Abstract
The Notch pathway is a highly conserved juxtacrine signaling mechanism that is important for many cellular processes during development, including differentiation and proliferation. Although Notch is important during ovarian follicle formation and early development, its functions during the gonadotropin-dependent stages of follicle development are largely unexplored. We observed positive regulation of Notch activity and expression of Notch ligands and receptors following activation of the luteinizing hormone-receptor in prepubertal mouse ovary. JAG1, the most abundantly expressed Notch ligand in mouse ovary, revealed a striking shift in localization from oocytes to somatic cells following hormone stimulation. Using primary cultures of granulosa cells, we investigated the functions of Jag1 using small interfering RNA knockdown. The loss of JAG1 led to suppression of granulosa cell differentiation as marked by reduced expression of enzymes and factors involved in steroid biosynthesis, and in steroid secretion. Jag1 knockdown also resulted in enhanced cell proliferation. These phenotypes were replicated, although less robustly, following knockdown of the obligate canonical Notch transcription factor RBPJ. Intracellular signaling analysis revealed increased activation of the mitogenic phosphatidylinositol 3-kinase/protein kinase B and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways following Notch knockdown, with a mitogen-activated protein kinase kinase inhibitor blocking the enhanced proliferation observed in Jag1 knockdown granulosa cells. Activation of YB-1, a known regulator of granulosa cell differentiation genes, was suppressed by Jag1 knockdown. Overall, this study reveals a role of Notch signaling in promoting the differentiation of preovulatory granulosa cells, adding to the diverse functions of Notch in the mammalian ovary.
Suppression of Notch signal using siRNA against Jag1 or Rbpj leads to suppression of differentiation and retention of proliferative capacity in mouse granulosa cells.
As key structural and functional units of the female gonad, ovarian follicles are responsible for the growth and ovulation of high-quality oocytes that ensure fertility, as well as for the production of steroid and peptide hormones important for reproductive physiology. The formation, growth, and function of these follicles are controlled by networks of intraovarian and endocrine signals. Local factors, acting through paracrine and juxtacrine mechanisms, are important for follicle formation and early development to the secondary follicle stage. These include, but are not limited to, estrogen (1–3), progesterone (4), Kit/Kit-ligand (5, 6), the transforming growth factor β superfamily (7–9), and the Notch signaling pathway (10–12). Following puberty and activation of the hypothalamic-pituitary-gonadal axis, follicle-stimulating hormone (FSH) from the pituitary drives follicular maturation through regulation of granulosa cell proliferation and differentiation (13). In response to FSH and activin signaling, rapid follicular growth is achieved through increased expression of cyclin D2 (Ccnd2) (14). FSH also induces gene expression changes in granulosa cells to make them responsive to the midcycle ovulatory luteinizing hormone (LH) surge (13). Hallmarks of these differentiating granulosa cells include increasing production of estrogen and progesterone through elevated expression of the rate-limiting enzymes in their biosynthesis Cyp19a1 and Cyp11a1, as well as expression of the membrane receptor for LH (Lhcgr) (13).
The highly conserved Notch signaling pathway is emerging as an important means of intrafollicular communication. As a cell contact-dependent pathway, Notch signaling requires binding of membrane bound Notch receptors to membrane-bound Jagged or Delta-like ligands on adjacent cells (15). This ligand-receptor interaction leads to a proteolytic cleavage cascade that ultimately liberates the Notch intracellular domain (NICD) of the receptor through action of the γ-secretase complex (16). NICD translocates to the nucleus and acts as a transcriptional activator following binding with the obligate cofactor RBPJκ (17). During development, activation of Notch signaling leads to pleiotropic effects, which include cell proliferation, specification, and differentiation, impacting organ formation and patterning (18).
The involvement of Notch signaling in follicles and granulosa cells has been demonstrated through pharmacological and genetic means in various culture and mouse models. Initial interest in Notch signaling in the ovary came from efforts to understand the molecular mechanisms behind the formation of primordial follicles, where pregranulosa cells interact closely with clusters of cytoplasmically connected oocytes, termed germ cell nests or syncitia, and eventually encapsulate a single germ cell within each follicle (19). Disruption of Notch signaling using the γ-secretase inhibitor, N-(N-(3,5-difluorophenacetyl)-l-alanyl)-S-phenylglycine t-butyl ester (DAPT), in cultured neonatal ovaries results in retention of germ cells in nests and reduced numbers of primordial follicles (10–12). Conditional knockout in mice of Notch2 in granulosa cells and Jag1 in oocytes results in formation of multioocytic follicles, which are postulated to be the result of incomplete germ cell nest breakdown, and these mice exhibit reduced fertility (11, 12). Characterization of infertile mice with a null mutation in the Notch receptor modifier Lunatic fringe (lfng) also reveals defects in follicle development and meiotic maturation of the ovulated oocyte (20), whereas an independently derived Lfng–/– mouse line is subfertile (21). Disruption of Hes1, a Notch target/effector gene (22), was shown to result in a decreased proportion of oocytes progressing to meiosis I, reduced oocyte survival following germ cell nest breakdown, and subsequent impaired fertility (23). Although various genetic models of ovarian Notch disruption present with reduced fertility or infertility, analysis of ovarian phenotypes has largely focused on follicle formation and early follicle growth and development (11, 20, 23).
Notch signaling molecules and effector genes have been shown to be expressed in gonadotropin-responsive follicles; however, their roles during later stages of follicle development are largely unexplored (24, 25). Gonadotropin interactions with underlying local signals are critical for control of follicular functions, including growth and differentiation (26). To better understand the dynamics of ovarian Notch signaling during the gonadotropin-dependent stages of follicle development, we used a Notch-responsive transgenic reporter mouse line to identify Notch activity in granulosa cells of all stages of follicular growth as well as in luteal cells. We found that Notch activity in the ovary is upregulated following activation of LHCGR by exogenous human chorionic gonadotropin (hCG). This net-positive regulation of Notch activity was accompanied by similarly increased expression of Notch2 and Jag1 following hCG stimulation. Although the ligand JAG1 was previously thought to be germ cell restricted, we found that JAG1 expression shifts to multiple types of steroidogenically active somatic cells following hormone stimulation. Using cultured primary granulosa cells, we identified a role for Jag1, through canonical Notch signaling, in regulating differentiation and proliferation of these cells. Together, these experiments demonstrate that, consistent with its known role in development, Notch signaling regulates the balance between differentiation and proliferation in ovarian granulosa cells.
Materials and Methods
Animal treatments and tissue collection
CD1 mice (Charles River Laboratories, Wilmington, MA) were maintained on a 12-hour light/dark cycle in controlled environmental condition with access to water and food ad libitum. A diet free from alfalfa and soybean was used to minimize levels of naturally occurring phytoestrogens (2919 for breeding pairs, 2916 for maintenance; Teklad Diets, Harlan, Madison, WI). The transgenic Notch responsive (TNR) EGFP reporter mice (27) were a generous gift from Nicholas Gaiano (Johns Hopkins University, Baltimore, MD) and were maintained on a CD1 background.
For hormone treatment, prepubertal postnatal day (PND) 19 CD1 mice were given an intraperitoneal (IP) injection of 5 IU pregnant mare’s serum gonadotropin (PMSG; Sigma Aldrich, St. Louis, MO) and, after 48 hours, were either euthanized or given an IP injection of 5 IU hCG (Sigma Aldrich, St. Louis, MO). Animals were euthanized at various time points following hCG as indicated in the “Results” section. All animal protocols were approved by the Institutional Animal Care and Use Committee of Northwestern University (Evanston, IL).
Immunologic detection on histological samples
Ovarian tissue samples were dissected in phosphate buffer saline and fixed overnight in 4% paraformaldehyde in phosphate buffer saline at 4°C. Samples were embedded in paraffin and sectioned at 5 μm for histological analysis. Immunohistochemistry (IHC), and immunofluorescence (IF), were performed as previously described (10). Primary antibodies used include a goat polyclonal anti-JAG1 (SC-6011; Santa Cruz Biotechnology, Dallas, TX), a rabbit monoclonal anti-P450SCC (14217; Cell Signaling Technology, Danvers, MA), and a rabbit polyclonal anti-GFP (A11122; Life Technology, Eugene, OR). Biotinylated conjugated secondary antibodies against goat (BA-5000) or rabbit (BA-1000) were purchased from Vector Laboratories (Burlingame, CA) and used with a Colorimetric Tyramide Signal Amplification kit (for GFP IHC; PerkinElmer, Waltham, MA) or a VECTASTAIN Elite ABC Kit (Vector Laboratories). Methyl green or 4′,6-diamidino-2-phenylindole were used as counterstains, as appropriate.
RNA isolation and quantitative real-time polymerase chain reaction
Ovaries were dissected, and the bursae were removed. Samples were immediately preserved in RNAlater reagent (Invitrogen, Carlsbad, CA) overnight at 4°C and then stored at –80°C prior to RNA extractions. Total RNA was extracted using RNeasy Plus Mini Kit (QIAGEN, Valencia, CA). For cultured granulosa cells, total RNA was extracted using Quick-RNA MicroPrep (Zymo Research, Irvine, CA). RNA was reverse transcribed to complementary DNA using SuperScript IV First-Strand Synthesis System (Invitrogen). Quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) using either an Applied Biosystems 7300 (Applied Biosystems) or BioRad CFX384 (Bio-Rad Laboratories, Hercules, CA) thermocycler. The comparative cycle threshold (28) method was used for relative quantification using Rpl19 (29) as an internal control. The sequences of primers used for qRT-PCR are provided in Supplemental Table 1 (15.1KB, docx) .
Primary granulosa cell culture and small interfering RNA knockdown
PND21 mice were given IP injection of 5 IU PMSG for 48 hours. Granulosa cells were collected by follicle puncture and cultured as previously described (30). Oocytes were removed with a 40-μm cell strainer (Fisher Scientific, Hampton, NH). Granulosa cells were cultured at 175,000 cells/well in 24-well plates in a humidified incubator at 37°C and 5% CO2 using a 1:1 ratio of Dulbecco’s modified Eagle medium:F-12 medium (Fisher Scientific) supplemented with 15 mM HEPES, pH 7.4, 5 mg/mL transferrin, 2 mg/mL insulin, 40 ng/mL hydrocortisone, 10% fetal bovine serum, and 100 U/mL penicillin/streptomycin (4F media). Cells were allowed to adhere and to reach 90% confluency for 96 hours prior to small interfering RNA (siRNA) transfection. siGENOME SMARTpool (GE Dharmacon, Lafayette, CO) was used at concentrations of 20 nM (siJag1, siScramble) or 40 nM (siRbpj, siScramble). siRNA transfection was done using DharmaFect I reagent (GE Dharmacon) in 4F media supplemented with 10% charcoal-stripped fetal bovine serum. RNA or protein was extracted 72 hours after transfection. The mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 (513000; Calbiochem, San Diego, CA) was added at a concentration of 4 μM, 24 hours following siRNA transfection for a total incubation time of 48 hours. The protein kinase B (AKT) inhibitor MK2206 (S1078; Selleck Chemicals, Houston, TX) was added at a concentration of 10 nM, 24 hours following siRNA transfection for a total incubation time of 48 hours.
Protein extraction and western blotting
Cells were lysed with ice-cold radioimmunoprecipitation assay buffer supplemented with 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 μg/mL pepstatin, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL antipain, and 20 U/mL benzonase nuclease (Sigma Aldrich) with freshly added Phosphatase Inhibitor Cocktail 2 (1% volume-to-volume ratio, Sigma Aldrich) and Phosphatase Inhibitor Cocktail 3 (1% volume-to-volume ratio, Sigma Aldrich). Ten micromolar of forskolin was added 1 hour prior to lysate collection. Total protein was measured by bicinchoninic assay (Thermo Fisher Scientific, Waltham, MA). Equal amounts of protein were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and blotted onto nitrocellulose membranes. Membranes were incubated overnight at 4°C in primary antibody and for 1 hour at room temperature with infrared dye conjugated secondary antibody. In addition to the antibodies listed previously, primary antibodies used include a rabbit polyclonal anti-RBPJ (25949; Abcam, Cambridge, MA), a rat monoclonal anti-NOTCH2 (C651.6DbHN; Developmental Studies Hybridoma Bank, Iowa City, IA), a rabbit monoclonal anti-GAPDH (2118; Cell Signaling Technology), a rabbit monoclonal anti-panAKT (4691; Cell Signaling Technology), a rabbit monoclonal anti-ERK1/2 (4695; Cell Signaling Technology), a rabbit monoclonal anti-phospho-AKT (Ser473, 4060; Cell Signaling Technology), a rabbit polyclonal anti-phospho-ERK1/2(Thr202/Tyr204, 9101; Cell Signaling Technology), a rabbit polyclonal anti-phospho-FOXO1 (Ser256, 9461; Cell Signaling Technology), a rabbit monoclonal anti-FOXO1 (2880; Cell Signaling Technology), a rabbit monoclonal anti-phospho-YB1 (Ser102, 2900; Cell Signaling Technology), and a rabbit monoclonal anti-YB1 (8475; Cell Signaling Technology). Infrared (IR) dye conjugated secondary antibodies were purchased from LI-COR Biosciences (IRDye 800CW Goat anti-Rabbit IgG 925-32211, IRDye 680RD Donkey anti-Rabbit IgG 926-68073, IRDye 800CW Donkey anti-Goat IgG 925-32214, IRDye 800CW Goat anti-Rat IgG 926-32219, IRDye 680RD Goat anti-Rat IgG 926-68076; Lincoln, NE). Membranes were washed and fluorescence signals were detected using a Syngene PXi multiapplication gel imaging system equipped with Epi LED illumination for infrared detection (Syngene, Frederick, MD).
Flow cytometry analysis for cell proliferation and apoptosis
Primary granulosa cells were cultured and transfected with siRNA as described previously. For proliferation and apoptosis analyses, cells were cultured at 875,000 cells/well in a six-well plate for 96 hours. Cells were then transfected with siRNA for 24 hours, after which they were trypsinized and replated in 24-well plates at subconfluent density of 175,000 cells/well. For apoptosis analysis, cells were harvested 48 hours after replating (72 hours post transfection) and processed for Annexin V and propidium iodide (PI) staining with a Dead Cell Apoptosis Kit with Annexin V Alexa Fluor 488 and PI (Invitrogen). For proliferation assays, 5-ethynyl-2′-deoxyuridine (EdU) was added to the culture media at a concentration of 10 μM 6 hours prior to cell harvest and at 42 hours following replating. A Click-iT EdU Alexa Fluor 647 Flow Cytometry kit was used for EdU detection (Invitrogen). Labeled cells were analyzed in a BD FACSAria II (BD Biosciences, San Jose, CA).
Estradiol-17β and progesterone enzyme-linked immunosorbent assay
Granulosa cells were cultured and transfected with siRNA as described previously in charcoal-stripped serum. At the end of culture, media was collected. Estradiol-17β (E2) levels were measured in undiluted media using an estradiol human enzyme-linked immunosorbent assay (ELISA; ALPCO, Salem, NH) with a range of detection of 10 to 1000 pg/mL. The intra-assay coefficient of variation (CV%) was 6.3 and the interassay CV% was 8.1. Progesterone (P4) levels were measured in 1:100 diluted media using P4 mouse and rat ELISA with a range of detection of 0.15 to 40 ng/mL (IBL, Minneapolis, MN). The intra-assay CV% was 3.9 and the interassay CV% was 9.0 for this assay. All hormone measurement was conducted at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Statistical analysis
Data are presented as means ± standard error of the mean. Experiments were performed using a minimum of three biological replicates and control groups, as stated. Differences between groups were calculated with GraphPad Prism (GraphPad Software, Inc, La Jolla, CA) using a Student t test or analysis of variance with Tukey post hoc, as appropriate. Differences were considered to be significant at a 95% confidence interval (P < 0.05).
Results
Identification of Notch active cells in unstimulated and gonadotropin-treated ovaries
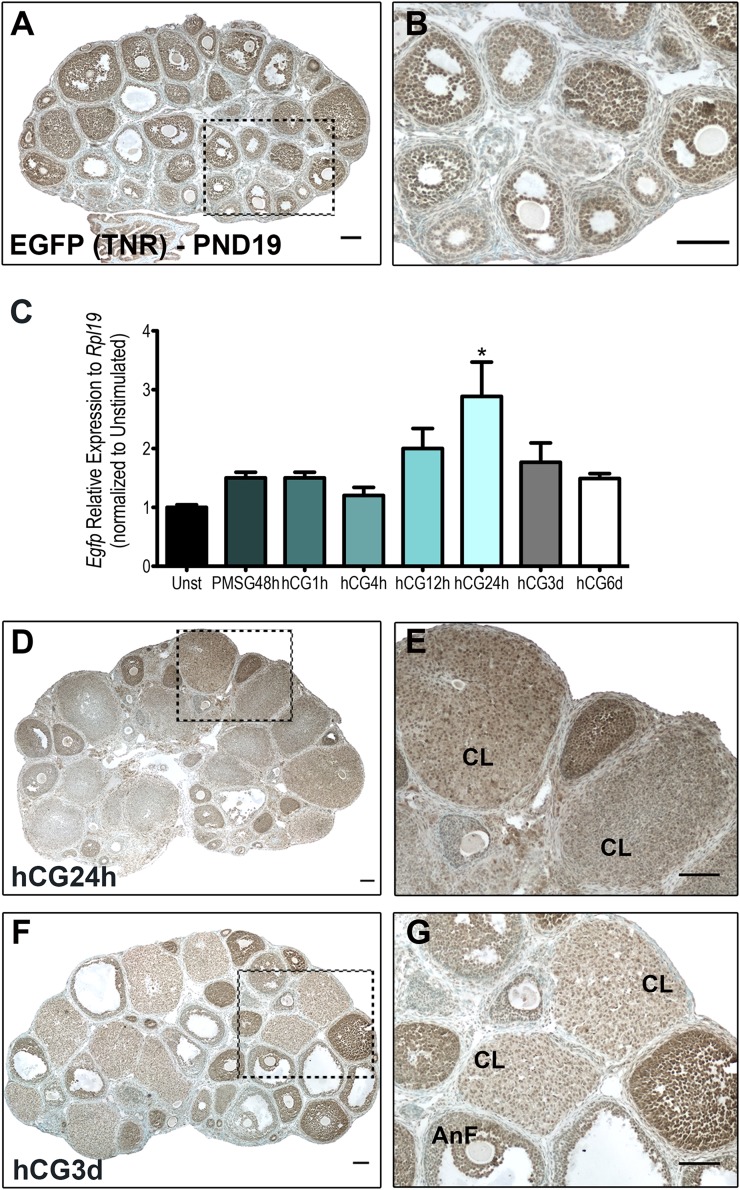
To investigate the localization of Notch active cells in the prepubertal ovary (PND19), we used the TNR reporter line that we had used previously to identify Notch active cells during ovarian follicle formation (12). Using IHC to detect the EGFP reporter, we observed that granulosa cells of growing follicles of various stages stained positively for EGFP, indicating that Notch signaling is active throughout follicular development (Fig. 1A and 1B). Of note, Notch activity was detected in antral follicles, a population of follicles in which continued growth and development will depend on pituitary gonadotropins (31). Because Notch signaling has been previously shown to regulate granulosa cells function (32), and gonadotropins are known to exert their functions through interactions with local signals within the ovary, these observations led us to investigate potential gonadotropin regulation of ovarian Notch activity.
Figure 1.
The transgenic Notch reporter shows activity and is dynamically regulated in gonadotropin-stimulated mouse ovaries. (A, B) In the prepubertal PND19 ovary, EGFP from the Notch reporter line was detected using IHC in granulosa cells of the growing follicle population, including late secondary and early antral follicles. Prepubertal animals were stimulated with PMSG to stimulate follicle growth, followed by hCG 48 hours later to stimulate ovulation. (C) Egfp expression was quantified using qRT-PCR in samples collected at different time points. Data are presented as values relative to Rpl19 and normalized to the unstimulated PND19 samples. Egfp expression was significantly upregulated 24 hours following hCG as compared with the unstimulated control. n = 5; *P < 0.05 compared with unstimulated (Unst). (D, E) Following hCG treatment (24 hours post hCG), Notch activity was detected in corpora lutea (CL) with some staining within the stroma. (F, G) In the luteinized ovary (3 days post hCG), Notch activity remained in CL and granulosa cells of antral follicles (AntF). Scale bars = 100 μm.
To determine whether Notch activity changes in the ovary following activation of follicle-stimulating hormone receptor (FSHR) and LHCGR, we quantified the Egfp reporter expression using qRT-PCR. Samples were collected at 48 hours post PMSG treatment and at various time points following hCG treatment. These time points were selected to capture the preovulatory state, the highly dynamic ovulatory cascade, and subsequent luteinization. Expression of the Egfp reporter was measured relative to that in PND19 unstimulated ovaries. As shown in Fig. 1C, Egfp expression was unchanged following PMSG stimulation, but was significantly upregulated by 24 hours post hCG.
We proceeded to identify the Notch active cells in luteinized ovaries by using IHC for the TNR EGFP reporter in sections from 24-hour and 3-day post-hCG ovaries (Fig. 1D–1G). Corpora lutea (CL) in these sections stained positively for EGFP, indicating that Notch activity remains following ovulation of the follicle and the loss of the oocyte. Although there appears to be some EGFP staining in the stroma at 24 hours post hCG (Fig. 1D and 1E), EGFP staining in the 3-day post-hCG ovary was restricted to follicular and CL boundaries. (Fig. 1F and 1G). These findings suggest that Notch signaling is dynamically regulated by hCG in the ovary during the periovulatory period.
Expression and localization of Notch signaling molecules in the ovary following hormone stimulation
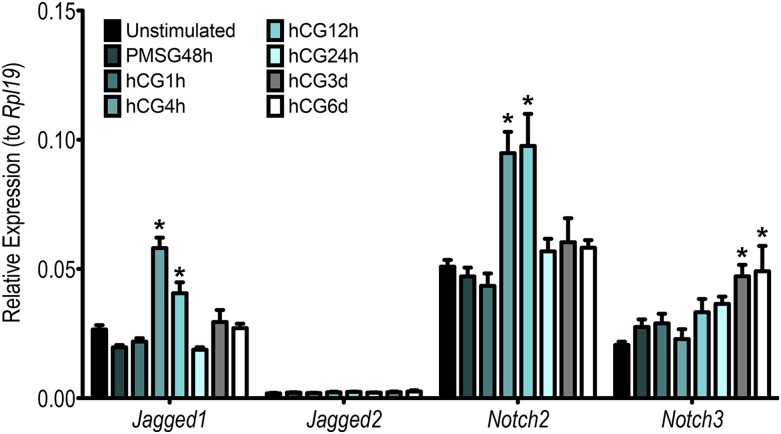
Having observed regulation of the TNR reporter by hCG, we characterized gene expression of the four Notch receptors and five Jagged and Delta-like ligands in ovaries following exogenous gonadotropin stimulation (Fig. 2; Supplemental Fig. 1 (38.8MB, tif) ). We focused on the expression of the ligands Jag1 and Jag2, as well as the receptors Notch2 and Notch3, as we previously found these ligands and receptors to be the most abundantly expressed in neonatal ovaries (12) and they have also been previously reported to be expressed in adult cycling ovaries (24, 25). Compared with unstimulated ovaries, expression of Notch signaling molecules did not change following PMSG treatment. By contrast, hCG exerts positive regulation on all Notch receptors and ligands, with the exception of Jag2, although the timing of the upregulation observed is transcript specific.
Figure 2.
Expression of Notch ligands and receptors is positively regulated by gonadotropins. Expression of Jag1, Jag2, Notch2, and Notch3 were quantified using qRT-PCR in samples collected after 48 hours of PMSG stimulation, followed by hCG stimulation for the times indicated. Data are presented as relative to Rpl19. Compared with unstimulated PND19 ovaries, Jag1 and Notch2 expression was transiently upregulated 4 to 12 hours post hCG stimulation. n = 10 for unstimulated samples, n = 5 for PMSG- and hCG-stimulated samples; *P < 0.05 compared with unstimulated. Modest upregulation of Notch3 was seen in luteinized ovaries (3 and 6 days post hCG), whereas Jag2 remained unchanged following exogenous gonadotropin stimulation.
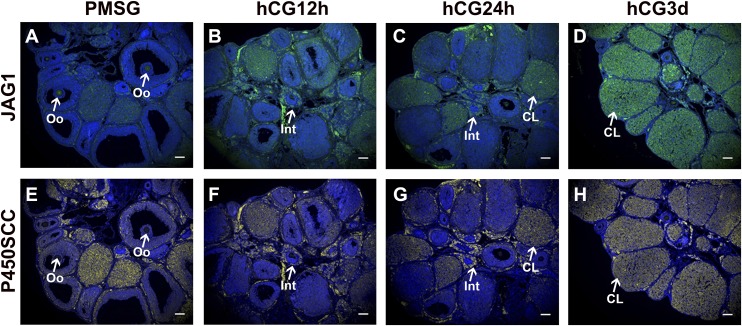
The regulation by hCG of Jag1 messenger RNA (mRNA) was surprising, given the previously reported localization of JAG1 protein expression to the oocyte (10, 12, 24). This led us to further characterize ovarian JAG1 localization following gonadotropin stimulation of PND19 mice (Fig. 3). Following PMSG stimulation, JAG1 was localized predominantly to oocytes of preovulatory follicles (Fig. 3A). Limited expression was also detected in somatic cells (Fig. 3A). However, within 12 hours post hCG treatment, JAG1 staining could be robustly detected within somatic cells of periovulatory follicles, the thecal layer, and a subset of interstitial cells (Fig. 3B). By 24 hours and 3 days post hCG treatment, JAG1 staining was detected in CL (Fig. 3C and 3D).
Figure 3.
JAG1 is localized to steroidogenically active somatic cells following hormone stimulation. JAG1 localization was detected using IF in (A) 48-hour post-PMSG, (B) 12-hour post-hCG, (C) 24-hour post-hCG, and (D) 3-day post-hCG ovarian sections. (A) In the PMSG 48-hour ovary, JAG1 was detected in oocytes (Oo; arrow) with some weaker signals in somatic cells. (E–H) Following hCG stimulation, JAG1 signals in somatic cells became more robust and localized to steroidogenically active structures as marked by P450 side-chain cleavage staining. Most notably, JAG1 was detected in thecal interstitial cells (Int; arrow) and CL (arrow). Scale bars = 100 μm.
To gain additional insights into the identity of the JAG1-expressing somatic cells following hormone stimulation, we probed consecutive ovarian sections from PMSG- and hCG-stimulated mice for the P450 side-chain cleavage (P450SCC) enzyme to mark steroid-producing cells (Fig. 3E–3H). Indeed, following hormone stimulation, JAG1 was localized to the same cell types that are marked by P450SCC staining. Most readily discernable in hCG 24-hour (Fig. 3C and 3G) and hCG 3-day (Fig. 3D and 3H) ovarian sections, JAG1 became localized to steroidogenically active thecal and thecal-interstitial cells, as well as CLs, following hCG stimulation.
Steroid biosynthesis and proliferation in cultured primary granulosa cells
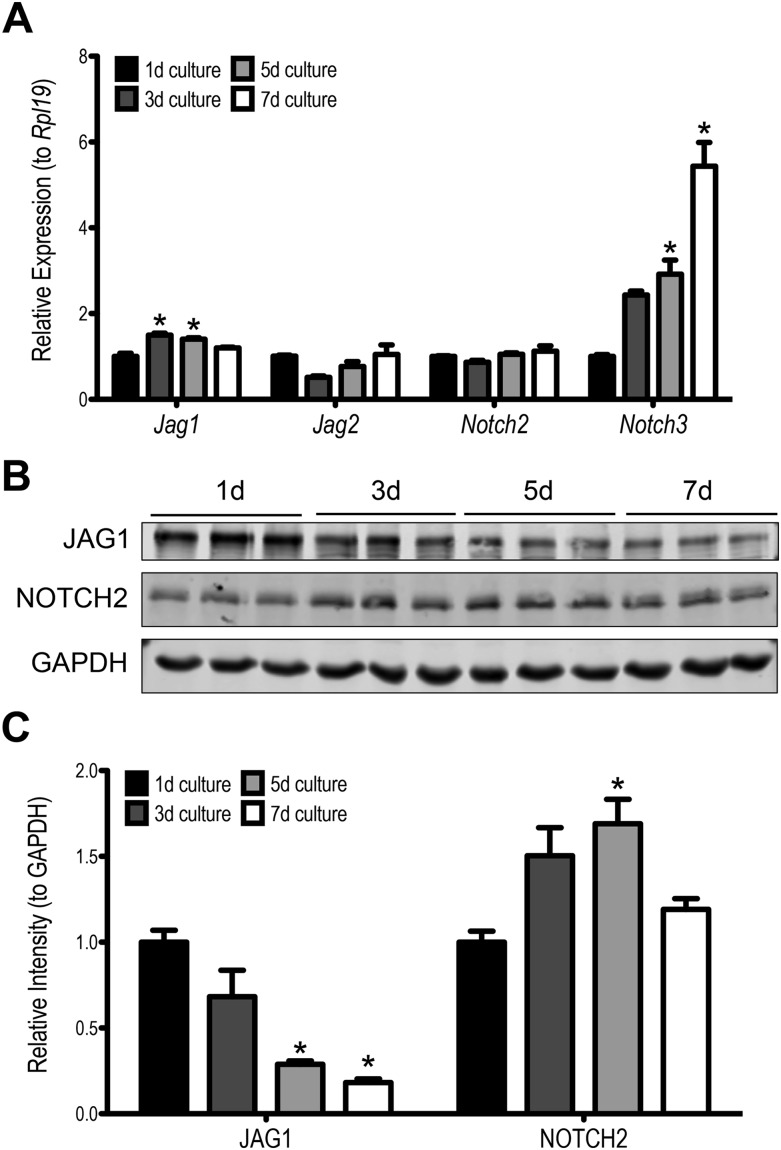
To better understand the potential functions for JAG1 in somatic cells during the gonadotropin-dependent stages of follicular development, we turned to primary cultures of mouse granulosa cells, a model extensively used to study gene function and gain mechanistic insights into the signaling pathways that regulate granulosa cell and follicular activities. Overtime, these cells are known to spontaneously differentiate under appropriate culture conditions (33). Most importantly for this study, these primary granulosa cells continue to express Notch ligands and receptors as detected by mRNA (Fig. 4A). The most abundantly expressed ligand and receptor, JAG1 and NOTCH2, are shown in western blot in Fig. 4B. JAG1 levels decrease beginning at day 5 of culture, whereas NOTCH2 levels show a modest transient upregulation at day 5 of culture (Fig. 4C).
Figure 4.
Notch ligands and receptors are expressed in cultured granulosa cells. Jag1 and Notch3 expression were found to be dynamically regulated throughout 7 days of granulosa cell culture. (A) Jag1 expression peaks at 3-day culture, whereas Notch3 expression continues to rise as the culture progresses. n = 3; *P ≤ 0.05 compared with 1-day culture. (B) The presence of JAG1 and NOTCH2 were confirmed using western blot. (C) Quantification of the western blot shows JAG1 levels decrease throughout the culture period beginning at day 5, whereas NOTCH2 levels transiently peak at day 5 of culture. n = 3, *P < 0.05 compared with 1-day culture.
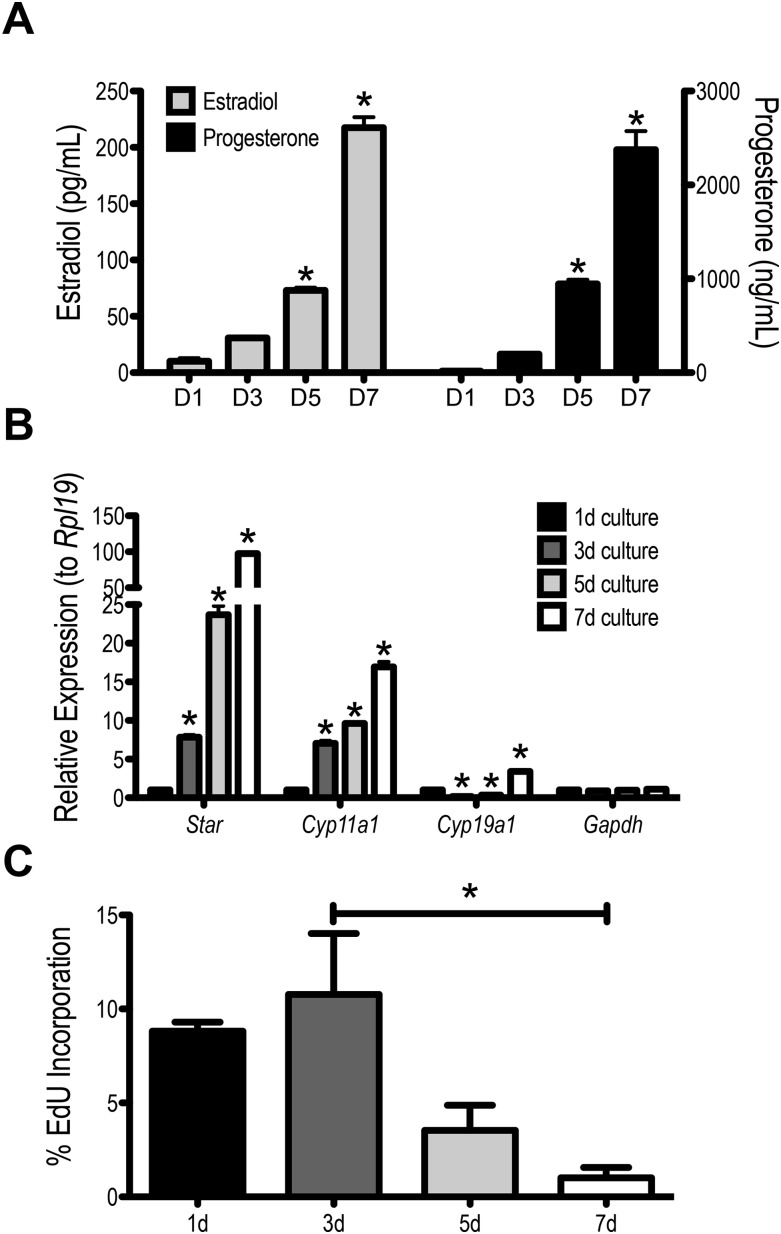
Figure 5A and 5B illustrates that primary granulosa cells cultured from PMSG-primed mouse ovaries exhibit characteristics of differentiating granulosa cells. These granulosa cells secrete E2 and P4 through increasing expression of the Star, Cyp19a1, and Cyp11a1 genes (34). The level of steroidogenic activity was increased as the culture period progressed. At the initiation of culture, these cells were proliferative, but the rate of proliferation decreased with time in culture, as they both differentiate and eventually become contact inhibited (Fig. 5C).
Figure 5.
PMSG-primed primary granulosa cells differentiate in culture. (A) Primary granulosa cells secrete estradiol and P4 throughout the 7-day culture period. The levels of E2 and P4 in the culture medium steadily increase throughout the culture period. (B) The increase in hormone production is consistent with increasing expression of Star, Cyp19a1, and Cyp11a1. (C) Primary granulosa cells are proliferative in culture as identified using an EdU incorporation assay. This proliferative potential diminishes as the culture progresses, and cells eventually become contact inhibited (data not shown). n = 3; *P < 0.05 compared with 1-day culture unless otherwise indicated.
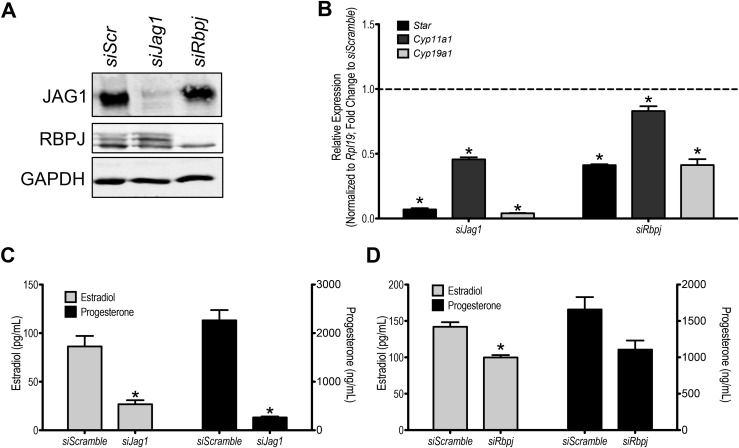
Characterization of steroidogenesis following Jag1 or Rbpj knockdown in cultured primary granulosa cells
To study the function of JAG1 in steroidogenically active granulosa cells, we used siRNA to knock down its expression. We also conducted a similar siRNA experiment targeting the obligatory transcriptional cofactor for canonical Notch signaling, Rbpj (15). siJag1 and siRbpj efficiently and specifically reduced mRNA and protein levels of their respective targets (Fig. 6A; Supplemental Fig. 2 (17.6MB, tif) ). mRNA measurements revealed reduced expression of genes encoding several of the factors and enzymes involved in steroid biosynthesis following Jag1 and Rbpj knockdown (Fig. 6B), including Star, Cyp19a1, and Cyp11a1. Confirming the gene expression findings, levels of E2 were significantly reduced in the culture medium of both Jag1 and Rbpj knockdown cells (Fig. 6C and 6D) 72 hours post transfection (a total of 7 days of culture). P4 levels, however, were found to be significantly reduced only in the Jag1 knockdown cell cultures (Fig. 6D).
Figure 6.
Steroid biosynthesis is suppressed following Jag1 and Rbpj knockdown in cultured granulosa cells. (A) The Notch ligand JAG1 and the canonical Notch signaling transcriptional cofactor RBPJ were efficiently and specifically decreased following siRNA transfection. (B) Expression of factors and enzymes involved in the steroid biosynthetic cascade was downregulated following Jag1 and Rbpj knockdown. The dashed line indicates the level of expression in siScramble samples. (C) Jag1 knockdown results in decreased levels of E2 and P4 in culture media. (D) Consistent with the less robust knockdown of Rbpj, only E2 levels were found to be significantly decreased in siRbpj samples. n = 3; *P < 0.05 compared with siScramble.
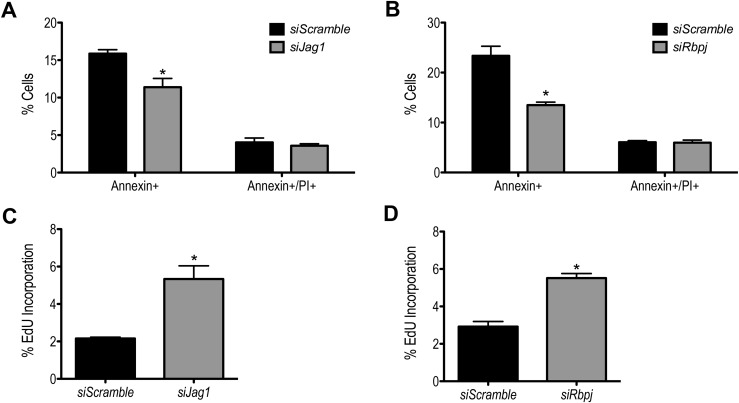
Proliferation and apoptosis in cultured primary granulosa cells following Jag1 or Rbpj knockdown
To ensure that the reduced levels of E2 and P4 in granulosa cell culture media following disruption of Notch signaling are not a consequence of compromised cell survival, and thus reduced cell number, we characterized apoptosis and cell death. Annexin V was used to label early apoptotic cells, whereas PI exclusion was used to measure dead cells. Our results showed that the percentage of dead cells (Annexin+/PI+) in siJag1 and siRbpj samples was not changed compared with the nontargeting siScramble (Fig. 7A and 7B). Surprisingly, knockdown of Jag1 or Rbpj resulted in fewer granulosa cells undergoing apoptosis (Fig. 7A and 7B).
Figure 7.
Knockdown of Jag1 and Rbpj in cultured granulosa cells results in the retention of proliferative potential. Apoptosis and proliferation assays were done 3 days following siRNA transfection, with a total culture period of 7 days. (A, B) As measured by Annexin V staining and PI exclusion assay, Jag1 or Rbpj knockdown did not result in increased cell death. Instead, Jag1 and Rbpj knockdown led to decreased cellular apoptosis. (C, D) An increased rate of cell proliferation was observed following Jag1 and Rbpj knockdown. n = 3; *P < 0.05 compared with siScramble.
As shown previously (Fig. 5), PMSG-primed primary granulosa cells exhibited hallmarks of differentiation, as they became highly steroidogenic and exited the cell cycle as the culture progressed. Suppression of steroidogenesis following Notch pathway knockdown suggests an impact on this differentiation process. To test whether cell proliferation is likewise affected following Jag1 and Rbpj knockdown, we measured actively proliferating cells using a flow cytometric-based EdU incorporation assay. As shown in Fig. 7C and 7D, knockdown of either Jag1 or Rbpj resulted in an increased rate of granulosa cell proliferation compared with controls.
Assessment of intracellular signaling pathways following Jag1 or Rbpj knockdown
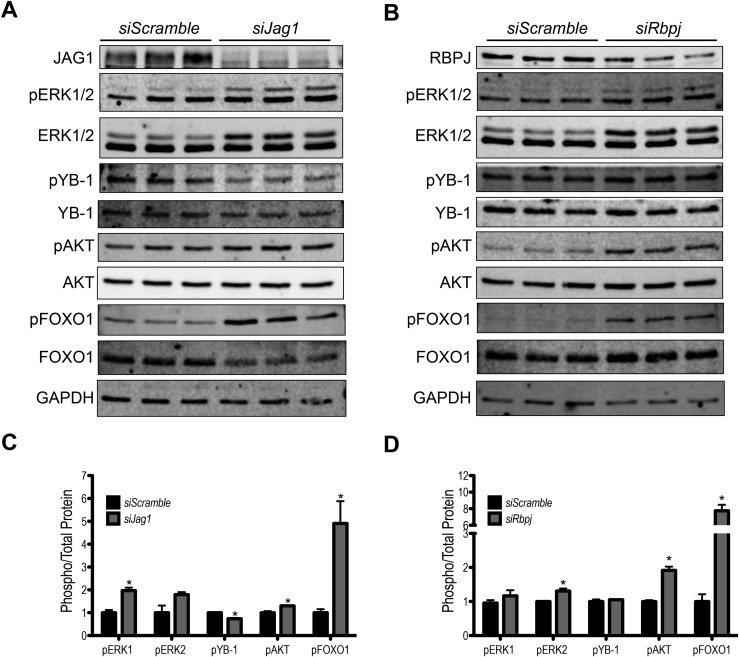
To begin uncovering mechanisms mediating the influence of Notch signaling on steroid biosynthesis and proliferation, we initially investigated expression of genes encoding transcription factors including Nr5a1 (Sf1), Nr5a2 (Lrh1), Gata4, and Creb, whose protein products positively regulate expression of Star, Cyp11a1, and Cyp19a1 (35–37). At the transcriptional level, expression of these factors is not changed following Jag1 or Rbpj knockdown (data not shown). The mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/AKT pathways are understood to regulate differentiated granulosa cell phenotypes in a cyclic adenosine monophosphate/protein kinase A (PKA)–dependent manner, including proliferation (14, 38, 39) and regulation of gene expression (40–43). In the granulosa cell culture system, MAPK/ERK and PI3K/AKT pathway activity decreases as the culture period progresses (Supplemental Fig. 3 (15.1MB, tiff) ), correlating with the decreasing proliferation observed in these cells (Fig. 5).
ERK1/2 and AKT phosphorylation levels are increased in Jag1 (Fig. 8A and 8C) or Rbpj (Fig. 8B and 8D) siRNA transfected samples compared with siScramble controls. A closer analysis of the 3-day period of siRNA transfection (siJag1 and siScramble samples; Supplemental Fig. 4 (24.1MB, tiff) ) reveals that the observed kinase pathway activity is the result of maintenance of phospho-ERK1/2 at levels comparable to early times of the culture, preventing the loss that occurs with normal differentiation. It has been reported that in granulosa cells, proliferation is achieved in part through transcription of the cell cycle gene Ccnd2 following inactivation of the transcriptional repressor FOXO1, downstream of the AKT pathway (14). We observed enhanced phosphorylation at Ser256 of FOXO1 following Jag1 or Rbpj knockdown, indicating its inactivation (Fig. 8C and 8D). Consistent with this result, Ccnd2 expression was increased more than twofold following Jag1 knockdown (Fig. 9A). Whereas Ccnd2 was not changed following Rbpj knockdown, another cell cycle regulator, cyclin-dependent kinase 1 (Cdk1), was found to be upregulated in these samples (as well as siJag1 samples; Fig. 9A and 9B).
Figure 8.
Jag1 or Rbpj knockdown is associated with activation of the MAPK and AKT pathways. To identify activity of the MAPK/ERK and PI3K/AKT pathways following Jag1 and Rbpj knockdown, phosphorylation of ERK1/2 (Thr202/Tyr204) and AKT (Ser473) was analyzed using (A, B) western blots. (C and D) Quantification of the blots is presented. Increased phosphorylation of ERK1/2 (Thr202/Tyr204) and AKT (Ser473) was observed in siJag1 and siRbpj lysates compared with the nontargeting siScramble. The total level of ERK1/2 was also elevated in siJag1 and siRBPJ compared with siScramble. Phosphorylation of the transcriptional repressor FOXO1 (Ser256) is higher in both siJag1 and siRbpj samples compared with siScramble. YB-1 activation (phosphorylation at Ser102) is unchanged following Rbpj knockdown but is downregulated in siJag1 samples compared with control. n = 3; *P < 0.05 compared with siScramble.
Figure 9.
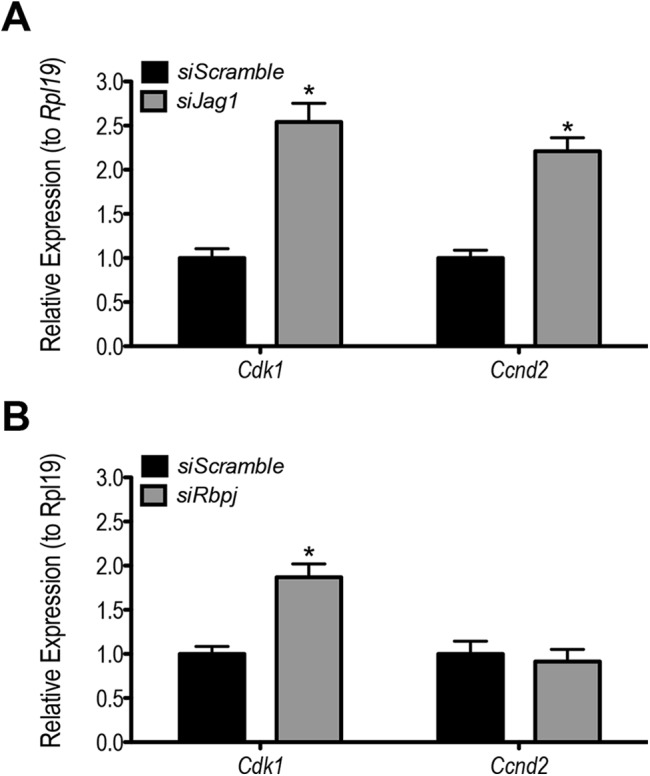
(A) Expression of cell cycle regulators is increased following knockdown of Jag1 or Rbpj. siJag1 resulted in upregulation of Cdk1 and Ccnd2 mRNA expression. (B) Cdk1 but not Ccnd2 was upregulated following Rbpj knockdown. n = 3; *P < 0.05 compared with siScramble.
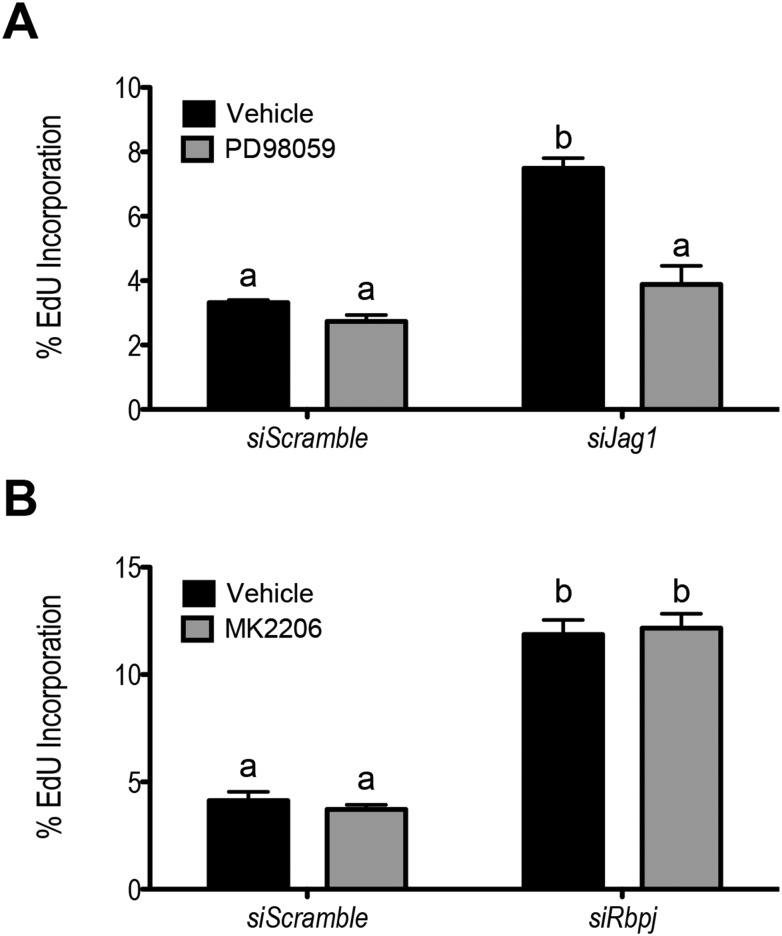
We proceeded to test the consequences of altered MAPK/ERK or PI3K/AKT activity following Notch disruption in granulosa cells. As MAPK/ERK pathway activity is more robustly retained following Jag1 knockdown, although the same is true for PI3K/AKT pathway activity following Rbpj knockdown (Fig. 8), we used the MEK inhibitor PD98059 in Jag1 knockdown cells and the AKT inhibitor MK2206 in Rbpj knockdown cells. Inhibitors were used at doses that normalized ERK phosphorylation or AKT phosphorylation to levels comparable to those observed siScramble samples (data not shown). The enhanced observed proliferation following Jag1 knockdown was returned to that of siScramble samples following addition of PD98059 (Fig. 10A). In contrast, AKT inhibition using MK2206 had no effect on siRbpj or siScramble granulosa cells (Fig. 10B). Neither inhibitor affected the reduced expression of steroid biosynthetic genes seen following Jag1 nor Rbpj knockdown.
Figure 10.
MAPK/ERK pathway inhibition abrogates enhanced granulosa cell proliferation following Jag1 knockdown. (A) Addition of 4 μM of MEK inhibitor PD98059 reduced the enhanced proliferation observed in siJag1 to that of the siScramble control. The baseline of granulosa cell proliferation in siScramble is unchanged by the addition of PD98059. (B) Inhibition of the AKT pathway using 10 nM of MK2206 did not impact proliferation in siRbpj or siScramble samples. n = 3; means not sharing the same letter are significantly different; P < 0.05.
We next sought to understand the observed suppression in steroid biosynthetic enzyme gene expression following Jag1 or Rbpj knockdown. Previous studies have shown that the MAPK pathway stimulates expression of granulosa cell genes involved in steroid biosynthesis through activation of the nucleic acid binding protein Y-Box–binding protein-1 (YB-1) (40). Indeed, following Jag1 knockdown, phosphorylation of YB-1 was decreased by 27% despite the observed activation of ERK1/2 (Fig. 8C). Downregulation of YB-1 phosphorylation was not observed following Rbpj knockdown (Fig. 8D), which may contribute to the less robust suppression in gene expression and steroidogenesis observed in the Rbpj-depleted granulosa cells.
Discussion
Progression through ovarian follicular development requires coordinated cell signaling and activation or repression of gene expression in a spatially and temporally restricted fashion. Although FSH and LH are indispensable for follicle growth, maturation, and eventual ovulation, their actions are achieved through interactions with local intraovarian signals. Stimulation of granulosa cell proliferation following FSHR activation requires coactivation by Smad2 and Smad3, which function redundantly in granulosa cells, by activin (14, 44), whereas FSH induction of preovulatory phenotypes in granulosa cells requires several concurrent paracrine cues, including, but not limited to, IGF1R, canonical Wnt, and ERβ signaling (45–47). Later during ovulation, EGF ligands and EGFR signaling are required to transduce LH actions to the oocyte (48). In this study, using the transgenic Notch reporter line, we showed that the Notch signaling pathway in the mouse ovary is active in gonadotropin-responsive follicles of all stages as well as in CL. Our data support previous reports that Notch signaling components are expressed in adult cycling ovaries (24, 25). The current study adds to that knowledge by providing evidence that ovarian Notch signaling is regulated by gonadotropins and is important for granulosa cell differentiation toward a steroidogenic phenotype.
We find the mRNA expression of several Notch receptors and ligands to be upregulated in response to hCG in vivo. Among these is the gene encoding the ligand Jag1, which was previously postulated to be a germ cell–specific Notch ligand (10, 12), with limited expression in the corpus luteum in adult cycling mice (24, 25). Although the periovulatory upregulation of Jag1 expression may signify its potential role during the ovulatory cascade, findings on the localization and identity of JAG1-expressing cells suggest an additional or alternative role. We found that following hormone stimulation, JAG1 becomes localized to both follicular, luteal, and interstitial somatic cells, structures that are identified to be steroidogenic through P450SCC staining. The transient upregulation of Jag1 mRNA likely signifies the initiation of Jag1 expression in somatic cells, as while mRNA levels return to baseline, protein IF indicates a substantial shift away from oocyte-restricted expression toward that in steroidogenic somatic cells.
To further investigate the role of somatic cell–expressed JAG1, we elected to use primary granulosa cells grown under conditions where the cells spontaneously differentiate toward a steroidogenic phenotype (33). We recognize that the cues promoting differentiation in this cell culture model are distinct from those operative in vivo during the periovulatory period. Nonetheless, there are clear phenotypic similarities in granulosa cells from these two models, and cell culture allows direct and transient approaches to manipulating the Notch signaling pathway. One unexpected observation in the cell culture model was the gradual decline in JAG1 levels with time. This may represent transendocytosis of the ligand with active Notch signaling (49, 50), compensation for enhanced Notch2 expression, or an increasing functional role for JAG2, which is reported to be expressed in granulosa cells of growing follicles (25, 32).
A role for Jag1 in promoting granulosa cell differentiation is supported by the observation that following depletion of Jag1, both E2 and P4 production are suppressed through reduced expression of factors and enzymes in the steroid biosynthetic cascade. Concurrent suppression of E2 and P4 following Jag1 or Rbpj knockdown can be explained in part by downregulation of Star, the cofactor responsible for initiating the steroid biosynthetic cascade by transporting cholesterol substrate to the inner mitochondria membrane. This, along with the in vivo findings regarding gonadotropin-regulated Notch activity, suggests that in the cycling ovary, Notch signaling promotes the differentiated granulosa cell phenotype to ensure an optimal response to the ovulatory cue. Additionally, because JAG1 continues to be detected and the Notch reporter is active in the CL, it is likely that Notch signaling also functions to promote and maintain the terminally differentiated luteal phenotype.
JAG1 localizes to somatic cells that are Notch active based on observations made in the transgenic Notch reporter mouse, which suggests that the action of JAG1 in these cells is achieved through canonical Notch signaling. Knockdown of the Notch obligate cofactor Rbpj in cultured granulosa cells results in similar steroidogenic suppression, albeit slightly less robust compared with that observed in the Jag1 knockdown. Although differences in the efficiency of Jag1 and Rbpj knockdown most likely explain the variance in the degree of suppression of steroid biosynthesis, it is important to note the role of Rbpj in transcriptional repression of Notch target genes. Prior to Notch activation and subsequent NICD binding to promoters and recruitment of transcriptional coactivators such as Maml, Rbpj suppresses gene expression through interactions with transcriptional corepressors and/or histone deacetylases (15, 17, 51). The compensatory effects due to the loss of target gene repression by Rbpj in the basal state has been demonstrated in Drosophila melanogaster in which the loss of Su(H), the homolog to mammalian Rbpj, leads to weaker phenotypes than the loss of Notch in several developmental processes, including bristle formation, wing development, and dorsal-ventral boundary formation (52–54). In murine fibroblast cells, p53 expression was found to be suppressed by the binding of Rbpj to its promoter in a cell cycle–dependent manner (51). Thus, depletion of Rbpj may lead to broader changes that result in a blunted phenotype compared with the depletion of Jag1, the specific ligand that appears to initiate the Notch signal in these differentiating granulosa cells.
The in vivo findings of robust Notch activity in growing follicles and CL, as well as results from the in vitro functional studies, raise questions regarding the identities of the sending and receiving cells engaged in this contact-dependent signaling. Our previous work (12) concerning the role of Notch signaling during germ cell nest breakdown posits a model in which the ligand JAG1 from the oocytes interacts with the NOTCH2 receptor on intimately associated granulosa cells. Once follicles are recruited to the growing pool, however, deposition of the zona pellucida matrix is likely to create a partial physical barrier to contact-dependent signaling. Also, as the follicle expands, the most distal mural granulosa cells are far removed from contact with the oocyte. Thus, the most parsimonious explanation for the observation of continued Notch activity in growing follicles, CL, and cultured granulosa cells is the expression of both Notch ligand(s) and receptor(s) by granulosa cells (and later luteal cells), allowing for ligand-receptor interaction in a trans-manner between neighboring cells. This is consistent with the somatic cell localization of JAG1 following hormone stimulation, additional reports of ligand and receptor expression in granulosa cells throughout the entirety of follicular development (32), and the observation that the Notch reporter remains active in granulosa cells cultured from TNR ovaries (our unpublished observations).
Notch activity in multilayer follicles (and later CL) likely is maintained through lateral induction, where the Notch signal is propagated among adjacent cells through positive feedback regulation of Notch ligand expression following Notch receptor transactivation (55). It is reasonable to speculate that Notch ligands in the oocyte act to initiate signaling within the first layers of granulosa cells, which in turn relay Notch signals to more distal granulosa cells by way of lateral induction. Physiologically, the importance of such lateral induction mechanisms in Notch signaling can be seen in the developing inner ear where Jag1 maintains a patch of sensory progenitor cells (56). Ectopic expression of NICD in the nonsensory epithelium was shown to cause increased Jag1 expression, which was in turn sufficient to induce expression of sensory progenitor markers (57). Jag1 and Notch2 are also coexpressed in cells of the peripheral lens epithelium where they function to promote fibroblast growth factor–dependent lens fiber differentiation (58). Finally, Jag1 has been identified as a direct canonical Notch target gene in cardiac neural crest, where Notch signaling is responsible for the differentiation of smooth muscle cells required for the assembly of the multilayered arterial wall (59). These examples demonstrate the importance of lateral induction of Notch signaling in regulating differentiation events in diverse systems.
The suppressed steroid biosynthesis observed following Jag1 or Rbpj knockdown is similar to that reported in DAPT-treated luteal cells (60–62) and in response to Mamld1 knockdown in mouse Leydig tumor cells (63). In terminally differentiated luteal cells, reduced P4 secretion due to Notch inhibition resulted in cellular apoptosis (60, 61). In contrast to these studies, Jag1 or Rbpj knockdown in differentiating granulosa cells not only caused a reduction in the fraction of apoptosing cells, but also led to an increase in the percentage of cells in the S-phase of the cell cycle, indicating a retention of proliferative potential. These differences are not entirely surprising because, in contrast to differentiating granulosa cells, luteal cells lack the capacity to proliferate and require P4 for survival (64). Although proliferation and differentiation can happen concurrently, and both are hormonally driven by FSH in granulosa cells, these events are also well known to occur in an autonomous manner. An example is the Cyclind2 null mouse, in which follicles with severely impaired granulosa cell proliferation remain steroidogenically active and are able to mature and eventually terminally differentiate into a luteal state (although they lack the ability to be ovulated) (31).
It is likely that the diverse functions of Notch within the follicle are achieved through interactions with underlying intracellular signaling landscapes that are distinct at different developmental stages. Numerous studies have shown the importance of PI3K/AKT and MAPK/ERK signaling in promoting proliferation and gene expression in granulosa cells (14, 38–43). Activation of the PI3K/AKT and MAPK/ERK pathways resulting in increased expression of the cell cycle genes Ccnd2 and Cdk1 provides a molecular mechanism for the observed retention of proliferative potential in cultured granulosa cells following Jag1 or Rbpj knockdown. The MAPK/ERK pathway, however, can also promote expression of differentiation-associated genes, including Cyp19a1 and Cyp11a1 (40, 41, 43). Our results reveal that the enhanced activation of the MAPK/ERK pathway that is observed following Jag1 or Rbpj knockdown represents retention of a less differentiated phenotype, consistent with the cells being less steroidogenic and more proliferative. Indeed, inhibition of the MAPK/ERK pathway using the MEK inhibitor PD98059 following Jag1 knockdown normalized the proliferation rate to that observed in the siScramble control, which suggests that in cultured primary granulosa cells, the MAPK/ERK pathway is strongly associated with proliferation. More recently, it has been shown that expression of granulosa cell differentiation-associated genes through the MAPK/ERK pathway requires activation of the DNA- and RNA-binding protein YB-1 (40). We found that despite enhanced MAPK/ERK pathway activity, YB-1 phosphorylation is downregulated following Jag1 knockdown, which may begin to explain the observed suppression of steroidogenic gene expression, despite the increased activation of prodifferentiation intercellular signals.
It remains to be explored how disruption of canonical Notch signaling leads to alterations in signal transducers such as PI3K/AKT, MAPK/ERK, and YB-1 in granulosa cells. In this regard, there are examples of Notch signaling modulating differentiation and proliferation through interactions with intracellular kinase cascades. Interplay between Notch and receptor tyrosine kinase signaling has been found to be important in the decision-making process between self-renewal and differentiation in the various follicle somatic cell types of the Drosophila ovariole (65). A balance between Notch signaling and the MAPK/ERK pathway, downstream of the fibroblast growth factor receptor, in Sertoli cells is thought to be important in maintaining cyclical recruitment of quiescent prospermatogonia into spermatogenesis (66). Another example of cross-talk between Notch signaling and the MAPK pathway was observed in myotube differentiation from muscle precursors, where MKP-1, a MAPK phosphatase, is regulated in an Rbpj-dependent manner (67). Together, these examples and our data demonstrate that the Notch pathway has the potential to interact with diverse types of intracellular signals to regulate cellular activity.
In summary, we have described a key role for Notch signaling in promoting differentiation and in negatively regulating proliferation in mouse granulosa cells. Our findings suggest that activation of Notch signaling by the ligand Jag1 impacts multiple kinase cascades that are important in control of proliferation and promotion of gene expression leading to granulosa cell differentiation. Considering both our in vivo and in vitro data, we demonstrate that, like many other local factors important during the periovulatory period (45–47), Notch signaling promotes a differentiated phenotype that is initiated by gonadotropin signaling. Understanding how Notch signaling regulates the balance between differentiation and proliferation in granulosa cells promises further insights into the periovulatory period, where the maturing follicle needs to sustain an FSH-dependent differentiated state so as to optimally respond to LH and successfully ovulate a healthy fertilizable egg. Our finding that Notch signaling has a role in promoting the differentiation of granulosa cells adds to the growing and diverse functions for Notch in the mammalian ovary and in reproduction.
Acknowledgments
Financial Support: This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant P01 HD021921 (to K.E.M.), the Cellular and Molecular Basis of Disease Training Program through National Institutes of Health/Institute of General Medical Sciences Grant T32 GM008061 (to R.D.P.), and the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core through Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Grant P50-HD28934.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer; Catalog No. | Species Raised In; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| JAG1 | Goat anti-JAG1 | Santa Cruz Biotechnology; SC-6011 | Goat; polyclonal | 1:200 for western blot; 1:50 for IF | AB_649689 |
| P450SCC | Rabbit anti-P450SCC | Cell Signaling Technology; 14217 | Rabbit; monoclonal | 1:800 for IF | AB_2631970 |
| GFP | Rabbit anti-GFP | Life Technology; A11122 | Rabbit; polyclonal | 1:200 for IHC | AB_221569 |
| NOTCH2 | Rat anti-Notch2 | Developmental Studies Hybridoma Bank; C651.6DbHN | Rat; monoclonal | 1.8 μg/mL for western blot | AB_528412 |
| RBPJ | Rabbit anti-RBPJ | Abcam 25949 | Rabbit; polyclonal | 1:1000 for western blot | AB_778155 |
| GAPDH | Rabbit anti-GAPDH | Cell Signaling Technology; 2118 | Rabbit; monoclonal | 1:10,000 for western blot | AB_561053 |
| panAKT | Rabbit anti-panAKT | Cell Signaling Technology; 4691 | Rabbit; monoclonal | 1:1000 for western blot | AB_915783 |
| ERK1/2 | Rabbit anti-ERK1/2 | Cell Signaling Technology; 4695 | Rabbit; monoclonal | 1:1000 for western blot | AB_390779 |
| phosphoAKT (Ser473) | Rabbit anti-pAKT | Cell Signaling Technology; 4060 | Rabbit; monoclonal | 1:2000 for western blot | AB_2315049 |
| phosphoERK1/2 (Thr202; Tyr204) | Rabbit anti-pERK1/2 | Cell Signaling Technology; 9101 | Rabbit; polyclonal | 1:1000 for western blot | AB_331646 |
| FOXO1 | Rabbit anti-FOXO1 | Cell Signaling Technology; 2880 | Rabbit; monoclonal | 1:1000 for western blot | AB_2106495 |
| phosphoFOXO1 (Ser256) | Rabbit anti-pFOXO1 | Cell Signaling Technology; 9461 | Rabbit; polyclonal | 1:1000 for western blot | AB_329831 |
| YB-1 | Rabbit anti-YB1 | Cell Signaling Technology; 8475 | Rabbit; monoclonal | 1:1000 for western blot | AB_11179070 |
| phosphoYB-1 (Ser102) | Rabbit anti-pYB1 | Cell Signaling Technology; 2900 | Rabbit; monoclonal | 1:1000 for western blot | AB_2219273 |
| IgG rabbit | IRDye 800CW Goat anti-Rabbit IgG | LI-COR; 925-32211 | Goat; polyclonal | 1:5000 for western blot | AB_2651127 |
| IgG rabbit | IRDye 680RD Donkey anti-Rabbit IgG | LI-COR; 926-68073 | Donkey; polyclonal | 1:5000 for western blot | AB_10954442 |
| IgG goat | IRDye 800CW Donkey anti-Goat IgG | LI-COR; 925-32214 | Donkey; polyclonal | 1:5000 for western blot | AB_2687553 |
| IgG rat | IRDye 800CW Goat anti-Rat IgG | LI-COR; 926-32219 | Goat; polyclonal | 1:5000 for western blot | AB_1850025 |
| IgG rat | IRDye 680RD Goat anti-Rat IgG | LI-COR; 926-68076 | Goat; polyclonal | 1:5000 for western blot | AB_10956590 |
Abbreviation: IgG, immunoglobulin G; RRID, Research Resource Identifier.
Footnotes
- AKT
- protein kinase B
- Ccnd2
- cyclin D2
- Cdk1
- cyclin-dependent kinase 1
- CL
- corpora lutea
- CV%
- coefficient of variation
- DAPT
- N-(N-(3,5-difluorophenacetyl)-l-alanyl)-S-phenylglycine t-butyl ester
- E2
- estradiol-17β
- EdU
- 5-ethynyl-2′-deoxyuridine
- ELISA
- enzyme-linked immunosorbent assay
- ERK
- extracellular signal-regulated kinase
- FSH
- follicle-stimulating hormone
- FSHR
- follicle-stimulating hormone receptor
- hCG
- human chorionic gonadotropin
- IF
- immunofluorescence
- IHC
- immunohistochemistry
- IP
- intraperitoneal
- LH
- luteinizing hormone
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen-activated protein kinase kinase
- mRNA
- messenger RNA
- NICD
- Notch intercellular domain
- P4
- progesterone
- P450SCC
- P450 side-chain cleavage
- PI
- propidium iodide
- PI3K
- phosphatidylinositol 3-kinase
- PMSG
- pregnant mare’s serum gonadotropin
- PND
- postnatal day
- qRT-PCR
- quantitative real-time polymerase chain reaction
- siRNA
- small interfering RNA
- TNR
- transgenic Notch responsive
- YB-1
- Y-Box–binding protein-1.
References
- 1.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148(8):3580–3590. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol. 2009;202(3):407–417. [DOI] [PubMed] [Google Scholar]
- 3.Mu X, Liao X, Chen X, Li Y, Wang M, Shen C, Zhang X, Wang Y, Liu X, He J. DEHP exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms. J Hazard Mater. 2015;298:232–240. [DOI] [PubMed] [Google Scholar]
- 4.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144(8):3329–3337. [DOI] [PubMed] [Google Scholar]
- 5.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12(2):61–69. [DOI] [PubMed] [Google Scholar]
- 6.Jones RL, Pepling ME. KIT signaling regulates primordial follicle formation in the neonatal mouse ovary. Dev Biol. 2013;382(1):186–197. [DOI] [PubMed] [Google Scholar]
- 7.Kimura F, Bonomi LM, Schneyer AL. Follistatin regulates germ cell nest breakdown and primordial follicle formation. Endocrinology. 2011;152(2):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298(1):132–148. [DOI] [PubMed] [Google Scholar]
- 9.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148(5):1968–1976. [DOI] [PubMed] [Google Scholar]
- 10.Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology. 2009;150(2):1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Gridley T. Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol. 2013;11(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol. 2014;28(4):499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev. 1980;60(1):51–89. [DOI] [PubMed] [Google Scholar]
- 14.Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, Unterman TG, Lee EJ, Jameson JL, Hunzicker-Dunn M. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005;280(10):9135–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137(2):216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5(2):197–206. [DOI] [PubMed] [Google Scholar]
- 17.Kovall RA. More complicated than it looks: assembly of Notch pathway transcription complexes. Oncogene. 2008;27(38):5099–5109. [DOI] [PubMed] [Google Scholar]
- 18.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138(17):3593–3612. [DOI] [PubMed] [Google Scholar]
- 19.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234(2):339–351. [DOI] [PubMed] [Google Scholar]
- 20.Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132(4):817–828. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Norton CR, Gridley T. Not all lunatic fringe null female mice are infertile. Development. 2006;133(4):579. [DOI] [PubMed] [Google Scholar]
- 22.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134(7):1243–1251. [DOI] [PubMed] [Google Scholar]
- 23.Manosalva I, González A, Kageyama R. Hes1 in the somatic cells of the murine ovary is necessary for oocyte survival and maturation. Dev Biol. 2013;375(2):140–151. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J. Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev. 2001;109(2):355–361. [DOI] [PubMed] [Google Scholar]
- 25.Murta D, Batista M, Silva E, Trindade A, Mateus L, Duarte A, Lopes-da-Costa L. Differential expression of Notch component and effector genes during ovarian follicle and corpus luteum development during the oestrous cycle. Reprod Fertil Dev. 2015;27(7):1038–1048. [DOI] [PubMed] [Google Scholar]
- 26.Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57(1):195–220. [DOI] [PubMed] [Google Scholar]
- 27.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6(3):314–322. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 29.Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of follicle-stimulating hormone and luteinizing hormone receptor messenger RNAs in the rat ovary. Mol Endocrinol. 1991;5(10):1405–1417. [DOI] [PubMed] [Google Scholar]
- 30.Kipp JL, Kilen SM, Woodruff TK, Mayo KE. Activin regulates estrogen receptor gene expression in the mouse ovary. J Biol Chem. 2007;282(50):36755–36765. [DOI] [PubMed] [Google Scholar]
- 31.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384(6608):470–474. [DOI] [PubMed] [Google Scholar]
- 32.Vanorny DA, Mayo KE. The role of Notch signaling in the mammalian ovary. Reproduction. 2017;153(6):R187–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channing CP. Influences of the in vivo and in vitro hormonal environment upon luteinization of granulosa cells in tissue culture. Recent Prog Horm Res. 1970;26:589–622. [DOI] [PubMed] [Google Scholar]
- 34.Hsueh AJ, Adashi EY, Jones PB, Welsh TH Jr. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5(1):76–127. [DOI] [PubMed] [Google Scholar]
- 35.Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood). 2009;234(8):880–907. [DOI] [PubMed] [Google Scholar]
- 36.Shih M-CM, Chiu Y-N, Hu M-C, Guo I-C, Chung B-C. Regulation of steroid production: analysis of Cyp11a1 promoter. Mol Cell Endocrinol. 2011;336(1-2):80–84. [DOI] [PubMed] [Google Scholar]
- 37.Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142(3):977–986. [DOI] [PubMed] [Google Scholar]
- 38.Kayampilly PP, Menon KMJ. Follicle-stimulating hormone inhibits adenosine 5′-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology. 2009;150(2):929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayampilly PP, Menon KMJ. Inhibition of extracellular signal-regulated protein kinase-2 phosphorylation by dihydrotestosterone reduces follicle-stimulating hormone-mediated cyclin D2 messenger ribonucleic acid expression in rat granulosa cells. Endocrinology. 2004;145(4):1786–1793. [DOI] [PubMed] [Google Scholar]
- 40.Donaubauer EM, Hunzicker-Dunn ME. Extracellular signal-regulated kinase (ERK)-dependent phosphorylation of Y-box-binding protein 1 (YB-1) enhances gene expression in granulosa cells in response to follicle-stimulating hormone (FSH). J Biol Chem. 2016;291(23):12145–12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donaubauer EM, Law NC, Hunzicker-Dunn ME. Follicle-stimulating hormone (FSH)-dependent regulation of extracellular regulated kinase (ERK) phosphorylation by the mitogen-activated protein (MAP) kinase phosphatase MKP3. J Biol Chem. 2016;291(37):19701–19712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herndon MK, Law NC, Donaubauer EM, Kyriss B, Hunzicker-Dunn M. Forkhead box O member FOXO1 regulates the majority of follicle-stimulating hormone responsive genes in ovarian granulosa cells. Mol Cell Endocrinol. 2016;434:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan H-Y, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28(23):7001–7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, Stocco C. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27(3):511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, DeMayo FJ, Richards JS, Boerboom D. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 2010;24(8):3010–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deroo BJ, Rodriguez KF, Couse JF, Hamilton KJ, Collins JB, Grissom SF, Korach KS. Estrogen receptor beta is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol. 2009;23(7):955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-LC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. [DOI] [PubMed] [Google Scholar]
- 49.Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin Cell Dev Biol. 2012;23(4):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansson EM, Lanner F, Das D, Mutvei A, Marklund U, Ericson J, Farnebo F, Stumm G, Stenmark H, Andersson ER, Lendahl U. Control of Notch-ligand endocytosis by ligand-receptor interaction. J Cell Sci. 2010;123(Pt 17):2931–2942. [DOI] [PubMed] [Google Scholar]
- 51.Boggs K, Henderson B, Reisman D. RBP-Jkappa binds to and represses transcription of the p53 tumor suppressor gene. Cell Biol Int. 2009;33(3):318–324. [DOI] [PubMed] [Google Scholar]
- 52.Koelzer S, Klein T. A Notch-independent function of Suppressor of Hairless during the development of the bristle sensory organ precursor cell of Drosophila. Development. 2003;130(9):1973–1988. [DOI] [PubMed] [Google Scholar]
- 53.Morel V, Schweisguth F. Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 2000;14(3):377–388. [PMC free article] [PubMed] [Google Scholar]
- 54.Koelzer S, Klein T. Regulation of expression of Vg and establishment of the dorsoventral compartment boundary in the wing imaginal disc by Suppressor of Hairless. Dev Biol. 2006;289(1):77–90. [DOI] [PubMed] [Google Scholar]
- 55.Ross DA, Kadesch T. Consequences of Notch-mediated induction of Jagged1. Exp Cell Res. 2004;296(2):173–182. [DOI] [PubMed] [Google Scholar]
- 56.Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134(12):2369–2378. [DOI] [PubMed] [Google Scholar]
- 57.Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci USA. 2010;107(36):15792–15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol. 2009;332(1):166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manderfield LJ, High FA, Engleka KA, Liu F, Li L, Rentschler S, Epstein JA. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2012;125(2):314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hernandez F, Peluffo MC, Stouffer RL, Irusta G, Tesone M. Role of the DLL4-NOTCH system in PGF2alpha-induced luteolysis in the pregnant rat. Biol Reprod. 2011;84(5):859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Accialini P, Hernández SF, Bas D, Pazos MC, Irusta G, Abramovich D, Tesone M. A link between Notch and progesterone maintains the functionality of the rat corpus luteum. Reproduction. 2015;149(1):1–10. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Liu S, Peng L, Dong Q, Bao R, Lv Q, Tang M, Hu C, Li G, Liang S, Zhang C. Notch signaling pathway regulates progesterone secretion in murine luteal cells. Reprod Sci. 2015;22(10):1243–1251. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura M, Fukami M, Sugawa F, Miyado M, Nonomura K, Ogata T. Mamld1 knockdown reduces testosterone production and Cyp17a1 expression in mouse Leydig tumor cells. PLoS ONE. 2011;6(4):e19123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117–149. [DOI] [PubMed] [Google Scholar]
- 65.Johnston MJ, Bar-Cohen S, Paroush Z, Nystul TG. Phosphorylated Groucho delays differentiation in the follicle stem cell lineage by providing a molecular memory of EGFR signaling in the niche. Development. 2016;143(24):4631–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia TX, Hofmann M-C. NOTCH signaling in Sertoli cells regulates gonocyte fate. Cell Cycle. 2013;12(16):2538–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kondoh K, Sunadome K, Nishida E. Notch signaling suppresses p38 MAPK activity via induction of MKP-1 in myogenesis. J Biol Chem. 2007;282(5):3058–3065. [DOI] [PubMed] [Google Scholar]