Abstract
Body composition assessment provides a sharper picture of the human biological response to genetic and environmental influences than measures of body size and weight. Infant body composition is particularly important as a marker of fetal adaptation and developmental programming of subsequent health and disease, but until recently, the range of options for measuring infant body composition was relatively narrow. The purpose of this Toolkit: Methods in Human Biology review is to provide a comprehensive overview of methods of body composition methods currently used in infants 0 to 2 years of age, including anthropometric prediction equations, air displacement plethysmography (ADP), dual energy X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), isotope dilution, and magnetic resonance imaging (MRI). Information on the reliability, validity, and accuracy of the methods is provided. Unique aspects of infant physiology and behavior create challenges for body composition assessment, but this review provides guidance on suitable testing approaches and environments that may aid researchers in this important area of investigation.
Introduction
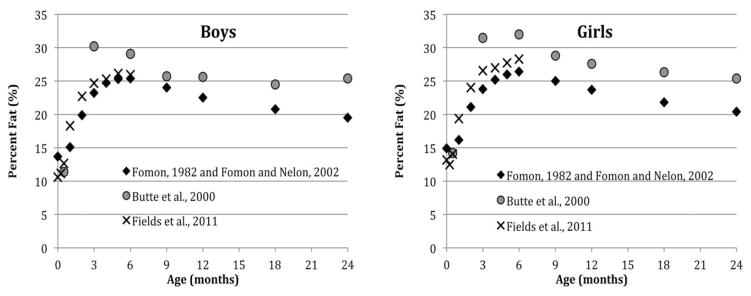
The allocation of energy to the growth of different tissues follows a complex, but fairly predictable, species-specific course (Pond and Mattacks, 1987), reflecting the changing adaptive value of absolute and relative increases in adipose tissue, muscle mass and other features over the course of development (Pond and Mattacks, 1987). In human infants, for example, adipose tissue growth is negligible until 24 to 25 weeks gestation (Ziegler et al., 1976). Fat mass increases rapidly during the third trimester of pregnancy, peaking near 6 postnatal months, after which the rate of fat mass (FM) growth slows relative to fat-free mass (FFM) growth, resulting in a decline in percent body fat (Fig. 1), as shown by existing infant body composition reference data (Butte et al., 2000; Fields et al., 2011; Fomon et al., 1982; Fomon and Nelson, 2002). Within these general patterns, however, there is considerable inter-individual variation in the timing and tempo of growth in different tissues during infancy, which relates to feeding mode and nutrient composition (de Zegher et al., 2012; Escribano et al., 2012), inflammation (Mak and Cheung, 2007; Martinez et al., 2012), the insulin/IGF-1 hormone axis (Madsen et al., 2011), genetic factors (Choh et al., 2011; Demerath et al., 2007), and other influences.
Fig. 1.
Percent body fat during infancy in boys and girls: selected reference data.
Human biologists have a strong interest in individual and population variation in infant body composition because it reflects human developmental plasticity in response to environmental cues (Wells, 2012a; Yajnik et al., 2003). Evolutionary life history theory (Hill, 1993) predicts that under conditions of energy deficit, adipose tissue stores are utilized first, buffering linear growth, lean tissue accrual, and continuing brain development. Although lean tissues including bone and muscle are critical for later reproductive success and work capacity, they are costly to synthesize. When energy constraints are lifted, increased metabolic efficiency strongly favors the preferential replenishment of body fat stores, regardless of diet composition (Dulloo and Girardier, 1992). This response is adaptive when energy supply continues to be limited, but may incur reproductive costs in the longer term (lower FFM), as well as increased health risks (higher percent body fat) when energy is continuously abundant. Such adaptive tradeoffs (the “allocation game”) (Wells, 2012b) between short-term survival and long-term capacity may have particular significance in infancy because it is a period of nutritional vulnerability, with historically high mortality and selective pressure. Another important area of human biological research on infant body composition frames it as an early and potentially modifiable risk factor for subsequent metabolic disease and other outcomes (e.g., (Forsen et al., 2000; Ibanez et al., 2006)), and an aspect of the developmental origins of health and disease. For instance, low birth weight may pose health risks both because it is an indicator of low muscle mass which tracks into later ages, reducing metabolic capacity, and also because it predicts catch-up growth associated with elevated adipose tissue mass and increased metabolic load (Wells, 2012a). Different interventions may be appropriate where risk for a particular disease stems primarily from poor lean body mass accrual, or in excess adipose tissue, or both.
There have been a number of excellent review articles and book chapters focusing on the assessment of infant body composition published since 2000 (Ellis, 2007; Koo, 2000; Rigo, 2006; Sopher et al., 2005; Wells and Fewtrell, 2006). The reader is also referred to Heymsfield et al. (2005), a comprehensive and accessible reference manual covering the theory and practice of all major body composition methods in children and adults. The primary goal of the present review is to provide an updated overview of methods specifically appropriate for infants less than two years of age, with emphasis on studies of existing methods adapted for infants (e.g., air displacement plethysmography (ADP), dual energy X-ray absorptiometry (DXA), bio-electric impedance (BIA), isotope dilution and total body water (TBW), and magnetic resonance imaging (MRI), in the context of the unique physiological and behavioral features of infancy.
Gold standards in infant body composition assessment
The only direct method for assessing composition of the body is through direct chemical carcass analysis. Valuable reference data in a small number of human subjects using chemical analysis are available for the fetus (stillborn infants) and neonate (Ziegler et al., 1976). However, such direct estimates are nearly nonexistent for infants beyond the neonatal period and by nature are not representative of the spectrum of normal infant body composition variation (Koo, 2000). While entailing a number of assumptions itself, a four-component densitometry-based body composition model is generally considered the most appropriate reference method against which to validate simpler methods in pediatric populations (Sopher et al., 2005), and is considered the “gold standard” in body composition assessment (Baumgartner et al., 1991).
The four-component molecular model approach is based on division of the body mass into FM, and within the FFM: TBW, bone mineral, and protein; a residual component of soft tissue minerals and glycogen (carbohydrate) is calculated from constants (Butte et al., 2000; Fomon et al., 1982). Body volume, body mass, and the assumed constant densities of each of these components can be used to calculate the individual mass of each component. The total body mass is measured using a scale, body water is measured using isotope dilution (e.g., 2H2O, 3H2O), bone mineral mass is measured using DXA, and the total body volume is measured using air displacement plethysmography (ADP, in the case of infants) or underwater weighing in older children able to comply with the breath hold. Assumed constant densities for fat (0.9007 g/cm3), water (0.99371 g/cm3 at 36°C), bone mineral (2.982 g/ cm3), and a known relationship of potassium to nitrogen for protein are then used to calculate the FFM and FM components (Wang et al., 2005). The 4C model by Lohman (1989) is commonly used as follows:
where Db is body density (g/cm3); W is water content of the body in liters expressed as a fraction of body mass, and B is bone mineral content in kilograms expressed as a fraction of body mass. As the number of measurements increases, the measurement errors also increase, but the reduction in error by using more complex models has been shown to be greater than that from the additive effect of the individual measurement errors (Wang et al., 2005). Another important issue for the infant population is subject compliance. The four-component model involves numerous tests and a long protocol (e.g., ~4 h) which can be highly demanding for parent and infant, with greater likelihood of failure than for a single test.
The reason why a multicomponent model is desirable for the creation of reference data and for validation of new techniques in infancy is that the components of FFM are changing greatly during the “chemical maturation” of the body, a concept originally introduced by Moulton (1923). Specifically, FFM density increases from early infancy (approximately 1.06–1.07 g/cm3) to adulthood (1.1 g/cm3), due to a decrease in TBW (including a reciprocal reduction in the proportion of extracellular water and an increase in intracellular water as mean cell size increases), and increases in the proportion of bone mineral. Sex and age-specific reference values for TBW and its composition during infancy (and later childhood) are from (Butte et al., 2000; Fomon et al., 1982), and with some adjustments by (Schoeller, 2005), and compiled in (Heymsfield et al., 2005). Table 1 presents sex and age-specific FFM components for infancy adapted from (Heymsfield et al., 2005). The keystone work in using a multi-compartment model in the evaluation of body composition in infants was performed by Butte et al. (2000). This elegant work established gender normative body composition data for the first 2 years of life in 76 infants. Body water was determined by deuterium dilution, total body potassium by whole body counting and bone by DXA which were used in a multicompartment approach to determine body composition starting at 0.5, 3, 6, 9, 12, and 24 months of age. The sheer number of subjects and tests in this study, considering the lower compliance among infants, is remarkable and it remains one of the few multicompartment infant body composition datasets available. Although preferred, the four-compartment model is impractical in subjects less than 5 years old unless the study is conducted in a body composition research facility, and regardless, drop-out rates can be expected to be high.
TABLE 1.
Fat-free mass composition of the reference boy and girl, 0 – 24 months
| Reference Boy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Age (mon | Composition (% of FFM) | TBK | FFM Density | ||||||
|
| |||||||||
| Protein | TBW | Extracellular Water | Intracellular Water | Bone mineral | Soft tissue mineral | Carbohydrate | |||
| 0 | 15.0 | 80.6 | 49.3 | 31.3 | 3.0 | 0.7 | 0.6 | 49.0 | 1.063 |
| 1 | 15.1 | 80.5 | 48.4 | 32.1 | 3.0 | 0.7 | 0.6 | 50.1 | 1.064 |
| 2 | 15.4 | 80.3 | 47.4 | 32.9 | 3.0 | 0.7 | 0.6 | 51.2 | 1.065 |
| 3 | 15.6 | 80.0 | 46.4 | 33.6 | 3.0 | 0.7 | 0.6 | 52.2 | 1.065 |
| 4 | 15.8 | 79.9 | 45.8 | 34.1 | 3.0 | 0.7 | 0.6 | 53.5 | 1.066 |
| 5 | 15.9 | 79.7 | 45.2 | 34.5 | 3.0 | 0.7 | 0.6 | 53.6 | 1.066 |
| 6 | 16.0 | 79.6 | 44.7 | 34.9 | 3.0 | 0.7 | 0.6 | 54.1 | 1.066 |
| 9 | 16.4 | 79.3 | 43.5 | 35.8 | 3.0 | 0.7 | 0.6 | 55.5 | 1.068 |
| 12 | 16.6 | 79.0 | 42.5 | 36.5 | 3.0 | 0.7 | 0.6 | 56.5 | 1.068 |
| 18 | 17.1 | 78.5 | 40.8 | 37.7 | 3.1 | 0.7 | 0.6 | 58.2 | 1.070 |
| 24 | 17.4 | 78.1 | 39.6 | 38.5 | 3.2 | 0.7 | 0.6 | 59.3 | 1.072 |
| Reference Girl | |||||||||
|
| |||||||||
| 0 | 15.0 | 80.6 | 49.3 | 31.3 | 3.0 | 0.7 | 0.6 | 49.0 | 1.064 |
| 1 | 15.2 | 80.5 | 48.3 | 32.1 | 3.0 | 0.7 | 0.6 | 50.2 | 1.064 |
| 2 | 15.5 | 80.2 | 47.1 | 33.1 | 3.0 | 0.7 | 0.6 | 51.5 | 1.065 |
| 3 | 15.8 | 79.9 | 46.0 | 33.9 | 3.0 | 0.7 | 0.6 | 52.7 | 1.066 |
| 4 | 15.9 | 79.7 | 45.2 | 34.5 | 3.0 | 0.7 | 0.6 | 53.5 | 1.066 |
| 5 | 16.1 | 79.5 | 44.6 | 34.9 | 3.0 | 0.7 | 0.6 | 54.2 | 1.067 |
| 6 | 16.3 | 79.4 | 44.0 | 35.4 | 3.0 | 0.7 | 0.6 | 54.8 | 1.067 |
| 9 | 16.6 | 79.0 | 42.7 | 36.4 | 3.0 | 0.7 | 0.6 | 56.3 | 1.068 |
| 12 | 16.9 | 78.8 | 41.6 | 37.1 | 3.0 | 0.7 | 0.6 | 57.4 | 1.069 |
| 18 | 17.2 | 78.4 | 40.3 | 38.1 | 3.0 | 0.7 | 0.6 | 58.8 | 1.070 |
| 24 | 17.4 | 78.2 | 39.5 | 38.7 | 3.0 | 0.7 | 0.6 | 59.6 | 1.071 |
Adapted with permission from Appendix Tables A2 and A3 in Wang et al., Human Body Composition, 2nd edition, Human Kinetics Press, 2005; data from Fomon et al., 1982, and Fomon and Nelson, 2002.
Considerations in selecting a body composition assessment method
The first consideration in selecting an assessment method is to determine what components of body composition are relevant to the planned investigation. For example, if one is primarily concerned with measurement of total adiposity or total fat- free tissue, then a two-component approach which determines the fat-free mass (FFM) and calculates fat mass (FM) may be sufficient. Here, the options would include isotope dilution to assess FFM (from TBW), or densitometry, using body volume and assumed constant densities of the FM and the FFM. However, one must be confident that the prediction equations linking TBW or body volume to FM and FFM accurately account for the changes in the density and hydration of FFM during infancy mentioned above. Anthropometry has been extensively used in infants and is more appropriate for field studies where measurement of body volume and/or TBW is not possible. If the components of FFM (particularly water, protein, and osseous mineral) are likely to be far from the assumptions used in these simple models, and/or if the prediction equations were not developed in a similar population to that under investigation, then ideally a subset of subjects would undergo a combination of measures including TBW and possibly bone mineral content using DXA, in addition to densitometry (a three- or four-component model) from which a population-specific prediction equation can be developed for subsequent use, using the optimal combination of measurements providing maximum validity and feasibility for the particular population under investigation. If one aims to distinguish determinants or health outcomes associated with anatomic-level body composition (organ growth, adipose tissue distribution) then an imaging method will be required. In the case of infant research studies, DXA is increasingly used to assess regional adipose tissue and lean mass, while for anatomic-level analysis, magnetic resonance imaging (MRI) is most often used, as computed tomography (CT) involves significant subject exposure to ionizing radiation. When choosing a method, a number of additional considerations must be made, including feasibility and cost, as well as validation in an appropriate population, availability of reference data, precision, and reliability. Repeated measurements can only be planned for increments in which the expected rate of change in body composition components exceeds the measurement error of the method. The following sections will review the strengths and weaknesses of currently-used research methods for infant body composition assessment: anthropometry, isotope dilution, BIA, ADP, DXA, TBW, and MRI.
Anthropometry
Anthropometry provides an inexpensive, portable, and versatile proxy for infant body composition assessment, and a variety of combinations of weight, length, circumferences, and skinfolds can discriminate between fat and lean tissue and subcutaneous fat distribution variation in infants at the group level (e.g., Yajnik et al., 2003). In terms of equipment and standard operating principles, body weight should be measured if possible on a calibrated electronic baby scale to the nearest gram. Recumbent length and crown-rump length are measured to the nearest millimeter on a length board, with careful positioning using two staff members, one keeping the infant’s head against the fixed headboard (often the mother), and the other to help extend the legs fully, while keeping the heels firmly against the moveable footboard. Body circumferences can be measured with a standard nonstretchable plastic measuring tape to the nearest millimeter. Circumferences that are frequently measured include head (fronto-occipital), chest (at the level of the nipples), abdomen (recumbent, at the level of the largest cross-sectional area), mid-upper arm, mid-thigh, and mid-calf. Skinfolds should be collected in duplicate as long as the measures are within 1 mm of each other. In the event they are not, a third measure is taken with the two closest measures averaged. Typically, Lange or Harpenden calipers are used, with the most common sites including biceps, triceps, subscapular, suprailiac, and quadriceps femoris thicknesses. The caliper should be applied and the measurement read after three seconds to standardize the degree of tissue compression. Intraobserver CVs for skinfolds in infants may be <3% (e.g., (de Bruin et al., 1995), but interobserver error is usually much higher (CV% =8.9% in our hands, 10% in (de Bruin et al., 1995); this aspect of measurement error is one of the major criticisms of skinfold measurements for body fat assessment in infancy, while body weight, length, and circumferences tend to have much higher reliability (Sopher et al., 2005). It is for this reason that the same person should obtain all skinfold measures for a given study if possible, although that is not possible in large and multi-center studies. Bony landmarks used to standardize the placement of calipers and measuring tapes are also more difficult to identify in the very young infant.
Anthropometric indices (BMI, ponderal index, and weight-for length z scores) can be used directly to compare relative adiposity within a study sample of similarly aged children; for example, skinfolds are widely used to capture peripheral (limb) as opposed to central (trunk) subcutaneous fat distribution (e.g., (Mueller and Reid, 1979) and are used as a ratio to compare individuals. Indeed, raw skin-fold data generally correlate well with other adiposity measures and are therefore useful for ranking individuals within a sample in terms of relative adiposity. In order to obtain a measure of whole-body FFM and FM, a variety of prediction equations have been developed in infants to relate anthropometrics to measured fat mass (Table 2). Dauncey et al. (1977) developed an equation based upon the theoretical assumption that subcutaneous fat is a uniform layer enveloping a series of cylinders (trunk, limbs) each having measurable length and circumference. Total body fat using this equation therefore represents subcutaneous fat only, yielding an overestimate of FFM because nonsubcutaneous fat is included within the FFM. The Dauncey method uses numerous anthropometric measures requiring rigorous measurer training and reliability testing which may be why it has not been used extensively. Weststrate and Deurenberg (1989) likewise used known anatomical and physiological properties to create a prediction equation using only age and skinfolds to estimate body density. These early studies did not use a reference method, and their validity and precision are unknown. Subsequent work has used a variety of reference methods and larger samples of infants to develop and validate simple anthropometric equations, typically including weight, length, sex, and a limited number of circumference and/or skinfolds. Validity is rather high, with R2 near 0.90 for many of these equations against FM and FFM measured using appropriate reference methods.
TABLE 2.
Selected anthropometric prediction equations for body fat mass (FM) and fat free mass (FFM) in infants
| Reference | Year | Body composition component |
N subjects | Term/preterm | Subject age range | Design | Reference method |
Equations | R2 | Cross validation R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dauncey et al. | 1977 | FM | N =27 | Term | 0–40 wks | Longitudinal | NA | FM =0.90 × [[(crownrump length-diameter of head) × (trunk circumference) × (subscpular skinfold)] +[(arm length × upper arm circumference) x triceps thickness] +[(supine -crown-rump length) × 1/2 (midthigh +calf circ) × (triceps skinfold)]] | NA | NA |
| Weststrate and Deurenberg | 1989 | Density | NA | NA | Designed for infants 0–1.99 yrs |
Theoretical | NA | Density =[1.1235 +(0.0016[age(mo)]0.5)] −0.0719 × log (biceps+ triceps +subscapular+ suprailiac) | NA | NA |
| Catalano et al. | 1995 | FM |
N =194 (development). N =65 (validation) |
Term | 0–72 h | Cross- sectional | TOBEC | FM =(0.39055 × birth weight) +(0.0453 × suprailiac skinfold) −(0.03237 × length) +0.54657 (various) | 0.78 | 0.84 |
| de Bruin et al. | 1995 | FM and FFM |
N =435 (development). N =110 (validation) |
NA | 1–11 mo | Cross- sectional | TOBEC | llnFM =−0.358 +1.499 [ln(weight × calf circumference)/length]. lnFM =−2.219 +1.176 [ln (weight × calf circumference × sq. root (sum of 3 skinfolds/length)]. lnFFM =0.433 +0.056 × [sq. root (weight × length)] |
0.87 0.89 0.95 |
0.81 0.86 0.92 |
| Koo et al. | 2004 | FM and FFM |
N =74 AGA. N =30 LGA N =16 SGA |
Term, Preterm | 0–1 wk | Cross- sectional | DXA | FM (SGA) =weight +length + sex +race + GA. FM (SGA) =weight +length +sex +race + GA +skinfold PC score. FM (AGA) =weight +length +sex +race + GA. FM (AGA) =weight +length +sex +race + GA +skinfold PC score. FM (LGA) =weight +length +sex +race + GA. FM (LGA) =weight +length +sex +race + GA +skinfold PC score. |
0.84 0.89 0.84 0.89 0.86 0.91 |
NR |
| Dung et al. | 2007 | DXA | N =118 | Preterm | Hospital discharge | Cross- sectional | DXA | FFM =0.05 Ht2/Iength +0.69 × weight +0.31 | 0.94 | 0.94 |
| Lingwood et al. | 2012 | FFM |
N =77 (birth). N =54 (2 weeks). N =55 (3 months). N =53 (4–5 months) |
Term | Birth, 6 wks, 3 mo, 4–5 mo | Longitudinal | ADP | FFM (birth) =0.507 +0.646 × weight −0.089 × sex +0.009 × length. FFM (6 weeks) =0.260 +0.528 × weight −0.125 × sex +0.022 × length. FFM (3 months) =−0.338 +0.434 × weight −0.177 × sex +0.041 × length. FFM (4–5 mo) =−0.044 +0.397 × weight −0.427 × sex +0.045 × length |
0.94 0.89 0.89 0.87 |
NA |
Particularly at or near birth, body weight and length alone or together explain a very large proportion of the variance in FM and/or FFM; addition of more error-prone and technically difficult anthropometrics or BIA increase the R2 relatively little. It is frequently reported that the use of skinfold thickness in infancy is controversial (Sopher et al., 2005); poor or no correlation was observed between body fat measured by skinfolds and isotope dilution (Kabir and Forsum, 1993) or MRI (Deans et al., 1989), and skinfolds improved the prediction (R2) of FM by only 2 to 5% over weight and length (de Bruin et al., 1995; Hammami et al., 2003; Koo et al., 2004c). Weight and length may provide adequate prediction of FM at the sample level and are thus quite useful for between-group comparisons, but they nonetheless still perform rather poorly at the individual level. For example, Lingwood et al. (2012) (Hammami et al., 2003) reported that for models using weight, length, and sex, individual limits of agreement with ADP-measured FFM were 6 to 10% at birth and 8 to 13% at later ages, and as high as 10% for ADP-measured percent body fat. When skinfolds were used instead of weight and length, percent fat was underestimated by 2 to 9%, with greater underestimation in fatter infants.
When considering use of anthropometric or other prediction equations for use in a given study, it is important that the age and ethnic composition of the source population for the equations is relevant for the proposed study. Most anthropometric body composition prediction models have been conducted in European ancestry/Caucasian infants, but there are known ethnic differences in neonatal body composition, with higher total lean mass in Caucasian than African American infants (Lampl et al., 2012). Equations using data derived only from European ancestry subjects may therefore provide biased estimates for infants from other race/ethnic groups. Additionally, many of the anthropometric prediction equations for TBW and body composition in infancy were developed before the recent increases in the prevalence of maternal obesity, gestational diabetes, and child obesity. It has been shown that with the increase in adiposity, the validity of FFM hydration assumptions of earlier equations may no longer be accurate (Chumlea et al., 2007) with existing prediction equations yielding particularly high overestimates of TBW in infancy (Wells et al., 2005). Updated prediction equations for TBW have been developed in a large cohort of infants and children (Wells et al., 2005).
Another issue is that, with some exceptions, most anthropometric prediction equations were developed in cross-sectional sectional studies at or near birth, or included a wide age range of infants in the models. There is intense interest in the field of the developmental origins of obesity in changes in relative weight and adiposity during infancy, and yet surprisingly little work has been conducted to compare concurrent changes in clinically available nutritional status indicators (e.g., weight-for-length z score (WLZ), ponderal index (PI)) and total body fat or lean mass in infants. Recent unpublished data from our laboratory comparing a number of selected anthropometric indices for their prediction of FM are provided in Table 3. The subjects included 97 term AGA infants seen at both 2 weeks and at 3 months of age for anthropometry and body composition assessment using ADP. The regression models presented were not optimized by determination of a “best subset” of all possible measurements, but rather were constructed only to compare the prediction values for a variety of easily obtained indices and measures. We observed first that these simple models (including only one anthropometric measure, sex, and exact age at measurement in each case) performed fairly poorly overall, regardless of which anthropometric variable was used (maximum partial R2 < 0.70). Second, the predictions improved with age (R2 increased, and CV% decreased at 3 months as compared to 2 weeks for the same metric). The inference is that the same prediction models are unlikely to be perform equally well at different ages even within the relatively short period of infancy, casting some doubt on the validity of longitudinal analyses of infant body composition using anthropometric proxies. PI and WLZ score are commonly thought to be superior to the simpler BMI index for the correction of weight for length and therefore better markers of relative adiposity in infancy. For instance, the WHO and CDC growth charts include BMI-for-age only for children greater than 2 years of age. However, BMIZ score was as good or better a predictor of FM than WLZ and especially PI at both 2 weeks and 3 months. PI is possibly a better measure of “thinness” and low lean mass (Yajnik, 2000) than it is an indicator of adiposity. Development of simple anthropometric prediction equations for ethnically diverse populations of infants beyond the neonatal period, and relevant to the prevailing body composition norms are areas for further investigation.
TABLE 3.
Comparison of selected anthropometric variables for association with fat mass (FM) estimated by air displacement plethysmography in term infants followed from 2 weeks to 3 months of age
| Anthropometric variable | 2 Weeks (mean FM =0.56 kg) | 3 Months (mean FM =1.54 kg) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Mean (range) | Partial R2/ total R2 | RMSE (FM kg) | CV% | Mean (range) | Partial R2/ Total R2 | RMSE (FM kg) | CV% | |
| BMIZa | −0.02 (−2.2 to 1.8) | 0.39/0.46 | 0.13 | 24.0 | −0.34 (−4.2 to 2.6) | 0.66/0.66 | 0.26 | 16.8 |
| WAZa | 0.12 (−1.9 to 1.9) | 0.33/0.38 | 0.15 | 27.4 | −0.05 (−2.2 to 2.4) | 0.66/0.68 | 0.25 | 16.4 |
| WLZa | −0.46 (−3.4 to 2.1) | 0.25/0.29 | 0.14 | 25.6 | −0.36 (−4.8 to 2.4) | 0.53/0.55 | 0.30 | 19.5 |
| Ponderal Index (kg/m3) | 26.1 (20.8 to 32.2) | 0.31/0.34 | 0.15 | 26.3 | 26.4 (18.2 to 33.2) | 0.43/0.46 | 0.33 | 21.3 |
| Triceps +subscapular skinfolds (mm) | 11.6 (8.0 to 17.5) | 0.39/0.43 | 0.14 | 24.6 | 15.7 (9.5 to 25.0) | 0.50/0.56 | 0.30 | 19.4 |
BMI z score (BMIZ), weight-for-age z score (WAZ), and Weight-for-Length z score (WFLZ) were calculated against the WHO 2006 growth standards. All anthropometric variables were positively associated with fat mass at P < 0.01 or lower. Each linear regression model included fat mass from ADP as the dependent variable (using Fomon et al., 1982 to estimate FFM from total body mass and density), and the anthropometric variable, infant sex, and exact age at measurement as the independent variables. There were no significant sex, anthropometric variable interactions at P < 0.05. Infants (N =97, 49% male) were born at ≥37.0 weeks gestation, had birth weight appropriate for gestational age (AGA), and were all seen within 1 week of the 2 week visit and within 2 weeks of the 3 month visit. (Demerath, unpublished data).
Isotope dilution
Total body water (TBW) can be measured using the dilution principle: that is, that the total body water volume is equal to the amount of tracer delivered divided by the concentration of the tracer in the body water after a period of equilibration (Schoeller, 2005). Although time-consuming, the procedure is rather straightforward in theory, and can be used in the field setting, with properly stored samples remaining quite stable until they can be sent to a central laboratory for analysis. The dose is typically a stable (nonradioactive), isotopically-labeled water such as deuterium oxide or oxygen-18 hydride, and is provided as a drink of water. Dose spillage is common in infants, although in a recent study two thirds of infants consumed 90% of their dose (Nielsen et al., 2011). Dose spillage can be minimized by using a syringe, but the key determinant of accuracy is accurately quantifying the dose consumed. Dilution can be measured in either saliva or urine. Urine collection by placing cotton balls in the infant’s diapers is particularly suitable for young infants. Typically, a baseline sample of either saliva or urine is collected before the dose is given and another is collected once the tracer concentration has equilibrated throughout the body water compartment to deduce dilution (typically by 2–4 hours postdose (Salazar et al., 1994; Traver et al., 2009)). Because body water is always in a state of turnover, and thus the tracer is indeed undergoing some metabolism during equilibrium, two approaches are used: either the “plateau method” which requires no food or drink be given during the equilibration period and assumes a constant plateau in tracer concentration is achieved, or the “back-extrapolation” method, which entails normal meal patterns, but requires a larger number of samples be collected over a period of days, from which a log-linear regression model is used to back-extrapolate the intercept concentration (Coward, 1988; Schoeller et al., 1985). This latter method has been found to be more accurate in infants (Davies and Wells, 1994). Depending on the half-life of the isotope, clearance may not be complete in the infant for 1 to 3 weeks’ time after the dose (Ellis, 2007).
The assumptions of TBW assessment using isotope dilution are that the tracer is distributed only in body water, the tracer is equally distributed in all water compartments, the rate of equilibration of the tracer is rapid, and neither the tracer nor body water undergoes metabolism during the equilibration period. Isotope dilution methods in general have excellent precision (1–2% variation between repeated measurements, if isotope-ratio mass spectrometry is used to detect the isotopes (Schoeller et al., 1985), and accuracy is also very good, with the uncertainty regarding the amount of non-aqueous exchange of the isotopes, leading to at most a 3 to 4% overestimation of TBW using deuterium oxide in comparison to gold standard dessication values for TBW in animals (e.g., Schoeller, 2005). Age-associated variability in isotopic proton exchange during infancy is provided by Wells et al. (1998). Even smaller error is associated with the remaining assumptions, provided standardized methods are followed (e.g., Butte et al., 2000). The assumptions involved in the use of TBW alone for assessment of FM and FFM are much less easily met; in particular, the assumption of constant hydration of the FFM does not hold in infancy. As mentioned above, hydration of the FFM is falling rapidly with age during this period before reaching a fairly stable 73% in adulthood. Reference FFM hydration values by month of age can be used to adjust TBW estimates of FFM. Recent evidence suggests that at least in neonates, the inter-individual variability of FFM hydration is relatively low (0.8% of mean hydration) (Eriksson et al., 2011); nonetheless, it is recommended that body volume (for instance, using ADP) is used alongside isotope dilution for infants, at least in a subset of the study population from which population-specific prediction equations linking FFM to TBW can be created. In summary, due to the time-consuming nature of the equilibration period, potential for spillage of the dose, difficulty in dosing and collecting serial samples from infants (fresh cotton balls for each data point), and sensitivity to FFM hydration for body composition estimation, isotope dilution methods can be challenging for this age group. However, it is a relatively inexpensive and portable method that can be used in the field setting, and directly estimates the most variable component of infant FFM. Another notable strength of the method is that unlike most techniques, isotope dilution can be used from birth to old age.
Bioelectrical impedance analysis (BIA)
Bioelectrical impedance analysis (BIA) measures opposition of body tissues to the flow of an alternating electric current in physiological conditions. This opposition (or impedance) is captured by measuring resistance (R) and reactance (Xc) of the electrical current and relates to the differing electrolyte and water content of tissues and the composition of cell membranes (Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement, 1996; Foster and Lukaski, 1996; Ellis et al., 1999). A tetrapolar method is the most common impedance method; for instance in the Impedimed SFB7 system (Impedimed, Brisbane, QLD, Australia), the current and detection electrodes are placed on the infant’s wrist and ankle (Lingwood et al., 2000); R and Xc values are detected by the detection electrodes, with the distance between current and detection electrodes being the length of the conductor (Chumlea and Sun, 2005).
BIA estimates body composition indirectly based on prediction equations relating bioelectrical parameters to total body water and lean mass as determined by reference methods, such as isotope dilution (Chumlea and Sun, 2005). Infant prediction equations include Fjeld et al. (1990) and Kushner et al. (1992). Accuracy of the prediction equations depends on numerous assumptions, including constant tissue hydration and homogenous conduction of the electrical current through a cylindrical body. The former assumption is poorly upheld in infancy; as mentioned above, body water compartments and the FFM hydration are undergoing rapid changes during infancy here that affect fluid distribution and electrolyte concentrations between the intra- and extracellular compartments, making estimates of body composition obtained from BIA imprecise for the first few years of life (Margutti et al., 2010; Savino et al., 2003). For these and other reasons, BIA body composition equations tend to have poor performance across different ethnic groups and populations (Dehghan and Merchant, 2008), including in infants (Tanabe et al., 2012). For instance, FM and percent FM from total body electrical conductivity (TOBEC), a whole-body variant of BIA no longer commercially available (Hashimoto et al., 2002), and from BIA using existing equations (Sen et al., 2010) had poor correlation with estimates from isotope dilution. In both preterm (Dung et al., 2007) and term (Hashimoto et al., 2002; Lingwood et al., 2012) infants, BIA improves the prediction of FFM and percent body fat only very slightly, if at all, beyond simple anthropometrics (weight and length). Lingwood has published a review focused on the strengths and weaknesses of BIA for TBW and body composition assessment in infants (Lingwood, 2013). In summary, BIA is a noninvasive, inexpensive, safe, and portable (Houtkooper et al., 1996) method of body composition assessment, but its application to the infant population is problematic, resulting in generally poor accuracy at the individual level.
Alternatively, if the target of investigation is infant body fluid level or nutritional status (e.g., in tracking infants with protein-energy malnutrition), and a qualitative or semi-quantitative determination is sufficient, methods such as bioelectrical impedance vector analysis (BIVA) (as in (Tanabe et al., 2012) are useful. BIVA uses R and Xc values (both adjusted for length) directly from the instrument (Piccoli et al., 1994), without reliance on any assumptions about their relationship to FFM. BIVA reference data are available for both neonates and older infants (Barbosa-Silva et al., 2005; L’Abee et al., 2010; Margutti et al., 2010; Savino et al., 2003). Multifrequency bioelectrical impedance spectroscopy (BIS), in which over 250 different frequencies are used, has also been examined in infants to distinguish between intracellular and extracellular water; interobserver error in extracellular and intracellular resistance measures (Re and Ri, respectively) is very good to excellent (ICC =0.97 for Re and 0.72 for Ri), as is intra-observer error (ICC for Re =0.97–0.99; ICC for Ri =0.61–0.85) in infants 0 to 6 months (Sesmero et al., 2005). BIS had acceptable validity against reference methods (DXA and TBK) in children 4 to 18 years (Ellis et al., 1999) but to our knowledge, validation studies have not been conducted in infants. The finding that greater inter-test movement variation was associated with greater measurement error (particularly for Ri) indicates caution in use of BIS for clinical tracking of intracellular fluid changes in infants (Sesmero et al., 2005).
Air displacement plethysmography (ADP)
The underlying principles of ADP originated in Germany and have been reported for almost a century (Murlin and Hoobler, 1913; Pfaundler, 1916). ADP, as operationalized by Cosmed, Inc. (the only human ADP equipment currently commercially available), measures body volume by detecting air pressure differences between a test chamber, in which the human subject is placed, to a reference chamber with controlled air pressure. Body density is then computed from body mass and volume, and uses the known density of fat (0.9007 g/ml) and age- and sex-specific FFM density coefficients (e.g., from Fomon et al., 1982; Fomon and Nelson, 2002) to determine total body FM, FFM, and percent body fat. The estimated average changes in fat-free density during the first few days of life due to rapid weight and water loss are also accounted for. Two review papers (Fields et al., 2002, 2005) and seminal works explaining its operating principles (Dempster and Aitkens, 1995) and its validity in humans (McCrory et al., 1995) indicate it can accurately measure body composition in children, adolescents and adults. In 2003 the first papers began to emerge showing the validity of ADP (i.e. Pea Pod; Cosmed) in infants as young as one week old (Urlando et al., 2003; Yao et al., 2003) and shortly thereafter validating it against known bovine phantoms (Sainz and Urlando, 2003) and 2H2O (Ma et al., 2004). These and the reports discussed below are summarized in Table 4.
TABLE 4.
Accuracy, reliability, and validity of air displacement plethysmography (ADP) and dual-energy X-ray absorptiometry (DXA) for measurement of infant fat mass
| Method | Subjects | Accuracy or reliability (CV% or ICC) | Validity (R2) | Comparison method for validation |
|---|---|---|---|---|
| ADP | ||||
| Urlando et al. (2003) | 5 Known-volume phantoms | <1% | 1.0 | – |
| Sainz et al. (2003) | 24 Known-composition phantoms | 2.58–18.1% | 0.99 | – |
| Yao et al. (2003) | 17 Infants (1 wk–4 m) | 2.95% | NR | – |
| Ma et al. (2004) | 80 Infants (0.4–22 wk) | 5.1% | 0.76 | 2H2O |
| DXA | ||||
| Hammami et al. (2002) | 4 Known-Composition Phantoms | CV% =5.9% | NR | – |
| Koo et al. (2004b) | 13 Piglets | ICC =0.98 | 0.99 | Direct Carcass Analysis |
| Rigo et al. (1998) | 21 Piglets | NR | 0.98 | Direct Carcass Analysis |
| Picaud et al. (1996) | 13 Piglets | CV% =5.35% | 0.94 | Direct Carcass Analysis |
| Godang et al. (2010) | 50 Infants (~3 d) | ICC =0.94 | NR | – |
| Venkataraman et al. (1992) | 12 Infants (1.5 d) | CV% =<1% | NR | – |
| Koo et al. (2004c) | 35 Infants (10 hr–1 yr) | ICC =0.99 | NR | – |
Air-displacement plethysmography (ADP), dual energy X-ray absorptiometry (DXA); NR, not reported; CV%, Coefficient of variation; ICC, intra-class correlation coefficient.
Urlando et al., 2003: Five volume phantoms (2, 4, 6, 8, and 10 kg) of known certified instruments traceable to the NIST and in accordance with MIL-I-44208 and MIL-STD-45662A standards were used. The CV for within-day (0.04%) and between day (0.04%) variability was calculated across volumes expected for infants with no one specific volume CV greater than 0.09%. Sainz et al., 2003: 24 known bovine phantoms ranging in both mass (1.3894–9.9516 kg) and %fat (2.1–34.4 %fat) were used. The phantoms were then sub-divided into fat categories ≤10%, 10 to ≤20%, 20 to ≤30%, >30%). The group mean CV decreased as the bovine fat % increased with the CV for the fat categories ranging from 18.1% for the <10% fat group and 2.58% for the 20 to ≤30% category. Yao et al., 2003: 17 infants (1 to 22 weeks) were tested three times over two days. The within-day reliability for %fat (fat mass was not reported) was 2.85% and the between-day reliability was 2.95%.
Ma et al., 2004: 80 infants (0.4–22 wk) were tested four times over two days. The reliability portion of the study looked at 36 infants while the validity (using 2H2O) studied 53 infants. The within-day reliability for %fat (fat mass was not reported) was 4.94% and the between-day reliability was 5.10%. Hammami et al., 2002: Four phantoms (1.5, 3, 5, and 7 kg) of known composition were scanned in triplicate at three centers. The CV reported is the average of the three centers for the 3 kg phantom which was chosen because of the focus of this paper. Koo et al., 2004: 13 piglets (n =8 < 8 kg (4,508 ±1,625 g) and n =5 >8 kg (14,564 ±4,211 g)) were scanned twice. Rigo et al., 1998: 21 piglets weighing 1,408–5,151 g were scanned. Picaud et al., 1996: 13 piglets weighing 1,471–5,507 g were scanned 3 times. Godang et al., 2010: 50 newborn infants weighing 3,408 ±474 g were scanned twice on the same day. Venkataraman et al., 1992: 12 infants were tested twice on the same day after repositioning. Koo et al., 2004: 35 infants weighing 1,954–11,564 g were scanned in duplicate.
Because ADP is a relatively new method for infant body composition measurement, we provide a fairly thorough review of reliability and validity studies below. There are approximately 35 published indexed articles using ADP in infants with the vast majority using it as an instrument to determine outcome variables, track growth trajectories, or understand the impact of various disease states on body composition. The first published paper reported the validity of ADP using five volume phantoms (2, 4, 6, 8, and 10 kg) of known certified instruments traceable to the NIST and in accordance with MIL-I-44208 and MIL-STD-45662A standards (Urlando et al., 2003). The linear regression equation between the measured and the actual volume produced the following regression (y =1.001x – 0.0366; R2 =1.0; SEE 1.1740 ml) with the regression not significantly deviating from the line of identity. The CV for both the within-day CV (0.04%) and between day (0.04%) variability was calculated across the five volumes expected for infants with no one specific volume CV greater than 0.09%. Following this was a validation of 24 known bovine phantoms ranging in both mass (1.3894–9.9516 kg) and %fat (2.1–34.4 %fat) (Sainz and Urlando, 2003). The phantoms were then sub-divided into fat categories (<10%, 10 to <20%, 20 to <30%, >30%). The group mean CV decreased as the bovine fat percentage increased with the CV of the fat categories ranging from 18.1% for the <10% fat group to 2.58% for the 20 to <30% category. The linear regression equation between measured and the actual bovine phantom %fat produced a regression (y =0.996x%fat – 0.119; SEE =0.60, R2 = 0.997) that did not deviate from the line of identity.
The next logical step was studying ADP in infants. Reliability was established in 17 infants (1 to 22 weeks) tested three times over two days (Yao et al., 2003). The within-day reliability for %fat was 2.85% and the between-day reliability was 2.95%. The Bland-Altman analysis (a test to demonstrate potential bias across the range of body fatness (Bland and Altman, 1986) showed tight 95% limits of agreement for both within-day (−2.0 to 1.2%fat) and between-day (−2.2 to 1.7%fat) variance; bias estimates were not reported.
The largest reliability and validation study to date (of which we are aware) was conducted in 80 infants (0.4–22 wk) tested four times over two days in the United States and China (Ma et al., 2004). The reliability study included 36 infants while the validity study (using 2H2O as the comparison method) included 53 infants. The within-day reliability for %fat was 4.94% and the between-day reliability was 5.10%. The Bland-Altman analysis again showed tight 95% limits of agreement for both within-day (−2.9 to 1.9%fat) and between-day variance (−2.7 to 3.1%fat) with no bias reported across the range of fatness. The validity portion of the study revealed a linear regression equation between ADP and 2H2O %fat (y = 0.851x –0.3.094, R2 =0.76; SEE =3.26%) that did not deviate from the line of identity. Yet, because the slope was appreciably less than 1.0, further evidence on ADP validation in human infants is desirable. The Bland-Altman analysis showed no bias across the range of fatness and 95% limits of agreement between methods at the individual level from −6.84 to +6.71 %fat. Recently, %fat estimated by ADP was validated against %fat estimated by 2H2O dilution in very low birth weight preterm infants, showing high precision (Test-retest error ~1.1 %fat) and good validity (R2 =0.63, SEE =1.65%), with no evidence of bias (Roggero et al., 2012). Accuracy, reliability, and validation information on ADP is provided in Table 4.
Taken together, the literature reveals ADP in infants to have excellent accuracy for volume estimation, to provide valid measures of FM and FFM using gold standard carcass analysis, and is highly reliable between repeat measurements. Due to the fact that the current equipment is limited to infant weight <8 kg, however, ADP is now only useful for infants less than ~6 months of age. ADP equipment is expensive and not portable, and also requires costly maintenance service. No special training is required for its use. Development of new ancillary equipment for the adult ADP allows valid measures for children 2 to 6 years (Fields and Allison, 2012), but the period from 6 months to 2 years remains a gap for use of this instrument. An advantage ADP has over DXA is the absence of radiation exposure, which allows researchers and clinicians to obtain serial body composition measures on individual infants followed over time (Fields et al., 2012). In addition, subject movement is less of an issue, because the volume measurements are obtained numerous times during the testing sequence so that movement artifacts are partially cancelled out, while movement artifact is a common reason for DXA scans in infants to fail quality control. However, the impact of crying has not been clearly established for infant ADP, and it provides no regional adipose tissue or lean mass quantification as does DXA. As in any two-compartment model, the estimation of FFM and FM rests on the accuracy of the FFM density assumption for the population under investigation. Longitudinal reference body composition data for term infants 0 to 6 months using ADP has been recently published (Fields et al., 2011).
Dual energy X-ray absorptiometry (DXA)
DXA works on the underlying principle that the different body tissues (fat, muscle, and bone) attenuate X-ray differently, with bone tissue resulting in almost complete attenuation and fat resulting in very little attenuation. All DXA devices irrespective of model and manufacturer measure the attenuation of X-rays pulsed at both a low and high energy synchronously, with each pixel ascribed an R value. The R value is the ratio of the low and high energy X-ray attenuation, with each tissue type possessing a characteristic R signature.
DXA is an attractive method for body composition assessment because it gives both whole body and regional estimates. In contrast to ADP, TBW, BIA, and anthropometry, DXA can delineate muscle from FFM, calculate bone mineral density/content and provide regional estimates for bone, fat and lean tissue, all of which the aforementioned methods cannot do. Considerable attention has been paid to establishing the validity, reliability of DXA for body composition estimation in adults and children, but studies specifically in populations <2 years old are scarce, perhaps due to concern over exposure to ionizing radiation. Keeping in mind radiation exposure varies by manufacture and scanning mode (i.e. adult is less), a typical infant DXA scan is ~0.00893 milliSieverts (~1 mR) which is equal to, or less than, 1 day’s exposure to daily background radiation. There may be increasing openness of Institutional Review Boards in the United States to allowing DXA in infant protocols, due to increased awareness of true radiation exposure levels and improvements in the scanners themselves. Because this technique is overlooked for infant assessment, we provide a thorough review of existing reliability and validation studies below.
Nonetheless, there remain limited body composition DXA data specifically in infants (birth—2 years) or typical infant weight ranges (i.e. piglet data) (Brunton et al., 1997; Butte et al., 2000; Godang et al., 2010; Koo, 2000; Koo et al., 1995, 2004a; Lapillonne et al., 1997a,b; Picaud et al., 1996; Rigo et al., 1998). Additional studies have been performed using DXA in infants as a method to diagnosis/improve specific disease/clinical states (Ahmad et al., 2010; Bolt et al., 2002; Henche et al., 2008; Weiler et al., 2008).
Accuracy, reliability, and validity information for DXA in infants is provided in Table 4. Two groups have reported the reliability in phantoms (Hammami et al., 2002) and piglets; (Koo et al., 2004a; Picaud et al., 1996) in three separate studies. The CV for a 3 kg phantom scanned in triplicate was 5.9% (Hammami et al., 2002). The derived CV was the average of the three scans on three different pencil beam scanners at three study centers in Belgium, the United States, and Germany, making the results somewhat difficult to interpret. Two studies reported very similar reliability in piglets using CV (5.35%) (Picaud et al., 1996) and the intra class correlation (ICC =0.98) (Koo et al., 2004a). Three studies have reported the reliability in infants ranging from 10 hours to 1 year old (Godang et al., 2010; Koo et al., 2004b; Venkataraman and Ahluwalia, 1992). CV of fat mass was <1% in twelve infants tested twice on the same day after repositioning (Venkataraman and Ahluwalia, 1992), with the ICC for within-day trials being 0.94 to 0.99 (Godang et al., 2010; Koo et al., 2004b). In general, these intraclass correlations and CVs are comparable to what is typically seen in the adult literature.
The validity of DXA in the human infant weight range is based predominately on direct carcass analysis of piglets. The limited number of studies in humans may be attributable to the radiation dose associated with DXA, albeit small. Of the three studies that have studied the validity of DXA using carcass analysis (Koo et al., 2004a; Picaud et al., 1996; Rigo et al., 1998), the weight range studied was between 1,471 and 14,564 g. Whole body Hologic QDR 2000+ had mean fat mass slightly higher (715 ±521 g) compared to direct carcass analysis (671 ±541) in 13 piglets (Koo et al., 2004a). DXA explains between 94% and 98% of the variation in direct carcass analysis fat mass (Koo et al., 2004a; Picaud et al., 1996; Rigo et al., 1998), with a standard error of the estimate of 31 g (Picaud et al., 1996). Biases were not reported by these studies.
To our knowledge, only one study to date has validated DXA in infants against another body composition technique (Fields et al., 2012). Fields et al. reported a significant (P < 0.001) group mean difference in 84 six-month-old infants in total fat mass derived by DXA (2,284 ±449 g) and ADP (1,921 ±492 g). The R2 (89%) and standard error of the estimate (148 g; unpublished data) was similar to the direct carcass data reported by others (Koo et al., 2004a; Picaud et al., 1996). Bland-Altman analysis showed bias across the weight ranging (r =0.281; P < 0.005) with DXA overestimating fat mass compared to ADP in all but two subjects. Bias in fat mass and fat-free mass estimates from DXA relative to the 4C model has also been observed to increase with increasing adiposity in older children (Sopher et al., 2004; Williams et al., 2006).
It is common practice for DXA to be used as the “reference” or “standard” to which other techniques are compared in pediatric populations (Cameron et al., 2004; Eisenmann et al., 2004; Elberg et al., 2004), but this approach has been brought into question. As early as 1997, Lapillonne et al. concluded that DXA was not ready to be used as a criterion or gold standard in pediatric populations (1997b). More recently, Shypailo et al. reanalyzed 1,384 pediatric scans (ages ranged from 1.7 to 17.2 years) using Hologic software V12.1 and compared results from the same individuals using the previous version of the software (V11.2) (Shypailo et al., 2008). Mean total fat mass increased significantly with the new software in boys (0.45 ±0.18 kg higher) and girls (1.0 ±0.39 kg higher). The authors concluded that the “still evolving technology of DXA warrants scrutiny and cannot yet be considered the standard” (Shypailo et al., 2008).
Differences in manufacturers, hardware, software algorithms, scan analysis and acquisition techniques all may affect body composition estimates with proportionally greater errors in smaller sizes (Koo et al., 2004a). In the most comprehensive study yet investigating sources of error affecting DXA measurements for infants, Koo et al. using both animal (piglets) and human (infants) models looked at the role of clothing (blanket and diaper) and movement on estimates of bone, fat and lean mass in the infant weight range (Koo et al., 2004b). The reliability in triplicate scans in piglets (0.99) and duplicate scans in infants (0.99) for fat mass was high. In an attempt to better understand the effect of a blanket or diaper on body composition five piglets were first scanned with no blanket and diaper (i.e. reference group), they were then scanned with only a blanket and again with both a blanket and diaper. No significant difference for fat, lean or any bone measure was observed for the blanket vs. referent condition, however, a significant (P < 0.01) difference was observed for the blanket plus diaper (3,436 ±2,846 vs. 3,391 ±2,836) condition for lean mass. In 29 pairs of infant scans (no movement vs. movement) motion artifact resulted in increased estimates (P < 0.05) for bone, lean and fat compartments which had previously been reported only for bone (Koo et al., 1995); these differences were of greater relative magnitude for smaller than larger infants. To maintain body temperature (and for some gentle restraint), infants can be swaddled, but the same size and type of blanket should be used for all scans (Koo et al., 1995). Little can be done to ensure the infant remains motionless throughout testing, but keeping the environment dark and quiet, with the infant as drowsy as possible, can help to ensure high compliance.
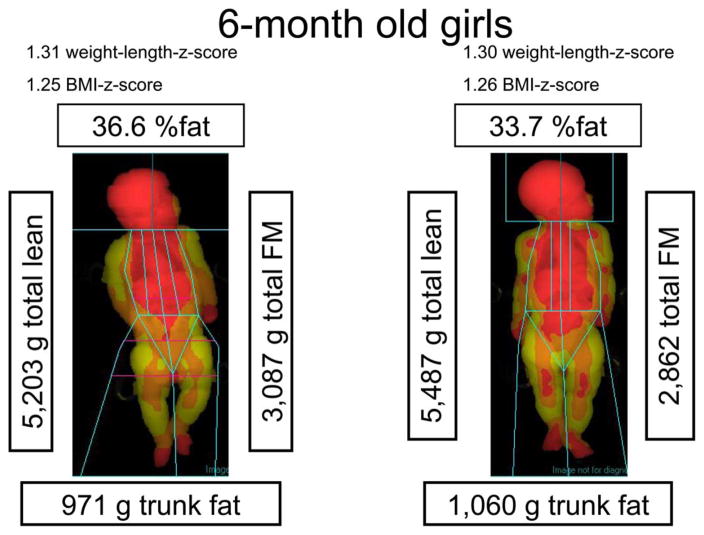
DXA represents a very useful technology for infant body composition particular because of its ability to provide regional estimates of adipose tissue and to decompose FFM into lean and bone. Figure 2 illustrates some of the strengths and weaknesses of DXA in two 6-month-old girls. Although their WLZ and BMI z scores are similar, DXA shows that their percent body fat is different, which information is theoretically important for documenting later health risks. Further, their trunk fat is approximately 1 kg, representing 30% of their total FM. No other widely available method allows this deep level of body composition analysis, other than imaging techniques (below). Nonetheless, in this non-compliant population, subject movement (as is somewhat evident in the head region in Fig. 2 subject on the left) may make the accuracy of DXA estimates of body composition at least as prone to error as other methods. In addition, DXA is a somewhat expensive, research-focused technology, requiring costly maintenance service, and radiologic certification for users. The radiation exposure, while low, limits serial investigations to track serial changes. Reference body composition data using DXA alone in infants has not yet been published.
Fig. 2.
Body composition in 2 six-month-old infants using dual-energy X-ray absorptiometry (DXA). Red =areas less than 31% fat; Orange =areas 31% fat to 55% fat; Yellow =areas greater than 55% fat.
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) utilizes the fact that hydrogen protons are abundant in all biological tissues and have a nonzero magnetic moment, causing them to align like small magnets when the individual is placed within the strong magnetic field of the MRI unit. A pulsed radio frequency field is applied to cause the protons to absorb energy, which energy is then released and detected as radio frequency signals once the radio frequency field is turned off. The spatial pattern of detected signals across the receiver coil during repeated pulses and relaxations of the protons is then used to create the cross-sectional images (Plewes and Kucharczyk, 2012). MRI is considered a reference method for total and regional body composition assessment, because it provides high resolution images, and if combined with contiguous or narrowly spaced multiple slice protocols, allows for direct assessment of tissue and organ volumes (Shen et al., 2005). MRI protocols have been developed that are rapid (approximately 5 min) and feasible for the infant population, and which provide estimates of adipose tissue volume that approximate the total body fat mass of the reference infant. In the first such study, interobserver CV% for subcutaneous AT was 2.4% and for internal (non-subcutaneous, including visceral) AT CV% was 17% (Harrington et al., 2002). Another study also found that MRI estimates of total body adipose tissue volume were similar to those for the reference infant (Olhager et al., 2003), with high (intra-observer) precision for total and subcutaneous adipose tissue (CV% =1.7% and 1.6%, respectively) but lower precision for non-subcutaneous (visceral) adipose tissue: 8.7% (Olhager et al., 2003). The higher intra- and inter-observer measurement errors for internal/visceral AT stem from the very small visceral adipose tissue volume in infants (approximately 100–300 ml found by Olhager et al., 2003 and 20–40 ml in Harrington et al., 2002). Infant MRI studies were the first to demonstrate that subcutaneous, but not intra-abdominal adipose tissue is reduced in infants with intrauterine growth restriction (Harrington et al., 2004).
MRI does not involve radiation exposure, making it well-suited for use in infants and children; however, body movement during image acquisition creates artifacts and therefore infants are ideally scanned while sleeping (for instance, after having been fed) (Shen et al., 2005). Due to difficulty in obtaining compliance in older infants, it is likely that MRI imaging will be feasible only in infants less than 6 months of age. Infants should be protected from scanner noise with earplugs, kept warm with blankets, and monitored for crying (using a pulse oximeter). Typically, a number of T1-weighted axial images are acquired across the entire body, with a special receiver coil being used to increase the signal to noise ratio. Whole-body contiguous-image MRI requires far fewer images in infants than in larger subjects, due to the infant’s small size, and thus data acquisition can be completed quickly (<10 min); contiguous image protocols preclude the need for estimation of volumes between images. Commercial software (e.g., Tomovision, Quebec, Canada) and freeware is available to assist in image postprocessing, including segmentation of the images to tag tissue and organ areas of interest. Due to normal variations in signal intensity across the field, MRI requires manual image segmentation (albeit computer-aided) and thus analysis is time-consuming. Areas are summed across images, and the resulting volumes may be converted to mass by multiplying by the appropriate tissue densities. For example, adipose tissue density in infants at birth was estimated to be 0.456 kg/l (Baker, 1969; McGowan, 1979) and between 1 and 5 months of age is approximately 0.67 kg/l (Kabir and Forsum, 1993).
Body composition assessment of the infant 0 to 6 months of age using MRI is a quasi-direct means of body composition, is quite feasible from a behavioral standpoint, is highly precise (at least for total body adipose tissue and organ volumes), allows numerous tissues and organs to be assessed, including visceral adipose tissue volume, requires many fewer assumptions to be met than do the methods reviewed above, and is available at an increasing number of research and clinical facilities. However, the of MRI is relatively high both for acquisition and for image processing, and therefore is not appropriate unless quantification of specific organ and tissue volumes is the focus of investigation.
Beyond MR imaging, MR spectroscopy (MRS), including chemical shift imaging and Dixon methods, provide added information on in vivo metabolism within tissues and organs, by allowing functional characteristics (oxygen uptake, blood flow) of tissues to be assessed from their chemical spectra. For instance, MRS can resolve and quantify intramyocellular and extramyocellular lipids within skeletal muscle. New methodological advances in MRS are also making it possible to rapidly and non-invasively assess brown adipose tissue (BAT) in infants (Hu et al., 2012). BAT is a highly vascularized tissue rich in mitochondria, is responsible for nonshivering thermogenesis, comprises a relatively high proportion of adipose tissue in infants, and may predict obesity and diabetes (Enerback, 2010). Causes and consequences of developmental variation in BAT in human populations is an area for future investigation. Due to cost and accessibility concerns, MRS will be typically used in basic human biological and clinical studies rather than in population-based research.
CONCLUSIONS
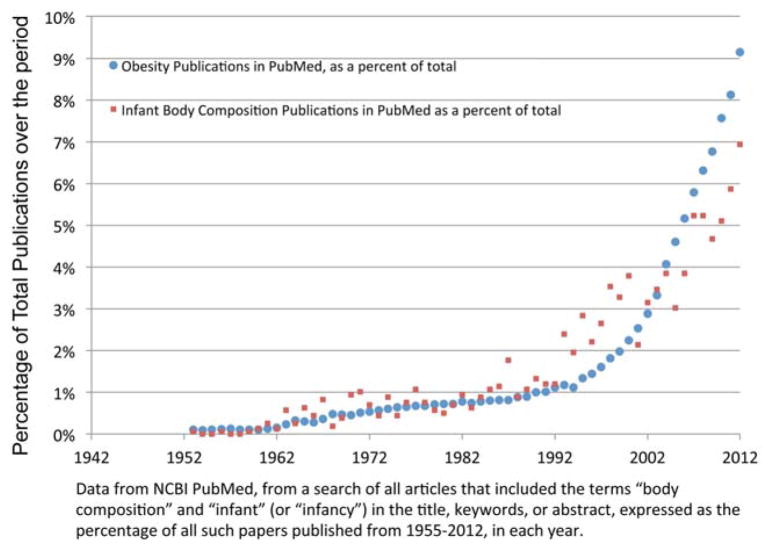
As in other health and nutrition related areas such as obesity, interest in infant body composition appears to be growing as evidenced by an increasing number of publications published per year (Fig. 3). Human biologists have a wide array of choices for infant body composition assessment, each of which has unique advantages and disadvantages. Infancy poses particular challenges for the researcher because (1) their body size and composition (including tissue densities) are changing rapidly compared to other phases of development, and (2) infants are unable to control their movements during a test session. To address the first issue, validation studies are ideally conducted before the initiation of the main research study, to quantify and adjust for potential biases in assessment. This is particularly important in under-studied populations where nutritional status (including obesity) may be expected to diverge from that of infants contributing to reference data. To address the second issue, which is one of lower reliability due to subject noncompliance, creation of a testing environment that soothes the infant (darkened rooms, blankets where possible, involvement of the parent) and taking extra time and providing training to data collection staff to become familiar with particular needs of the infant can greatly improve the success rate.
Fig. 3.
Publications on infant body composition per year 1955–2012.
LITERATURE CITED
- Ahmad I, Nemet D, Eliakim A, Koeppel R, Grochow D, Coussens M, Gallitto S, Rich J, Pontello A, Leu SY, Cooper DM, Waffarn F. Body composition and its components in preterm and term newborns: A cross-sectional, multimodal investigation. Am J Hum Biol. 2010;22:69–75. doi: 10.1002/ajhb.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GL. Human adipose tissue composition and age. Am J Clin Nutr. 1969;22:829–835. doi: 10.1093/ajcn/22.7.829. [DOI] [PubMed] [Google Scholar]
- Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN., Jr Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Heymsfield SB, Lichtman S, Wang J, Pierson RN., Jr Body composition in elderly people: effect of criterion estimates on predictive equations. Am J Clin Nutr. 1991;53:1345–1353. doi: 10.1093/ajcn/53.6.1345. [DOI] [PubMed] [Google Scholar]
- Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr. 1996;64(3 Suppl):524s–532s. doi: 10.1093/ajcn/64.3.524S. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bolt RJ, van Weissenbruch MM, Roos JC, Delemarre-van de Waal HA, Cranendonk A, Lafeber HN. Body composition in infants with chronic lung disease after treatment with dexamethasone. Acta Paediatrica. 2002;91:815–821. doi: 10.1080/08035250213220. [DOI] [PubMed] [Google Scholar]
- Brunton JA, Weiler HA, Atkinson SA. Improvement in the accuracy of dual energy X-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res. 1997;41:590–596. doi: 10.1203/00006450-199704000-00022. [DOI] [PubMed] [Google Scholar]
- Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47:578–585. doi: 10.1203/00006450-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Cameron N, Griffiths PL, Wright MM, Blencowe C, Davis NC, Pettifor JM, Norris SA. Regression equations to estimate percentage body fat in African prepubertal children aged 9 y. Am J Clin Nutr. 2004;80:70–75. doi: 10.1093/ajcn/80.1.70. [DOI] [PubMed] [Google Scholar]
- Choh AC, Curran JE, Odegaard AO, Nahhas RW, Czerwinski SA, Blangero J, Towne B, Demerath EW. Differences in the heritability of growth and growth velocity during infancy and associations with FTO variants. Obesity. 2011;19:1847–1854. doi: 10.1038/oby.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumlea WC, Schubert CM, Sun SS, Demerath E, Towne B, Siervogel RM. A review of body water status and the effects of age and body fatness in children and adults. J Nutr Health Aging. 2007;11:111–118. [PubMed] [Google Scholar]
- Chumlea WC, Sun SS. Bioelectrical impedance analysis. In: Heymsfield S, Lohman T, Wang H, editors. Human body composition. 2. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- Coward WA. The doubly-labelled-water (2Hx180) method principles and practice. Proc Nutr Soc. 1988;47:209–218. doi: 10.1079/pns19880037. [DOI] [PubMed] [Google Scholar]
- Dauncey M, Gandy G, Dairdner D. Assessment of total body fat in infancy from skinfold thickness measurements. Arch Dis Child. 1977;52:223. doi: 10.1136/adc.52.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PS, Wells JC. Calculation of total body water in infancy. Eur J Clin Nutr. 1994;48:490–495. [PubMed] [Google Scholar]
- de Bruin NC, van Velthoven KA, Stijnen T, Juttmann RE, Degenhart HJ, Visser HK. Body fat and fat-free mass in infants: new and classic anthropometric indexes and prediction equations compared with total-body electrical conductivity. Am J Clin Nutr. 1995;61:1195–1205. doi: 10.1093/ajcn/61.6.1195. [DOI] [PubMed] [Google Scholar]
- de Zegher F, Sebastiani G, Diaz M, Sanchez-Infantes D, Lopez-Bermejo A, Ibanez L. Body composition and circulating high-molecular-weight adiponectin and IGF-I in infants born small for gestational age: breast- versus formula-feeding. Diabetes. 2012;61:1969–1973. doi: 10.2337/db11-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans HE, Smith FW, Lloyd DJ, Law AN, Sutherland HW. Fetal fat measurement by magnetic resonance imaging. Br J Radiol. 1989;62:603–607. doi: 10.1259/0007-1285-62-739-603. [DOI] [PubMed] [Google Scholar]
- Dehghan M, Merchant AT. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr J. 2008;7:26. doi: 10.1186/1475-2891-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath EW, Choh AC, Czerwinski SA, Lee M, Sun SS, Chumlea WC, Duren D, Sherwood RJ, Blangero J, Towne B, Siervogel RM. Genetic and environmental influences on infant weight and weight change: the Fels Longitudinal Study. Am J Hum Biol. 2007;19:692–702. doi: 10.1002/ajhb.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- Dulloo AG, Girardier L. Influence of dietary composition on energy expenditure during recovery of body weight in the rat: implications for catch-up growth and obesity relapse. Metabolism. 1992;41:1336–1342. doi: 10.1016/0026-0495(92)90105-j. [DOI] [PubMed] [Google Scholar]
- Dung NQ, Fusch G, Armbrust S, Jochum F, Fusch C. Body composition of preterm infants measured during the first months of life: bioelectrical impedance provides insignificant additional information compared to anthropometry alone. EurJ Pediatr. 2007;166:215–222. doi: 10.1007/s00431-006-0232-y. [DOI] [PubMed] [Google Scholar]
- Eisenmann JC, Heelan KA, Welk GJ. Assessing body composition among 3- to 8-year-old children: anthropometry, BIA, and DXA. Obes Res. 2004;12:1633–1640. doi: 10.1038/oby.2004.203. [DOI] [PubMed] [Google Scholar]
- Elberg J, McDuffie JR, Sebring NG, Salaita C, Keil M, Robotham D, Reynolds JC, Yanovski JA. Comparison of methods to assess change in children’s body composition. Am J Clin Nutr. 2004;80:64–69. doi: 10.1093/ajcn/80.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KJ. Evaluation of body composition in neonates and infants. Semin Fet Neonatal Med. 2007;12:87–91. doi: 10.1016/j.siny.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Ellis KJ, Bell SJ, Chertow GM, Chumlea WC, Knox TA, Kotler DP, Lukaski HC, Schoeller D. Bioelectrical impedance methods in clinical research a follow-up to the NIH Technology Assessment Conference. Nutrition. 1999;15:874–880. doi: 10.1016/s0899-9007(99)00147-1. [DOI] [PubMed] [Google Scholar]
- Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–252. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Eriksson B, Lof M, Eriksson O, Hannestad U, Forsum E. Fat-free mass hydration in newborns: assessment and implications for body composition studies. Acta Paediatr. 2011;100:680–686. doi: 10.1111/j.1651-2227.2011.02147.x. [DOI] [PubMed] [Google Scholar]
- Escribano J, Luque V, Ferre N, Mendez-Riera G, Koletzko B, Grote V, Demmelmair H, Bluck L, Wright A, Closa-Monasterolo R. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU Childhood Obesity Programme. Int J Obes. 2012;36:548–553. doi: 10.1038/ijo.2011.276. [DOI] [PubMed] [Google Scholar]
- Fields DA, Allison DB. Air-displacement plethysmography pediatric option in 2–6 years old using the four-compartment model as a criterion method. Obesity. 2012;20:1732–1737. doi: 10.1038/oby.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DA, Demerath EW, Pietrobelli A, Chandler-Laney PC. Body composition at 6 months of life: comparison of air displacement plethysmography and dual-energy X-ray absorptiometry. Obesity. 2012;20:2302–2306. doi: 10.1038/oby.2012.102. [DOI] [PubMed] [Google Scholar]
- Fields DA, Gilchrist JM, Catalano PM, Gianni ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity. 2011;19:1887–1891. doi: 10.1038/oby.2011.11. [DOI] [PubMed] [Google Scholar]
- Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- Fields DA, Higgins PB, Radley D. Air-displacement plethysmography: here to stay. Curr Opin Clin Nutr Metab Care. 2005;8:624–629. doi: 10.1097/01.mco.0000171127.44525.07. [DOI] [PubMed] [Google Scholar]
- Fjeld CR, Fruendt-Thurne J, Schoeller D. Total body water measured by 18-O dilution and bioelectrical impedance in well and malnourished children. Pediatr Res. 1990;27:98–102. doi: 10.1203/00006450-199001000-00024. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35(5 Suppl):1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, Nelson SE. Body composition of the male and female reference infants. Annu Rev Nutr. 2002;22:1–17. doi: 10.1146/annurev.nutr.22.111401.145049. [DOI] [PubMed] [Google Scholar]
- Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- Foster KR, Lukaski HC. Whole-body impedance—what does it measaure. Am J Clin Nutr. 1996;64(Suppl):388S–396S. doi: 10.1093/ajcn/64.3.388S. [DOI] [PubMed] [Google Scholar]
- Godang K, Qvigstad E, Voldner N, Isaksen GA, Froslie KF, Notthellen J, Henriksen T, Bollerslev J. Assessing body composition in healthy newborn infants: reliability of dual-energy X-ray absorptiometry. J Clin Densitom. 2010;13:151–160. doi: 10.1016/j.jocd.2010.01.121. [DOI] [PubMed] [Google Scholar]
- Hammami M, Koo WW, Hockman EM. Body composition of neonates from fan beam dual energy X-ray absorptiometry measurement. J Parenter Enteral Nutr. 2003;27:423–426. doi: 10.1177/0148607103027006423. [DOI] [PubMed] [Google Scholar]
- Hammami M, Picaud JC, Fusch C, Hockman EM, Rigo J, Koo WW. Phantoms for cross-calibration of dual energy X-ray absorptiometry measurements in infants. J Am Coll Nutr. 2002;21:328–332. doi: 10.1080/07315724.2002.10719230. [DOI] [PubMed] [Google Scholar]
- Harrington TA, Thomas EL, Frost G, Modi N, Bell JD. Distribution of adipose tissue in the newborn. Pediatr Res. 2004;55:437–441. doi: 10.1203/01.PDR.0000111202.29433.2D. [DOI] [PubMed] [Google Scholar]
- Harrington TA, Thomas EL, Modi N, Frost G, Coutts GA, Bell JD. Fast and reproducible method for the direct quantitation of adipose tissue in newborn infants. Lipids. 2002;37:95–100. doi: 10.1007/s11745-002-0868-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Wong WW, Thomas AJ, Uvena-Celebrezze J, Huston-Pressley L, Amini SB, Catalano PM. Estimation of neonatal body composition: isotope dilution versus total-body electrical conductivity. Neonatology. 2002;81:170–175. doi: 10.1159/000051530. [DOI] [PubMed] [Google Scholar]
- Henche SA, Torres RR, Pellico LG. An evaluation of patterns of change in total and regional body fat mass in healthy Spanish subjects using dual-energy X-ray absorptiometry (DXA) Eur J Clin Nutr. 2008;62:1440–1448. doi: 10.1038/sj.ejcn.1602883. [DOI] [PubMed] [Google Scholar]
- Heymsfield S, Lohman T, Wang Z. Human body composition. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- Hill K. Life history theory and evolutionary anthropology. Evol Anthropol. 1993;2:78–88. [Google Scholar]
- Houtkooper LB, Lohman TG, Going SB, Howell WH. Why bioelectrical impedance analysis should be used for estimating adiposity. Am J Clin Nutr. 1996;64(3 Suppl):436s–448s. doi: 10.1093/ajcn/64.3.436S. [DOI] [PubMed] [Google Scholar]
- Hu HH, Tovar JP, Pavlova Z, Smith ML, Gilsanz V. Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging. 2012;35:938–942. doi: 10.1002/jmri.23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Ong K, Dunger DB, de Zegher F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J Clin Endocrinol Metab. 2006;91:2153–2158. doi: 10.1210/jc.2005-2778. [DOI] [PubMed] [Google Scholar]
- Kabir N, Forsum E. Estimation of total body fat and subcutaneous adipose tissue in full-term infants less than 3 months old. Pediatr Res. 1993;34:448–454. doi: 10.1203/00006450-199310000-00013. [DOI] [PubMed] [Google Scholar]
- Koo WW. Body composition measurements during infancy. Ann NY Acad Sci. 2000;904:383–392. doi: 10.1111/j.1749-6632.2000.tb06487.x. [DOI] [PubMed] [Google Scholar]
- Koo WW, Hammami M, Hockman EM. Validation of bone mass and body composition measurements in small subjects with pencil beam dual energy X-ray absorptiometry. J Am Coll Nutr. 2004a;23:79–84. doi: 10.1080/07315724.2004.10719346. [DOI] [PubMed] [Google Scholar]
- Koo WW, Hockman EM, Hammami M. Dual energy X-ray absorptiometry measurements in small subjects: conditions affecting clinical measurements. J Am Coll Nutr. 2004b;23:212–219. doi: 10.1080/07315724.2004.10719363. [DOI] [PubMed] [Google Scholar]
- Koo WW, Massom LR, Walters J. Validation of accuracy and precision of dual energy X-ray absorptiometry for infants. J Bone Miner Res. 1995;10:1111–1115. doi: 10.1002/jbmr.5650100716. [DOI] [PubMed] [Google Scholar]
- Koo WW, Walters JC, Hockman EM. Body composition in neonates: relationship between measured and derived anthropometry with dual-energy X-ray absorptiometry measurements. Pediatr Res. 2004c;56:694–700. doi: 10.1203/01.PDR.0000142587.59238.BD. [DOI] [PubMed] [Google Scholar]
- Kushner R, Schoeller D, Fjeld CR, Danford L. Is the impedance index (ht2 R) significant in predicting total body water. Am J Clin Nutr. 1992;56:835–839. doi: 10.1093/ajcn/56.5.835. [DOI] [PubMed] [Google Scholar]
- L’Abee C, Poorts-Borger PH, Gorter EH, Piccoli A, Stolk RP, Sauer PJ. The bioelectrical impedance vector migration in healthy infants. Clin Nutr. 2010;29:222–226. doi: 10.1016/j.clnu.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Lampl M, Lee W, Koo W, Frongillo EA, Barker DJ, Romero R. Ethnic differences in the accumulation of fat and lean mass in late gestation. Am J Hum Biol. 2012;24:640–647. doi: 10.1002/ajhb.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997a;86:196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Lapillonne A, Braillon PM, Delmas PD, Salle BL. Dual-energy X-ray absorptiometry in early life. Horm Res. 1997b;48(Suppl 1):43–49. doi: 10.1159/000191267. [DOI] [PubMed] [Google Scholar]
- Lingwood BE. Bioelectrical impedance analysis for assessment of fluid status and body composition in neonates–the good, the bad and the unknown. Eur J Clin Nutr. 2013;67(Suppl 1):S28–S33. doi: 10.1038/ejcn.2012.162. [DOI] [PubMed] [Google Scholar]
- Lingwood BE, Coghlan JP, Ward LC, Charles BG, Colditz PB. Measurement of extracellular fluid volume in the neonate using multiple frequency bio-impedance analysis. Physiol Meas. 2000;21:251–262. doi: 10.1088/0967-3334/21/2/305. [DOI] [PubMed] [Google Scholar]
- Lingwood BE, Storm van Leeuwen AM, Carberry AE, Fitzgerald EC, Callaway LK, Colditz PB, Ward LC. Prediction of fat-free mass and percentage of body fat in neonates using bioelectrical impedance analysis and anthropometric measures: validation against the PEA POD. Br J Nutr. 2012;107:1545–1552. doi: 10.1017/S0007114511004624. [DOI] [PubMed] [Google Scholar]
- Lohman TG. Assessment of body composition in children. Pediatr Exerc Sci. 1989;1:19–30. doi: 10.1123/pes.1.1.19. [DOI] [PubMed] [Google Scholar]
- Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, Wong WW, Nommsen-Rivers L, Dewey KG. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79:653–660. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- Madsen AL, Larnkjaer A, Molgaard C, Michaelsen KF. IGF-I and IGFBP-3 in healthy 9 month old infants from the SKOT cohort: breast-feeding, diet, and later obesity. Growth Horm IGF Res. 2011;21:199–204. doi: 10.1016/j.ghir.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Mak RH, Cheung W. Cachexia in chronic kidney disease: role of inflammation and neuropeptide signaling. Curr Opin Nephrol Hypertens. 2007;16:27–31. doi: 10.1097/MNH.0b013e3280117ce7. [DOI] [PubMed] [Google Scholar]
- Margutti AV, Monteiro JP, Camelo JS., Jr Reference distribution of the bioelectrical impedance vector in healthy term newborns. Br J Nutr. 2010;104:1508–1513. doi: 10.1017/S000711451000245X. [DOI] [PubMed] [Google Scholar]
- Martinez JA, Cordero P, Campion J, Milagro FI. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc. 2012;71:276–283. doi: 10.1017/S0029665112000055. [DOI] [PubMed] [Google Scholar]
- McCrory MA, Gomez TD, Bernauer EM, Mole PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- McGowan AR. The fat and water content of the dead infants skin-fold. Pediatr Res. 1979;13:1304–1306. doi: 10.1203/00006450-197911000-00021. [DOI] [PubMed] [Google Scholar]
- Moulton CR. Age and chemical development in mammals. J Biolog Chemi. 1923;57:79–97. [Google Scholar]
- Mueller W, Reid R. A multivariate analysis of fatness and relative fat patterning. AmJ Phyl Anthropol. 1979;50:199–208. doi: 10.1002/ajpa.1330500208. [DOI] [PubMed] [Google Scholar]
- Murlin J, Hoobler B. The energy metabolism of normal and marasmic children with special reference to the specific gravity of the child’s body. Proc Soc Exp Biol Med. 1913;11:115–116. [Google Scholar]
- Nielsen SB, Wells JC, Slater C, Fewtrell MS, Reilly JJ. Administering labelled water to exclusively breast-fed infants in studies involving stable isotope dilution techniques. Isotopes Environ Health Stud. 2011;47:18–25. doi: 10.1080/10256016.2011.554980. [DOI] [PubMed] [Google Scholar]
- Olhager E, Flinke E, Hannerstad U, Forsum E. Studies on human body composition during the first 4 months of life using magnetic resonance imaging and isotope dilution. Pediatr Res. 2003;54:906–912. doi: 10.1203/01.PDR.0000088064.63106.5E. [DOI] [PubMed] [Google Scholar]
- Pfaundler M. Korpermass-studien an kindern. Von korpervolumen und der korperdichte. Ztschr f Kinderheilk. 1916;14:123–127. [Google Scholar]
- Picaud JC, Rigo J, Nyamugabo K, Milet J, Senterre J. Evaluation of dual-energy X-ray absorptiometry for body-composition assessment in piglets and term human neonates. Am J Clin Nutr. 1996;63:157–163. doi: 10.1093/ajcn/63.2.157. [DOI] [PubMed] [Google Scholar]
- Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int. 1994;46:534–539. doi: 10.1038/ki.1994.305. [DOI] [PubMed] [Google Scholar]
- Plewes DB, Kucharczyk W. Physics of MRI: a primer. J Magn Reson Imaging. 2012;35:1038–1054. doi: 10.1002/jmri.23642. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The anatomy of adipose tissue in captive Macaca monkeys and its implications for human biology. Folia Primatol. 1987;48:164–185. doi: 10.1159/000156293. [DOI] [PubMed] [Google Scholar]
- Rigo J. Body composition during the first year of life. Nestle Nutr Workshop Ser Paediatr Programme. 2006;58:65–76. doi: 10.1159/000095021. discussion 76–68. [DOI] [PubMed] [Google Scholar]
- Rigo J, Nyamugabo K, Picaud JC, Gerard P, Pieltain C, De Curtis M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J Pediatr Gastroenterol Nutr. 1998;27:184–190. doi: 10.1097/00005176-199808000-00011. [DOI] [PubMed] [Google Scholar]
- Roggero P, Gianni ML, Amato O, Piemontese P, Morniroli D, Wong WW, Mosca F. Evaluation of air-displacement plethysmography for body composition assessment in preterm infants. Pediatr Res. 2012;72:316–320. doi: 10.1038/pr.2012.75. [DOI] [PubMed] [Google Scholar]
- Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am J Clin Nutr. 2003;77:364–370. doi: 10.1093/ajcn/77.2.364. [DOI] [PubMed] [Google Scholar]
- Salazar G, Infante C, Vio F. Deuterium equilibration time in infant’s body water. Eur J Clin Nutr. 1994;48:475–481. [PubMed] [Google Scholar]
- Savino F, Grasso G, Cresi F, Oggero R, Silvestro L. Bioelectrical impedance vector distribution in the first year of life. Nutrition. 2003;19:492–496. doi: 10.1016/s0899-9007(02)00947-4. [DOI] [PubMed] [Google Scholar]
- Schoeller D. Hydrometry. In: Heymsfield S, Lohman T, Wang Z, editors. Human body composition. 2. Champaign, IL: Human Kinetics; 2005. pp. 35–50. [Google Scholar]
- Schoeller D, Kushner R, Taylor P, Dietz WH, Bandini L. Measurement of total body water: isotope dilution techniques. In: Roche AF, editor. Body composition assessments inyouth and adults sixth ross conferences on medical research. Columbus, OH: Ross Laboratories; 1985. pp. 124–129. [Google Scholar]
- Sen B, Mahalanabis D, Kurpad AV, Shaikh S, Bose K. Total body water and fat-free mass: evaluation of equations based on bioelectrical impedance analysis in infants and young children in India. Br J Nutr. 2010;104:256–264. doi: 10.1017/S0007114510000498. [DOI] [PubMed] [Google Scholar]
- Sesmero MA, Mazariegos M, Pedron C, Jones J, Solomons NW. Bio-impedance electrical spectroscopy in the first six months of life: some methodologic considerations. Nutrition. 2005;21:567–573. doi: 10.1016/j.nut.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Shen W, Liu H, Punyanitya M, Chen J, Heymsfield SB. Pediatric obesity phenotyping by magnetic resonance methods. Curr Opin Clin Nutr Metabol Care. 2005;8:595–601. [PMC free article] [PubMed] [Google Scholar]
- Shypailo RJ, Butte NF, Ellis KJ. DXA: can it be used as a criterion reference for body fat measurements in children? Obesity. 2008;16:457–462. doi: 10.1038/oby.2007.81. [DOI] [PubMed] [Google Scholar]
- Sopher A, Shen E, Peitrobeilli A. Pediatric body composition methods. In: Heymsfield S, Lohman T, Wang Z, editors. Human body composition. 2. Champaign, IL: Human Kinetics; 2005. pp. 129–140. [Google Scholar]
- Sopher AB, Thornton JC, Wang J, Pierson RN, Jr, Heymsfield SB, Horlick M. Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics. 2004;113:1285–1290. doi: 10.1542/peds.113.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe RF, de Azevedo ZM, Fonseca VM, Peixoto MV, dos Anjos LA, Gaspar-Elsas MI, Moore DC, Ramos EG. Distribution of bioelectrical impedance vector values in multi-ethnic infants and pre-school children. Clin Nutr. 2012;31:144–148. doi: 10.1016/j.clnu.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Traver LA, Martinez FE, Ferriolli E, Marchini JS, Monteiro JP, Pfrimer K, Sanchez AP, de Oliveira T, Ducatti C, Camelo JS., Jr Deuterium equilibrium time in saliva of newborn infants. J Pediatr Gastroenterol Nutr. 2009;48:471–474. doi: 10.1097/MPG.0b013e31818bba05. [DOI] [PubMed] [Google Scholar]
- Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53:486–492. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- Venkataraman PS, Ahluwalia BW. Total bone mineral content and body composition by x-ray densitometry in newborns. Pediatrics. 1992;90:767–770. [PubMed] [Google Scholar]
- Wang Z, Shen W, Withers R, Hemysfield S. Multicomponent molecular-level models of body composition analysis. In: Hemysfield S, Lohman T, Wang Z, editors. Human body composition. Champaign, IL: Human Kinetics; 2005. pp. 163–176. [Google Scholar]
- Weiler HA, Fitzpatrick-Wong SC, Schellenberg JM. Bone mass in First Nations, Asian and white newborn infants. Growth Dev Aging. 2008;71:35–43. [PubMed] [Google Scholar]
- Wells JC. Body composition in infants: evidence for developmental programming and techniques for measurement. Rev Endocr Metab Disord. 2012a;13:93–101. doi: 10.1007/s11154-012-9213-9. [DOI] [PubMed] [Google Scholar]
- Wells JC. The evolution of human adiposity and obesity: where did it all go wrong? Dis Model Mech. 2012b;5:595–607. doi: 10.1242/dmm.009613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC, Fewtrell MS. Measuring body composition. Arch Disease Child. 2006;91:612–617. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC, Fewtrell MS, Davies PS, Williams JE, Coward WA, Cole TJ. Prediction of total body water in infants and children. Arch Disease Child. 2005;90:965–971. doi: 10.1136/adc.2004.067538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC, Ritz P, Davies PS, Coward WA. Factors affecting the 2H to 18O dilution space ratio in infants. Pediatr Res. 1998;43:467–471. doi: 10.1203/00006450-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Wesstrate J, Deurenberg P. Body composition in children proposal for a method for calculating body fat percentage or skinfold-thickness. Am J Clin Nutr. 1989;50:1104–1115. doi: 10.1093/ajcn/50.5.1104. [DOI] [PubMed] [Google Scholar]
- Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr. 2006;83:1047–1054. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- Yajnik C. Interactions of perturbations in intrauterine growth and growth during childhood on the risk of adult-onset disease. Proc Nutr Soc. 2000;59:257–265. doi: 10.1017/s0029665100000288. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- Yao M, Nommsen-Rivers L, Dewey K, Urlando A. Preliminary evaluation of a new pediatric air displacement plethysmograph for body composition assessment in infants. Acta Diabetol. 2003;40(Suppl 1):S55–S58. doi: 10.1007/s00592-003-0027-9. [DOI] [PubMed] [Google Scholar]
- Ziegler EE, O’Donnell AM, Nelson SE, Fomon SJ. Body composition of the reference fetus. Growth. 1976;40:329–341. [PubMed] [Google Scholar]