Key Points
Fingolimod could be efficient to treat GVHD of the central nervous system.
Further research should explore the use of fingolimod and other sphingosine-1-phosphate receptor agonists to prevent or treat GVHD.
Introduction
Graft-versus-host disease (GVHD) involving the central nervous system (CNS) is a rare complication after allogeneic hematopoietic cell transplantation (allo-HCT). CNS GVHD is largely misunderstood, has limited therapeutic options, and often leads to dismal outcomes.1,2 Targeting lymphocyte trafficking with sphingosine-1-phosphate receptors (S1PR) agonists is a promising approach to treat GVHD, which have proven efficient in several preclinical models.3,4 Here, we describe the case of a 66-year-old patient with severe CNS GVHD treated successfully with fingolimod (FTY720), a first-in-class, orally bioavailable S1PR agonist approved by the US Food and Drug Administration in 2010 for the treatment of relapsing forms of multiple sclerosis (MS).
Methods
Our patient provided written consent for the publication of this case report. Cognition was assessed with the Mini-Mental Status Examination (MMSE) and the frontal assessment battery (FAB). The MMSE is a widely used, reliable, and reproducible clinical tool to assess cognitive impairment.5 MMSE comprises simple questions and problems in 8 categories: orientation to time, orientation to place, registration, attention and calculation, recall, language, repetition, and complex commands. The FAB is a bedside cognitive and behavioral evaluation of frontal lobe functions and has been shown to be a reliable and consistent test with great sensitivity to detect frontal lobe dysfunction. It consists of 6 problems evaluating conceptualization, mental flexibility, programming, sensitivity to interference, inhibitory control, and environmental autonomy.6 Lymphocyte count and immunophenotypic analyses of T- and B-cell subsets were performed as previously described.7
Results and discussion
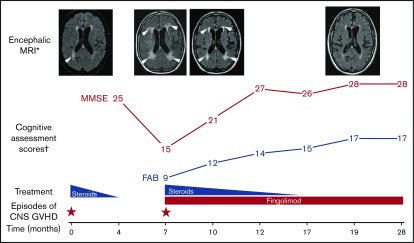
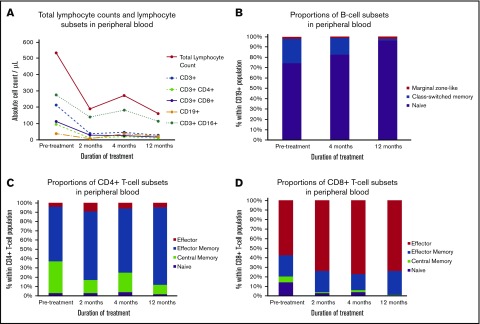
Our patient was initially diagnosed with acute myeloblastic leukemia that needed 2 cycles of induction chemotherapy to achieve complete remission. He then underwent allo-HCT from an HLA-matched, unrelated donor (peripheral blood stem cells) after reduced-intensity conditioning containing fludarabine and busulfan. GVHD prophylaxis associated anti-thymocyte globulin, ciclosporin, and methotrexate. Our patient had a distant history of traumatic subdural hematoma, but no other neurological history. Neither relevant pretransplant comorbidity nor familial history were noted. Two months after his transplant, he developed steroid-responsive, grade II gastrointestinal acute GVHD (neither skin nor liver involvement was observed). Ten months after allo-HCT, our patient was admitted to the emergency department with a 3-week history of drowsiness and apathy. Physical examination revealed diffuse cognitive impairment as well as frontal lobe involvement (widened-base gait, dysexecutive syndrome, grasping reflex). The remainder of the examination was unremarkable. We did not observe signs classically associated with acute or chronic GVHD. He was not receiving any medications potentially responsible for his symptoms. Cerebrospinal fluid (CSF) analysis ruled out bacterial, viral (herpes simplex virus [HSV], varicella-zoster virus, human herpesvirus 6 [HHV6], HHV7, HHV8, Epstein-Barr virus, cytomegalovirus, adenovirus, JC virus, enterovirus), and fungal (candida, Cryptococcus) infections of the CNS. CSF counts were unremarkable per morphology (0 cells/µL) and flow cytometry studies (absence of leukemic blasts). Glucose and protein levels in the CSF were in the normal range. Blood analyses were negative for the following tests: electrolyte abnormalities, liver function tests abnormalities, anti-neuronal antibodies, and vitamin B1, B3, and B6 deficiencies. Thyroid tests were normal. Electroencephalography was unremarkable. Brain magnetic resonance imaging (MRI) showed hyperintensities of the centrum ovale and the lateral ventricles without gadolinium enhancement. Chimerism studies of the peripheral blood revealed 100% of T cells of donor origin. Corticosteroids were initiated at 1 mg/kg and all symptoms improved within 3 days and resolved within 2 weeks. Steroids were tapered over 4 months. Unfortunately, similar symptoms recurred 3 months after steroids were discontinued. An identical work-up was performed and was again unremarkable. Another course of steroids was initiated, but this time fingolimod was added at 0.5 mg per day. We observed no acute toxicity, in particular, no arrhythmia. Neurological symptoms receded gradually on treatment, and within 2 weeks, our patient could be discharged. Steroids were slowly tapered and discontinued after 8 months. At the last follow-up evaluation, he had been treated with fingolimod for 21 months (12 months with fingolimod alone) and remained free of CNS GVHD. Importantly, we did not observe any infectious complications on Pneumocystis and HSV prophylaxis with cotrimoxazole and valacyclovir. Cognitive assessments and MRI scans at different time points are displayed in Figure 1. Evolution during treatment with fingolimod and steroids of total lymphocyte counts and main lymphocyte subsets are graphed in Figure 2.
Figure 1.
Clinical and morphologic response to fingolimod and steroids in a patient with CNS GVHD. *MRI images are T2-FLAIR weighted; arrowheads indicate main periventricular lesions, which gradually disappeared on treatment. †Scores were not evaluated at the time of the first episode of CNS GVHD. Any MMSE score ≥24 points (out of 30) indicates normal cognition. Any FAB score ≥16 points (out of 18 points) indicates normal executive functions. T0, time of the first episode of CNS GVHD (10 months after allo-HCT).
Figure 2.
Immunophenotyping analysis of immune cells in the peripheral blood of a patient treated with fingolimod and corticosteroids for CNS GVHD. (A) Total lymphocyte counts, lymphocyte subsets absolute counts and changes in subset proportions of B and T cells were determined by flow cytometry. Fingolimod and corticosteroids were associated with lymphopenia and decreased B-cell, T-cell, and natural killer cell absolute counts. Treatment with fingolimod and corticosteroids was followed by an increase in the proportion of naive B cells (B), CD4+ (C), and CD8+ (D) effector memory T cells in the peripheral blood. T0, time of treatment initiation with fingolimod and corticosteroids to treat the second episode of CNS GVHD. Pretreatment samples were drawn at the time of diagnosis of the second episode of CNS GVHD, 17 months after allo-HCT.
CNS GVHD is a very rare and neglected entity, reflected by the paucity of data available today regarding this complication. A retrospective analysis from the Memorial Sloan Kettering Cancer Center8 revealed that out of 1484 patients who received an allo-HCT, only 7 patients (0.5%) developed CNS or peripheral nervous system GVHD. CNS GVHD is commonly characterized by clinical, radiological, and pathological involvement of the CNS in the absence of another cause and with response to immunosuppressive therapy. Despite the lack of histological proof, the dramatic response to immunosuppressive therapy in our patient was felt to be highly consistent with the diagnosis of CNS GVHD. MRI findings in our case (diffuse periventricular white matter lesions) were also consistent with previous reports of CNS GVHD.
There is little insight into the pathogenesis and treatment of CNS GVHD. Common findings in biopsy-proven CNS GVHD include CD3+ interstitial and/or perivascular lymphocytic infiltrates as well as activated microglia.2 Standard immunosuppressive treatments, such as steroids and mycophenolate mofetil, have shown modest efficacy to treat CNS GVHD, achieving only partial or transient responses in most patients.1 Given the efficacy of fingolimod to treat MS, known to be mainly a T-cell–mediated autoimmune disease of the CNS, we hypothesized it could also be a potent treatment for CNS GVHD. Fingolimod acts as a high-affinity agonist for S1PR, hence inducing aberrant internalization of the receptor by lymphocytes. Lack of S1PR membrane expression leads to the entrapment of lymphocytes within lymph nodes, preventing their egress into the peripheral blood. In MS, this is thought to ultimately prevent several pathogenic immune subsets, such as naive CD4+ T cells and memory B cells, from migrating to the peripheral blood and the CNS. In line with published data,9,10 we observed in our patient’s peripheral blood an increase in the proportion of naive B cells and effector memory CD4+ cells (Figure 2B-C). Although most studies3,11-13 emphasized inhibition of lymphocyte egress as the main mechanism responsible for the anti-GVHD effects of fingolimod, Taylor et al reported contrasting effects of the drug in a murine model of acute GVHD.14 In this study, fingolimod did not uniformly trap effector T cells in all secondary lymphoid organs. In addition, it did not prevent their migration to targets organs, such as the liver or lungs.14 Equally arguing against effects restricted to the S1PR/ sphingosine-1-phosphate (S1P) pathway, Ntranos et al observed that fingolimod could impair the functionality of CD8+ effector T cells, and that it could occur independently of the S1PR/S1P pathway.15 In addition to immune cells, fingolimod has been shown to interact directly with CNS-specific cell types, such as astrocytes.16 In summary, the effects of fingolimod are manifold and extend well beyond lymphocyte trafficking.
In conclusion, this is the first report on the efficacy and safety of fingolimod to treat CNS GVHD and, more broadly, the first report in human GVHD. Fingolimod was well tolerated in our patient and acted as a steroid-sparing agent. We hope this report will stimulate further research into the role of fingolimod to treat or prevent GVHD after allo-HCT.
Acknowledgment
The authors would like to thank the neurology team of the Lille University Hospital for their help in the care of this patient.
Authorship
Contribution: J.G. and I.Y.-A. collected and analyzed the data, and wrote the preliminary versions of the manuscript; P. Vermersch, P.C., P. Varlet, V.C., and L.M. contributed to the redaction of the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ibrahim Yakoub-Agha, Department of Hematology, Allogeneic Stem Cell Transplantation Unit, Centre Hospitalier Universitaire de Lille, Rue Michel Polonovski, F-59037 Lille Cedex, France; e-mail: ibrahim.yakoubagha@chru-lille.fr.
References
- 1.Kamble RT, Chang CC, Sanchez S, Carrum G. Central nervous system graft-versus-host disease: report of two cases and literature review. Bone Marrow Transplant. 2007;39(1):49-52. [DOI] [PubMed] [Google Scholar]
- 2.Saad AG, Alyea EP III, Wen PY, Degirolami U, Kesari S. Graft-versus-host disease of the CNS after allogeneic bone marrow transplantation. J Clin Oncol. 2009;27(30):e147-e149. [DOI] [PubMed] [Google Scholar]
- 3.Huu DL, Matsushita T, Jin G, et al. . FTY720 ameliorates murine sclerodermatous chronic graft-versus-host disease by promoting expansion of splenic regulatory cells and inhibiting immune cell infiltration into skin. Arthritis Rheum. 2013;65(6):1624-1635. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Q, Ma S, Lin D, et al. . The S1P1 receptor-selective agonist CYM-5442 reduces the severity of acute GVHD by inhibiting macrophage recruitment. Cell Mol Immunol. 2015;12(6):681-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922-935. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621-1626. [DOI] [PubMed] [Google Scholar]
- 7.Yakoub-Agha I, Saule P, Depil S, et al. . A high proportion of donor CD4+ T cells expressing the lymph node-homing chemokine receptor CCR7 increases incidence and severity of acute graft-versus-host disease in patients undergoing allogeneic stem cell transplantation for hematological malignancy. Leukemia. 2006;20(9):1557-1565. [DOI] [PubMed] [Google Scholar]
- 8.Delios AM, Rosenblum M, Jakubowski AA, DeAngelis LM. Central and peripheral nervous system immune mediated demyelinating disease after allogeneic hemopoietic stem cell transplantation for hematologic disease. J Neurooncol. 2012;110(2):251-256. [DOI] [PubMed] [Google Scholar]
- 9.Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology. 2011;76(8 suppl 3):S20-S27. [DOI] [PubMed] [Google Scholar]
- 10.Claes N, Dhaeze T, Fraussen J, et al. . Compositional changes of B and T cell subtypes during fingolimod treatment in multiple sclerosis patients: a 12-month follow-up study. PLoS One. 2014;9(10):e111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y-M, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest. 2003;111(5):659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka H, Ohtsuki M, Shimano K, et al. . Immunosuppressive activity of FTY720, sphingosine 1-phosphate receptor agonist: II. Effect of FTY720 and FTY720-phosphate on host-versus-graft and graft-versus-host reaction in mice. Transplant Proc. 2005;37(1):107-109. [DOI] [PubMed] [Google Scholar]
- 13.Song J, Ito T, Matsuda C, et al. . Regulation of donor T cells in the tolerant rats to graft-versus-host disease by FTY720 following small bowel transplantation. Transplant Proc. 2006;38(10):3181-3183. [DOI] [PubMed] [Google Scholar]
- 14.Taylor PA, Ehrhardt MJ, Lees CJ, et al. . Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD). Blood. 2007;110(9):3480-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ntranos A, Hall O, Robinson DP, et al. . FTY720 impairs CD8 T-cell function independently of the sphingosine-1-phosphate pathway. J Neuroimmunol. 2014;270(1-2):13-21. [DOI] [PubMed] [Google Scholar]
- 16.Choi JW, Gardell SE, Herr DR, et al. . FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2011;108(2):751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]