Abstract
Endometriosis is a common gynecologic disorder characterized by ectopic endometrial tissue growth outside the uterine cavity. Although usually occurring in pelvic organs, endometrial lesions may involve urinary tract. Renal endometriosis is extremely rare and it has only occasionally been reported in the past. We report two cases of patients with renal cystic lesions, incidentally found at imaging techniques during oncologic follow-up for gastric sarcoma and melanoma, initially misinterpreted as complicated haemorrhagic cysts and then histologically characterized as renal localizations of extragenital endometriosis.
Keywords: Renal endometriosis, Extragenital endometriosis, Kidney, Complicated renal cyst, Endometrioma, Shading sign
Introduction
Endometriosis is a common gynecologic disorder characterized by ectopic endometrial tissue growth outside the uterine cavity. The incidence rate is about 6% to 10% in women of reproductive age (1). Infertility has been reported in 37% of patients in a recent epidemiological research (2). The ectopic endometrial tissue focuses commonly in the pelvic organs, it seldom occurs outside of the reproductive organs, when it is termed extragenital endometriosis (3–5). Urinary tract endometriosis is uncommon, accounting for only 1.2% of all cases, with the bladder being the most frequently affected organ (6). Endometrial lesions involving the bladder are found in about 85% of cases of urinary endometriosis and are located within the ureter in about 15%; whereas endometriomas involving the kidneys and urethra are extremely rare, accounting for less than 1% of urinary tract endometriosis (7). Thus, renal endometriosis is a rare manifestation of this condition, which has only occasionally been reported in the past, with approximately 24 cases documented in the literature to date (7). Conversely, complicated cysts of kidney are frequent incidental findings encountered during imaging follow-up of oncologic patients (8).
We report two cases of oncologic patients with renal endometriosis which were preoperatively misinterpreted as complicated cysts of kidney. To our knowledge, there are only few cases of asymptomatic renal endometriomas with haemorrhagic cystic appearance described in the literature (9, 10). We report our experience to raise awareness about imaging findings of renal endometriosis in order to avoid a misinterpretation of imaging findings of this extremely rare condition.
Case presentation
Case 1
In January 2014, a 40-years-old woman was admitted to our University Hospital “P. Giaccone”, Palermo, Italy for further investigation about a gastric ulcerated neoformation discovered and biopsied during endoscopy performed for dysphagia and bleeding (11). The patient previously underwent a laparoscopic left ovariectomy because of ovarian endometriosis. At the time of hospital admission, there was no history of abdominal pain or gross haematuria. Furthermore, the menstrual pattern was normal and there was no history of recent dyspaureunia, dysmenorrhea, or leucorrhoea.
The ultrasonography (US) of the abdomen showed some cystic formations in the left kidney, not reported in abdomen US performed two years before. Then, contrast enhanced Computed Tomography (CECT) of abdomen was performed. It showed a small (maximum diameter 2.5 cm) gastric polypoid lesion with a slight and homogeneous contrast enhancement located in the antrum, appearing suspicious for tumor of stomach. CECT showed also multiple hyperattenuating nodular cortical lesions (maximum diameter 1.8 cm), contiguous among themselves with “bunch of grapes” appearance, both in upper and mid poles of the left kidney (Figure 1 A, C). On the arterial and portal venous phases, despite the evaluation of different areas of interest within the lesions, it has never been observed significant enhancement of the renal lesions themselves (Figure 1 B). These were considered compatible with hemorrhagic cysts but it was not possible to exclude other diagnosis. Hystopathology of the gastric tumor revealed an advanced gastric cancer and a Magnetic Resonance (MR) of the abdomen was performed in order to characterize the renal findings, revealing left renal cortical lesions, tending to confluence, hyperintense on T1-weighted images and with low signal on T2-weighted images. No significant enhancement was demonstrated on post-contrast phases (Figure 2). However, due to the medical history of gastric cancer, after a multidisciplinary discussion between oncologists, surgeons and radiologists, a CT-guided biopsy was suggested.
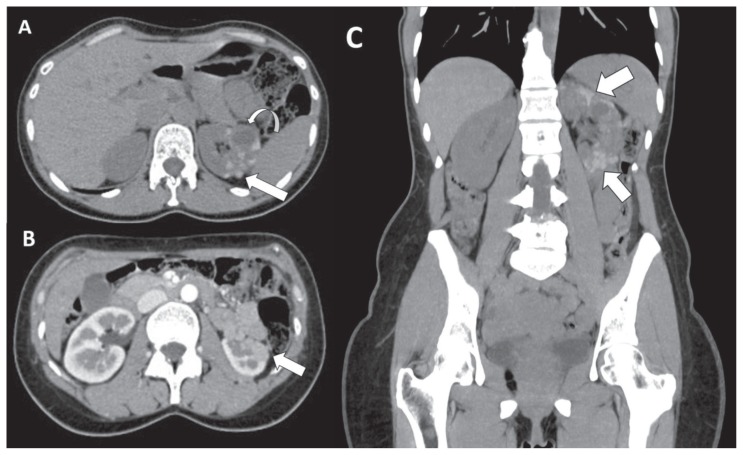
Figure 1.
40-years-old woman with renal endometriosis -Technique: Abdominal CT scans (120 kV, modulated mA, 0,6 mm slice thickness, 80 ml of Ultravist 370) -Findings: A: Axial unenhanced CT image shows multiple hyperattenuating lesions (maximum diameter 1.8 cm, 67 HU) (arrow). A simple cyst coexists on the mid pole (maximum size of 2.4 cm) (curved arrow). B: Axial contrast enhanced CT image on corticomedullary phase demonstrates slight enhancement of the renal lesions (80 HU) interpreted as “pseudoenhancement” (arrow). C: Coronal reformatted unenhanced CT image of the abdomen depicts multiple hyperattenuating renal lesions in upper and lower poles of the left kidney, contiguous among themselves with “bunch of grapes” appearance, related to endometriotic lesions (arrow).
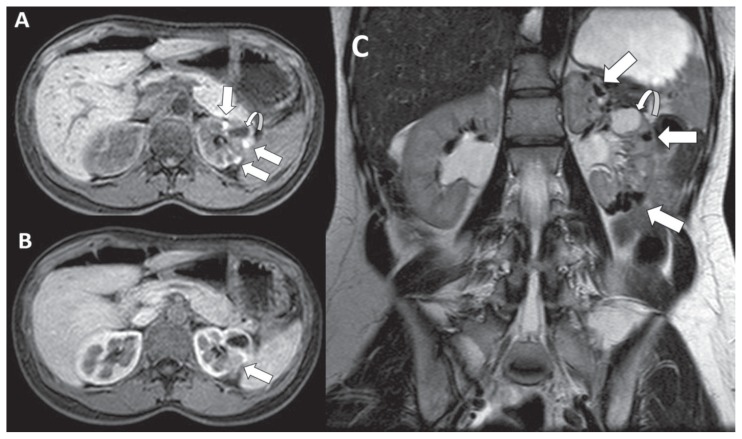
Figure 2.
40-years-old woman with renal endometriosis - Technique: Abdominal MRI scans - Findings: A: pre-contrast three-dimensional fat-suppressed T1-weighted gradient-recalled-echo axial image shows multiple hyperintense left renal cortical lesions, tending to confluence (arrows). A simple hypointense cyst coexists on the mid pole (curved arrow). B: three-dimensional fat-suppressed T1-weighted gradient-recalled-echo axial image after paramagnetic contrast administration demonstrates no abnormal enhancement within the renal lesions (arrow). C: coronal turbo spin-echo T2-weighted image demonstrates the presence of deoxyhaemoglobin and intracellular methaemoglobin within the endometriotic lesions, which appear entirely hypointense (shading sign) (arrows). A simple hyperintense cyst coexists on the mid pole (maximum size of 2.4 cm) (curved arrow).
Case 2
In September 2016, a 39-years-old woman with newly diagnosed skin melanoma was admitted to our Department of Radiology for staging work-up. Since the age of 22, the patient had suffered from ovarian endometriosis, successfully regressed after 8 years of treatment with hormonal therapy. At the time of diagnosis of melanoma, there was no history of abdominal pain or gross hematuria.
CECT of the abdomen showed a left kidney reduced in size (maximum diameter 8 cm), with multiple millimetric spontaneously hyperattenuating cortical lesions, contiguous among themselves with “bunch of grapes” appearance, with no significant enhancement after contrast medium injection (Figure 3) considered as haemorrhagic cysts. However, medical history of skin melanoma led the clinicians to request a CT-guided biopsy to exclude the diagnosis of renal metastases.
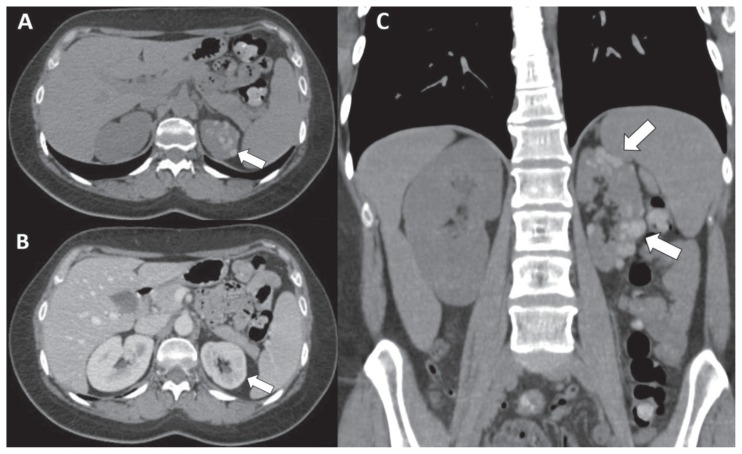
Figure 3.
39-years-old woman with endometriotic lesions of left kidney - Technique: Abdominal CT scans (120 kV, modulated mA, 0,6 mm slice thickness, 120 ml of Iomeron 350) - Findings: A: Axial unenhanced CT image shows multiple millimetric hyperattenuating renal lesions (72 HU) (arrow). B: Axial CT image on portal venous phase demonstrates not significant enhancement of the renal lesions (86 HU) (arrow) interpreted as “pseudoenhancement”. C: Coronal reformatted unenhanced CT image of the abdomen depicts multiple hyperattenuating renal lesions in upper and lower poles of the left kidney with “bunch of grapes” appearance, related to renal endometriosis (arrows).
In both patients, a CT-guided biopsy of renal lesions was performed: in both cases, pathological examination revealed fibrocollagenous cysts lined by endometrial epithelium, glands and stromal tissue, along with areas of haemorrhage, hemosiderin and macrophages. Immunohistochemistry showed both stromal and epithelial cells were focally positive for estrogen (ER) and progesterone receptors (PR). Based on these features, a diagnosis of endometriosis involving the left kidney was given. Patients were asymptomatic and did not need any hormonal or surgical therapy for renal lesions. During the follow-up, 6 months after the first imaging examination, no clinical changes occured, neither hematuria in the urinalysis or modification of CECT findings.
Discussion
The pathogenesis of endometriosis is controversial and includes ectopic transplantation theory, metaplasia of coelomic epithelium, autoimmunity, bloodlymphatic embolism and embryonic theory (12). Renal endometriosis is an extremely rare find of this disease, which may remain asymptomatic for several years and discovered incidentally, although it might present as vague nonspecific symptoms (13). The presence of genitourinary symptomatology depends on the extent, depth and location of the ectopic endometrium: this cyclically thickens and sheds in response to the changing levels of ovarian sex hormones, resulting in the growth of fibrous tissue and formation of the endometrioma in the kidney. Repeated periodic bleeding may lead to haemorrhagic cysts, that gradually increase in the renal tissue or invade the renal capsule, causing abdominal pain (6). Gross haematuria is clinically manifest when the lesions break into the renal calyces, while blood clots and deciduous endometrium may cause ureteral obstructions, which may evoke renal colic pain (13). Rarely, renal endometriosis can result in asymptomatic kidney dysfunction if a large endometriotic lesion involves directly the renal pelvis, resulting in hydronephrosis (6). Our cases were accidentally discovered in two asymptomatic patients on abdominal imaging performed for other indications. The absence of symptoms was probably related to the fact the endometriotic lesions were confined within the renal cortex with no involvement of the calyces. An early diagnosis of renal endometriosis is primarily dependent on the knowledge of a medical history of endometriosis. Persisting urinary symptoms in women with endometriosis or undergone gynaecological surgery, should suggest the diagnosis of urinary tract endometriosis. However, cyclic hormonal changes may affect both stromal and glandular components in endometriosis, making the radiological and pathological interpretation challenging.
The imaging features of renal endometriosis do not facilitate accurate diagnosis. Although US is useful to differentiate solid from cystic lesions and contrast enhanced US increases the accuracy of the examination in the evaluation of lesions of the urinary tract (14), US provides just little diagnostic information to identify renal endometriosis.
CECT is commonly helpful to detect extragenital endometriosis. Renal endometriosis is usually located in the parenchyma and may have the appearance of multilobular cyst, polypoid bulge or just scar tissue deformity. Unenhanced CT may show lesions of several size, from a few millimetres to 3 cm in diameter, with clear and irregular borders. Lesion density is usually uniformly hypodense, but it changes with the menstrual cycle. When ectopic endometrium bleeding occurs during the menstrual period, the density of lesions may increase or appear uneven. Renal cysts show attenuation value between 0 and 20 HU on unenhanced CT, while renal masses with 20–70 HU values (“danger zone”) require further investigation, since renal cell carcinomas have typically these attenuation values (15). Moreover, renal lesions with HU values greater than 70 HU on unenhanced CT are cystic lesions with haemorrhagic content in 99% of cases (15). Furthermore, it is possible to observe a “pseudoenhancement” (15), an increase in attenuation values between 10 and 20 HU as a consequence of beam hardening, as we observed in our cases. CECT may show enhancement in the central part of endometriotic lesions and enhancement in the surrounding proliferative fibromuscular tissue (13).
MR imaging, compared to CT, despite the higher costs and longer acquisition times, shows superior contrast resolution that could lead to an easier determination of renal endometriosis extention and may help in differential diagnosis from haemorrhagic cysts. Typically, endometrioma of kidney appears as a cystic mass, with occasional internal septa or blood. The solid components of renal endometrioma usually show hypointensity or isointensity on T1-weighted and hypointensity on T2-weighted images. No significant contrast enhancement is generally seen. MR demonstrates the haemorrhagic nature of the lesions, with areas of increased and decreased signal intensity, which may be seen on T1- and T2-weighted images (16). In particular, the “shading sign” is a typical MR finding of endometriotic lesions, that helps to differentiate these latter from other blood containing lesions (e.g. haemorrhagic cysts). This sign consists of loss of signal intensity on T2-weighted images of lesions, which appear hyperintense on T1-weighted images; it may involve only a small portion or the entire cyst. The low signal on T2-weighted sequences is due to high concentration of protein and iron within the endometrioma because of recurrent hemorrhages. The degree of shading can vary from faint to complete signal loss. Conversely, marked loss of signal intensity on T2-weighted images is not usually seen in haemorrhagic cysts. Without recurrent haemorrhages and concentration of haemoglobin derivatives (deoxyhaemoglobin and methaemoglobin), viscosity of the cyst remains lower and shading sign is unlikely to be observed (17). Diffusion weighted imaging is a MR sequence which provides functional information by probing the random motion of water molecules in biological tissues, also with a quantitative assessment of diffusivity calculated on the ADC map (18–23). Endometriomas tipically show low ADC values mainly due to “T2 blackout effects”, related to the low T2-weigthed signal intensity of the lesions (24). To our knowledge, patient of “Case 1” is the first documented report in the English literature describing MR features of renal endometriosis with haemorrhagic cystic appearance.
Regarding the possible role of nuclear medicine procedures, while FDG-positron emission tomography (PET) plays a crucial role in oncologic diseases of the urinary tract (25), it does not seem to be useful for detection and staging of endometriosis, since endometriotic lesions do not show hypermetabolic activity (26).
The recurrence of symptoms, usually correlated with patients’ menstrual cycles, may lead to the adoption of medical approaches, including hormonal therapies such as GnRH agonists and oral contraceptives, while surgical therapeutic strategies, in particular laparoscopic management, should be considered for large renal endometriomas or persistent obstructive uropathy (6, 27, 28). These surgical procedures should to be performed by a surgical team experienced in general, gynaecological, adrenal and urological laparoscopic surgery also in urgency setting because we well know the possible intraoperative complications of this kind of surgery as bowel, bladder, ureteric and vascular injuries (29–35).
Conclusion
Our cases are two examples of asymptomatic patients with history of ovarian endometriosis and incidental detection of renal endometriotic lesions, subsequently histopathological examined. These patients do not need any therapy for renal lesions being asymptomatic and unchanged on the subsequent imaging examinations. From literature data most of the previously reported examples of endometriosis of kidney are found in symptomatic patients.
In conclusion, renal endometriosis is a rare entity that may be asymptomatic or clinically controversial and the diagnosis is possible only in presence of an appropriate clinical assessment. CECT and MR may be helpful in staging the disease process and for differential diagnosis from other blood containing lesions, although definitive diagnosis requires histological confirmation by identifying endometrial glands and stroma within the renal lesions.
Footnotes
Supporting foundations
The authors state that this work has not received any funding.
Informed consent statement
The patient provided informed written consent.
Conflict-of-interest statement
The authors state they have no conflict of interests.
References
- 1.Ajayi A, Ajayi V, Oyetunji I, et al. Endometriosis and Assisted Reproductive Technology: A Review Article. Med Clin Rev. 2017;3:1. [Google Scholar]
- 2.Eisenberg VH, Weil C, Chodick G, et al. Epidemiology of endometriosis: a large population-based database study in a 2-million-member health care provider. BJOG: Int J Obstet Gy. 2017 doi: 10.1111/1471-0528.14711. [DOI] [PubMed] [Google Scholar]
- 3.Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449–66. doi: 10.1097/GRF.0b013e3181e0ea6e. [DOI] [PubMed] [Google Scholar]
- 4.Calagna G, Perino A, Chianetta D, Vinti D, Triolo MM, Rimi C, Cucinella G, Agrusa A. Primary umbilical endometrioma: Analyzing the pathogenesis of endometriosis from an unusual localization. Taiwan J Obstet Gynecol. 2015 Jun;54(3):306–12. doi: 10.1016/j.tjog.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Agrusa A, Romano G, Di Buono G, Frazzetta G, Chianetta D, Sorce V, Billone V, Cucinella G, Gulotta G. Acute appendicitis and endometriosis: Retrospective analysis in emergency setting. Giornale Italiano di Ostetricia e Ginecologia. 2013;35(6):728–732. [Google Scholar]
- 6.Shook TE, Nyberg LM. Endometriosis of the urinary tract. Urology. 1988;31:1–6. doi: 10.1016/0090-4295(88)90560-2. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Thorpe P, Ramsay JW, et al. Endometriosis of ureter. Br J Urol. 1992;69:495–8. doi: 10.1111/j.1464-410x.1992.tb15595.x. [DOI] [PubMed] [Google Scholar]
- 8.Galia M, Albano D, Narese D, Patti C, Chianca V, Di Pietto F, et al. Whole-body MRI in patients with lymphoma: collateral findings. Radiol Med. 2016;121:793–800. doi: 10.1007/s11547-016-0658-x. [DOI] [PubMed] [Google Scholar]
- 9.Hajdu SI, Koss LG. Endometriosis of the kidney. Am J Obstet Gynecol. 1970;106:314. doi: 10.1016/0002-9378(70)90284-x. [DOI] [PubMed] [Google Scholar]
- 10.Balci O, Karatayli R, Capar M. An incidental coexistence of Mayer–Rokitasnky–Kuster syndrome with pelvic ectopic kidney and perirenal endometrioma. Saudi Med J. 2008;29:13450–13451. [PubMed] [Google Scholar]
- 11.Romano G, Agrusa A, Amato G, De Vita G, Frazzetta G, Chianetta D, Sorce V, Di Buono G, Gulotta G. Endoscopic sclerotherapy for hemostasis of acute esophageal variceal bleeding. G Chir. 2014 Mar-Apr;35(3–4):61–4. [PMC free article] [PubMed] [Google Scholar]
- 12.Signorile PG, Baldi A. Endometriosis: new concepts in the pathogenesis. Int J Biochem Cell Biol. 2010;42:778–80. doi: 10.1016/j.biocel.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Yohannes P. Ureteral endometriosis. J Urol. 2003;170:20–5. doi: 10.1097/01.ju.0000054836.32660.9e. [DOI] [PubMed] [Google Scholar]
- 14.Caruso G, Salvaggio G, Campisi A, Melloni D, Midiri M, Bertolotto M, Lagalla R. Bladder tumor staging: comparison of contrast-enhanced and gray-scale ultrasound. AJR Am J Roentgenol. 2010;194:151–6. doi: 10.2214/AJR.09.2741. [DOI] [PubMed] [Google Scholar]
- 15.Galia M, Albano D, Bruno A, Agrusa A, Romano G, Di Buono G, et al. Imaging features of solid renal masses Br J Radiol 20179020170077. 10.1259/bjr.20170077(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gougoutas CA, Siegelman ES, Hunt J, et al. Pelvic endometriosis: various manifestations and MR imaging findings. AJR Am J Roentgenol. 2000;175:353–8. doi: 10.2214/ajr.175.2.1750353. [DOI] [PubMed] [Google Scholar]
- 17.Glastonbury CM. The shading sign. Radiology. 2002;224:199–201. doi: 10.1148/radiol.2241010361. [DOI] [PubMed] [Google Scholar]
- 18.Albano D, Patti C, Lagalla R, Midiri M, Galia M. Whole-body MRI, FDG-PET/CT, and bone marrow biopsy, for the assessment of bone marrow involvement in patients with newly diagnosed lymphoma. J Magn Reson Imaging. 2017;45:1082–9. doi: 10.1002/jmri.25439. [DOI] [PubMed] [Google Scholar]
- 19.Albano D, Sinagra E, Patti C, Narese D, Agrusa A, Di Buono G, et al. Caecal leiomyoma detected by whole-body MRI in a patient with hodgkin lymphoma: First case report. G Chir. 2017;38:27–32. doi: 10.11138/gchir/2017.38.1.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pozzi G, Albano D, Messina C, Angileri SA, Al-Mnayyis A, Galbusera F, et al. Solid bone tumors of the spine: Diagnostic performance of apparent diffusion coefficient measured using diffusion-weighted MRI using histology as a reference standard. J Magn Reson Imaging. 2017 doi: 10.1002/jmri.25826. (in press) [DOI] [PubMed] [Google Scholar]
- 21.Robba T, Chianca V, Albano D, Clementi V, Piana R, Linari A, et al. Diffusion-weighted imaging for the cellularity assessment and matrix characterization of soft tissue tumour. Radiol Med. 2017 doi: 10.1007/s11547-017-0787-x. (in press) [DOI] [PubMed] [Google Scholar]
- 22.Albano D, Patti C, La Grutta L, Agnello F, Grassedonio E, Mulè A, et al. Comparison between whole-body MRI with diffusionweighted imaging and PET/CT in staging newly diagnosed FDG-avid lymphomas. Eur J Radiol. 2016;85:313–8. doi: 10.1016/j.ejrad.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Galia M, Albano D, Tarella C, Patti C, Sconfienza LM, Mulè A, et al. Whole body magnetic resonance in indolent lymphomas under watchful waiting: the time is now. Eur Radiol. 2017 doi: 10.1007/s00330-017-5071-x. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Albano D, La Grutta L, Grassedonio E, Patti C, Lagalla R, Midiri M, Galia M. Pitfalls in whole body MRI with diffusion weighted imaging performed on patients with lymphoma: what radiologists should know. Magn Res Imaging. 2016;34:922–31. doi: 10.1016/j.mri.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Alongi P, Caobelli F, Gentile R, Stefano A, Russo G, Albano D, et al. Recurrent bladder carcinoma: clinical and prognostic role of 18 F-FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:224–33. doi: 10.1007/s00259-016-3500-8. [DOI] [PubMed] [Google Scholar]
- 26.Fastrez M, Nogarède C, Tondeur M, Sirtaine N, Rozenberg S. Evaluation of 18FDG PET-CT in the diagnosis of endometriosis: a prospective study. Reprod Sci. 2011;18:540–4. doi: 10.1177/1933719110392060. [DOI] [PubMed] [Google Scholar]
- 27.Granese R, Perino A, Calagna G, Saitta S, De Franciscis P, Colacurci N, Triolo O, Cucinella G. Gonadotrophin-releasing hormone analogue or dienogest plus estradiol valerate to prevent pain recurrence after laparoscopic surgery for endometriosis: a multi-center randomized trial. Acta Obstet Gynecol Scand. 2015 Jun;94(6):637–45. doi: 10.1111/aogs.12633. Epub 2015 Mar 29. [DOI] [PubMed] [Google Scholar]
- 28.Mainini G, Torella M, Di Donna MC, Esposito E, Ercolano S, Correa R, Cucinella G, Stradella L, Luisi A, Basso A, Cerreto FV, Cicatiello R, Matteo M, De Franciscis P. Nonhormonal management of postmenopausal women: effects of a red clover based isoflavones supplementation on climacteric syndrome and cardiovascular risk serum profile. Clin Exp Obstet Gynecol. 2013;40(3):337–41. [PubMed] [Google Scholar]
- 29.Agrusa A, di Buono G, Chianetta D, Sorce V, Citarrella R, Galia M, Vernuccio L, Romano G, Gulotta G. Three-dimensional (3D) versus two-dimensional (2D) laparoscopic adrenalectomy: A case-control study. Int J Surg. 2016 Apr;28( Suppl 1):S114–7. doi: 10.1016/j.ijsu.2015.12.055. Epub 2015 Dec 18. [DOI] [PubMed] [Google Scholar]
- 30.Agrusa A, Romano G, Salamone G, Orlando E, Di Buono G, Chianetta D, Sorce V, Gulotta L, Galia M, Gulotta G. Large cavernous hemangioma of the adrenal gland: Laparoscopic treatment. Report of a case. Int J Surg Case Rep. 2015;16:150–3. doi: 10.1016/j.ijscr.2015.09.040. Epub 2015 Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano G, Agrusa A, Chianetta D, Frazzetta G, Sorce V, Di Buono G, Gulotta G. Laparoscopic management of adrenal tumors: A four-year experience in a single center. Minerva Chirurgica. 2014;69(2) Suppl 1:125–129. [Google Scholar]
- 32.Agrusa A, Romano G, Chianetta D, De Vita G, Frazzetta G, Di Buono G, Sorce V, Gulotta G. Right diaphragmatic injury and lacerated liver during a penetrating abdominal trauma: case report and brief literature review. World J Emerg Surg. 2014 Apr 28;9:33. doi: 10.1186/1749-7922-9-33. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cocchiara G, Cajozzo M, Amato G, Mularo A, Agrusa A, Romano G. Terminal ligature of inferior thyroid artery branches during total thyroidectomy for multinodular goiter is associated with higher postoperative calcium and PTH levels. J Visc Surg. 2010 Oct;147(5):e329–32. doi: 10.1016/j.jviscsurg.2010.08.020. Epub 2010 Oct 16. [DOI] [PubMed] [Google Scholar]
- 34.Cucinella G, Perino A, Romano G, Di Buono G, Calagna G, Sorce V, Gulotta L, Triolo M, Billone V, Gulotta G, Agrusa A. Endometrial cancer: Robotic versus Laparoscopic treatment. Preliminary report Giornale Italiano di Ostetricia e Ginecologia. 2015;37(6):283–287. [Google Scholar]
- 35.Cucinella G, Calagna G, Romano G, Di Buono G, Gugliotta G, Saitta S, Adile G, Manzone M, Accardi G, Perino A, Agrusa A.Robotic versus laparoscopic sacrocolpopexy for apical prolapse: a case-control study G Chir 2016. May–June373113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]