05 as significant from two-tailed Student t test.
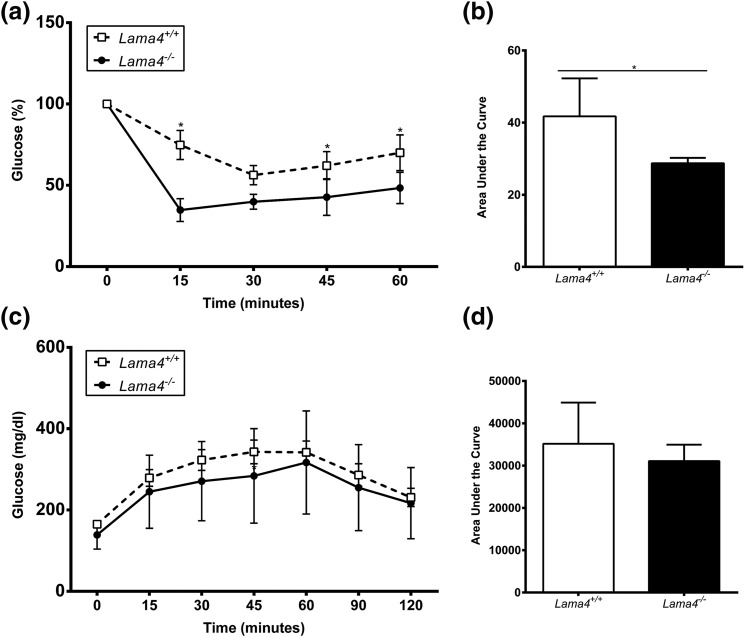
Lama4−/− mice exhibit significantly improved insulin sensitivity compared with WT mice
To define the effects of LAMA4 on systemic insulin sensitivity, Lama4+/+ and Lama4−/− mice were fed a 45% HFD for 7 weeks, and the glucose response to systemic insulin administration was evaluated. Interestingly, Lama4−/− mice exhibited significantly improved insulin sensitivity compared with Lama4+/+ mice [Fig. 5(a) and 5(b)]. Glucose tolerance was similar between the two groups [Fig. 5(c) and 5(d)].
Figure 5.
Insulin sensitivity is improved in HFD-fed Lama4−/−. (a) Insulin tolerance test was performed after 6 hours of fasting and (b) the area under the curve (AUC) was calculated for Lama4−/− and Lama4+/+ mice at 15 weeks of age fed HFD for 7 weeks (Lama4−/− mice, n = 5; Lama4+/+ mice, n = 8). Data are presented from three independent experiments as mean ± standard deviation (SD) with *P < 0.05 as significant from two-tailed Student t test. (c) Glucose tolerance test was performed after 6 hours of fasting and the (d) AUC was calculated for Lama4−/− and Lama4+/+ mice at 13 weeks of age fed HFD for 5 weeks (Lama4−/− mice, n = 9; Lama4+/+ mice, n = 13). Data are presented from five independent experiments as mean ± SD with *P < 0.05 as significant. A two-way repeated-measures ANOVA with one-factor repetition was followed by a pairwise multiple-comparison posttest (Holm-Sidak method).
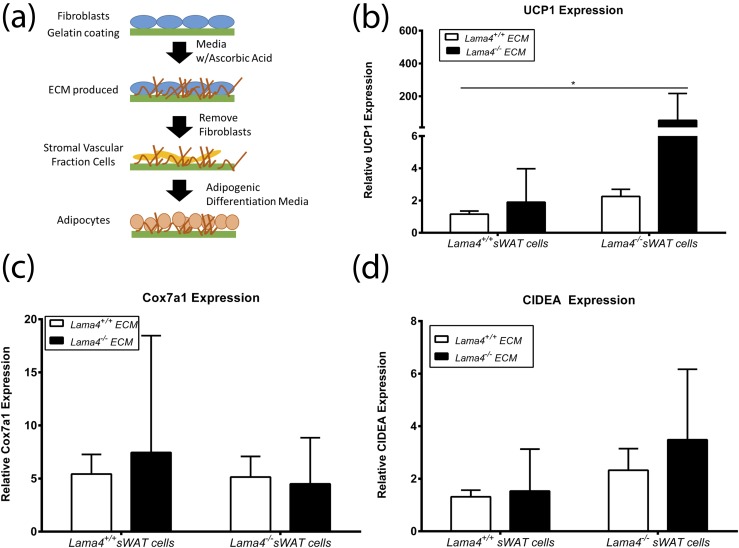
Lama4−/− ECM results in greater UCP1 expression by Lama4−/− cells
An in vitro approach (35, 36) was used to directly examine interactions between LAMA4-deficient ECM and adipocytes [Fig. 6(a)]. Fibroblasts isolated from Lama4−/− and Lama4+/+ mice were cultured in conditions that enhance ECM production. After culture, the fibroblasts were lysed, leaving behind a network of ECM proteins with (Lama4+/+ fibroblasts) and without (Lama4−/− fibroblasts) LAMA4. Adherent cells derived from the stromal vascular fraction of adipose tissue from Lama4−/− and Lama4+/+ mice were cultured on the ECM and induced to differentiate into adipocytes [Fig. 6(a)]. Lama4−/− adipocytes grown on Lama4−/− ECM expressed UCP1 at significantly higher levels than Lama4+/+ cells grown on Lama4+/+ ECM [Fig. 6(b)]. Gene expression of Cox7a1 and CIDEA was not significantly increased [Fig. 6(c) and 6(d)]. Overall, maximal UCP1 expression was observed with Lama4−/− cells grown on Lama4−/− ECM. Adipocytes lacking the ability to make LAMA4 cultured on matrix lacking LAMA4 results in maximum expression of UCP1, showing a direct adipocyte effect of ECM lacking LAMA4.
Figure 6.
UCP1 expression is increased in cells cultured on Lama4−/− ECM. (a) Illustration of the in vitro generation of Lama4−/− and Lama4+/+ ECM and cell culture. (b) Relative UCP1 gene expression by cells on Lama4−/− or Lama4+/+ ECM, with three to six biological replicates per group and three technical replicates per biological sample. The UCP1 data are from three independent experiments (n = 15). (c) Relative Cox7a1 gene expression by cells on Lama4−/− or Lama4+/+ ECM, with six biological replicates per group and three technical replicates per biological sample. The data are from two independent experiments (n = 12). (d) Relative CIDEA gene expression by cells on Lama4−/− or Lama4+/+ ECM, with six biological replicates per group and three technical replicates per biological sample. The Cox7a1 and CIDEA data are from two independent experiments (n = 12). Data are presented as mean ± standard error of the mean with * P < 0.05 as significant from Kruskal-Wallis one-way ANOVA on ranks followed by all pairwise multiple-comparison procedures (Dunn method).
Discussion
Cell behavior is regulated by basement membranes. For example, basement membranes provide signals to stems cells that enable cells to remain quiescent or induce differentiation (37), and basement membranes influence tumor growth and metastasis (38). The composition of the basement membrane varies throughout the body, and alterations in composition contribute to a number of pathologies. Several human diseases, including congenital muscular dystrophy, Pierson syndrome, osteoarthritis, Alport syndrome, and Goodpasture syndrome, are characterized by abnormalities in the laminin family of proteins, a primary component of the basement membrane (39).
Every adipocyte is surrounded by a thin basement membrane. However, how this basement membrane influences adipocyte function is unknown. The studies presented here demonstrate that deletion of a single basement membrane component (LAMA4) leads to increased adipocyte expression of UCP1. This results in increased EE and a lean mouse phenotype. Mice lacking LAMA4 are resistant to obesity despite food consumption equal to that of WT controls and exhibiting the same level of locomotor activity. In fact, when LAMA4 null mice are challenged with HFD, they maintain a weight similar to chow-fed WT controls. In addition, this phenotype is recapitulated in vitro with adipocytes grown on ECM lacking LAMA4, suggesting an adipose-autonomous effect.
There is great interest in the activation of brown or beige fat cells to increase EE as a possible treatment of obesity. Brown adipocytes were previously thought to contribute to thermogenesis in small mammals, with limited function in humans. More recently, studies have shown that brown fat in humans may contribute to whole-body EE. The exact lineages of these cells is not clear, as some studies suggest that certain brown and white adipose depots in humans may exhibit a beige gene expression program (22, 40–42). The origin of beige cells is controversial, with evidence for transdifferentiation as well as potential beige progenitor cell populations. In this work, we did not investigate how beige cells are recruited but instead investigated the presence of beige cells in sWAT. In Lama4−/− mice, UCP1 expression was upregulated at room temperature in sWAT on both a chow diet and HFD, even in the absence of cold exposure or hormonal beige inducers. Cox7a1 and CIDEA are two genes that have enriched expression in beige adipocytes (22). On HFD, Lama4−/− mice had Cox7a1 expression significantly increased. CIDEA, however, did not experience this upregulation in HFD conditions. Beige adipocytes were not observed in eWAT of LAMA4 knockout mice, consistent with other beige adipose studies (43).
Three temperature conditions were selected for evaluating the LAMA4 mice. Cold exposure activates BAT and beige adipocytes in sWAT (44–47). At room temperature, UCP1 was upregulated in sWAT from Lama4−/− mice without exogenous induction. This is in contrast to other studies at room temperature that suggested beige cells in sWAT require induction (43, 48). Interestingly, at thermoneutral conditions, mice on a standard chow diet had the same EE, whereas the Lama4−/− mice on HFD exhibited increased EE even at thermoneutrality. Targeting the basement membrane may result in increased EE independent of environmental temperature.
On a chow diet or HFD, there were no differences between UCP1 expression in the BAT of Lama4−/− and Lama4+/+ mice, suggesting that the improved metabolic consequences result from the changes observed in sWAT. When examining Lama4−/− mice on HFD, sWAT UCP1 expression was ∼10% of the WT BAT UCP1 expression. Although UCP1 expression is lower, if one takes into account that sWAT is approximately five to six times greater in mass than BAT, then the 10% increase in UCP1 in sWAT could conservatively produce an additional 60% contribution in thermogenesis to the overall energy balance. The UCP1 gene expression, in combination with UCP1 immunohistochemistry, and increased EE support a substantial thermogenic contribution from beige cells in sWAT physiologically relevant to the Lama4−/− mice lean phenotype.
The mechanism by which LAMA4 deficiency leads to UCP1 expression is not known. An adipose-autonomous effect was observed using ECM-based in vitro studies. The properties of the ECM that influence cell behavior include composition, mechanical properties, and structure. The composition of the LAMA4 ECM is clearly different from WT ECM. Synthetic hydrogels containing peptides derived from laminin α1, AG73 (an α6 integrin-engaging ligand), and C16 (an α5/vβ1/3 integrin-engaging ligand) were shown to positively influence expression of UCP1 by adipocytes in vitro and resulted in enhanced thermogenesis in mice following implantation of these hydrogels (25). However, these peptides did not include LAMA4 structures and were from laminin isoforms not present in adipose basement membrane. It is also possible that the absence of LAMA4 also alters basement membrane assembly, resulting in different structure and mechanical properties. In previous work, UCP1 expression was shown to be influenced by substrate stiffness in three-dimensional culture environments (26). Future studies could examine the mechanical properties and structural features of the adipose basement membrane to determine their contribution to UCP1 expression and thermogenesis in vivo. Additional understanding of ECM’s ability to induce beige adipocytes could provide new strategies for tissue engineering of functional beige/brown fat.
In summary, basement membrane in adipose tissue directly influences adipocyte function. Resistance to obesity by Lama4−/− mice occurred with a simultaneous increase in EE. Additional focus on why Lama4−/− mice develop increased adipocyte beiging could lead to novel therapeutics that increase the thermogenic contribution of sWAT.
Acknowledgments
We acknowledge Dr. Graeme Bell for resource support with metabolic cage studies and Ms. Veronica Ibarra and Ms. April Wanagas for assistance with UCP1 staining.
Parts of this study were presented in abstract form at the American Diabetes Association Conference, Boston, MA, June 2015; the Endocrinology Society Conference, Boston, MA, April 2016; and the Levine-Riggs Diabetes Research Symposium in Long Beach, CA, March 2016. Parts of this study were included in the doctoral thesis of M.K.V.
Financial Support: This work was funded, in part, by the University of Chicago Diabetes Research and Training Center (National Institutes of Health Grant P30 DK020595), the University of Chicago Institute for Translational Medicine (National Institutes of Health Grant CTSA UL1 TR000430), the Pritzker Institute of Biomedical Science and Engineering, National Science Foundation Grant EEC-1157041, and Merit Review Award 5 I01 BX000418-06 from the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Author Contributions: M.K.V., E.M.B., and R.N.C.: conceptualization, methodology, writing of original draft, review and editing, supervision. M.K.V. and H.Y.: formal analysis. M.K.V., A.B., H.Y., M.C.M., F.Y., and A.G.: investigation. H.Y., E.M.B., and R.N.C.: resources. M.K.V. and F.Y.: visualization. M.K.V.: project administration. E.M.B. and R.N.C.: funding acquisition.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| UCP1 | Anti‐UCP1 antibody | Abcam, ab10983 | Rabbit; polyclonal | 5.2 μg/mL | AB_2241462 |
Footnotes
- BAT
- brown adipose tissue
- DEXA
- dual-energy X-ray absorptiometry
- DMEM
- Dulbecco’s modified Eagle medium
- ECM
- extracellular matrix
- EE
- energy expenditure
- eWAT
- epididymal white adipose tissue
- FBS
- fetal bovine serum
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- ITT
- insulin tolerance test
- LAMA4
- laminin α4
- sWAT
- subcutaneous white adipose tissue
- UCP1
- uncoupling protein 1
- VCO2
- carbon dioxide production
- VO2
- oxygen consumption
- WAT
- white adipose tissue
- WT
- wild-type.
References
- 1.Mariman ECM, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67(8):1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clément K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Divoux A, Clément K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev. 2011;12(5):e494–e503. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. [DOI] [PubMed] [Google Scholar]
- 6.Reggio S, Rouault C, Poitou C, Bichet JC, Prifti E, Bouillot JL, Rizkalla S, Lacasa D, Tordjman J, Clément K. Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J Clin Endocrinol Metab. 2016;101(6):2578–2587. [DOI] [PubMed] [Google Scholar]
- 7.Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM, van Kuppevelt T, Meirovitz A, Pisano C, Li JP, van der Vlag J, Vlodavsky I, Elkin M. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2011;61(1):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J Histochem Cytochem. 2012;60(12):976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Scott HA, Monickaraj F, Xu J, Ardekani S, Nitta CF, Cabrera A, McGuire PG, Mohideen U, Das A, Ghosh K. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J. 2015;30(2):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27(1–2):93–127. [DOI] [PubMed] [Google Scholar]
- 11.Domogatskaya A, Rodin S, Tryggvason. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–553. [DOI] [PubMed] [Google Scholar]
- 12.Aumailley M, Krieg T. Laminins: a family of diverse multifunctional molecules of basement membranes. J Invest Dermatol. 1996;106(2):209–214. [DOI] [PubMed] [Google Scholar]
- 13.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–332. [DOI] [PubMed] [Google Scholar]
- 14.Noro A, Sillat T, Virtanen I, Ingerpuu S, Bäck N, Konttinen YT, Korhonen M. Laminin production and basement membrane deposition by mesenchymal stem cells upon adipogenic differentiation. J Histochem Cytochem. 2013;61(10):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang G, Greenspan DS. ECM roles in the function of metabolic tissues. Trends Endocrinol Metab. 2012;23(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uriel S, Huang J-J, Moya ML, Francis ME, Wang R, Chang SY, Cheng M-H, Brey EM. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29(27):3712–3719. [DOI] [PubMed] [Google Scholar]
- 17.Vaicik MK, Thyboll Kortesmaa J, Movérare-Skrtic S, Kortesmaa J, Soininen R, Bergström G, Ohlsson C, Chong LY, Rozell B, Emont M, Cohen RN, Brey EM, Tryggvason K. Laminin α4 deficient mice exhibit decreased capacity for adipose tissue expansion and weight gain. PLoS One. 2014;9(10):e109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima I, Yamaguchi T, Ozutsumi K, Aso H. Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. Differentiation. 1998;63(4):193–200. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima I, Muroya S, Tanabe R, Chikuni K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biol Cell. 2002;94(3):197–203. [DOI] [PubMed] [Google Scholar]
- 20.Niimi T, Kumagai C, Okano M, Kitagawa Y. Differentiation-dependent expression of laminin-8 (alpha 4 beta 1 gamma 1) mRNAs in mouse 3T3-L1 adipocytes. Matrix Biol. 1997;16(4):223–230. [DOI] [PubMed] [Google Scholar]
- 21.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2008;284(2):1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartesaghi S, Hallen S, Huang L, Svensson PA, Momo RA, Wallin S, Carlsson EK, Forslöw A, Seale P, Peng XR. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol. 2015;29(1):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lidell ME, Betz MJ, Enerbäck S. Brown adipose tissue and its therapeutic potential. J Intern Med. 2014;276(4):364–377. [DOI] [PubMed] [Google Scholar]
- 25.Tharp KM, Jha AK, Kraiczy J, Yesian A, Karateev G, Sinisi R, Dubikovskaya EA, Healy KE, Stahl A. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes. 2015;64(11):3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaicik MK, Morse M, Blagajcevic A, Rios J, Larson J, Yang F, Cohen RN, Papavasiliou G, Brey EM. Hydrogel-based engineering of beige adipose tissue. J Mater Chem B Mater Biol Med. 2015;3(40):7903–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22(4):1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castelló-Cros R, Cukierman E. Stromagenesis during tumorigenesis: characterization of tumor-associated fibroblasts and stroma-derived 3D matrices. Methods Mol Biol. 2009;522:275–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J Vis Exp. 2010;(44):2033. [DOI] [PMC free article] [PubMed]
- 30.Yu G, Wu X, Kilroy G, Halvorsen YD, Gimble JM, Floyd ZE. Isolation of murine adipose-derived stem cells. Methods Mol Biol. 2010;702:29–36. [DOI] [PubMed] [Google Scholar]
- 31.Aune UL, Ruiz L, Kajimura S. Isolation and differentiation of stromal vascular cells to beige/brite cells. J Vis Exp. 2013;(73). [DOI] [PMC free article] [PubMed]
- 32.Overton JM. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes. 2010;34(Suppl 2):S53–S58. [DOI] [PubMed] [Google Scholar]
- 33.Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods. 2011;9(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castillo M, Hall JA, Correa-Medina M, Ueta C, Kang HW, Cohen DE, Bianco AC. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes. 2011;60:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermette M, Trottier V, Ménard V, Saint-Pierre L, Roy A, Fradette J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28(18):2850–2860. [DOI] [PubMed] [Google Scholar]
- 36.Ramanathan A, Karuri N. Proteolysis of decellularized extracellular matrices results in loss of fibronectin and cell binding activity. Biochem Biophys Res Commun. 2015;459(2):246–251. [DOI] [PubMed] [Google Scholar]
- 37.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104. [DOI] [PubMed] [Google Scholar]
- 38.Devergne O, Tsung K, Barcelo G, Schüpbach T. Polarized deposition of basement membrane proteins depends on phosphatidylinositol synthase and the levels of Phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2014;111(21):7689–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci. 2010;67(17):2879–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7(11):e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, Pedersen BK, Møller K, Scheele C. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17(5):798–805. [DOI] [PubMed] [Google Scholar]
- 42.Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013;98(9):E1448–E1455. [DOI] [PubMed] [Google Scholar]
- 43.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22(4):546–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298(6):E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 45.Ashwell M, Jennings G, Richard D, Stirling DM, Trayhurn P. Effect of acclimation temperature on the concentration of the mitochondrial ‘uncoupling’ protein measured by radioimmunoassay in mouse brown adipose tissue. FEBS Lett. 1983;161(1):108–112. [DOI] [PubMed] [Google Scholar]
- 46.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–942. [DOI] [PubMed] [Google Scholar]
- 47.Rosell M, Kaforou M, Frontini A, Okolo A, Chan Y-W, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306(8):E945–E964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):9–15. [DOI] [PubMed] [Google Scholar]