Abstract
Nonalcoholic fatty liver disease (NAFLD) is a growing epidemic worldwide, particularly in countries that consume a Western diet, and can lead to life-threatening conditions such as cirrhosis and hepatocellular carcinoma. With increasing prevalence of NAFLD in both children and adults, an understanding of the factors that promote NAFLD development and progression is crucial. Environmental agents, including endocrine-disrupting chemicals (EDCs), which have been linked to other diseases, may play a role in NAFLD development. Increasing evidence supports a developmental origin of liver disease, and early-life exposure to EDCs could represent one risk factor for the development of NAFLD later in life. Rodent studies provide the strongest evidence for this link, but further studies are needed to define whether there is a causal link between early-life EDC exposure and NAFLD development in humans. Elucidating the molecular mechanisms underlying development of NAFLD in the context of developmental EDC exposures may identify biomarkers for people at risk, as well as potential intervention and/or therapeutic opportunities for the disease.
The references cited in this mini-review were selected from manuscripts found through a PubMed search, without a publication year cutoff. References found in these publications provided additional articles to search and cite.
Nonalcoholic fatty liver disease (NAFLD) is a spectrum disorder beginning with accumulation of fat, progressing to fibrosis and hepatic damage, and culminating in carcinogenic transformation. The first stage of fat accumulation, also known as steatosis or fatty liver, is reversible (1–4). Fat accumulation can be assessed by examining liver tissue sections for microsteatosis (fat droplets do not impinge on the nucleus) and macrosteatosis (fat droplets are large enough to displace or distort the nucleus) (5), by staining neutral lipids with Oil Red O, or by quantifying liver triglycerides. This stage can also include macrophage infiltration and inflammation (representing an advanced stage of steatosis), which can lead to irreversible fibrosis/cirrhosis and is termed nonalcoholic steatohepatitis (NASH). Fibrosis can be identified by staining with Masson trichrome or Picrosirius red (5). The last stage in NAFLD progression is hepatocellular carcinoma (HCC), and in the absence of cirrhosis, the main risk factors for HCC are NAFLD and metabolic syndrome (6). Upon damage to the liver due to disease, the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio will increase in the serum. This serum ratio is used as a diagnostic test for liver disease (7), and an AST/ALT blood ratio >1 has also been shown to be a risk factor for NAFLD (8).
Other risk factors for developing NAFLD include obesity, type 2 diabetes, dyslipidemia, and insulin resistance, which are hallmarks of metabolic syndrome (9). These associations highlight the fact that NAFLD is a complex disease that involves crosstalk between multiple organs (10). NAFLD is widespread in countries where a Western diet is typically consumed (11), and the prevalence of NAFLD and obesity is increasing in parallel (12). In the United States, NAFLD prevalence is ∼30% in studies using imaging to diagnose NAFLD or noninvasive biomarkers that comprise the United States fatty liver index and the NAFLD fibrosis score (12–14). Analysis of data from the National Health and Nutrition Examination Survey revealed that subjects with NAFLD were more likely to be male or Mexican American (12, 15, 16), providing evidence of sex and ethnic disparities in NAFLD. Paradoxically, being male or Mexican American was also protective against advanced fibrosis (12). Recent studies indicate that genetics may underlie observed ethnic differences (17).
Another risk factor for NAFLD is exposure to toxic chemicals. Liver disease caused by exposures to chemicals has been termed “toxicant-associated fatty liver disease” (18). The liver is the body’s natural first line of defense against becoming poisoned, as it filters harmful chemicals out of the blood for excretion, while also detoxifying harmful chemicals through several mechanisms involving oxidations and conjugations; therefore, the liver is at increased risk of injury inflicted by toxin exposure. A recent report inventoried chemicals that promote liver disease and found 123 chemicals in a range of classes, including endocrine-disrupting chemicals (EDCs), to be harmful to the liver (18). Some EDCs have also been classified as “metabolism-disrupting chemicals” that can promote the development of metabolic syndrome, a risk factor for NAFLD (19). EDCs can alter the endocrine milieu of an organism, leading to adverse alterations of endocrine function, and can work through a variety of mechanisms, including direct binding to hormone receptors, altering signaling molecules upstream of those receptors, or modifying the epigenome, poising the system for aberrant gene expression (20, 21). A comprehensive review of the physiological, cellular, molecular, and epigenetic changes attributable to direct EDC exposure in a variety of target tissues was recently published by the Endocrine Society (21). Although several studies have shown a link between adult exposure to EDCs and liver disease in rodents and humans (4), the present review focuses on developmental exposure to EDCs and liver disease.
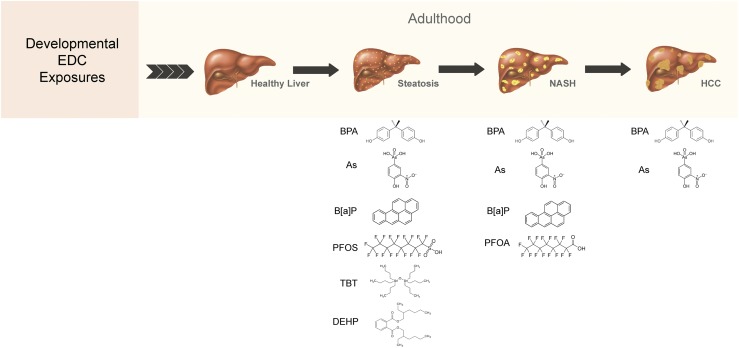
During gestational development, a suboptimal environment can lead to increased susceptibility to disease in the offspring later in life. This observation was first made in 1989 when a report showed that increased birth weight was associated with increased risk for heart disease, hypertension, and diabetes later in adulthood (22, 23). This phenomenon is now termed the developmental origins of health and disease paradigm and has been extended to a variety of diseases, including liver disease. For example, early-life exposure to famine was associated with an increase in the prevalence of NAFLD in adulthood (24). Several other gestational exposures have been shown to increase risk for NAFLD, including a maternal high-fat diet, hyperglycemia, hyperinsulinemia, obesity, hyperlipidemia, and gestational diabetes mellitus (25). These maternal changes can impair placental function and lead to alterations in fetal liver responses that promote a pathological phenotype in offspring (25). In this mini-review, we highlight rodent studies examining the link between developmental and gestational exposures of EDCs on NAFLD development/progression (Fig. 1), identify potential mechanisms underlying this link (Table 1) (26–45), and discuss the translational relevance of such exposures to NAFLD development in humans.
Figure 1.
Developmental exposure to endocrine-disrupting chemicals and the development of NAFLD in adulthood. Rodent studies have shown that early-life exposure to BPA, TBT, B[a]P, PFOS, PFOA, DEHP, and As is associated with hepatic steatosis, hepatic inflammation (one aspect of NASH), and/or HCC. As, arsenic; B[a]P, benzo[a]pyrene; BPA, bisphenol A; DEHP, bis(2-ethylhexyl) phthalate; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; TBT, terbutyltin.
Table 1.
Summary of Developmental Exposures to EDCs and NAFLD Progression in Rodent Studies
| Developmental Exposure | Liver Phenotype | Species | Metabolic Abnormalities | Potential Mechanisms | Challenge |
|---|---|---|---|---|---|
| BPA (26–31) | Hepatic steatosis, NASH, and HCC | Wistar, Sprague Dawley rats (steatosis and NASH), C57BL/6 mice (HCC) | Elevated serum triglycerides, free fatty acids, and ALT | Hepatic mitochondrial dysfunction and oxidative stress; epigenetic modifications | High-fat diet exacerbated the steatosis and metabolic phenotypes |
| TBT (32–36) | Hepatic steatosis | C57BL/6 mice | Increased adipose depot weight and adipose cell size | Crosstalk with peripheral systems (activation of PPAR/RXR) | ND |
| B[a]P (4, 37–39) | Hepatic steatosis and NASH | C57BL/6 mice | ND | Activation of AHR | ND |
| PFOS (40) | Hepatic steatosis | Wistar rats | Increased liver triglycerides | ND | ND |
| PFOA (41) | NASH | CD-1 mice | ND | Hepatic mitochondrial dysfunction | High-fat diet did not affect the response to PFOA |
| DEHP (42) | Hepatic steatosis | CD-1 mice | Delayed hepatic metabolic maturation | ND | ND |
| Arsenic (43–45) | Hepatic steatosis, NASH, and HCC | Swiss Webster mice (steatosis and NASH); C3H/HeN mice (HCC) | Hyperglycemia, increased serum triglycerides, cholesterol, LDL, and HDL; increased liver cholesterol, free fatty acids, and triglycerides | Hepatic mitochondrial dysfunction and oxidative stress; epigenetic modifications | ND |
Abbreviations: AHR, aryl hydrocarbon receptor; B[a]P, benzo[a]pyrene; BPA, bisphenol A; DEHP, bis(2-ethylhexyl) phthalate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ND, not determined; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PPAR, peroxisome proliferator–activated receptor; RXR, retinoid X receptor; TBT, tributyltin.
Early Life Exposure to EDCs and NAFLD Progression
Xenoestrogens
Bisphenol A (BPA) is a weakly estrogenic chemical that is widely used in manufacturing polycarbonate plastics and epoxy resins, in food packaging, and in toys. BPA exposure is ubiquitous and is continuous, with >90% of the U.S. population exhibiting detectable levels of BPA in their urine (46–48). In adult humans, BPA is rapidly metabolized with a half-life of 4 to 5 hours, but the metabolic rate is much slower in the fetus and in infants (49, 50), suggesting that exposure during early life may be more detrimental than during adulthood. Importantly, the Environmental Protection Agency has set the safety level for BPA at 50 μg/kg/d, and the following studies demonstrate physiological effects in the liver at, or below, this level.
In a recent study, Wistar rat pups were exposed to 40 μg/kg/d BPA from gestational day (GD) 0 to weaning at postnatal day (PND) 21 (26). Exposed male offspring exhibited mild hepatic steatosis at 15 weeks of age, which progressed to moderate hepatic steatosis, accompanied by elevated serum triglycerides, serum-free fatty acids, and serum ALT levels at 26 weeks of age compared with corn oil–exposed rats (26). Interestingly, accumulating evidence suggests that exposure to a high-fat diet increases the severity of NAFLD in BPA-exposed animals. For example, male Wistar rats exposed to 50 μg/kg/d BPA from GD 0 to weaning at PND 21 exhibited increased liver triglycerides, liver free fatty acids, and serum ALT levels at 27 weeks of age compared with corn oil–exposed rats (27). Feeding BPA-exposed rats a high-fat diet at weaning resulted in increased liver weight/body weight ratio, liver triglycerides, liver cholesterol, liver free fatty acids, and serum AST/ALT, as well as hepatic inflammation/fibrosis at 27 weeks compared with corn oil–exposed rats fed a high-fat diet (27), indicating a more severe phenotype after high-fat diet challenge. In a separate study, Sprague Dawley rats were exposed to corn oil or 100 μg/kg/d BPA from GD 6 to weaning at PND 21, followed by challenge with control diet or high-fat diet until PND 110 (28). Perinatal BPA exposure increased hepatic steatosis, whereas perinatal BPA plus a high-fat diet caused an even greater increase in hepatic steatosis and resulted in the highest fat/lean ratio in adult offspring compared with the other treatment groups (28). Interestingly, these effects did not occur in female offspring (28).
Perinatal BPA exposure has also been linked to the development of HCC, the final endpoint of hepatic disease progression. Wild-type a/a C57BL/6J mice were fed a diet supplemented with BPA (0 ng, 50 ng, or 50 mg/kg diet) that commenced prior to mating through gestation and lactation (29). At 10 months of age, 23% (18 of 78) of the offspring presented with preneoplastic or neoplastic lesions, with the highest incidence seen in the 50 mg/kg treatment group (29). Interestingly, BPA exposure was associated with altered DNA methylation in tumor vs nontumor tissue (30, 31), suggesting an epigenetic mechanism of BPA action as discussed elsewhere in this review.
Organotins
The organotin tributyltin (TBT) was widely used as an antifouling agent for aquatic vessels in the 1960s. The compound was banned after the discovery that it had severe effects on marine life, including alterations in sex ratios resulting in reproductive deficiencies. However, TBT is a stable compound with a half-life of ∼578 days in sediment (51), providing ample opportunity for TBT bioaccumulation in the marine food chain. In fact, it has been detected in fish prepared for human consumption (52–58). Studies have also identified house dust as a source of human TBT exposure (59, 60), with estimated rates of intake higher for children than adults (60). In addition to the reproductive effects on marine life, TBT exposure also increases adiposity, leading to its investigation as an “obesogen” in mammals (61).
In a mouse model, exposure to TBT in utero led to alterations in adipose depots without altering whole body weight, which was accompanied by fatty liver disease. Pregnant C57BL/6J mice were injected with TBT [0.05 or 0.5 mg/kg body weight (bw)] or vehicle from embryonic day 12 to embryonic day 20, and at PND 0 livers, testes, and adipose tissue of the offspring all show significantly increased fat accumulation as assessed by Oil Red O staining. At 10 weeks of age, no change in body weight was observed, but the males displayed increased epididymal adipose weight (32). In a follow-up study, in utero TBT exposure (5.42 nM, 54.2 nM, and 542 nM) was extended from 7 days prior to mating until delivery. These animals displayed a dose-dependent increase in fat accumulation and an increase in expression of genes involved in lipid maintenance and metabolism in the liver at 8 weeks of age (33).
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are chemicals that are released into the environment from both natural (generated from volcanic activity, open burning, and natural seepage of petroleum or coal deposits) and anthropogenic (generated from the incomplete combustion of coal, oil, gas, wood, garbage, and tobacco) sources. The emitted PAHs can form or bind to particles in the air, which serves as a major source of PAH exposure. Ingesting meat and other foods cooked or grilled at high temperatures is another source of exposure. PAH exposure is widespread in the U.S. population as evidenced by measurement of PAH metabolites in urine, with higher PAH metabolite levels in smokers vs nonsmokers (62).
To date, only one study examined the effect of early-life PAH, in this case benzo[a]pyrene (B[a]P) exposure on NAFLD progression in adulthood. Pregnant C57BL/6J dams were treated by oral gavage with sesame oil (control) or 2 mg/kg B[a]P from GD 7 to GD 16 (37). B[a]P-exposed female offspring exhibited increased hepatic steatosis and evidence of inflammatory infiltrate in the liver at 7.5 to 8 months of age (37). Importantly, this effect was dependent on availability of glutathione, which plays a role in detoxification of B[a]P metabolites and of reactive oxygen species produced during B[a]P metabolism (37).
Perfluorinated chemicals
Perfluorinated chemicals (PFCs) are a large group of synthetic compounds that have the unique property of being able to repel oil, grease, and water. As such, they have been used to make nonstick cookware, stain-resistant sofas and carpets, waterproof clothes and mattresses, food packaging, and fire-fighting materials. Perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are two of the most highly produced PFCs in the United States. Although PFC use was restricted in 2015, exposure is still ubiquitous (63). Furthermore, PFCs are slowly eliminated from the body with a biological half-life of ∼2 to 8 years depending on the specific compound (64, 65).
Few studies have examined the effect of early-life PFC exposure on NAFLD progression. In one study, pregnant Wistar rats were treated, 0.5 mg/kg/d PFOS or 1.5 mg/kg/d PFOS from GD 0 to PND 21, with vehicle control (40). At 22 weeks of age, PFOS-exposed offspring exhibited increased Oil Red O staining and increased liver triglycerides, particularly at the 1.5 mg/kg dose (40). In another study, pregnant CD-1 mice were treated with 0, 0.01, 0.1, 0.3, or 1 mg/kg PFOA by oral gavage from GD 1 to GD 17 (41). PFOA-exposed mice exhibited dose-related increases in hepatic inflammation at PND 21 and PND 91, regardless of exposure to control diet or high-fat diet (41).
Phthalates
Phthalates are a group of chemicals that are used as liquid plasticizers to impart flexibility and resilience to plastics, and as solubilizing and stabilizing agents in other applications. There are numerous products that contain phthalates, including polyvinyl chloride plastics (e.g., plastic bags, garden hoses, inflatable toys, medical tubing) and flooring, automotive plastics, detergents, lubricating oils, medical devices and pharmaceuticals, and personal care products. Importantly, phthalates are not chemically bound to the plastics to which they are added, and they can readily leach into the environment. Similar to other EDCs discussed in this review, phthalate exposure is widespread with measurable levels of many phthalate metabolites in the general U.S. population (63).
Only one study has examined the link between developmental phthalate exposure and NAFLD in adulthood. Pregnant CD-1 mice were orally treated with corn oil, 25 mg/kg bw bis(2-ethylhexyl) phthalate (DEHP), or 100 mg/kg bw DEHP from GD 11 to GD 19 (42). At PND 21, both male and female offspring that were exposed to DEHP exhibited increased hepatic steatosis compared with those exposed to corn oil (42). Evidence of delayed hepatocyte metabolic maturation was also observed in both male and female DEHP-exposed offspring (42), suggesting that early-life DEHP exposure may have long-term consequences for hepatic metabolic function.
Arsenic
Arsenic is a naturally occurring element that is widely distributed in the Earth’s crust and is most commonly found in high levels in groundwater (e.g., deep-well water) (66). Arsenic has been detected in human hair, nails, blood, and urine and has an estimated biological half-life of 4 days (for inorganic arsenic) (67). Exposure to arsenic can manifest in a variety of conditions, including cardiovascular and lung disease as well as neuronal defects. According to the International Agency for Research on Cancer, arsenic is 1 of 120 agents classified as a group I carcinogen. The potential health hazards of developmental arsenic and susceptibility to NAFLD and to HCC have recently begun to be explored in rodents.
In Swiss Webster mice exposed to 100 parts per billion sodium arsenite from embryonic day 6 until parturition, sodium arsenite induced a fatty liver phenotype, as evidenced by Oil Red O staining, compared with sodium chloride controls (43). This was accompanied by hyperglycemia, as well as higher cholesterol, low-density lipoprotein, and high-density lipoprotein levels. Fibrosis was not increased in exposed animals, nor ALT, AST, or alkaline phosphatase, indicating that NAFLD is present without full-blown NASH (43).
In a follow-up report, the same group investigated additional treatment paradigms; in addition to in utero exposure they included a postnatal only exposure, and an in utero plus 13 weeks of postnatal exposure (IU+). The authors quantitated the level of NAFLD present using an NAFLD activity score, which considers both fat accumulation and fibrosis (68). Both the in utero and IU+ groups displayed increased NAFLD activity score values compared with unexposed animals whereas the postnatal only exposure group was not different from unexposed animals (44). The same trend was evident in the liver/body weight ratio as well as liver triglyceride levels. Additionally, the IU+ group had significantly higher liver cholesterol, liver free fatty acids, and serum triglycerides. The IU+ animals also displayed insulin resistance where no other group did and they weighed more than any other group (44). An assessment of metabolites in the liver revealed a potential defect in the tricarboxylic acid cycle, which was supported by a decrease in isocitrate dehydrogenase activity in livers from in utero and IU+ groups (44). This model is especially interesting, in that the postnatal only exposure group does not display a phenotype, which implies that alterations that occur during development are crucial to disease onset and that continued postnatal exposure exacerbates the phenotype. This model also replicates the human environment very well, because human fetuses exposed in utero will likely continue to be exposed postnatally.
In a model for spontaneous hepatocellular carcinoma (C3H/HeN mice), developmental arsenic exposure promotes carcinogenesis (45). Animals were exposed to 85 ppm sodium arsenite from E8 to E18 and allowed to age 74 to 84 weeks. Fifty-one percent of arsenic-exposed mice developed tumors compared with 41% of controls, and tumors in arsenic-exposed animals grew to be ∼92 mm2, whereas tumors in control animals grew to be ∼76 mm2. Interestingly, developmental arsenic exposure increased the proportion of tumors containing mutations in the proto-oncogene H-ras (45). Transcriptomic analysis revealed altered expression of genes related to oxidative stress, which were accompanied by changes in histone modifications (45), implying that epigenetic modifications may contribute to the arsenic-mediated increase in HCC development (discussed later).
Potential Mechanisms of EDC-Mediated NAFLD Development
Collectively, the data from animal studies provide evidence that developmental EDC exposure enhances susceptibility to NAFLD and HCC later in life; however, the underlying molecular mechanisms of this phenomenon remain a central question. One potential mode of EDC action is regulation of nuclear receptor signaling (4, 21). For example, some PAHs can bind the aryl hydrocarbon receptor (AHR) (38) and constitutive activation of AHR in mice induced spontaneous hepatic steatosis (39). AHR binding to other EDCs, including dioxins and polychlorinated biphenyls, also promotes hepatic steatosis (4). Note that nuclear receptor–mediated effects of EDCs on liver function cannot always be easily predicted. As discussed previously, PFC exposure has been linked to increased steatosis and PFCs can interact with the nuclear receptors estrogen receptor and peroxisome proliferator–activated receptor (PPAR) α (69–71). However, activation of these receptors generally results in decreased steatosis (4). In a methionine choline–deficient diet-induced model of steatohepatitis, PPARα deficiency provoked more severe steatosis and hepatitis (72), which can be ameliorated with a PPARα agonist (73). This phenotype is likely hepatocyte-autonomous, as liver-specific knockout of PPARα also results in increased steatosis (74). As such, PPARα agonists have been proposed as a useful treatment of NAFLD (75). These observations suggest that an alternative mechanism, other than activation of nuclear receptor signaling, may account for the EDC effects observed in vivo.
Recent evidence suggests that alteration of mitochondrial energetics, gene expression, and morphology may play a key role in the pathogenesis of NAFLD (76). For example, dysfunctional mitochondrial energetics is associated with disrupted fat oxidation, leading to accumulation of lipids, inflammation, and impaired insulin signaling, which hastens the transition from steatosis to NASH (77). Interestingly, pharmacological agents that improve mitochondrial function may be potential therapies to treat NAFLD (78). Studies have demonstrated that early-life EDC exposures disrupt mitochondrial function in the liver. Longitudinal assessment of mitochondrial function in rats perinatally exposed to BPA revealed decreased mitochondrial respiratory complex activity as early as 3 weeks of age and a progressive decrease in mitochondrial respiratory complex activity leading to hepatic oxidative stress by 15 and 26 weeks of age, concomitant with increased steatosis (26). Challenge with a high-fat diet exacerbates BPA-mediated steatosis that may be attributed to altered hepatic β-oxidation, as evidenced by decreased expression of β-oxidation–related genes in the liver of exposed rats (28). The balance of β-oxidation, along with lipid uptake/release, and triglyceride synthesis helps to preserve energy homeostasis in the liver, and disruption of these processes may lead to NAFLD development. Finally, mitochondria can exhibit structural alterations in response to hepatotoxic injury, and perinatal PFOA exposure induced abnormal mitochondrial proliferation and morphologies, as well as hepatic inflammation, in one study (41).
EDC-mediated disruption of epigenetic mechanisms may also contribute to NAFLD progression. Heavy metals, such as arsenic, are some of the best characterized toxicants known to aberrantly affect DNA methylation patterns, particularly in the context of carcinogenicity (79). For example, sodium arsenite treatment induced malignant transformation of rat liver cells (80). Genomic DNA hypomethylation was observed in the arsenic-transformed cells, which was highly correlated with dose-dependent increases in malignant transformation as assessed by xenograft tumor formation (80). Similar results were also reported in a subsequent in vivo study in which adult male 129/SvJ mice were treated with control (unaltered water) or 45 ppm of sodium arsenite in the drinking water for 48 weeks (81). Arsenic-exposed animals exhibited increased steatosis, altered expression of genes associated with hepatocarcinogenesis, and global DNA hypomethylation in the liver (81). These direct exposure studies suggest that arsenic-mediated epigenetic modifications may be a mechanism for developmental arsenic exposure; however, it is possible that direct and developmental exposures may have distinct mechanisms of action (44). Interestingly, developmental BPA exposure has been linked to epigenetic modifications in hepatic tumor tissue (30, 31). Dose-dependent changes in DNA methylation of one target, STAT3, was observed in the liver of 3-week- old mice exposed to BPA before the onset of tumorigenesis, implicating Stat3 as a potential early biomarker for increased risk of liver tumors (30). Furthermore, this candidate biomarker may also be relevant for human health, as DNA methylation of STAT3 varied with liver tissue BPA levels in human fetal liver samples (30). Additional studies provide further support for disruption of epigenetic processes in the liver due to developmental BPA exposure (30, 82–86). Taken together, these studies suggest that EDC-mediated epigenetic disruption may play a causal role in the developmental origins of NAFLD.
It is becoming increasingly clear that NAFLD development involves extrahepatic organs and regulatory pathways (10). Therefore, early-life exposures to EDCs may contribute to NAFLD development in adulthood by modulating the crosstalk between the liver and peripheral organs, such as adipose tissue, the gut, the hypothalamus, and the intestine. As previously discussed, obesity is a major risk factor for NAFLD in humans (9). Adipose tissue is a source of free fatty acids that are delivered to the liver for triacylglycerol synthesis, as well as of adipokines and proinflammatory cytokines that participate in the pathogenesis of NAFLD (10). Multiple studies have shown that TBT stimulates adipogenesis (32–36, 87), likely through activation of PPAR/retinoid X receptor signaling. For example, perinatal TBT exposure increased white adipose tissue depots, adipocyte size, and adipocyte number in mice (33). Perinatal BPA exposure also stimulates adipogenesis (88), as well as metabolic processes (89–93), glucose homeostasis (94), and insulin signaling (95), which all may involve dysfunction of regulatory pathways in peripheral organs. However, EDCs could exert metabolic effects without directly inducing obesity by triggering mechanisms that are distinct, but overlap, those induced by a high-fat diet (96, 97).
Unraveling the mechanisms underlying adult-onset disease by developmental EDC exposure can be complicated by the fact that adverse effects in target organs may be dose-dependent. For example, more robust hepatic transcriptomic effects were observed in adult CD-1 mice treated with low-dose BPA compared with high-dose BPA (98). While this study illustrates dose-dependent effects of adult BPA exposure, a similar relationship has been reported for developmental BPA exposure and metabolic alterations (99–101). These studies are consistent with the well-documented nonmonotonic response curves that characterize EDCs (21). Another potential complication for mechanistic studies is the fact that EDC exposures in humans predominantly occur as a mixture of chemicals. Studies have shown that chronic exposure to an EDC cocktail (beginning prior to gestation and continued throughout adulthood) induced sex-dependent hepatic metabolic changes in exposed offspring (97, 102, 103). To date, no studies have examined the effects of perinatal EDC mixtures on development of liver disease in adulthood, and future studies in this area are warranted.
Translational Relevance of Early-Life Exposure to EDCs
Although the aforementioned rodent studies suggest that developmental exposures to EDCs might promote NAFLD in adulthood (Fig. 1), there is limited human epidemiological data to support this link. Most of the available human studies have focused on adult EDC exposures (4); however, exposure to BPA and dioxins has been associated with altered serum activity of the liver enzymes AST/ALT in children (104, 105), suggesting a detrimental effect of early-life EDC exposure on liver function.
Numerous studies have measured EDCs (or their metabolites/biomarkers) in maternal blood and urine during pregnancy, in amniotic fluid, in placenta, in cord blood, and in breast milk, demonstrating that exposures can occur prenatally (via placental transfer) and postnatally (via breast milk) (106, 107). EDCs such as BPA, PFCs, and arsenic have also been measured in fetal organs, including liver (108–111). To assess fetal risk of exposure, both maternal and fetal samples should be collected, where possible, because maternal EDC levels do not always correlate with fetal EDC levels. PFCs exhibit the strongest evidence for a correlation between maternal blood and cord blood/placenta levels (112–125), but both correlation and lack of correlation have been shown for PAH (and its biomarkers) (126–129) and phthalate metabolites (130, 131). Timing of sample collection is another factor to take into consideration, as the correlation between maternal PFC levels and cord blood PFC levels is strongest when the maternal sample is taken closer to delivery (119). Finally, another factor to take into consideration is the geographic area/region of the subject population. Higher cord blood/placenta levels of PAH/biomarkers have been measured in newborns of women living near electronic waste recycling facilities and newborns of women using large amounts of smoky coal for heating and cooking compared with newborns of women living in control regions (132, 133). Furthermore, PAH biomarker levels were highest in newborns of women who resided within 1 mile of the World Trade Center, intermediate in newborns of women who were employed within 1 mile of the World Trade Center, and lowest in newborns of women who lived and worked outside of this radius during the release of toxic pollutants from the World Trade Center fires in 2001 (134).
To date, no longitudinal studies of developmental origins of NAFLD due to EDC exposure have been conducted in humans, but there is evidence that developmental EDC exposure is associated with other health outcomes later in life. For example, higher levels of PAH/biomarkers in cord blood are associated with neurodevelopmental/behavioral disorders (135–141) and decreased body weight (139, 142) in children. Interestingly, the association between developmental PAH exposure and neurodevelopmental disorders is dependent on timing of sample collection (e.g., before vs after closing of a coal-fired power plant) (138, 139) and may be exacerbated by other factors, including exposure to tobacco smoke and material hardship (136, 143). One caveat to performing similar longitudinal studies for adverse liver outcomes is the fact that NAFLD typically takes years to develop in adults, with increased prevalence due to aging (144). However, the prevalence of pediatric NAFLD is on the rise (145, 146), making longitudinal studies in humans more feasible. Inclusion of populations at increased risk for NAFLD, including Hispanics and males (15, 16, 145), in these studies is also warranted.
Conclusions
The prevalence of NAFLD and its associated diseases is rapidly rising in both adults and children and thus may become a significant health and economic burden in the future. Although studies have shown that early-life factors, such as maternal nutrition, play a role in NAFLD development, the potential contribution of developmental EDC exposure to the development and progression of NAFLD represents a significant gap in our knowledge. Rodent studies provide the strongest evidence for this link, but only for a small percentage of chemicals with known association to NAFLD based on adult exposures. As such, additional rodent studies are warranted to expand our knowledge of relevant EDC exposures (particularly taking the effect of mixtures into consideration), to elucidate the molecular mechanisms underlying development of NAFLD, and to identify biomarkers for people at risk, as well as possible intervention/therapeutics for the disease. Future areas of investigation should also consider the role that early-life EDC exposure plays in the setting of other risk factors, such as obesity and metabolic syndrome.
Acknowledgments
Financial Support: This work was supported by National Institute of Environmental Health Sciences Grants R01ES23206, P30ES023512, and U01ES026719 to Dr. Cheryl Walker (Baylor College of Medicine).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHR
- aryl hydrocarbon receptor
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- B[a]P
- benzo[a]pyrene
- BPA
- bisphenol A
- bw
- body weight
- DEHP
- bis(2-ethylhexyl) phthalate
- EDC
- endocrine-disrupting chemical
- GD
- gestational day
- HCC
- hepatocellular carcinoma
- IU+
- in utero plus 13 weeks of postnatal exposure
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- PAH
- polycyclic aromatic hydrocarbon
- PFC
- perfluorinated chemical
- PFOA
- perfluorooctanoic acid
- PFOS
- perfluorooctane sulfonic acid
- PND
- postnatal day
- PPAR
- peroxisome proliferator–activated receptor
- TBT
- tributyltin.
References
- 1.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11(1):451–496. [DOI] [PubMed] [Google Scholar]
- 2.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10(11):656–665. [DOI] [PubMed] [Google Scholar]
- 3.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis—new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10(11):627–636. [DOI] [PubMed] [Google Scholar]
- 4.Foulds CE, Treviño LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 2017;13(8):445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar V, Abbas AK, Aster JC, Robbins SL. Robbins Basic Pathology, 9th ed. Philadelphia, PA: Elsevier/Saunders; 2013. [Google Scholar]
- 6.Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124–131;e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44(6):1249–1253. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–1362. [DOI] [PubMed] [Google Scholar]
- 9.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–84. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Ji X, Wang Q, Li JZ. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD) [published online ahead of print 22 June 2017]. Protein Cell. doi: 10.1007/s13238-017-0436-0. [DOI] [PMC free article] [PubMed]
- 11.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. [DOI] [PubMed] [Google Scholar]
- 12.Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, Nguyen MH. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12(3):e0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41(1):65–76. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(5):274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherif ZA, Saeed A, Ghavimi S, Nouraie SM, Laiyemo AO, Brim H, Ashktorab H. Global epidemiology of nonalcoholic fatty liver disease and perspectives on US minority populations. Dig Dis Sci. 2016;61(5):1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sookoian S, Pirola CJ. Genetic predisposition in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2017;23(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Eryani L, Wahlang B, Falkner KC, Guardiola JJ, Clair HB, Prough RA, Cave M. Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicol Pathol. 2015;43(4):482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, Shen J, Foulds CE, Coarfa C, O’Malley BW, Shilatifard A, Walker CL. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol. 2016;30(8):856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the Endocrine Society’s second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. [DOI] [PubMed] [Google Scholar]
- 24.Wang N, Chen Y, Ning Z, Li Q, Han B, Zhu C, Chen Y, Xia F, Jiang B, Wang B, Wang X, Jensen MD, Lu Y. Exposure to famine in early life and nonalcoholic fatty liver disease in adulthood. J Clin Endocrinol Metab. 2016;101(5):2218–2225. [DOI] [PubMed] [Google Scholar]
- 25.Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol. 2017;14(2):81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y, Xia W, Zhu Y, Li X, Wang D, Liu J, Chang H, Li G, Xu B, Chen X, Li Y, Xu S. Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicol Lett. 2014;228(2):85–92. [DOI] [PubMed] [Google Scholar]
- 27.Wei J, Sun X, Chen Y, Li Y, Song L, Zhou Z, Xu B, Lin Y, Xu S. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J Endocrinol. 2014;222(3):313–325. [DOI] [PubMed] [Google Scholar]
- 28.Strakovsky RS, Wang H, Engeseth NJ, Flaws JA, Helferich WG, Pan YX, Lezmi S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol. 2015;284(2):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, Yang J, Dolinoy DC. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect. 2014;122(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinhouse C, Bergin IL, Harris C, Dolinoy DC. Stat3 is a candidate epigenetic biomarker of perinatal bisphenol A exposure associated with murine hepatic tumors with implications for human health. Epigenetics. 2015;10(12):1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinhouse C, Sartor MA, Faulk C, Anderson OS, Sant KE, Harris C, Dolinoy DC. Epigenome-wide DNA methylation analysis implicates neuronal and inflammatory signaling pathways in adult murine hepatic tumorigenesis following perinatal exposure to bisphenol A. Environ Mol Mutagen. 2016;57(6):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grün F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol. 2006;20(9):2141–2155. [DOI] [PubMed] [Google Scholar]
- 33.Chamorro-García R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121(3):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira-Fernandes A, Vanparys C, Hectors TL, Vergauwen L, Knapen D, Jorens PG, Blust R. Unraveling the mode of action of an obesogen: mechanistic analysis of the model obesogen tributyltin in the 3T3-L1 cell line. Mol Cell Endocrinol. 2013;370(1-2):52–64. [DOI] [PubMed] [Google Scholar]
- 35.Kanayama T, Kobayashi N, Mamiya S, Nakanishi T, Nishikawa J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor γ/retinoid X receptor pathway. Mol Pharmacol. 2005;67(3):766–774. [DOI] [PubMed] [Google Scholar]
- 36.Yanik SC, Baker AH, Mann KK, Schlezinger JJ. Organotins are potent activators of PPARγ and adipocyte differentiation in bone marrow multipotent mesenchymal stromal cells. Toxicol Sci. 2011;122(2):476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortiz L, Nakamura B, Li X, Blumberg B, Luderer U. In utero exposure to benzo[a]pyrene increases adiposity and causes hepatic steatosis in female mice, and glutathione deficiency is protective. Toxicol Lett. 2013;223(2):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, Gonzalez FJ, Xie W. A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology. 2010;139(2):653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, Wei J, Lin Y, Jiang Y, Wang Y, Shu B, Xu B, Xu S. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28(9):532–542. [DOI] [PubMed] [Google Scholar]
- 41.Quist EM, Filgo AJ, Cummings CA, Kissling GE, Hoenerhoff MJ, Fenton SE. Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol Pathol. 2015;43(4):546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maranghi F, Lorenzetti S, Tassinari R, Moracci G, Tassinari V, Marcoccia D, Di Virgilio A, Eusepi A, Romeo A, Magrelli A, Salvatore M, Tosto F, Viganotti M, Antoccia A, Di Masi A, Azzalin G, Tanzarella C, Macino G, Taruscio D, Mantovani A. In utero exposure to di-(2-ethylhexyl) phthalate affects liver morphology and metabolism in post-natal CD-1 mice. Reprod Toxicol. 2010;29(4):427–432. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Soria P, Broka D, Quach S, Hardwick RN, Cherrington NJ, Camenisch TD. Fetal exposure to arsenic results in hyperglycemia, hypercholesterolemia, and nonalcoholic fatty liver disease in adult mice. J Toxicol Health. 2104;1:1. [Google Scholar]
- 44.Ditzel EJ, Nguyen T, Parker P, Camenisch TD. Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ Health Perspect. 2016;124(2):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nohara K, Tateishi Y, Suzuki T, Okamura K, Murai H, Takumi S, Maekawa F, Nishimura N, Kobori M, Ito T. Late-onset increases in oxidative stress and other tumorigenic activities and tumors with a Ha-ras mutation in the liver of adult male C3H mice gestationally exposed to arsenic. Toxicol Sci. 2012;129(2):293–304. [DOI] [PubMed] [Google Scholar]
- 46.Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15(10):1281–1287. [DOI] [PubMed] [Google Scholar]
- 47.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol. 2015;49(19):11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson TA, Twaddle NC, Roegge CS, Callicott RJ, Fisher JW, Doerge DR. Concurrent determination of bisphenol A pharmacokinetics in maternal and fetal rhesus monkeys. Toxicol Appl Pharmacol. 2013;267(1):41–48. [DOI] [PubMed] [Google Scholar]
- 50.Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol. 2013;47(21):12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakultantimetha A, Keenan HE, Beattie TK, Bangkedphol S, Cavoura O. Bioremediation of tributyltin contaminated sediment: degradation enhancement and improvement of bioavailability to promote treatment processes. Chemosphere. 2011;83(5):680–686. [DOI] [PubMed] [Google Scholar]
- 52.Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. a review. Environ Int. 2008;34(2):292–308. [DOI] [PubMed] [Google Scholar]
- 53.Airaksinen R, Rantakokko P, Turunen AW, Vartiainen T, Vuorinen PJ, Lappalainen A, Vihervuori A, Mannio J, Hallikainen A. Organotin intake through fish consumption in Finland. Environ Res. 2010;110(6):544–547. [DOI] [PubMed] [Google Scholar]
- 54.Kucuksezgin F, Aydin-Onen S, Gonul LT, Pazi I, Kocak F. Assessment of organotin (butyltin species) contamination in marine biota from the Eastern Aegean Sea, Turkey. Mar Pollut Bull. 2011;62(9):1984–1988. [DOI] [PubMed] [Google Scholar]
- 55.Jadhav S, Bhosale D, Bhosle N. Baseline of organotin pollution in fishes, clams, shrimps, squids and crabs collected from the west coast of India. Mar Pollut Bull. 2011;62(10):2213–2219. [DOI] [PubMed] [Google Scholar]
- 56.Rastkari N, Mesdaghinia A, Yunesian M, Ahmadkhaniha R. Butyltin compounds in fish commonly sold in north of Iran. Bull Environ Contam Toxicol. 2012;88(1):74–77. [DOI] [PubMed] [Google Scholar]
- 57.Filipkowska A, Złoch I, Wawrzyniak-Wydrowska B, Kowalewska G. Organotins in fish muscle and liver from the Polish coast of the Baltic Sea: is the total ban successful? Mar Pollut Bull. 2016;111(1-2):493–499. [DOI] [PubMed] [Google Scholar]
- 58.Ashraf MW, Salam A, Mian A. Levels of organotin compounds in selected fish species from the Arabian Gulf. Bull Environ Contam Toxicol. 2017;98(6):811–816. [DOI] [PubMed] [Google Scholar]
- 59.Fromme H, Mattulat A, Lahrz T, Rüden H. Occurrence of organotin compounds in house dust in Berlin (Germany). Chemosphere. 2005;58(10):1377–1383. [DOI] [PubMed] [Google Scholar]
- 60.Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S. Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch Environ Contam Toxicol. 2010;58(4):901–907. [DOI] [PubMed] [Google Scholar]
- 61.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int J Androl. 2012;35(3):437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. Available at: https://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf. Accessed 23 October 2017.
- 63.Centers for Disease Control and Prevention Fourth Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. Available at: https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf.
- 64.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell MH, Waterland RL, Wong F. Calculation of chemical elimination half-life from blood with an ongoing exposure source: the example of perfluorooctanoic acid (PFOA). Chemosphere. 2015;129:210–216. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198(3):243–252. [DOI] [PubMed] [Google Scholar]
- 67.Shakoor MB, Nawaz R, Hussain F, Raza M, Ali S, Rizwan M, Oh SE, Ahmad S. Human health implications, risk assessment and remediation of As-contaminated water: a critical review. Sci Total Environ. 2017;601-602:756–769. [DOI] [PubMed] [Google Scholar]
- 68.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 69.Maloney EK, Waxman DJ. trans-Activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 1999;161(2):209–218. [DOI] [PubMed] [Google Scholar]
- 70.Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol Sci. 2011;120(1):42–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henry ND, Fair PA. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J Appl Toxicol. 2013;33(4):265–272. [DOI] [PubMed] [Google Scholar]
- 72.Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARα-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38(1):123–132. [DOI] [PubMed] [Google Scholar]
- 73.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39(5):1286–1296. [DOI] [PubMed] [Google Scholar]
- 74.Montagner A, Polizzi A, Fouché E, Ducheix S, Lippi Y, Lasserre F, Barquissau V, Régnier M, Lukowicz C, Benhamed F, Iroz A, Bertrand-Michel J, Al Saati T, Cano P, Mselli-Lakhal L, Mithieux G, Rajas F, Lagarrigue S, Pineau T, Loiseau N, Postic C, Langin D, Wahli W, Guillou H. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65(7):1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015;62(3):720–733. [DOI] [PubMed] [Google Scholar]
- 76.Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metab. 2017;28(4):250–260. [DOI] [PubMed] [Google Scholar]
- 77.Patterson RE, Kalavalapalli S, Williams CM, Nautiyal M, Mathew JT, Martinez J, Reinhard MK, McDougall DJ, Rocca JR, Yost RA, Cusi K, Garrett TJ, Sunny NE. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab. 2016;310(7):E484–E494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metab. 2017;S1550-4131(17)30487–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brocato J, Costa M. Basic mechanics of DNA methylation and the unique landscape of the DNA methylome in metal-induced carcinogenesis. Crit Rev Toxicol. 2013;43(6):493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci USA. 1997;94(20):10907–10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H, Li S, Liu J, Diwan BA, Barrett JC, Waalkes MP. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis. 2004;25(9):1779–1786. [DOI] [PubMed] [Google Scholar]
- 82.Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53(5):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Y, Xia W, Wang DQ, Wan YJ, Xu B, Chen X, Li YY, Xu SQ. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia. 2013;56(9):2059–2067. [DOI] [PubMed] [Google Scholar]
- 84.Kim JH, Sartor MA, Rozek LS, Faulk C, Anderson OS, Jones TR, Nahar MS, Dolinoy DC. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics. 2014;15(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Esterik JC, Vitins AP, Hodemaekers HM, Kamstra JH, Legler J, Pennings JL, Steegenga WT, Lute C, Jelinek J, Issa JP, Dollé ME, van der Ven LT. Liver DNA methylation analysis in adult female C57BL/6J×FVB mice following perinatal exposure to bisphenol A. Toxicol Lett. 2015;232(1):293–300. [DOI] [PubMed] [Google Scholar]
- 86.Anderson OS, Kim JH, Peterson KE, Sanchez BN, Sant KE, Sartor MA, Weinhouse C, Dolinoy DC. Novel epigenetic biomarkers mediating bisphenol A exposure and metabolic phenotypes in female mice. Endocrinology. 2017;158(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shoucri BM, Martinez ES, Abreo TJ, Hung VT, Moosova Z, Shioda T, Blumberg B. Retinoid X receptor activation alters the chromatin landscape to commit mesenchymal stem cells to the adipose lineage. Endocrinology. 2017;158(10):3109–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Hüppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009;117(10):1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabaton NJ, Canlet C, Wadia PR, Tremblay-Franco M, Gautier R, Molina J, Sonnenschein C, Cravedi JP, Rubin BS, Soto AM, Zalko D. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ Health Perspect. 2013;121(5):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Esterik JC, Dollé ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, van der Ven LT. Programming of metabolic effects in C57BL/6J×FVB mice by exposure to bisphenol A during gestation and lactation. Toxicology. 2014;321:40–52. [DOI] [PubMed] [Google Scholar]
- 91.Tremblay-Franco M, Cabaton NJ, Canlet C, Gautier R, Schaeberle CM, Jourdan F, Sonnenschein C, Vinson F, Soto AM, Zalko D. Dynamic metabolic disruption in rats perinatally exposed to low doses of bisphenol-A. PLoS One. 2015;10(10):e0141698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ilagan Y, Mamillapalli R, Goetz LG, Kayani J, Taylor HS. Bisphenol-A exposure in utero programs a sexually dimorphic estrogenic state of hepatic metabolic gene expression. Reprod Toxicol. 2017;71:84–94. [DOI] [PubMed] [Google Scholar]
- 93.Susiarjo M, Xin F, Stefaniak M, Mesaros C, Simmons RA, Bartolomei MS. Bile Acids and tryptophan metabolism are novel pathways involved in metabolic abnormalities in BPA-exposed pregnant mice and male offspring. Endocrinology. 2017;158(8):2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.García-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, Nadal A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One. 2014;9(6):e100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galyon KD, Farshidi F, Han G, Ross MG, Desai M, Jellyman JK. Maternal bisphenol A exposure alters rat offspring hepatic and skeletal muscle insulin signaling protein abundance. Am J Obstet Gynecol. 2017;216(3):290.e1–290.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Labaronne E, Pinteur C, Vega N, Pesenti S, Julien B, Meugnier-Fouilloux E, Vidal H, Naville D, Le Magueresse-Battistoni B. Low-dose pollutant mixture triggers metabolic disturbances in female mice leading to common and specific features as compared to a high-fat diet. J Nutr Biochem. 2017;45:83–93. [DOI] [PubMed] [Google Scholar]
- 98.Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H, Martin PG, Mselli-Lakhal L. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology. 2012;55(2):395–407. [DOI] [PubMed] [Google Scholar]
- 99.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, Zhou Z, Lv Z, Xia W, Chen X, Xu S. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology. 2011;152(8):3049–3061. [DOI] [PubMed] [Google Scholar]
- 101.Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, vom Saal FS, Taylor JA. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol. 2013;42:256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naville D, Labaronne E, Vega N, Pinteur C, Canet-Soulas E, Vidal H, Le Magueresse-Battistoni B. Metabolic outcome of female mice exposed to a mixture of low-dose pollutants in a diet-induced obesity model. PLoS One. 2015;10(4):e0124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naville D, Pinteur C, Vega N, Menade Y, Vigier M, Le Bourdais A, Labaronne E, Debard C, Luquain-Costaz C, Bégeot M, Vidal H, Le Magueresse-Battistoni B. Low-dose food contaminants trigger sex-specific, hepatic metabolic changes in the progeny of obese mice. FASEB J. 2013;27(9):3860–3870. [DOI] [PubMed] [Google Scholar]
- 104.Khalil N, Ebert JR, Wang L, Belcher S, Lee M, Czerwinski SA, Kannan K. Bisphenol A and cardiometabolic risk factors in obese children. Sci Total Environ. 2014;470-471:726–732. [DOI] [PubMed] [Google Scholar]
- 105.Mocarelli P, Marocchi A, Brambilla P, Gerthoux P, Young DS, Mantel N. Clinical laboratory manifestations of exposure to dioxin in children. A six-year study of the effects of an environmental disaster near Seveso, Italy. JAMA. 1986;256(19):2687–2695. [PubMed] [Google Scholar]
- 106.Faniband M, Lindh CH, Jönsson BA. Human biological monitoring of suspected endocrine-disrupting compounds. Asian J Androl. 2014;16(1):5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitro SD, Johnson T, Zota AR. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep. 2015;2(4):367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cao XL, Zhang J, Goodyer CG, Hayward S, Cooke GM, Curran IH. Bisphenol A in human placental and fetal liver tissues collected from Greater Montreal area (Quebec) during 1998–2008. Chemosphere. 2012;89(5):505–511. [DOI] [PubMed] [Google Scholar]
- 109.Mamsen LS, Jönsson BAG, Lindh CH, Olesen RH, Larsen A, Ernst E, Kelsey TW, Andersen CY. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ. 2017;596-597:97–105. [DOI] [PubMed] [Google Scholar]
- 110.Fowler PA, Drake AJ, O’Shaughnessy PJ, Bhattacharya S, Raab A, Sinclair KD, Feldmann J, Meharg AA. Comment on “Effects of arsenite during fetal development on energy metabolism and susceptibility to diet-induced fatty liver diseases in male mice” and “Mechanisms underlying latent disease risk associated with early-life arsenic exposure: current trends and scientific gaps”. Environ Health Perspect. 2016;124(6):A99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drake AJ, O’Shaughnessy PJ, Bhattacharya S, Monteiro A, Kerrigan D, Goetz S, Raab A, Rhind SM, Sinclair KD, Meharg AA, Feldmann J, Fowler PA. In utero exposure to cigarette chemicals induces sex-specific disruption of one-carbon metabolism and DNA methylation in the human fetal liver. BMC Med. 2015;13(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen F, Yin S, Kelly BC, Liu W. Chlorinated polyfluoroalkyl ether sulfonic acids in matched maternal, cord, and placenta samples: a study of transplacental transfer. Environ Sci Technol. 2017;51(11):6387–6394. [DOI] [PubMed] [Google Scholar]
- 113.Chen F, Yin S, Kelly BC, Liu W. Isomer-specific transplacental transfer of perfluoroalkyl acids: results from a survey of paired maternal, cord sera, and placentas. Environ Sci Technol. 2017;51(10):5756–5763. [DOI] [PubMed] [Google Scholar]
- 114.Govarts E, Remy S, Bruckers L, Den Hond E, Sioen I, Nelen V, Baeyens W, Nawrot TS, Loots I, Van Larebeke N, Schoeters G. Combined effects of prenatal exposures to environmental chemicals on birth weight. Int J Environ Res Public Health. 2016;13(5):E495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L, Li J, Lai J, Luan H, Cai Z, Wang Y, Zhao Y, Wu Y. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing Prenatal Exposure Study. Sci Rep. 2016;6(1):21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ode A, Rylander L, Lindh CH, Källén K, Jönsson BA, Gustafsson P, Olofsson P, Ivarsson SA, Rignell-Hydbom A. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ Sci Pollut Res Int. 2013;20(11):7970–7978. [DOI] [PubMed] [Google Scholar]
- 117.Porpora MG, Lucchini R, Abballe A, Ingelido AM, Valentini S, Fuggetta E, Cardi V, Ticino A, Marra V, Fulgenzi AR, De Felip E. Placental transfer of persistent organic pollutants: a preliminary study on mother-newborn pairs. Int J Environ Res Public Health. 2013;10(2):699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee YJ, Kim MK, Bae J, Yang JH. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere. 2013;90(5):1603–1609. [DOI] [PubMed] [Google Scholar]
- 119.Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol. 2012;46(16):9071–9079. [DOI] [PubMed] [Google Scholar]
- 120.Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, Wu Y. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int. 2011;37(7):1206–1212. [DOI] [PubMed] [Google Scholar]
- 121.Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. Placental transfer of perfluorinated compounds is selective—a Norwegian mother and child sub-cohort study. Int J Hyg Environ Health. 2012;215(2):216–219. [DOI] [PubMed] [Google Scholar]
- 122.Kim SK, Lee KT, Kang CS, Tao L, Kannan K, Kim KR, Kim CK, Lee JS, Park PS, Yoo YW, Ha JY, Shin YS, Lee JH. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut. 2011;159(1):169–174. [DOI] [PubMed] [Google Scholar]
- 123.Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003 2006. Environ Sci Technol. 2014;48(16):9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H, Giesy JP. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011;45(17):7465–7472. [DOI] [PubMed] [Google Scholar]
- 125.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112(11):1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iyer S, Perera F, Zhang B, Chanock S, Wang S, Tang D. Significant interactions between maternal PAH exposure and haplotypes in candidate genes on B[a]P-DNA adducts in a NYC cohort of non-smoking African-American and Dominican mothers and newborns. Carcinogenesis. 2014;35(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Topinka J, Milcova A, Libalova H, Novakova Z, Rossner P Jr, Balascak I, Sram RJ. Biomarkers of exposure to tobacco smoke and environmental pollutants in mothers and their transplacental transfer to the foetus. Part I: Bulky DNA adducts. Mutat Res. 2009;669(1–2):13–19. [DOI] [PubMed] [Google Scholar]
- 128.Wang S, Chanock S, Tang D, Li Z, Jedrychowski W, Perera FP. Assessment of interactions between PAH exposure and genetic polymorphisms on PAH-DNA adducts in African American, Dominican, and Caucasian mothers and newborns. Cancer Epidemiol Biomarkers Prev. 2008;17(2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Whyatt RM, Jedrychowski W, Hemminki K, Santella RM, Tsai WY, Yang K, Perera FP. Biomarkers of polycyclic aromatic hydrocarbon-DNA damage and cigarette smoke exposures in paired maternal and newborn blood samples as a measure of differential susceptibility. Cancer Epidemiol Biomarkers Prev. 2001;10(6):581–588. [PubMed] [Google Scholar]
- 130.Li LX, Chen L, Meng XZ, Chen BH, Chen SQ, Zhao Y, Zhao LF, Liang Y, Zhang YH. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One. 2013;8(5):e62526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate. 2003;83(1):22–24. [DOI] [PubMed] [Google Scholar]
- 132.Xu X, Yekeen TA, Xiao Q, Wang Y, Lu F, Huo X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ Pollut. 2013;182:63–69. [DOI] [PubMed] [Google Scholar]
- 133.Mumford JL, Lee X, Lewtas J, Young TL, Santella RM. DNA adducts as biomarkers for assessing exposure to polycyclic aromatic hydrocarbons in tissues from Xuan Wei women with high exposure to coal combustion emissions and high lung cancer mortality. Environ Health Perspect. 1993;99:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perera FP, Tang D, Rauh V, Lester K, Tsai WY, Tu YH, Weiss L, Hoepner L, King J, Del Priore G, Lederman SA. Relationships among polycyclic aromatic hydrocarbon-DNA adducts, proximity to the World Trade Center, and effects on fetal growth. Environ Health Perspect. 2005;113(8):1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perera F, Phillips DH, Wang Y, Roen E, Herbstman J, Rauh V, Wang S, Tang D. Prenatal exposure to polycyclic aromatic hydrocarbons/aromatics, BDNF and child development. Environ Res. 2015;142:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vishnevetsky J, Tang D, Chang HW, Roen EL, Wang Y, Rauh V, Wang S, Miller RL, Herbstman J, Perera FP. Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol Teratol. 2015;49:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, Sowa A. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res Int. 2015;22(5):3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang D, Lee J, Muirhead L, Li TY, Qu L, Yu J, Perera F. Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. PLoS One. 2014;9(3):e91966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tang D, Li TY, Chow JC, Kulkarni SU, Watson JG, Ho SS, Quan ZY, Qu LR, Perera F. Air pollution effects on fetal and child development: a cohort comparison in China. Environ Pollut. 2014;185:90–96. [DOI] [PubMed] [Google Scholar]
- 140.Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, Rauh V, Phillips DH. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect. 2011;119(8):1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang D, Li TY, Liu JJ, Zhou ZJ, Yuan T, Chen YH, Rauh VA, Xie J, Perera F. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ Health Perspect. 2008;116(5):674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tang D, Li TY, Liu JJ, Chen YH, Qu L, Perera F. PAH-DNA adducts in cord blood and fetal and child development in a Chinese cohort. Environ Health Perspect. 2006;114(8):1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Perera FP, Tang D, Rauh V, Tu YH, Tsai WY, Becker M, Stein JL, King J, Del Priore G, Lederman SA. Relationship between polycyclic aromatic hydrocarbon-DNA adducts, environmental tobacco smoke, and child development in the World Trade Center cohort. Environ Health Perspect. 2007;115(10):1497–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gong Z, Tas E, Yakar S, Muzumdar R. Hepatic lipid metabolism and non-alcoholic fatty liver disease in aging. Mol Cell Endocrinol. 2017;455:115–130. [DOI] [PubMed] [Google Scholar]
- 145.Goyal NP, Schwimmer JB. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin Liver Dis. 2016;20(2):325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bush H, Golabi P, Younossi ZM. Pediatric non-alcoholic fatty liver disease. Children (Basel). 2017;4(6):E48. [DOI] [PMC free article] [PubMed] [Google Scholar]