Abstract
Gonadotropin-releasing hormone (GnRH) neurons are critical to many aspects of fertility regulation, from producing episodic release critical to both sexes, to providing a central signal to induce the ovulatory cascade in females. This year saw progress through the rejection, and occasional support, of hypotheses in understanding how GnRH neurons contribute to these processes. This brief review provides one laboratory’s view of new insights into possible roles for these cells in development, adult reproductive function, and what may go wrong with GnRH neurons in some cases of infertility.
The Moenter laboratory rejected hypotheses about GnRH neurons many times this year; 2017 was thus a rather good year.
In his book The Logic of Scientific Discovery (often abbreviated LSD), Karl Popper makes the argument that science progresses when ideas can be proposed and “falsified” or rejected (1). Sometimes this happens with efficient rapidity; sometimes ideas persist for years, decades, or centuries before a field moves on. In the past year, my laboratory has rejected several hypotheses posited about the function of gonadotropin-releasing hormone (GnRH) neurons, thus advancing our understanding of the central regulation of fertility.
GnRH neurons link the processing power of the brain to the downstream reproductive system. There are ∼800 of these cells in mice and ∼2500 in humans. This small population is neither nuclear nor laminar in vertebrates, but is rather scattered from the diagonal band of Broca through the preoptic area and medial basal hypothalamus (2). These cells project mainly to circumventricular organs, primarily the median eminence, where GnRH is released near the pituitary portal vessels. GnRH stimulates the anterior pituitary gonadotropes to produce and release luteinizing hormone (LH) and follicle-stimulating hormone, which subsequently activate the gonadal functions of gametogenesis and steroidogenesis. Steroid hormones feed back to the hypothalamus to control the release of GnRH and at the pituitary to control response to this hormone.
In adult males and during most of the female reproductive cycle, the release of GnRH is episodic, with sharp increases observed in portal blood that are coincident with pulses of LH release into the periphery (3). Episodic release in both sexes is regulated by homeostatic steroid negative feedback. This episodic pattern is interrupted in females in the late follicular phase when sustained exposure to elevated estradiol results in a switch in feedback mode of this steroid to positive, inducing a prolonged surge of GnRH and LH, which triggers ovulation (4). This brief review attempts to integrate findings published by our laboratory during the last year into the broader picture of the neuroendocrine control of fertility, addressing questions from pulses to surges to infertility.
Do GnRH Neurons Outsource Pulse Generation?
Since the description of pulsatile LH release in the early 1970s (5), reproductive endocrinologists have searched for the origin of this episodic activity within the brain. Coordinated release of GnRH and LH (6) and electrical activity within the hypothalamus that is coincident with LH release were identified within about a decade of this search (7). The advent of green fluorescent protein–identified GnRH neurons at the turn of the century enabled studies of these cells using electrophysiologic approaches in brain slices (8, 9). In both males and females, GnRH neurons exhibited peaks and nadirs in action potential firing activity that occurred with frequency similar to pulsatile LH release; further, these patterns in firing activity were regulated by in vivo steroid treatment in an expected manner (i.e., steroid feedback reduced frequency of peaks in firing rate) (10–12).
Despite these advances, gaps remain in our understanding of this fundamental process, particularly with regard to the source and steroid regulation of episodic activity. Although GnRH release in vivo and firing rate in brain slices are both similarly affected by steroids, GnRH neurons themselves do not express detectable levels of most steroid receptors, with the exception of estrogen receptor (ER) β (13). Observations that ERα mediates most feedback effects of estradiol (14, 15) led to the postulate that feedback was conveyed via ERα-sensitive afferents. Kisspeptin neurons of the anteroventral periventricular and arcuate areas of the hypothalamus are likely candidates for this role (16). These cells express ERα and other steroid receptors (17), and the neuromodulator kisspeptin is perhaps the strongest stimulator of GnRH neuron activity and release (18, 19). Of particular interest with regard to pulse generation are the arcuate kisspeptin neurons, also known as KNDy cells, as they coexpress kisspeptin, neurokinin B, and dynorphin. KNDy neurons were postulated to be a potential source of the GnRH pulse generator based on opposing effects of steroids on neurokinin B and dynorphin expression and observation of the effects of knocking out dynorphin or its receptor on LH release (20). Subsequent studies of the action potential firing rate of KNDy neurons supported short-term stimulation of firing by agonists of neurokinin 3 receptor (NK3R), inhibition of firing by dynorphin, and steroid modulation of both of these responses (21–23).
These studies all used short-term recordings, leaving unanswered the questions of whether KNDy neurons generate firing patterns that fluctuate with intervals similar to LH/GnRH pulse generation in vivo and if any such rhythms are modulated steroid feedback. To address this, we made extracellular recordings of green fluorescent protein–identified KNDy neurons in brain slices from adult male mice that were intact, castrated, or castrated and treated with estradiol or dihydrotestosterone (DHT) (24). The firing rate of KNDy neurons exhibited marked peaks and nadirs over several hours of recording. Peaks in firing rate occurred more frequently in castrated than intact mice, suggesting a role for gonadal factors in regulating this rhythm. Indeed, replacement of either estradiol or DHT in vivo restored peak frequency to intact levels.
To begin to examine the role of the peptides neurokinin B and dynorphin in generating this rhythm, we antagonized NK3R and the κ-opioid receptor (KOR), respectively. Treatment of brain slices from castrate males with the NK3R antagonist SB222200 reduced the frequency of peaks of firing activity to intact levels, but interestingly did not eliminate firing. This suggests a role for the increased neurokinin B (NKB) expression and response to NKB observed in castrate animals in increasing firing peak and potentially LH pulse and frequency, but also indicates other mechanisms beyond activation of NK3R can generate rhythmic output from KNDy neurons. Treatment of brain slices from intact mice with the KOR antagonist norbinaltorphimine had no effect on long-term firing pattern, consistent with observation of its lack of effect on short-term firing rate (22). This dose of norbinaltorphimine did effectively block effects of exogenous dynorphin on KNDy neuron firing. Together, the observations that KNDy neurons fire in an episodic manner and that this pattern is regulated by steroid feedback are consistent with a role for these neurons in GnRH pulse generation and steroid regulation. Of interest in this regard, ongoing studies examining LH pulse patterns in mice in which ERα is selectively deleted from kisspeptin cells reveal pulse frequency is increased in these mice compared with controls (25). This indicates that despite similar mean LH levels between these groups, negative feedback on pulse generation may be mediated through estradiol action on kisspeptin neurons (26).
The long-term firing pattern of KNDy neurons in brain slices and their steroid regulation is similar to that of GnRH neurons from the same animal models (10, 11), with the exception of DHT inhibiting the former and activating the latter. This difference in steroid regulation suggests other steroid-sensitive afferents also play an important role in sculpting the ultimate pulsatile output of GnRH neurons. How KNDy neurons generate episodic activity is a question that remains to be answered. Although NKB can locally increase frequency of KNDy neuron output in castrate mice, the local inhibitory role of dynorphin in terms of firing activity is called into question. Of note, in vivo studies with the same KOR antagonist revealed a reduced frequency of hypothalamic multiunit activity and associated with LH release (27). Together, these findings suggest a revised hypothesis in which dynorphin may play an inhibitory role in pulse generation but that the neurocircuitry involved in this response extends beyond local KNDy-KNDy connections.
Do Estradiol-Induced and Proestrous LH and GnRH Surges Differ?
Episodic GnRH release at different frequencies is needed to provide proper gonadotropin input to the ovary to promote development of a dominant follicle(s). The high estradiol production from these follicles ultimately switches the feedback mode to positive, activating GnRH neurons. To study estradiol feedback mode switches, our laboratory makes frequent use of ovariectomized, estradiol-replaced (OVX+E) mice. LH levels in these mice are suppressed in the morning compared with OVX-only mice, indicative of estradiol negative feedback, and increased in the late afternoon, indicative of positive feedback (28). The activity of GnRH neurons in brain slices exhibits these same shifts. This activity repeats on a daily basis as long as estradiol levels remain in the high physiologic range, consistent with early observations of a daily signal for ovulation (29). Although this model provides the opportunity to examine the switch in feedback mode without an accompanying change in estradiol level, it does not recapitulate all of the steroid and other changes of the typical reproductive cycle. In particular, progesterone has been implicated in increasing LH surge amplitude, potentially at the central level (30, 31). Indeed, as we began to move into comparisons of estradiol-induced vs proestrous animal models for work on stress and the cycle (discussed later), we also observed that LH surge amplitude was lower in OVX+E than proestrous mice (32).
We hypothesized that the different amplitude of estradiol-induced and proestrous LH surges was attributable to the inability of estradiol alone to increase GnRH neuron activity to the same extent and the hormonal milieu of proestrus. We rejected this hypothesis based on the following findings. First, overall GnRH neuron firing rate was similarly increased in proestrous and OVX+E mice. Second, LH response to an early afternoon injection of GnRH was greater in proestrous than OVX+E mice. Together, these observations suggest reduced LH surge amplitude in the OVX+E model is more likely attributable to reduced pituitary sensitivity than gross changes in GnRH neuron activity. Similarly, observations of GnRH surges in sheep found the preovulatory and estradiol-induced surges were similar in amplitude and duration (4, 33). Further, studies of anteroventral periventricular kisspeptin neurons, which are postulated to convey estradiol positive feedback to GnRH neurons, found that proestrous changes in firing activity, and underlying mechanisms were recapitulated in OVX+E mice (34, 35). Does this mean there is no central role for progesterone? It does not. Positive feedback is rare in physiology, and given the importance of ovulation for survival of species, there may be multiple central neural mechanisms that can lead to the same output. Further, for species survival, ovulation must be paired with mating. Prior progesterone exposure is needed for full estrous behavior in many species and also delays the LH surge induced by estradiol (36, 37). Thus, although estradiol alone can likely recapitulate final central neuroendocrine hormone output to the pituitary, progesterone may be involved in some of the other aspects critical for fertility.
Don’t Judge a Book by Its Cover: The Surprisingly Active Childhood of GnRH Neurons
The reproductive system attains the mature functions discussed previously at more advanced ages than other aspects of physiology. Female reproductive cycles, steroidogenesis, and gametogenesis do not achieve adult levels until well into the pubertal transition. For this reason, a prevailing view has been that the reproductive neuroendocrine system was also quiescent. An exception to this is minipuberty of infancy in old-world primates, a period lasting several months in which LH and follicle-stimulating hormone are elevated (38). In mice, by contrast, there is a male-specific neonatal increase in testosterone that lasts less than a day and appears to be GnRH independent (39); gonadotropins are also low for the first 2 weeks postnatal before sporadic increases are observed (40).
Our first hint that GnRH neurons may be flying under the radar of this low-gonadotropin cover came from measurements of GnRH release using fast-scan cyclic voltammetry with electrodes positioned in the median eminence of brain slices from male mice. GnRH release frequency before birth, within a day of birth, and at 1 week of age was greater than has been observed in adults from any species (41). This made us rethink the concept of a quiescent neuroendocrine circuitry before puberty and consider a more general theme in developmental neuroscience, specifically the role of neuronal activity in sculpting its own inputs. If high-frequency GnRH release is indicative of high action potential firing rate, prepubertal GnRH neuron activity may be critical for adult reproductive outcomes. Because this system develops differently in females and males, we hypothesized that not only are GnRH neurons more active during development, but also that this activity is sexually differentiated (42). Action potential firing rate of GnRH neurons in brain slices from both sexes was examined at 1, 2, 3, and 4 weeks of age and in adults (>6 weeks of age). We could not reject either of these hypotheses; GnRH neuron firing rate was indeed higher before puberty than in adults of both sexes. Further, firing rate in females was greater than males at 2 and 3 weeks of age. These findings support and extend the study showing high-frequency GnRH release in postnatal mice and suggest a neural rather than endocrine output role of early GnRH neuron activity. An ongoing study in the laboratory using these same animal models and examining the development of GABAergic transmission to GnRH neurons suggests the frequency of transmission is also greater during development and that this peaks at younger ages in females than males (43), providing preliminary support for this concept.
The developmental profile of GnRH neuron activity is of interest with regard to the concept of developmental origins of health and disease, which has gained increasing popularity in the past couple of decades with the discoveries of epigenetic regulation. A developmental origin of polycystic ovary syndrome (PCOS), the most common form of endocrine in fertility, has been postulated for some time (44). Women with PCOS exhibit oligo/anovulation, hyperandrogenemia, and persistent elevation in LH, and presumably GnRH, release frequency rather than the typical steroid feedback regulation described previously (45). Increasing evidence supports early pubertal alterations in LH release in hyperandrogenemic girls and those with PCOS (46, 47). To study PCOS, many investigators have made use of female offspring from mothers treated with androgens during gestation (48, 49). Although no animal model perfectly recapitulates a human disease, prenatally androgenized (PNA) females from several species exhibit reproductive neuroendocrine characteristics similar to those in women with PCOS.
We thus extended our examination of GnRH neuron activity to PNA mice, which, as adults, exhibit increased GnRH neuron activity, LH pulse frequency, and disrupted cycles. Based on the increased activity of GnRH neurons in adults, we hypothesized that activity would be increased during development. We rejected this hypothesis, as activity in PNA females was lower during development, in particular at 3 weeks of age (42). Interestingly, the ongoing work on GABAergic transmission suggests this is elevated in PNA females at this same age (43). GABA is typically excitatory in GnRH neurons (50); thus, this finding is leading us to investigate if PNA alters the GnRH neuron response to GABA. Together, these findings have and continue to reshape how we think about GnRH neurons, in particular their roles in the central nervous system that are not directly related to activation of the pituitary. They also provide evidence that factors from the internal or external environment, such as androgens, can adjust the prepubertal development of the GnRH network, potentially altering adult fertility.
Does Stress Always Shut Down Fertility?
More often than a developmental origin, temporary disruption of the reproductive neuroendocrine system may arise from the perturbations of daily life, including diet, exercise, and stress exposure. These psychosocial stressors are often milder than the severe stresses that have been shown to inhibit the reproductive system. Exposure to psychosocial stress is often linked in public perception to infertility, but is that always the case? When we began to investigate psychosocial stress, we initially hypothesized daily exposure would disrupt estrous cycles in mice (32). We once again rejected our hypothesis when 2 hours of daily restraint did not alter cyclicity. We altered our approach to test if a single exposure to stress could affect the LH surge. This was chosen as a point of high potential disruption of reproduction; blocking or disrupting the LH surge may result in ovulation and/or fertility at an unexpected/inappropriate time. We developed a layered psychosocial stress paradigm that produced consistently increased corticosterone for 5 hours. When exposed to this stress paradigm on midmorning of proestrus, most animals did not have an LH surge at lights out, nor was there evidence for the surge occurring with a 1-day delay. Despite disruption of the surge, animals continued to cycle. This makes sense, as the estradiol rise that drives the cornification of vaginal epithelium would have occurred that morning; further, the effects of the single stress exposure are expected to dissipate fairly quickly and thus unlikely to disrupt subsequent reproductive tract events that produce the cyclical changes we observe. These observations may be relevant to women who have difficulty conceiving despite outwardly normal cycles.
The observations of cornified epithelium and increased uterine mass both suggest the proestrous estradiol rise did occur in the cycling mice, but we also specifically tested if stress could interfere with the estradiol-induced surge. We applied the stress paradigm at the same time of day in OVX+E mice; the LH surge was disrupted in all mice. The relative strength of the disruption of the estradiol-induced (complete in all mice tested) vs proestrous (>50% but several mice unaffected) surge returns us to the previous question about potential differences between these surges. It is possible that the presence of other ovarian factors potentially makes the proestrous surge more resilient; this may be at the pituitary level but could also be centrally mediated. Another possible explanation is that the negative feedback of estradiol provided by the constant-release implant is stronger than that during the cycle and that this affects the subsequent switch in modes. In this regard, stronger pulsatile GnRH release was observed just before the preovulatory GnRH surge than before the estradiol-induced surge in sheep (4, 33). Finally, it may be that the diurnal timing, although maintained, is shifted among these models. If this is the case, application of stress at the same time of day may interact with the surge-generating process at different points with different susceptibility to disruption.
The previous paragraphs very intentionally used the word disrupt, rather than block, for the LH surge. We did not look for the LH surge throughout the entire day, thus we can only claim not to have observed it within a few hours of the typical onset. Another reason for caution is that in ongoing studies of the action of corticotropin-releasing hormone (CRH) on the firing activity of GnRH neurons, data suggest that the response to CRH depends upon the presence of estradiol and also the CRH receptor (CRHR) that is activated (51). Response to CRH was examined in OVX vs OVX+E mice in the late afternoon when estradiol has a positive-feedback effect. No change in firing rate was observed in GnRH neurons from OVX mice, but the response to CRH depended upon dose, with lower doses activating and higher doses inhibiting GnRH neuron activity. This was attributable to an increase in firing in response to specific agonists of CRHR1 and a decrease in firing in response to specific agonists of CRHR2. These different actions of CRH, in combination with the minority report of observed stimulatory effects of stress on reproduction, open the door to the postulate that stress exposure in a high estradiol milieu advances the surge.
Can a Long Story Be Made Short?
Much of the previously discussed work examines the firing pattern of GnRH or KNDy neurons, in particular looking for long-term trends that resemble episodic release of LH in vivo. Neurosecretion, however, typically happens on a much shorter time scale of tens to hundreds of milliseconds. This year, we thus began to examine how the short-term firing pattern of these neurons was altered by the various treatments. To do this, we devised an algorithm that examined how action potentials would be gathered into short-term bursts, as the definition of a burst was altered by iteratively increasing the time between events for inclusion within a burst (increase by 10-ms increments from a 10-ms intraburst interval). In most cells and treatments, this yielded a peak intraburst interval at which the most bursts were observed. This interval was then used to analyze characteristics of the bursts including frequency, duration, and spikes per burst. Interestingly, both GnRH and KNDy neurons typically increase their overall firing rate by increasing the frequency of bursts, with increased duration being a second mechanism observed in some experiments. At a very rudimentary level, increasing burst frequency indicates the cells initiate action potential firing more frequently, whether by shifts in intrinsic properties, altered synaptic input, or a combination of these. Increased burst duration, although observed less often, suggests that when firing is initiated through whatever mechanism, it persists longer; this is more likely attributable to a change in the properties of the postsynaptic cell. Notably, both GnRH and KNDy neurons exhibit these groupings of action potentials into bursts. Although the pattern is clearly evident, it is quite different from what is observed in other forebrain neurons in the cortex, hippocampus, and thalamus. Whether these different short-term patterns are related to the precision of timing required to carry out the functions of these various brain regions remains to be determined.
Conclusions: Rejection Has Its Advantages
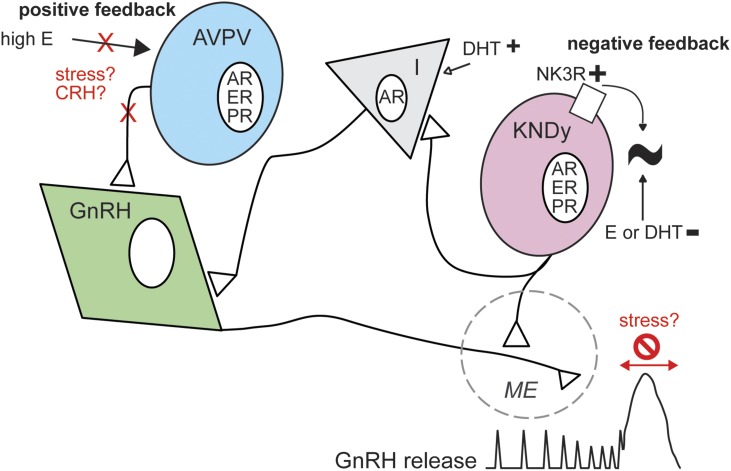
The paucity and anatomy of GnRH neurons have long helped them remain enigmatic (Fig. 1). Application of modern neuroscience methodologies has helped reveal more and more about the function and regulation of these neurons throughout the reproductive lifespan and in certain conditions of infertility. In addition to the work discussed in this review from our laboratory, many others have made substantial contributions over the past year, by no means limited to this list (52–56). No amount of experimentation can prove a hypothesis is correct, but rejection of hypotheses pushes us toward new ways of thinking, advancing our understanding of reproductive neuroendocrine function.
Figure 1.
Schematic of possible regulation of the reproductive neuroendocrine system in adults. In males and during most of the cycle, episodic GnRH release and negative feedback prevail (right). KNDy neurons produce rhythmic firing output that is regulated by estrogens and androgens and activation of NK3R, but whether this rhythm arises in these cells remains to be determined. In females, high estradiol (E) induces positive feedback, likely through anteroventral periventricular (AVPV) kisspeptin neurons. Stress may interrupt this process, possibly by elevating CRH, which may block or move the surge mode of release. AR, androgen receptor; I, interneuron; ME, median eminence; PR, progesterone receptor.
Acknowledgments
I thank the trainees and laboratory managers of the Moenter laboratory who did and made this work possible: Caroline Adams, Tova Berg, Laura Burger, R. Anthony DeFazio, Eden Dulka, Chayarndorn Phumsatitpong, Marina Silveira, Charlotte Vanacker, Elizabeth Wagenmaker, and Luhong Wang. I would also like to prevail upon my colleagues in the field for their patience in not citing all of their interesting work while fulfilling the request to review the work we published in this journal over the past year.
Financial Support: This work was supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD41469, R37 HD34860, and P50 HD28934.
Acknowledgments
Disclosure Summary: The author has nothing to disclose.
Footnotes
- CRH
- corticotropin-releasing hormone
- CRHR
- corticotropin-releasing hormone receptor
- DHT
- dihydrotestosterone
- ER
- estrogen receptor
- GnRH
- gonadotropin-releasing hormone
- KNDy
- neurons coexpressing kisspeptin, neurokinin B, and dynorphin
- KOR
- κ-opioid receptor
- LH
- luteinizing hormone
- NK3R
- neurokinin 3 receptor NKB, neurokinin B
- OVX
- ovariectomized
- OVX+E
- ovariectomized estradiol-replaced
- PCOS
- polycystic ovary syndrome
- PNA
- prenatally androgenized.
References
- 1.Popper KR. The Logic of Scientific Discovery. New York: Basic Books; 1959. [Google Scholar]
- 2.Silverman AJ, Livne I, Witkin JW. The gonadotropin-releasing hormone (GnRH) neuronal systems: immunocytochemistry and in situ hybridization In: Knobil E, Neill JD, eds. The Physiology of Reproduction. 2nd ed New York, NY: Raven Press, Ltd, 1994; 1683–1706. [Google Scholar]
- 3.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130(1):503–510. [DOI] [PubMed] [Google Scholar]
- 4.Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129(3):1175–1182. [DOI] [PubMed] [Google Scholar]
- 5.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87(5):850–853. [DOI] [PubMed] [Google Scholar]
- 6.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260. [DOI] [PubMed] [Google Scholar]
- 8.Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19(6):2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–419. [DOI] [PubMed] [Google Scholar]
- 10.Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147(3):1474–1479. [DOI] [PubMed] [Google Scholar]
- 11.Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74(5):931–937. [DOI] [PubMed] [Google Scholar]
- 12.Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144(3):823–831. [DOI] [PubMed] [Google Scholar]
- 13.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141(9):3506–3509. [DOI] [PubMed] [Google Scholar]
- 14.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17(6):1039–1053. [DOI] [PubMed] [Google Scholar]
- 15.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149(11):5328–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. [see comment] N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 17.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruka KA, Burger LL, Moenter SM. Both estrogen and androgen modify the response to activation of neurokinin-3 and κ-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology. 2016;157(2):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 24.Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM. Long-term recordings of arcuate nucleus kisspeptin neurons reveal patterned activity that is modulated by gonadal steroids in male mice. Endocrinology. 2017;158(10):3553–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr., Moenter SM. Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor α in adult female mice. J Neurosci. In press. [DOI] [PMC free article] [PubMed]
- 26.Dubois SL, Acosta-Martínez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102(43):15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. [DOI] [PubMed] [Google Scholar]
- 30.Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104(5):1247–1255. [DOI] [PubMed] [Google Scholar]
- 31.Micevych P, Sinchak K. Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology. 2008;149(6):2739–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagenmaker ER, Moenter SM. Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology. 2017;158(8):2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127(3):1375–1384. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, DeFazio RA, Moenter SM. Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro. 2016;3(3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Myers MG Jr., Moenter SM. Estrogen receptor α (ERα) expression in anteroventral periventricular nucleus (AVPV) kisspeptin neurons is required for estradiol regulation of intrinsic excitability of these cells. 99th Annual Meeting of the Endocrine Society; April 1–4, 2017; Orlando, FL. Abstract SH03-8.
- 36.Karsch FJ, Legan SJ, Ryan KD, Foster DL. Importance of estradiol and progesterone in regulating LH secretion and estrous behavior during the sheep estrous cycle. Biol Reprod. 1980;23(2):404–413. [DOI] [PubMed] [Google Scholar]
- 37.Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59(1):93–118. [DOI] [PubMed] [Google Scholar]
- 38.Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153(2):782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michael SD, Kaplan SB, Macmillan BT. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. J Reprod Fertil. 1980;59(1):217–222. [DOI] [PubMed] [Google Scholar]
- 41.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dulka EA, Moenter SM. Prepubertal development of gonadotropin-releasing hormone (GnRH) neuron activity is altered by sex, age and prenatal androgen exposure. Endocrinology. 2017;158(11):3943–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berg T, Moenter SM. Prenatal androgenization alters prepubertal development of gonadotropin-releasing hormone (GnRH) neuronal network function and connectivity. Program No. 60.15. 2016 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2016. [Google Scholar]
- 44.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17(10):2573–2579. [DOI] [PubMed] [Google Scholar]
- 45.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins JS, Beller JP, Burt Solorzano C, Patrie JT, Chang RJ, Marshall JC, McCartney CR. Blunted day-night changes in luteinizing hormone pulse frequency in girls with obesity: the potential role of hyperandrogenemia. J Clin Endocrinol Metab. 2014;99(8):2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ. Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril. 2006;85(4):1049–1056. [DOI] [PubMed] [Google Scholar]
- 48.Moore AM, Campbell RE. Polycystic ovary syndrome: understanding the role of the brain. Front Neuroendocrinol. 2017;46:1–14. [DOI] [PubMed] [Google Scholar]
- 49.Roland AV, Moenter SM. Reproductive neuroendocrine dysfunction in polycystic ovary syndrome: insight from animal models. Front Neuroendocrinol. 2014;35(4):494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phumsatitpong C, Moenter SM. Estradiol-dependent stimulation and suppression of gonadotropin-releasing hormone (GnRH) neuron firing activity by corticotropin-releasing hormone (CRH) in female mice. Endocrinology. In press. [DOI] [PMC free article] [PubMed]
- 52.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Rønnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu C, Messina A, Somm E, Miraoui H, Kinnunen T, Acierno J Jr, Niederländer NJ, Bouilly J, Dwyer AA, Sidis Y, Cassatella D, Sykiotis GP, Quinton R, De Geyter C, Dirlewanger M, Schwitzgebel V, Cole TR, Toogood AA, Kirk JMW, Plummer L, Albrecht U, Crowley WF Jr, Mohammadi M, Tena-Sempere M, Prevot V, Pitteloud N. KLB, encoding β-Klotho, is mutated in patients with congenital hypogonadotropic hypogonadism. EMBO Mol Med. 2017;9(10):1379–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padilla SL, Qiu J, Nestor CC, Zhang C, Smith AW, Whiddon BB, Rønnekleiv OK, Kelly MJ, Palmiter RD. AgRP to Kiss1 neuron signaling links nutritional state and fertility [published correction appears in Proc Natl Acad Sci USA. 2017;114(17):E3584]. Proc Natl Acad Sci USA. 2017;114(9):2413–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iremonger KJ, Porteous R, Herbison AE. Spike and neuropeptide-dependent mechanisms control GnRH neuron nerve terminal Ca(2+) over diverse time scales. J Neurosci. 2017;37(12):3342–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Messina A, Langlet F, Chachlaki K, Roa J, Rasika S, Jouy N, Gallet S, Gaytan F, Parkash J, Tena-Sempere M, Giacobini P, Prevot V. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat Neurosci. 2016;19(6):835–844. [DOI] [PubMed] [Google Scholar]