Abstract
Objective
To characterize exercise behaviors among adolescents with anorexia nervosa (AN), atypical AN, or bulimia nervosa (BN), and determine associations between exercise and medical risk.
Study design
Cross-sectional electronic medical records of all patients evaluated by the Eating Disorder Program at Stanford between January 1997 and February 2011 were retrospectively reviewed.
Results
1,083 subjects (961 females, 122 males; mean age 15.6) met eligibility criteria. Most patients (89.7%) reported exercise (mean 7.0 hours per week over mean 5.4 days per week) prior to presentation. Running (49.9%), calisthenics (40.7%), walking (23.4%), soccer (20.9%), and swimming (18.2%) were the most common exercises; a majority (60.6%) reported team sport participation. Males were less likely to report team exercise (p=0.005). Bradycardia (heart rate <50) at presentation was associated with team sport participation (adjusted odds ratio [AOR] 1.66, 95% confidence interval [CI] 1.02–2.72) and hours of exercise per week (AOR 1.05, 95% CI 1.02–1.09), controlling for diagnosis, sex, age, duration of illness, rate of weight loss, and percent median body mass index (%mBMI).
Discussion
Adolescents with AN, atypical AN, and BN reported high levels of exercise. Females reported more team sport participation. Greater exercise frequency and team sports participation were associated with bradycardia. Further studies assessing the relationship between exercise and bradycardia may help inform the medical management of adolescents with these eating disorders who are more physically active.
Keywords: Exercise, physical activity, team sports, anorexia nervosa, atypical anorexia nervosa, bulimia nervosa, bradycardia, QTc, cardiovascular
Introduction
Eating disorders (EDs) including anorexia nervosa (AN), atypical AN, and bulimia nervosa (BN) are major public health concerns with the highest mortality rates among psychiatric diagnoses (Arcelus, Mitchell, Wales, & Nielsen, 2011). EDs commonly present in adolescence and are associated with serious medical consequences including bradycardia, hypotension, electrolyte disturbances, gastrointestinal manifestations, low bone density, increased fracture risk, altered body composition, and amenorrhea (Campbell & Peebles, 2014; Nagata et al., 2017a; Nagata, Golden, Leonard, Copelovitch, & Denburg, 2017; Nagata et al., 2017b).
Exercise behaviors among adolescents with AN, atypical AN, or BN (hereafter referred to as AN or BN for simplicity) are not well characterized, and are relatively understudied compared to nutritional and food-related aspects of EDs (Giel et al., 2016; Noetel, Dawson, Hay, & Touyz, 2017). For instance, using the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013) exercise is not a listed diagnostic criterion in the diagnosis of AN whereas subtype classification is based on dietary behavior including restricting or binge-eating (Gummer et al., 2015). There is a wide range in the reported prevalence of exercise among adolescents with EDs, from 16.7% to 85.3%, according to a recent systematic review (Fietz, Touyz, & Hay, 2014). Controversy in the literature regarding how to define clinically significant exercise in EDs and what standard terminology to use (for instance, “excess exercise,” “compulsive exercise,” or “obligatory exercise”) may further contribute to the barriers for researching this topic (Gummer et al., 2015; Noetel et al., 2017).
Certain sports and exercise activities have been shown to be associated with an increased risk for EDs. For instance, aesthetic sports such as gymnastics and figure skating are associated with an increased risk for EDs given the importance of appearance and body image in these sports (Joy, Kussman, & Nattiv, 2016; Thiemann et al., 2015). In addition, sports involving weight classes and routine weight checks such as wrestling and rowing also have been associated with the development of an ED (Joy et al., 2016). Although these certain sports have been identified as high risk for the development of EDs, the rates of participation in other types of exercise and sports in adolescents with EDs are not well characterized. Previous literature has also described the female athlete triad, which refers to the interrelationship among energy availability, menstrual function, and bone mineral density (International Olympic Committee Medical Commission Work Group Women in Sport, 2005; Joy et al., 2014; Mountjoy et al., 2014; Nattiv et al., 2007), and highlights a large overlap of sports and EDs in female athletes. However, less research on EDs has focused on males (Mitchison, Hay, Slewa-Younan, & Mond, 2014; Murray, Griffiths, & Mond, 2016), where exercise may be more centrally implicated in illness presentations of AN (Murray, Griffiths, Rieger, & Touyz, 2014; Nurkkala et al., 2016) and sex differences in the frequency, duration, and types of exercise remain largely unknown.
High levels of exercise and physical activity among patients with EDs are associated with several adverse outcomes. When present prior to admission and after discharge, exercise has been shown to be associated with poorer treatment outcomes (Dalle Grave, Calugi, & Marchesini, 2008) and a longer course of treatment (Solenberger, 2001). In the setting of AN, high levels of exercise are associated with a longer duration of illness (Kostrzewa et al., 2013), a higher rate of dropouts (El Ghoch et al., 2013), and a greater risk of relapse (Strober, Freeman, & Morrell, 1997). Although exercise is associated with bradycardia among athletes (Guasch & Mont, 2017), to our knowledge, previous studies have not investigated the effect of exercise on bradycardia and vital sign abnormalities in the setting of AN or BN. Determining the association between exercise and medical risk is particularly important given the lack of standardized guidelines for the management of exercise in adolescents with AN or BN (Noetel et al., 2017). In addition, the relationship between exercise and the corrected QT interval (QTc) is not well characterized, particularly among adolescents with AN or BN. The QTc is the measure of the time between the start of the Q wave and the end of the T wave on the patient’s electrocardiogram, corrected for heart rate, which represents the electrical depolarization and repolarization of the ventricles of the heart (Postema & Wilde, 2014). A lengthened QTc interval may be associated with increased risk for a ventricular arrhythmia and sudden death (Padfield et al., 2016; Postema & Wilde, 2014). Previous studies in non ED populations demonstrated lengthening (Meher, Bhattacharjee, Rampal, Kapoor, & Sharma, 2014), shortening (Chinushi et al., 2012; Srinath C.G., 2011), or mixed results (Berger et al., 2011) of the QTc post-exercise.
Therefore, the objectives of this study were 1) to characterize the frequency, duration, and types of exercise in adolescents with AN or BN; 2) to identify sex differences in exercise behaviors and hours per week of exercise; and 3) to determine the association between exercise behaviors and vital sign abnormalities (such as bradycardia and hypotension) at presentation. We hypothesized that males would engage in more hours per week of exercise than females and that more hours per week of exercise would be associated with bradycardia among adolescents with EDs such as AN or BN.
Methods
Study population
Medical records of all patients presenting for an initial evaluation to the Eating Disorders Program at Lucile Packard Children’s Hospital, Stanford between January 1997 and February 2011, were retrospectively reviewed. Although patients were initially diagnosed using DSM-IV criteria, their clinical and psychological characteristics were re-reviewed and they were classified into a DSM-5 ED diagnosis for this study (American Psychiatric Association, 2013). Inclusion criteria included adolescents ages 10 to 19 based on the World Health Organization definition of adolescence (World Health Organization, 2014); having a DSM-5 ED diagnosis of AN, atypical AN, or BN; and availability of one or more exercise variables based on retrospective review of their medical record.
Study design
Assessments were completed by clinical staff in the Stanford Eating Disorders Program for the purposes of medical care. Demographic characteristics (sex, age), anthropometric measurements (height, weight), and clinical characteristics (duration of illness, vital signs, QTc interval) of eligible subjects were recorded from the medical record for this retrospective cross-sectional study.
The exercise questions regarding type of exercise, exercise frequency (hours per week and days per week), and team involvement in the past 30 days came from a standardized semi-structured clinical interview assessment that clinicians ask to all new patients at intake to the Stanford Eating Disorders Program. This information was extracted from the medical record as has been done in previous chart reviews on exercise in EDs (Solenberger, 2001), using a codebook and dual entry by two trained independent chart assessors from the research team. Behaviors were reported by both the adolescents and their caregivers. Several studies have validated the use of self-reported exercise obtained via interview in adolescents, including self-report of exercise frequency and up to three month recall, with 52–63% validity with heart rate monitoring and 72% validity with observation (Cale, 1994; Sallis, Buono, Roby, Micale, & Nelson, 1993; Sallis et al., 1996; Weston, Petosa, & Pate, 1997). If subjects reported multiple exercise types, the type and frequency of each was recorded. A subject who reported involvement with a team for any of their reported exercise types was considered to participate in team exercise.
DSM-IV diagnostic criteria were assessed by a psychologist or psychiatrist using a standardized clinical diagnostic interview which included information on behaviors such as binge frequency and compensatory behavior frequency, and this information was recorded in the medical record. Subjects were reassigned to DSM-5 criteria based on key changes from DSM-IV to DSM-5 criteria (such as no longer requiring <85% of expected body weight for AN, and changes in frequency of binge/purge behaviors in BN). One investigator reassigned DSM-5 criteria based on a standardized protocol agreed upon by the research team, and a second investigator checked these changes.
The duration of illness documented in the medical record was based on self-report of time of onset of symptoms. Rate of weight loss was calculated as the total weight loss (kg) divided by the duration of illness (months). Body mass index (BMI, kg/m2) was calculated and median BMI defined as the 50th percentile BMI for age and sex using the Centers for Disease Control and Prevention growth curves (Centers for Disease Control, C.D.C., 2000). Percentage median BMI (%mBMI) was defined as the patient’s BMI at presentation divided by the median BMI multiplied by 100 (Golden et al., 2015). Medical risk was based on the recommended admission criteria for the medical management of EDs from the Society for Adolescent Health and Medicine (Golden et al., 2015). Bradycardia was defined as heart rate<50 and systolic hypotension was defined as systolic blood pressure<90 (Golden et al., 2015).
The study was approved by the Stanford University Human Subjects Research and Institutional Review Board Committee.
Statistical analysis
Statistical analyses were conducted with STATA 15.0. Unadjusted differences between males and females in demographic characteristics, exercise characteristics, and health measures were calculated using independent samples t-tests for continuous variables and Pearson’s chi-square tests for categorical variables. Cohen’s d was used to calculate effect sizes of differences. Linear regression analyses were used to identify associations with clinical characteristics at presentation (heart rate, QTc interval, and systolic blood pressure) and DSM-5 diagnosis (AN was grouped with atypical AN since %mBMI is in the model), sex, age, duration of illness (log-transformed due to skewness), rate of weight loss, %mBMI, hours per week of exercise (continuous), and team exercise participation. Logistic regression analysis was used to identify associations with bradycardia and systolic hypotension and DSM-5 diagnosis, sex, age, duration of illness, rate of weight loss, %mBMI, hours per week of exercise (continuous), and team exercise participation. P<0.05 was considered statistically significant.
Results
Overall, 1,083 of 1,528 subjects (961 females and 122 males) met eligibility criteria and demographic characteristics of the sample are reported in Table 1. Mean %mBMI was 78.9 (95% confidence interval [CI] 78.4–79.4) for AN, 101.4 (95% CI 99.3–103.4) for atypical AN, and 106.0 (95% CI 103.6–108.5) for BN. Nearly all patients (89.7%) reported exercise in the month prior to presentation. Subjects exercised for a mean 7.0 hours per week over a mean of 5.4 days per week. Males were less likely than females to report team exercise (47.5% vs 62.2%, p=0.005). The most frequent exercise activities were running (49.9%), calisthenics (40.7%), walking (23.4%), soccer (20.9%), and swimming (18.2%) (Figure 1).
Table 1.
Demographic, exercise, and health characteristics of sample by sex, unadjusted comparisons
| Na | Total | Male | Female | Effect size of difference | p | |
|---|---|---|---|---|---|---|
| N | 1,083 | 1,083 | 122 | 961 | ||
| Mean or % (95% CI)b | Mean or % (95% CI)b | Mean or % (95% CI)b | Cohen’s d (95% CI)b | |||
| Demographic | ||||||
| DSM-5 diagnosis | 1,083 | <0.001c | ||||
| Anorexia nervosa | 71.4 (68.6–74.0)% | 73.0 (64.3–80.2)% | 71.2 (68.2–74.0)% | 0.04 (−0.15–0.23) | ||
| Atypical anorexia nervosa | 7.8 (6.3–9.5)% | 15.6 (10.1–23.3)% | 6.8 (5.3–8.5)% | −0.26 (−0.45– −0.07) | ||
| Bulimia nervosa | 20.9 (18.5–23.4)% | 11.5 (6.9–18.6)% | 22.1 (19.5–24.8)% | 0.33 (0.14–0.52) | ||
| Age, years | 1,083 | 15.63 (15.50 – 15.75) | 15.38 (14.99–15.78) | 15.66 (15.53–15.79) | −0.13 (−0.32–0.05) | 0.163d |
| Duration illness (months) | 1,076 | 16.52 (15.48–17.55) | 15.67 (11.85–19.49) | 16.62 (15.56–17.69) | −0.05 (−0.24–0.13) | 0.569d |
| Rate of weight loss (kg/month) | 1,025 | 1.57 (1.47–1.68) | 2.26 (1.84–2.68) | 1.48 (1.38–1.59) | 0.45 (0.25–0.64) | <0.001d |
| Exercise | ||||||
| Type of exercise | 875 | 0.005c | ||||
| Team | 60.6 (57.3–63.8)% | 47.5 (37.7–57.5)% | 62.2 (58.8–65.6)% | 0.30 (0.09–0.51) | ||
| No team | 39.4 (36.2–42.7)% | 52.5 (42.5–62.3)% | 37.8 (34.4–41.2)% | −0.30 (−0.51– −0.09) | ||
| Total days per week in prior month | 780 | 5.44 (5.27–5.61) | 5.58 (5.09–6.08) | 5.42 (5.24–5.60) | 0.07 (−0.15–0.29) | 0.545d |
| Total hours per week in prior month | 515 | 6.96 (6.34–7.58) | 8.30 (5.44–11.15) | 6.82 (6.20–7.44) | 0.21 (−0.09–0.50) | 0.169d |
| Health | ||||||
| BMI, kg/m2 | 1,083 | 17.41 (17.21–17.63) | 17.27 (16.72–17.82) | 17.43 (17.21–17.67) | −0.05 (−0.23–0.14) | 0.632d |
| %mBMIe | 1,083 | 86.28 (85.32–87.24) | 85.99 (83.50–88.48) | 86.32 (85.29–87.35) | −0.02 (−0.21–0.17) | 0.829d |
| Heart rate | 1,082 | 59.95 (59.01–60.90) | 58.37 (55.11–61.64) | 60.15 (59.17–61.14) | −0.11 (−0.30–0.08) | 0.241d |
| QTc interval | 1,025 | 392.86 (382.56–393.58) | 388.07 (382.56–393.58) | 393.48 (391.58–395.39) | −0.18 (−0.38–0.01) | 0.061d |
| QTc interval > 450 ms | 1,025 | 2.6 (1.8–3.8)% | 2.6 (0.8–7.8)% | 2.6 (1.8–3.9)% | 0.00 (−0.20–0.18) | 0.960c |
| Systolic blood pressure | 1,081 | 104.78 (104.12–105.44) | 107.91 (105.78–110.05) | 104.38 (103.69–105.07) | 0.32 (0.13–0.51) | <0.001d |
| Diastolic blood pressure | 1,080 | 60.77 (60.29–61.26) | 60.48 (59.03–61.93) | 60.81 (60.30–61.32) | −0.04 (−0.23–0.15) | 0.671d |
Different N’s are due to missing data
CI = confidence interval
Pearsons’ chi square test
Independent samples T-test
%mBMI = percentage median body mass index
Figure 1.
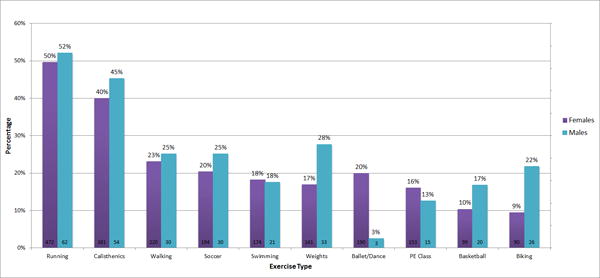
Frequency of exercise type, by sex
In multivariate linear regression analysis (Table 2), lower heart rate at presentation was associated with team exercise participation (B= −4.83, p=0.001), hours of exercise per week (B= −0.42, p<0.001), and older age (B= −1.02, p=0.011), controlling for sex, duration of illness (log-transformed), rate of weight loss, %mBMI, and ED diagnosis. In a similar analysis using logistic regression, bradycardia (heart rate <50) at presentation was associated with team exercise participation (adjusted odds ratio [AOR] 1.66, 95% confidence interval [CI] 1.02–2.72) and hours per week (continuous variable) of exercise (AOR 1.05, 95% CI 1.02–1.09), controlling for the same covariates (Appendix A). In multivariate linear regression analysis, longer QTc interval at presentation was associated with fewer approximate hours of exercise per week (B= −0.49, p=0.021)and BN diagnosis (B= −14.84, p=0.006), controlling for sex, age, duration of illness (log-transformed), rate of weight loss, %mBMI, and team exercise participation (Table 2).
Table 2.
Linear regression analysis of exercise and other covariates associated with vital signs at presentation, adjusted models
| B (95% CI)a | p | R2 | ||
|---|---|---|---|---|
| Heart rate | AN, including atypical (vs. BN) | −0.63 (−5.29 to 4.04) | 0.792 | 0.13 |
| Female (vs. male) | −0.30 (−5.72 to 5.11) | 0.913 | ||
| Age | −1.02 (−1.80 to −0.23) | 0.011 | ||
| Duration of illness (log-transformed) | 1.53 (−0.84 to 3.90) | 0.207 | ||
| Rate of weight loss | −1.53 (−0.85 to 3.90) | 0.052 | ||
| %mBMIb | 0.09 (−0.03 to 0.21) | 0.124 | ||
| Exercise: hours per week | −0.42 (−0.61 to −0.23) | <0.001 | ||
| Team | −4.83 (−7.79 to −1.86) | 0.001 | ||
| QTc interval | AN, including atypical (vs. BN) | −14.84 (−25.30 to −4.39) | 0.006 | 0.08 |
| Female (vs. male) | 3.61 (−8.12 to 15.34) | 0.546 | ||
| Age | −0.78 (−2.50 to 0.94) | 0.372 | ||
| Duration of illness (log-transformed) | −0.69 (−5.90 to 4.51) | 0.793 | ||
| Rate of weight loss | −0.36 (−2.91 to 2.19) | 0.784 | ||
| %mBMIb | 0.11 (−0.15 to 0.38) | 0.385 | ||
| Exercise: hours per week | −0.49 (−0.91 to −0.07) | 0.021 | ||
| Team | −5.77 (−12.21 to 0.68) | 0.079 | ||
| Systolic blood pressure | AN, including atypical (vs. BN) | 0.94 (−2.30 to 4.19) | 0.568 | 0.20 |
| Female (vs. male) | −7.10 (−10.86 to −3.33) | <0.001 | ||
| Age | 0.93 (0.38 to 1.47) | 0.001 | ||
| Duration of illness (log-transformed) | −1.41 (−3.06 to 0.25) | 0.095 | ||
| Rate of weight loss | −1.25 (−2.07 to −0.44) | 0.003 | ||
| %mBMIb | 0.31 (0.23 to 0.39) | <0.001 | ||
| Exercise: hours per week | −0.02 (−0.16 to 0.11) | 0.768 | ||
| Team | 1.01 (−1.05 to 3.08) | 0.334 |
95% CI = 95% confidence interval
%mBMI = percentage median body mass index
Appendix B reviews differences between patients who had documentation of responses to exercise-related questions in the medical record and those who did not have sufficient data in the medical record to be included in analyses. There were no significant differences in in diagnosis, age, duration of illness, rate of weight loss, or %mBMI between the males in the sample and males missing exercise data. There were no significant differences in diagnosis, duration of illness, or %mBMI between the females in the sample and females missing exercise data, although females with exercise data were slightly older (15.65 vs 15.27 years) and had a lower rate of weight loss (1.5 vs 1.75 kg/month).
Discussion
The vast majority of adolescents with AN or BN in this sample engaged in regular exercise prior to presentation. Females reported more team sport participation than males. Hours of exercise per week and team exercise participation were independently associated with bradycardia. Characterizing exercise activity in adolescents with AN or BN and identifying associations with bradycardia are particularly important given that there are no current guidelines on the management of exercise in EDs including AN or BN (Noetel et al., 2017).
Approximately 90% of patients with AN and BN in this study reported exercise in the month prior to presentation. This is higher than previous estimates of 16.7 to 85.3% reported in a recent systematic review of the exercise literature (Fietz et al., 2014). The largest sample size among the 10 articles included in the systematic review was 398 adolescents (Colleen Stiles-Shields, Labuschagne, Goldschmidt, Doyle, & Le Grange, 2012), and most of the studies had samples sizes less than 100 subjects. Our study is the largest clinical sample we are aware of to date to report exercise data in adolescents with AN and BN.
We also determined sex differences in exercise behaviors. Overall, compared to females, males were less likely to participate in team sports. This finding suggests that males with AN or BN may have been more internally or individually driven in their exercise habits. While we did not have sufficient data in this retrospective sample to accurately assess whether exercise behaviors were compulsive in quality, sex differences in exercise compulsions deserve further examination in future studies. Males may also typically endorse more muscularity-oriented body concerns (McCreary & Sasse, 2000; Olivardia, Pope Jr., Borowiecki III, & Cohane, 2004), which may necessitate specific muscle-building exercise patterns, rather than engagement in conventional team sports. Rather than restricting food as a primary means of pursuing thinness, males may have increased exercise activity in an effort to increase muscle mass (Murray et al., 2016). Reflecting this, our data revealed that males with AN or BN reported using weights more than their female counterparts.
There may be several reasons that adolescent females with AN or BN reported more team participation than adolescent males. Aesthetic team sports with high risk for disordered eating such as gymnastics have higher participation among females than males. One study estimated a 42% prevalence of EDs in female aesthetic sports athletes (Sundgot-Borgen & Torstveit, 2004). Furthermore, studies have shown a higher rate of disordered eating among female athletes compared to male athletes in team endurance sports such as cross-country running where low body mass is seen as advantageous. One study found that the prevalence of EDs in endurance sports was 24% in females and 9% in males (Sundgot-Borgen & Torstveit, 2004). In addition, in weight class sports (crew, wrestling) females have reported a higher rate of disordered eating compared to males (35.3% vs 17.1%) (Rosendahl, Bormann, Aschenbrenner, Aschenbrenner, & Strauss, 2009). Finally, some of the muscularity-oriented exercise behaviors more commonly observed in adolescent males, such as weightlifting, may be less commonly a team-oriented activity (Bal, Singh, & Singh, 2010).
We found that more hours per week of exercise and participation in team sports were independently associated with clinically significant bradycardia warranting admission to the hospital among adolescents with AN or BN. Some team sports may normalize disordered eating behaviors. For instance, one study of 2,532 high school wrestlers showed that 72% engaged in at least one potentially harmful weight loss method (including fasting, restricting, vomiting, heated wrestling rooms, and saunas) each week of wrestling season (Kiningham & Gorenflo, 2001). One study of 274 female undergraduates found that females who participated in team sports had higher levels of ED symptomatology than those who did not (Holm-Denoma, Scaringi, Gordon, Van Orden, & Joiner, 2009). Team sports may also push adolescents to train harder in terms of exercise; with a trend in early specialization in sports, team sports may now involve year-round training, participation on multiple teams on the same sport, and focused participation in a single sport at a young age (Myer et al., 2015).
Exercise and athleticism have been shown to be associated with bradycardia in previous studies; this is thought to be due to increased vagal tone as well as physiological adaption to the increased stroke volume being generated by a well-conditioned cardiac muscle (Carrick-Ranson et al., 2012; Guasch & Mont, 2017). However, most studies have not examined associations between bradycardia and exercise in the context of AN or BN and malnutrition, wherein autonomically mediated changes have occurred and the cardiac muscle has often lost mass (Biadi et al., 2001; Galetta et al., 2003; Nudel, Gootman, Nussbaum, & Shenker, 1984). Because there are no published guidelines for the medical management of exercise in AN or BN with relation to vital sign abnormalities (Noetel et al., 2017), it is important to continue research in this field to inform clinical care. Degree of bradycardia has been shown to be directly correlated with severity of malnutrition (Spaulding-Barclay, Stern, & Mehler, 2016). In the setting of bradycardia, additional exercise may contribute to even lower heart rates, and the medical significance of this in populations with AN or BN is unclear.
We found that shorter QTc intervals were associated with increased exercise in this sample. In general, the QT interval is affected by the heart rate and the relative activity of sympathetic and parasympathetic nerves (Meher et al., 2014). We used the corrected QT interval (QTc) in our analyses which corrects for heart rate. The effect of exercise on QTc is highly variable as some studies have demonstrated lengthening (Meher et al., 2014), shortening (Chinushi et al., 2012; Srinath C.G., 2011), or mixed results (Berger et al., 2011) of the QTc post-exercise. Increased sympathetic nerve activity and catecholamine secretion may be associated with QT interval shortening (Chinushi et al., 2012). Autonomic imbalance with sympathetic or parasympathetic modulation has been observed in adolescents with AN and may explain the association between exercise and QTc in this population (Koschke et al., 2010). However, only 2.6% of our sample had a prolonged QTc >450 ms, so the association between exercise and QTc in this sample may have limited clinical significance.
Limitations of this cross-sectional study included the retrospective analysis of medical records, which precluded causal inferences. Data were limited to what information was gathered by clinicians in the medical record, and there was the possibility for selection bias given that we only included subjects who had documentation of responses to exercise-related questions in the medical record; however, there were no clinically significant differences in diagnosis, age, duration of illness, rate of weight loss, or %mBMI between the sample and those missing exercise data (Appendix B). A slightly higher proportion of adolescent boys had exercise data, and this may further reflect sex differences, as boys with AN or BN may present with more muscularity-oriented concerns than girls and therefore may be more likely to discuss exercise at intake (Murray et al., 2016). Moreover, exercise data was not drawn from psychometrically validated self-report measures, and all data came from standardized clinician-administered semi-structured interviews. However, semi-structured clinical interviews are deemed acceptable methods of assessment for eating disorder psychopathology, and in some instances may even be preferable to self-report methods (Carter, Aime, & Mills, 2001). There is a need to develop a brief, low-cost, and practical measure of exercise in adolescents that can be used in the clinical setting to inform the medical management of AN and BN. Nonetheless, the exercise information obtained by self-report during a medical interview is important because it remains the current standard by which most clinical decisions are based, and we believe the size of this sample as well as the detail of clinical information collected make this a valuable contribution to the literature. Adolescents with AN or BN may underreport exercise because they may attempt to minimize their ED severity or have previously received recommendations to restrict their exercise. Further, patients’ perceptions of exercise activity and what was even considered exercise may have been distorted by their ED-related cognitions (Bratland-Sanda & Sundgot-Borgen, 2013). We lacked direct collection of exercise data via methods such as accelerometry (Gummer et al., 2015), did not evaluate the role of exercise in ED psychopathology, did not have healthy controls, and did not have longitudinal exercise data so are only able to comment on exercise activity at the time of presentation. Although we calculated an estimated rate of weight loss, subjects’ weight loss may not occur at a steady rate over the whole duration of illness.
Nonetheless, this study had several strengths. We had a large clinical sample that included 122 males, who are generally an understudied population in the literature on AN and BN. Although a minority of our treatment-seeking sample (11.3%) was male, this sex imbalance was consistent with estimates of males in other treatment-seeking samples making up about 5–15% of the ED population (Andersen & Holman, 1997). Other strengths of the study included systematic data collection of clinical characteristics by clinicians in a specialized ED program using standardized protocols. Finally, this study used recently established DSM-5 criteria to classify ED diagnoses of AN, atypical AN, and BN (American Psychiatric Association, 2013).
The current findings that exercise is associated with bradycardia may inform future guidelines for the medical management of AN and BN. Because current medical admission criteria for AN and BN in adolescents are based on bradycardia (heart rate <50) (Golden et al., 2015), adolescents with AN or BN engaging in exercise, particularly if the exercise activity is to be increased, should have regular medical monitoring of their heart rate. Because %mBMI was also significantly associated with bradycardia, it is important to ensure that nutritional rehabilitation adequately accounts for the energy exerted during exercise. Although beyond the scope of this study, other potential areas for development of exercise guidelines for AN/BN include the involvement of an interdisciplinary team, screening for and management of exercise-related psychopathology, and the development of an individualized exercise plan with a graded program (Cook et al., 2016).
A high proportion of adolescents with AN or BN engaged in regular exercise prior to presentation. Females were more likely to report team sports participation. Greater exercise frequency and team sports participation were independent risk factors for bradycardia. Future research should evaluate if the bradycardia or QTc changes associated with exercise confers a significantly higher risk of arrhythmias or sudden death in adolescents with AN or BN. Additional studies assessing sex differences in exercise behaviors and the relationship between exercise and bradycardia in the setting of AN or BN and malnutrition may help inform the medical management of physically active adolescents with AN or BN.
Acknowledgments
Funding Source: Supported by the National Institutes of Health (K23DK100558 to RP, K23 MH115184 to SM, and 5R01HD08216602 to NG); the Pediatric Scientist Development Program (American Academy of Pediatrics and American Pediatric Society) to JN; and the Hilda and Preston Davis Foundation to RP.
Appendix A. Logistic regression analysis of exercise and other covariates associated with bradycardia, adjusted models
| AORb (95% CI)c | p | ||
|---|---|---|---|
| Bradycardia (heart rate <50) | |||
| AN, including atypical (vs. BN) | 2.18 (0.93 to 5.10) | 0.072 | |
| Female (vs. male) | 0.86 (0.36 to 2.04) | 0.731 | |
| Age | 1.06 (0.93 to 1.20) | 0.390 | |
| Duration of illness (log-transformed) | 1.05 (0.71 to 1.55) | 0.810 | |
| Rate of weight loss | 1.18 (0.98 to 1.43) | 0.086 | |
| %mBMIa | 0.98 (0.96 to 1.00) | 0.126 | |
| Exercise: hours per week | 1.05 (1.02 to 1.09) | 0.001 | |
| Team | 1.66 (1.02 to 2.72) | 0.043 |
%mBMI = percentage median body mass index
Adjusted odds ratio, adjusted for all other variables in the model
95% CI = 95% confidence interval
Appendix B. Comparison of characteristics of eligiblea adolescents with and without exercise data
| Males (n=155)
|
Females (n=1,373)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| With exercise data | Missing exercise data | Effect size of difference | p
|
With exercise data | Missing exercise data | Effect size of difference | p
|
|
|
|
|
|||||||
| N=1,528 | 122 (78.7%) | 33 (21.3%) | 961 (70.0%) | 412 (30.0%) | ||||
| Mean or % (95% CI)b | Mean or % (95% CI)b | Cohen’s d (95% CI)b | Mean or % (95% CI)b | Mean or % (95% CI)b | Cohen’s d (95% CI)b | |||
| DSM-5 Diagnosis | 0.688 | 0.746 | ||||||
| Anorexia nervosa | 71.4 (68.7–74.0)% | 72.7 (54.3–85.7)% | −0.01 (−0.39–0.37) | 71.2 (68.3–74.0)% | 68.9 (64.3–73.2)% | −0.05 (−0.17–0.07) | ||
| Atypical anorexia nervosa | 7.7 (6.3–9.5)% | 18.2 (8.0–36.1)% | 0.07 (−0.31–0.46) | 6.8 (5.3–8.5)% | 7.5 (5.3–10.5)% | 0.03 (−0.09–0.15) | ||
| Bulimia nervosa | 20.8 (18.5–23.4)% | 9.1 (2.8–25.9)% | −0.07 (−0.46–0.31) | 22.0 (19.5–24.8)% | 23.5 (19.7–27.9)% | 0.04 (−0.08–0.15) | ||
| Age, years | 15.41 (15.01 – 15.81) | 15.19 (14.46 – 15.93) | −0.10 (−0.48–0.28) | 0.611 | 15.65 (15.52 – 15.78) | 15.27 (15.09 – 15.44) | −0.20 (−0.31– −0.08) | <0.001 |
| Duration illness (months) | 16.51 (15.48 – 17.55) | 15.79 (10.24 – 21.33) | 0.00 (−0.38–0.39) | 0.990 | 16.61 (15.54–17.67) | 15.51 (14.18–15.44) | −0.07 (−0.19–0.05) | 0.241 |
| Rate of weight loss | 2.25 (1.83 – 2.67) | 2.32 (1.53 – 3.11) | 0.03 (−0.38–0.44) | 0.878 | 1.50 (1.39–1.61) | 1.75 (1.54–1.96) | 0.14 (0.02–0.26) | 0.024 |
| %mBMIc | 85.92 (84.05 – 88.29) | 87.12 (82.94 – 91.29) | 0.09 (−0.30–0.47) | 0.650 | 86.32 (85.29 – 87.35) | 87.10 (85.59–88.62) | 0.05 (−0.07–0.16) | 0.408 |
Patients with AN, atypical AN, or BN who were 10–19 years old
95% CI = 95% confidence interval
%mBMI = percentage median body mass index
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Andersen AE, Holman JE. Males with eating disorders: Challenges for treatment and research. Psychopharmacology Bulletin. 1997;33(3):391–397. [PubMed] [Google Scholar]
- Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Archives of General Psychiatry. 2011;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- Bal BS, Singh B, Singh O. Achievement motivation and locus of control of university level and team sport players- a prognostic study. Journal of Physical Education and Sports Management. 2010;1(3):33–36. [Google Scholar]
- Berger WR, Gow RM, Kamberi S, Cheung M, Smith KR, Davis AM. The QT and corrected QT interval in recovery after exercise in children. Circulation Arrhythmia and Electrophysiology. 2011;4(4):448–455. doi: 10.1161/CIRCEP.110.961094. [DOI] [PubMed] [Google Scholar]
- Biadi O, Rossini R, Musumeci G, Frediani L, Masullo M, Ramacciotti CE, Mariani M. Cardiopulmonary exercise test in young women affected by anorexia nervosa. Italian Heart Journal : Official Journal of the Italian Federation of Cardiology. 2001;2(6):462–467. [PubMed] [Google Scholar]
- Bratland-Sanda S, Sundgot-Borgen J. Eating disorders in athletes: Overview of prevalence, risk factors and recommendations for prevention and treatment. European Journal of Sport Science. 2013;13(5):499–508. doi: 10.1080/17461391.2012.740504. [DOI] [PubMed] [Google Scholar]
- Cale L. Self-report measures of children’s physical activity: Recommendations for future development and a new alternative measure. Health Education Journal. 1994;53(4):439–453. [Google Scholar]
- Campbell K, Peebles R. Eating disorders in children and adolescents: State of the art review. Pediatrics. 2014;134(3):582–592. doi: 10.1542/peds.2014-0194. [DOI] [PubMed] [Google Scholar]
- Carrick-Ranson G, Doughty RN, Whalley GA, Walsh HJ, Gamble GD, Baldi JC. The larger exercise stroke volume in endurance-trained men does not result from increased left ventricular early or late inflow or tissue velocities. Acta Physiologica (Oxford, England) 2012;205(4):520–531. doi: 10.1111/j.1748-1716.2012.02430.x. [DOI] [PubMed] [Google Scholar]
- Carter JC, Aime AA, Mills JS. Assessment of bulimia nervosa: A comparison of interview and self-report questionnaire methods. The International Journal of Eating Disorders. 2001;30(2):187–192. doi: 10.1002/eat.1071. pii. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control C.D.C. Growth charts. 2000 Retrieved from http://www.cdc.gov/growthcharts/
- Chinushi M, Sato A, Iijima K, Suzuki K, Hiroshi F, Izumi D, Aizawa Y. Exercise-related QT interval shortening with a peaked T wave in a healthy boy with a family history of sudden cardiac death. Pacing and Clinical Electrophysiology : PACE. 2012;35(8):e239–42. doi: 10.1111/j.1540-8159.2012.03363.x. [DOI] [PubMed] [Google Scholar]
- Colleen Stiles-Shields E, Labuschagne Z, Goldschmidt AB, Doyle AC, Le Grange D. The use of multiple methods of compensatory behaviors as an indicator of eating disorder severity in treatment-seeking youth. The International Journal of Eating Disorders. 2012;45(5):704–710. doi: 10.1002/eat.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook BJ, Wonderlich SA, Mitchell JE, Thompson R, Sherman R, McCallum K. Exercise in eating disorders treatment: Systematic review and proposal of guidelines. Medicine and Science in Sports and Exercise. 2016;48(7):1408–1414. doi: 10.1249/MSS.0000000000000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Grave R, Calugi S, Marchesini G. Compulsive exercise to control shape or weight in eating disorders: Prevalence, associated features, and treatment outcome. Comprehensive Psychiatry. 2008;49(4):346–352. doi: 10.1016/j.comppsych.2007.12.007. [DOI] [PubMed] [Google Scholar]
- El Ghoch M, Calugi S, Pellegrini M, Milanese C, Busacchi M, Battistini NC, Dalle Grave R. Measured physical activity in anorexia nervosa: Features and treatment outcome. The International Journal of Eating Disorders. 2013;46(7):709–712. doi: 10.1002/eat.22140. [DOI] [PubMed] [Google Scholar]
- Fietz M, Touyz S, Hay P. A risk profile of compulsive exercise in adolescents with an eating disorder: A systematic review. Advances in Eating Disorders. 2014;2(3):241–263. doi: 10.1080/21662630.2014.894470. [DOI] [Google Scholar]
- Galetta F, Franzoni F, Prattichizzo F, Rolla M, Santoro G, Pentimone F. Heart rate variability and left ventricular diastolic function in anorexia nervosa. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine. 2003;32(6):416–421. doi: 10.1016/s1054-139x(03)00048-x. doi:S1054139X0300048X [pii] [DOI] [PubMed] [Google Scholar]
- Giel KE, Hermann-Werner A, Mayer J, Diehl K, Schneider S, Thiel A, GOAL study group Eating disorder pathology in elite adolescent athletes. The International Journal of Eating Disorders. 2016;49(6):553–562. doi: 10.1002/eat.22511. [DOI] [PubMed] [Google Scholar]
- Golden NH, Katzman DK, Sawyer SM, Ornstein RM, Rome ES, Garber AK, Kreipe RE. Update on the medical management of eating disorders in adolescents. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine. 2015;56(4):370–375. doi: 10.1016/j.jadohealth.2014.11.020. [DOI] [PubMed] [Google Scholar]
- Guasch E, Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nature Reviews Cardiology. 2017;14(2):88–101. doi: 10.1038/nrcardio.2016.173. [DOI] [PubMed] [Google Scholar]
- Gummer R, Giel KE, Schag K, Resmark G, Junne FP, Becker S, Teufel M. High levels of physical activity in anorexia nervosa: A systematic review. European Eating Disorders Review : The Journal of the Eating Disorders Association. 2015;23(5):333–344. doi: 10.1002/erv.2377. [DOI] [PubMed] [Google Scholar]
- Holm-Denoma JM, Scaringi V, Gordon KH, Van Orden KA, Joiner TE., Jr Eating disorder symptoms among undergraduate varsity athletes, club athletes, independent exercisers, and nonexercisers. The International Journal of Eating Disorders. 2009;42(1):47–53. doi: 10.1002/eat.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Olympic Committee Medical Commission Work Group Women in Sport. International olympic committee medical commission position stand on the female athlete triad. 2005 doi: 10.1002/eat.20232. [DOI] [PubMed] [Google Scholar]
- Joy E, De Souza MJ, Nattiv A, Misra M, Williams NI, Mallinson RJ, Borgen JS. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Current Sports Medicine Reports. 2014;13(4):219–232. doi: 10.1249/JSR.0000000000000077. [DOI] [PubMed] [Google Scholar]
- Joy E, Kussman A, Nattiv A. 2016 update on eating disorders in athletes: A comprehensive narrative review with a focus on clinical assessment and management. British Journal of Sports Medicine. 2016;50(3):154–162. doi: 10.1136/bjsports-2015-095735. [DOI] [PubMed] [Google Scholar]
- Kiningham RB, Gorenflo DW. Weight loss methods of high school wrestlers. Medicine and Science in Sports and Exercise. 2001;33(5):810–813. doi: 10.1097/00005768-200105000-00021. [DOI] [PubMed] [Google Scholar]
- Koschke M, Boettger MK, Macholdt C, Schulz S, Yeragani VK, Voss A, Bar KJ. Increased QT variability in patients with anorexia nervosa–an indicator for increased cardiac mortality? The International Journal of Eating Disorders. 2010;43(8):743–750. doi: 10.1002/eat.20765. [DOI] [PubMed] [Google Scholar]
- Kostrzewa E, van Elburg AA, Sanders N, Sternheim L, Adan RA, Kas MJ. Longitudinal changes in the physical activity of adolescents with anorexia nervosa and their influence on body composition and leptin serum levels after recovery. PloS One. 2013;8(10):e78251. doi: 10.1371/journal.pone.0078251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreary DR, Sasse DK. An exploration of the drive for muscularity in adolescent boys and girls. Journal of American College Health : J of ACH. 2000;48(6):297–304. doi: 10.1080/07448480009596271. [DOI] [PubMed] [Google Scholar]
- Meher A, Bhattacharjee M, Rampal P, Kapoor R, Sharma R. Effect of isometric exercise on QTc interval. Journal of Clinical and Diagnostic Research : JCDR. 2014;8(8):BC01–4. doi: 10.7860/JCDR/2014/9533.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison D, Hay P, Slewa-Younan S, Mond J. The changing demographic profile of eating disorder behaviors in the community. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-943. 943-2458-14-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Ljungqvist A. The IOC consensus statement: Beyond the female athlete triad–relative energy deficiency in sport (RED-S) British Journal of Sports Medicine. 2014;48(7):491–497. doi: 10.1136/bjsports-2014-093502. [DOI] [PubMed] [Google Scholar]
- Murray SB, Griffiths S, Mond JM. Evolving eating disorder psychopathology: Conceptualising muscularity-oriented disordered eating. The British Journal of Psychiatry : The Journal of Mental Science. 2016;208(5):414–415. doi: 10.1192/bjp.bp.115.168427. [DOI] [PubMed] [Google Scholar]
- Murray SB, Griffiths S, Rieger E, Touyz S. A comparison of compulsive exercise in male and female presentations of anorexia nervosa: What is the difference? Advances in Eating Disorders. 2014;2(1):65–70. doi: 10.1080/21662630.2013.839189. [DOI] [Google Scholar]
- Myer GD, Jayanthi N, Difiori JP, Faigenbaum AD, Kiefer AW, Logerstedt D, Micheli LJ. Sport specialization, part I: Does early sports specialization increase negative outcomes and reduce the opportunity for success in young athletes? Sports Health. 2015;7(5):437–442. doi: 10.1177/1941738115598747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata JM, Golden NH, Peebles R, Long J, Leonard MB, Carlson JL. Assessment of sex differences in bone deficits among adolescents with anorexia nervosa. International Journal of Eating Disorders. 2017a;50(4):352–58. doi: 10.1002/eat.22626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata JM, Golden NH, Leonard MB, Copelovitch L, Denburg MR. Assessment of sex differences in fracture risk among patients with anorexia nervosa: A population-based cohort study using the health improvement network. Journal of Bone and Mineral Research : The Official Journal of the American Society for Bone and Mineral Research. 2017;32(5):1082–1089. doi: 10.1002/jbmr.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata JM, Golden NH, Peebles R, Long J, Murray SB, Leonard MB, Carlson JL. Assessment of sex differences in body composition among adolescents with anorexia nervosa. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine. 2017b;60(4):455–459. doi: 10.1016/j.jadohealth.2016.11.005. doi:S1054-139X(16)30857-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP, American College of Sports Medicine American college of sports medicine position stand. the female athlete triad. Medicine and Science in Sports and Exercise. 2007;39(10):1867–1882. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- Noetel M, Dawson L, Hay P, Touyz S. The assessment and treatment of unhealthy exercise in adolescents with anorexia nervosa: A delphi study to synthesize clinical knowledge. The International Journal of Eating Disorders. 2017 doi: 10.1002/eat.22657. [DOI] [PubMed] [Google Scholar]
- Nudel DB, Gootman N, Nussbaum MP, Shenker IR. Altered exercise performance and abnormal sympathetic responses to exercise in patients with anorexia nervosa. The Journal of Pediatrics. 1984;105(1):34–37. doi: 10.1016/s0022-3476(84)80352-2. [DOI] [PubMed] [Google Scholar]
- Nurkkala M, Keranen AM, Koivumaa-Honkanen H, Ikaheimo TM, Ahola R, Pyky R, Korpelainen R. Disordered eating behavior, health and motives to exercise in young men: Cross-sectional population-based MOPO study. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3162-2. 483-016-3162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivardia R, Pope HG, Jr, Borowiecki JJ, III, Cohane GH. Biceps and body image: The relationship between muscularity and self-esteem, depression, and eating disorder symptoms. Psychology of Men & Masculinity. 2004;5(2):112–120. doi: 10.1037/1524-9220.5.2.112. [DOI] [Google Scholar]
- Padfield GJ, Escudero CA, DeSouza AM, Steinberg C, Gibbs K, Puyat JH, Krahn AD. Characterization of myocardial repolarization reserve in adolescent females with anorexia nervosa. Circulation. 2016;133(6):557–565. doi: 10.1161/CIRCULATIONAHA.115.016697. [DOI] [PubMed] [Google Scholar]
- Postema PG, Wilde AA. The measurement of the QT interval. Current Cardiology Reviews. 2014;10(3):287–294. doi: 10.2174/1573403X10666140514103612. doi:CCR-EPUB-60490 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl J, Bormann B, Aschenbrenner K, Aschenbrenner F, Strauss B. Dieting and disordered eating in german high school athletes and non-athletes. Scandinavian Journal of Medicine & Science in Sports. 2009;19(5):731–739. doi: 10.1111/j.1600-0838.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Medicine and Science in Sports and Exercise. 1993;25(1):99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Strikmiller PK, Harsha DW, Feldman HA, Ehlinger S, Stone EJ, Woods S. Validation of interviewer- and self-administered physical activity checklists for fifth grade students. Medicine and Science in Sports and Exercise. 1996;28(7):840–851. doi: 10.1097/00005768-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Solenberger SE. Exercise and eating disorders: A 3-year inpatient hospital record analysis. Eating Behaviors. 2001;2(2):151–168. doi: 10.1016/s1471-0153(01)00026-5. S1471-0153(01)00026-5 [pii] [DOI] [PubMed] [Google Scholar]
- Spaulding-Barclay MA, Stern J, Mehler PS. Cardiac changes in anorexia nervosa. Cardiology in the Young. 2016;26(4):623–628. doi: 10.1017/S104795111500267X. [DOI] [PubMed] [Google Scholar]
- Srinath CG, R S. Cardiovascular response to isometric handgrip exercise test in obese and normal weight young adults. Int J of Biomed Research. 2011;11:554–60. [Google Scholar]
- Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. The International Journal of Eating Disorders. 1997;22(4):339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. doi:10.1002/(SICI)1098-108X(199712)22:43.0.CO;2-N [pii] [DOI] [PubMed] [Google Scholar]
- Sundgot-Borgen J, Torstveit MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clinical Journal of Sport Medicine : Official Journal of the Canadian Academy of Sport Medicine. 2004;14(1):25–32. doi: 10.1097/00042752-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Thiemann P, Legenbauer T, Vocks S, Platen P, Auyeung B, Herpertz S. Eating disorders and their putative risk factors among female german professional athletes. European Eating Disorders Review : The Journal of the Eating Disorders Association. 2015;23(4):269–276. doi: 10.1002/erv.2360. [DOI] [PubMed] [Google Scholar]
- Weston AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Medicine and Science in Sports and Exercise. 1997;29(1):138–143. doi: 10.1097/00005768-199701000-00020. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Health for the world’s adolescents: A second chance in the second decade. Geneva, Switzerland: World Health Organization Press; 2014. [Google Scholar]


