Abstract
Recent epidemiological studies have identified an association between male infertility and increased cancer risk, however the underlying etiology for the shared risk has not been investigated. It is likely that much of the association between the two disease states can be attributed to underlying genetic lesions. In this article we review the reported associations between cancer and spermatogenic defects, and through database searches we identify candidate genes and gene classes that could explain some of the observed shared genetic risk. We discuss the importance of fully characterizing the genetic basis for the relationship between cancer and male infertility and propose future studies to that end.
Keywords: Cancer, Male infertility, Genetics
Introduction
Male infertility is a common disease affecting up to 6% of men in North America and at least 30 million men worldwide (1). In addition to the increasing fraction of men with poor sperm quality, the lower end of the fertility spectrum is affected with a significantly reduced overall health condition (2–4). The majority of pre-existing co-morbidities, such as obesity, chronic diseases, cardiovascular disease and metabolic syndrome, likely have a direct impact on reproductive outcomes and even life expectancy (2, 4–9). These comorbid conditions not only impact the well-being of affected men but the health risks may also transmit to their progeny (10, 11).
In some cases, the manifestation of infertility may portend a future health concern. For example, testicular cancer risk increases up to 20-fold among men with abnormal semen parameters, and the risk is 52% higher among their first-degree relatives as well (12–16). It has been proposed that various cancer phenotypes may co-occur in men with reproductive disorders due to shared pathophysiology rather than as a result of a direct metabolic intervention (17). Addressing the cancer incidence among men with poor semen parameters and/or infertility may prove challenging, as linking reproductive disorders with late onset malignancies is largely dependent on the availability and access to long-term cancer and mortality registries. A few large observational cohort studies have reached the goal, predominantly reporting the risk of prostate cancer in infertile men with mixed results (18). Most strikingly, Eisenberg et al. mined claims data for 76,083 infertile men based in the U.S. and found a 49% increased risk across a broad range of cancers (n = 18) compared to a control cohort (19). Furthermore, elevated risk of all cancers (SIR 2.9, 95% CI 1.4–5.4) was highlighted in cases of azoospermia, the most severe manifestation of male infertility (20). A recent evaluation of 10,511 men with a semen analysis as well as 63,891 siblings revealed a two-fold risk of any-site cancer and three-fold risk of acute lymphoblastic leukemia in siblings of oligozoospermic men compared with siblings of fertile controls (21). The reported findings may indicate the existence of shared pathophysiological pathways not only between male infertility and testicular cancer, as the most studied example, but potentially also a wide spectrum of other malignancies. However, the full range of co-morbid cancers and the underlying genetic mechanisms remain to be elucidated.
Genetics and genomics of male infertility
In spite of clear evidence for genetic causes of male infertility, the genetic architecture of this condition has largely remained elusive, and few variants have been confirmed as causative in male reproductive disorders, including Yq microdeletions that contribute to as much as 18% of severe oligozoospermia and non-obstructive azoospermia (NOA) cases (22), Klinefelter’s syndrome present in nearly 15% of men with severe spermatogenic defects (23) and mutations in the CFTR gene responsible for 78% of cases with congenital bilateral absence of the vas deferens. Genome-wide association studies (GWAS) have shed some additional light on the common genetic factors identifying a few susceptibility loci ((24–26); reviewed in (27)). However, GWAS studies are notoriously known to be limited to variants of low effect size (odds ratio <1.5) at intermediate frequency, and, historically, have only explained a small fraction of heritability of complex traits (28). Similar to research in other complex diseases, primary attention in male infertility has now shifted towards the low-frequency variants (minor allele frequency, MAF<5%) of large effect. Rare CNV studies have shown that men with spermatogenic failure feature a burden of rare CNVs that involves the autosomes and both sex chromosomes, and recurrent CNVs affecting specific genes can be reproducibly associated in well-powered studies (29)((30). In the case of NOA, a single exome-wide association study targeting rare variants in 962 cases and 1348 healthy controls in the discovery stage reported a variant in a DNA mismatch repair gene MSH5 that increased the risk of the disease (31). Candidate-gene based studies targeting pathways known to be essential for reproductive success have significantly expanded the list of potentially important infertility genes (reviewed in (32)), however more loci are expected to be found among the 50% of male infertility cases designated as idiopathic. For example, based on human transcriptome analysis in the Human Protein Atlas (www.proteinatlas.org), testis is the site of elevated expression for 2200 genes across all human tissues rendering them potentially sensitive to genetic disruptions. Additional insight into the identity and functional effect of genes essential for reproductive success can be drawn from research on mouse models and suggests the potential pool size of the disease genes yet to be discovered in the human. According to the Mouse Genome Informatics database (MGI; http://www.informatics.jax.org/) which integrates genomic and biologic data acquired from mouse model experiments, altogether 666 genes are known to lead to male infertility when disrupted in mice. For 531 of these genes, an orthologous locus in the human is known.
Genetics of cancer
Cancer is the predominant health burden affecting approximately 39.6% of men and women at some stage in their life (National Cancer Institute, NCI; https://www.cancer.gov/about-nci/budget) and has gained proportionally large scientific attention and funding. The budget of NCI alone is $5.4 billion in fiscal year 2017 which is boosting cancer research and rewarding the scientific and patient community with an extensive data on cancer-driving variants. Depending on the type of the malignancy, a multitude of variants can be detected per tumor with melanoma, colorectal and lung cancer positioned at the top of the list (over 100 non-synonymous mutations per tumor) (33). The somatic cancer variants tend to be recurring within the same genes and 95% of the mutations observed in common solid tumors are single-base substitution.
An expert-curated database of somatic mutations, Catalogue of Somatic Mutations in Cancer (COSMIC v82; https://cancer.sanger.ac.uk/cosmic) includes a continuously updated list of manually reviewed and well-studied genes repeatedly reported and confirmed to be altered in cancer. Although the number of genes relevant in cancer biology is certainly higher, the curated list is an accurate collection of 202 annotated cancer genes currently confirmed to be associated with cancer (August 2017). Based on the classification developed by Vogelstein et al., 116 out of the 202 (57%) curated genes are designated as cancer ‘driver genes’ promoting the malignant progression as an oncogene (n = 52) or as a tumor suppressor (n = 64) (COSMIC database; (33)). These cancer drivers can further be classified based on the core molecular pathway disrupted in the disease; 48% of the genes act in cell survival system, 44% in cell fate and 7% have an impact on genome maintenance. These same processes are known to be essential for the normal progression of spermatogenesis. Balanced fate decision of spermatogonial stem cells determines the maintenance of a sufficient pool of self-renewing and differentiating stem cells necessary for continuous spermatogenesis (34). Through multiple stages of mitosis during germ cell development, DNA integrity is protected by the mechanisms of DNA repair (35), and regulated apoptotic processes of differentiating germ cells ensure that the most vital cells reach the final mature phase of spermatozoa (36). Disruption in any of these pathways would be expected to lead to excessive loss or damage of germ cells and the associated expression of male infertility.
Shared genetic etiology in male infertility and cancer
Although several epidemiologic studies have arisen in recent years indicating an increased susceptibility of infertile men to comorbid cancer, to our knowledge, no genetic screens have been performed to investigate a shared genetic cause. Recent studies integrating the omics and literature predicted a significant genetic overlap not only for male infertility but also female reproductive disorders and particular types of cancer (37, 38). However, the extent of this overlap and which genes to study further is currently unknown.
Mouse model data, as an extensive experimental resource, could be applied to infer disease relationships and estimate whether, and what type of genes would be expected to have a pleiotropic effect. Searching the MGI database for human disease-related loci reveals 1194 genes that have been linked to various types of cancer and 666 genes to male infertility in a mouse model. Intersection of these two lists highlights 64 shared loci, which corresponds to 10% of all male infertility genes and may underlie susceptibility to both phenotypes in mice. For a similar estimate in humans, intersection was taken of 531 loci causing male infertility in mice (MGI database) and having a known ortholog in humans; and the 202 manually curated human cancer genes from the COSMIC database (COSMIC “classic” genes). This intersection identifies twenty five genes that may confer risk of experiencing male infertility and cancer in humans (Figure 1; Table 1). This overlap is highly non-random: there is a five-fold enrichment of COSMIC cancer genes in the MGI male infertility list compared to genes that are not on the MGI list (4.7% versus 0.95%, OR=5.12, p< 5 × 10−10 by Fisher Exact Test).
Figure 1. Identification of candidate genes associated with susceptibility to male infertility and cancer.
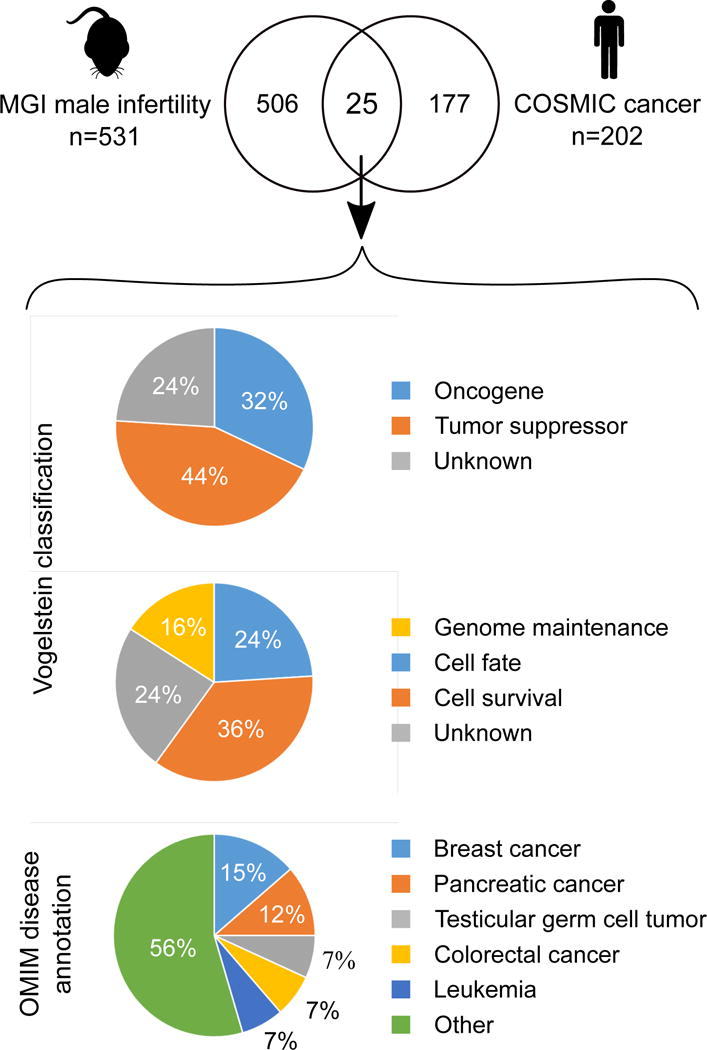
Human homologs to mouse male infertility genes were intersected with a curated list of known cancer genes (the COSMIC “classic” list). The observed overlap of 25 genes is highly non-random: there is a five-fold enrichment of COSMIC cancer genes in the MGI male infertility list compared to genes that are not on the MGI list (4.7% versus 0.95%, OR=5.12, p< 5 × 10−10 by Fisher Exact Test). We further characterized these 25 candidates by their putative role in cancer. Vogelstein classification of cancer driver genes from (33).
Table 1.
Candidate genes for male infertility and cancer co-morbidity.
| Human gene | Vogelstein classificationa
|
Hereditary cancer predisposition syndromeb | Human infertility disease | ||
|---|---|---|---|---|---|
| Cancer driver | Core pathway | Process | |||
| AR | Oncogene | Transcriptional Regulation | Cell Fate | – | Androgen insensitivity (39) |
| ATM | TSG | DNA Damage Control | Genome Maintenance | Ataxia telangiectasia | NOA (64) |
| BRCA1 | TSG | DNA Damage Control | Genome Maintenance | Hereditary breast cancer and ovarian cancer syndrome | – |
| BRCA2 | TSG | DNA Damage Control | Genome Maintenance | Hereditary breast cancer and ovarian cancer syndrome | NOA, oligozoospermia (65) |
| CDKN2A | TSG | Cell Cycle/Apoptosis | Cell Survival | Hereditary melanoma pancreatic cancer syndrome | – |
| CTNNB1 | Oncogene | APC | Cell Fate | – | – |
| DICER1 | Unknown | Unknown | Unknown | – | Idiopathic infertility (66) |
| DNMT3A | Oncogene | Chromatin Modification | Cell Fate | – | – |
| ESR1 | Unknown | Unknown | Unknown | – | Male infertility (67) |
| FGFR3 | Oncogene | PI3K; RAS; STAT | Cell Survival | – | – |
| H3F3A | Oncogene | Chromatin Modification | Cell Fate | – | – |
| H3F3B | Unknown | Unknown | Unknown | – | – |
| KIT | Oncogene | PI3K; RAS; STAT | Cell Survival | Familial gastrointestinal stromal tumor | Oligospermia (68) |
| MLH1 | TSG | DNA Damage Control | Genome Maintenance | Muir Torre syndrome, Lynch syndrome | NOA, oligozoospermia (58) |
| PPM1D | Unknown | Unknown | Unknown | – | – |
| PRKACA | Unknown | Unknown | Unknown | – | – |
| PTCH1 | TSG | HH | Cell Fate | Basal cell cancers, Gorlin syndrome | – |
| RB1 | TSG | Cell Cycle/Apoptosis | Cell Survival | Retinoblastoma | – |
| RET | Oncogene | RAS; PI3K | Cell Survival | Endocrine cancer predisposition syndromes MEN2 | – |
| SMAD4 | TSG | TGF-b | Cell Survival | Juvenile polyposis | – |
| STAT3 | Unknown | Unknown | Unknown | – | – |
| STK11 | TSG | mTOR | Cell Survival | Peutz-Jeghers syndrome | – |
| TSHR | Oncogene | PI3K; MAPK | Cell Survival | – | – |
| VHL | TSG | PI3K; RAS; STAT | Cell Survival | von Hippel-Lindau syndrome (VHL) | Infertility with VHL (69) |
| WT1 | TSG | Chromatin Modification | Cell Fate | Wilms’ tumor syndrome | NOA, cryptorchidism (70, 71) |
All of the 25 ‘male infertility-cancer genes’ have been established as known factors in cancer progression and nineteen have been designated as cancer drivers (44% tumor suppressors; 32% oncogenes) according to the Vogelstein et al. classification (33) (Figure 1). Out of the three main systems affected in cancer as well as spermatogenic failure, cell survival is disrupted most frequently, followed by cell fate and genome maintenance similar to the pattern observed among all curated COSMIC cancer genes. Based on the disease annotation in Online Mendelian Inheritance in Man database (OMIM; https://www.omim.org/), these ‘male infertility-cancer genes’ can give rise to a wide range of 38 different types of malignancies with breast and pancreatic cancers being the most common (Figure 1). Four of the genes are associated with the development of male reproductive cancers, including testicular germ cell tumor (KIT, FGFR3, STK11) and Sertoli-Leydig cell tumors in the presence of goiter (DICER1).
Importantly, nine out of 25 of the intersected cancer genes have previously been linked to impaired reproductive success in humans (Table 1). For example, polymorphisms in androgen receptor (AR) are a well-known cause of not only prostate cancer but also heritable androgen insensitivity syndrome, in which male differentiation and spermatogenesis are impaired (39). Similarly, Wilms Tumor 1 (WT1) is a critical factor in male sex determination, and mutations in WT1 may cause germ cell loss in addition to a spectrum of tumors and other syndromic features (40, 41). Mutations in VHL gene may lead to male infertility related to von Hippel-Lindau disease (VHL) in humans, which is confirmed in the mouse model of the VHL disease characterized by multiple tumors and small testis with reduced sperm counts (42).
Although no genomic alterations have been linked to infertility in men for other genes in the list, there is ample evidence for a critical role of several additional genes arising from mouse models in germline biology. For example, the role of DICER1, a regulator of small non-coding RNAs, in successful differentiation of male germline, has been well documented by several groups, showing germ cell loss and male infertility upon testis-specific DICER1 knock-out in mice (43, 44). An interesting candidate gene pair of H3F3A and H3F3B, both of which code for the histone variant H3.3, has been implicated in male and female infertility in mice due to impaired regulation of chromatin dynamics (45, 46). Both RB1 and RET proteins are required for the maintenance of undifferentiated spermatogonial stem cell pool (47, 48), and CTNNB1 and SMAD4 for the regulation of Sertoli and Leydig cell signaling in testis (49–51). Lastly, knockdown of STK11 (also known as LKB1) and particularly its major splice variant LKB1(S) in mice leads to defects in spermiogenesis and spermiation (52, 53).
Heritable susceptibility to cancer and male infertility
The genetic etiology of cancer is unique in the context of the observed mutational spectrum largely comprised of somatic variants. However, approximately 5–10% of cancers are caused by hereditary lesions that have heightened attention for germline testing for cancer susceptibility. A multitude of hereditary cancer predisposition syndromes are currently known, and a wide range of malignancies develop as a result of germline mutations in singleton genes, largely in an autosomal dominant manner (reviewed in (54)). Inherited risk of ovarian and breast cancer determined by variants in BRCA1 and BRCA2 is a well-known example (55). It is feasible that a single deleterious germline mutation in a gene essential for cell survival or genome maintenance could confer heritable risk to both male infertility and cancer. Among the 25 male infertility and cancer intersection genes, alterations in 13 loci are known to underlie a specific hereditary cancer predisposition syndrome (Table 1).
The most common etiology of hereditary cancers is defects in DNA repair genes, which are essential for accurate DNA mismatch repair, base excision repair, double-strand break repair and nucleotide excision repair (56). DNA repair genes are also fundamental for maintaining the genomic integrity and stability in the environment of frequent DNA damage in the early stages of the male germline (35). Continuous mitotic cell divisions of developing germ cells lead to DNA replication stress, and mitochondrial activity and various environmental toxicants (e.g. cigarette smoking and exposure to radiation) induce DNA breakage and base modifications via excessive levels of reactive oxygen species. Oxidative stress-related DNA fragmentation is significantly more frequent in subfertile men and leads to decreased sperm motility and fertilizing potential (57). Insufficiencies in DNA repair pathways due to genetic alterations may thus confer heritable predisposition to increased risk of impaired reproductive success and cancer. Numerous mutation analyses have been conducted showing the increased risk of reproductive and cancer disorders independently associated with defects in DNA repair pathways (Table 2). The link between genetic susceptibility to cancer and infertility has been noted previously for some of the genes, including MLH1 and ERCC1 (17). DNA variants in MLH1, which is involved in mismatch repair systems, are known to cause the hereditary cancer disorder ‘Lynch syndrome’ and also confer susceptibility to azoospermia and oligozoospermia (58, 59). Interestingly, an identical mutation C8092A (rs3212986) in the DNA base excision repair gene ERCC1 has independently been linked to both idiopathic azoospermia (60) and various types of cancer, including breast carcinoma, head and neck carcinoma and adult glioma (61–63). No studies have performed a mutational screening of these genes in male infertility cases with cancer as a comorbid state. Of note, there is relatively low overlap between the genes in Table 2 and Table 1 (only MSH1 and ATM). This likely reflects the fact that cancer gene list used to construct Table 1 (the COSMIC “classic” genes) have been identified through the observation of recurrent driver mutations in large numbers of tumors (>300); genes in Table 2 have been identified as having germline cancer risk mutations. These DNA repair genes with germline risk mutations may be less likely to be observed as having somatic mutations in cancer. Nonetheless, the distinction underscores the fact that both germline and somatic mutations confer risk for cancer, and could, in principle, confer risk for infertility as well.
Table 2.
DNA repair genes independently implicated in male infertility and hereditary cancer.
| Gene | Function | Male infertility type | Representative references | Cancer type | Representative references |
|---|---|---|---|---|---|
| MLH1 | DNA mismatch repair | Azoospermia, oligozoospermia | (58) | Various | (72, 73) |
| MLH3 | DNA mismatch repair | Azoospermia, oligozoospermia | (74) | Colorectal cancer | (75, 76) |
| MSH5 | DNA mismatch repair | Azoospermia, oligozoospermia | (31, 58, 74) | Various | (77, 78) |
| PMS2 | DNA mismatch repair | Azoospermia, oligozoospermia | (58) | Various | (79, 80) |
| ATM | DNA damage response | Azoospermia | (64) | Various | (81, 82) |
| XRCC1 | DNA base excision repair | Azoospermia | (83) | Various | (84, 85) |
| ERCC1 | DNA base excision repair | Azoospermia | (60) | Various | (61–63) |
| LIG4 | DSB repair | Male infertility | (86) | Various | (87, 88) |
| XPA | DNA base excision repair | Male infertility | (89) | Various | (90, 91) |
| RAG1 | V(D)J recombination | Male infertility | (86) | Various | (92, 93) |
DSB, double strand break
Conclusions
A growing body of data derived from epidemiological studies indicates an increased risk of cancer in men with spermatogenic defects. As highlighted here, a number of shared biological processes could account for a shared etiology of male infertility and cancer, including cell survival, cell fate, and genome maintenance. While the examples cited above represent only a small fraction of genes likely to be involved in both tumorigenesis and failed spermatogenesis, they are intended to illustrate the basic cellular processes whose disruption could explain the relationship between the two conditions.
A more complete understanding of the unifying mechanisms for male infertility and cancer will require large scale, whole genome studies in both fields. As genomic data continue to accumulate for both disease classes, increased understanding of the underlying mechanisms, shared genetic etiologies and potential risk to offspring will pave the way for germline screening for cancer and infertility susceptibility loci toward improved patient care.
Capsule.
Multiple epidemiological studies have identified an association between male infertility and increased cancer risk. This article reviews the current literature and discusses potential shared genetic links between the two conditions.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health (R01HD078641).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salonia A, Matloob R, Gallina A, Abdollah F, Sacca A, Briganti A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol. 2009;56:1025–31. doi: 10.1016/j.eururo.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latif T, Kold Jensen T, Mehlsen J, Holmboe SA, Brinth L, Pors K, et al. Semen quality is a predictor of subsequent morbidity. A Danish cohort study of 4,712 men with long-term follow-up. Am J Epidemiol. 2017 doi: 10.1093/aje/kwx067. [DOI] [PubMed] [Google Scholar]
- 5.Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32:18–31. doi: 10.1093/humrep/dew284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen JM, Herning H, Aschim EL, Hjelmesaeth J, Mala T, Hanevik HI, et al. Body Mass Index Is Associated with Impaired Semen Characteristics and Reduced Levels of Anti-Mullerian Hormone across a Wide Weight Range. PLoS One. 2015;10:e0130210. doi: 10.1371/journal.pone.0130210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieniek JM, Kashanian JA, Deibert CM, Grober ED, Lo KC, Brannigan RE, et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil Steril. 2016;106:1070–5. doi: 10.1016/j.fertnstert.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 8.Lotti F, Corona G, Degli Innocenti S, Filimberti E, Scognamiglio V, Vignozzi L, et al. Seminal, ultrasound and psychobiological parameters correlate with metabolic syndrome in male members of infertile couples. Andrology. 2013;1:229–39. doi: 10.1111/j.2047-2927.2012.00031.x. [DOI] [PubMed] [Google Scholar]
- 9.Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol. 2009;170:559–65. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]
- 10.Fullston T, Ohlsson Teague EM, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27:4226–43. doi: 10.1096/fj.12-224048. [DOI] [PubMed] [Google Scholar]
- 11.McPherson NO, Fullston T, Bakos HW, Setchell BP, Lane M. Obese father’s metabolic state, adiposity, and reproductive capacity indicate son’s reproductive health. Fertil Steril. 2014;101:865–73. doi: 10.1016/j.fertnstert.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Raman JD, Nobert CF, Goldstein M. Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol. 2005;174:1819–22. doi: 10.1097/01.ju.0000177491.98461.aa. discussion 22. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RE, Hanson HA, Patel DP, Johnstone E, Aston KI, Carrell DT, et al. Cancer risk in first- and second-degree relatives of men with poor semen quality. Fertil Steril. 2016;106:731–8. doi: 10.1016/j.fertnstert.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh TJ, Croughan MS, Schembri M, Chan JM, Turek PJ. Increased risk of testicular germ cell cancer among infertile men. Arch Intern Med. 2009;169:351–6. doi: 10.1001/archinternmed.2008.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, et al. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ. 2000;321:789–92. doi: 10.1136/bmj.321.7264.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson HA, Anderson RE, Aston KI, Carrell DT, Smith KR, Hotaling JM. Subfertility increases risk of testicular cancer: evidence from population-based semen samples. Fertil Steril. 2016;105:322–8 e1. doi: 10.1016/j.fertnstert.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril. 2015;104:48–55. doi: 10.1016/j.fertnstert.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Walsh TJ. Male reproductive health and prostate cancer risk. Curr Opin Urol. 2011;21:506–13. doi: 10.1097/MOU.0b013e32834bdf14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. 2015;193:1596–601. doi: 10.1016/j.juro.2014.11.080. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril. 2013;100:681–5. doi: 10.1016/j.fertnstert.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson RE, Hanson HA, Lowrance WT, Redshaw J, Oottamasathien S, Schaeffer A, et al. Childhood Cancer Risk in the Siblings and Cousins of Men with Poor Semen Quality. J Urol. 2017;197:898–905. doi: 10.1016/j.juro.2016.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krausz C, Forti G, McElreavey K. The Y chromosome and male fertility and infertility. Int J Androl. 2003;26:70–5. doi: 10.1046/j.1365-2605.2003.00402.x. [DOI] [PubMed] [Google Scholar]
- 23.Tuttelmann F, Werny F, Cooper TG, Kliesch S, Simoni M, Nieschlag E. Clinical experience with azoospermia: aetiology and chances for spermatozoa detection upon biopsy. Int J Androl. 2011;34:291–8. doi: 10.1111/j.1365-2605.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 24.Aston KI, Carrell DT. Genome-wide study of single-nucleotide polymorphisms associated with azoospermia and severe oligozoospermia. J Androl. 2009;30:711–25. doi: 10.2164/jandrol.109.007971. [DOI] [PubMed] [Google Scholar]
- 25.Hu Z, Li Z, Yu J, Tong C, Lin Y, Guo X, et al. Association analysis identifies new risk loci for non-obstructive azoospermia in Chinese men. Nat Commun. 2014;5:3857. doi: 10.1038/ncomms4857. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Xu J, Zhang H, Sun J, Sun Y, Wang Z, et al. A genome-wide association study reveals that variants within the HLA region are associated with risk for nonobstructive azoospermia. Am J Hum Genet. 2012;90:900–6. doi: 10.1016/j.ajhg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aston KI. Genetic susceptibility to male infertility: news from genome-wide association studies. Andrology. 2014;2:315–21. doi: 10.1111/j.2047-2927.2014.00188.x. [DOI] [PubMed] [Google Scholar]
- 28.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes AM, Aston KI, Thompson E, Carvalho F, Goncalves J, Huang N, et al. Human spermatogenic failure purges deleterious mutation load from the autosomes and both sex chromosomes, including the gene DMRT1. PLoS Genet. 2013;9:e1003349. doi: 10.1371/journal.pgen.1003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang N, Wen Y, Guo X, Li Z, Dai J, Ni B, et al. A Screen for Genomic Disorders of Infertility Identifies MAST2 Duplications Associated with Nonobstructive Azoospermia in Humans. Biol Reprod. 2015;93:61. doi: 10.1095/biolreprod.115.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni B, Lin Y, Sun L, Zhu M, Li Z, Wang H, et al. Low-frequency germline variants across 6p22.2-6p21.33 are associated with non-obstructive azoospermia in Han Chinese men. Hum Mol Genet. 2015;24:5628–36. doi: 10.1093/hmg/ddv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Genetics of Male Infertility. Curr Urol Rep. 2016;17:70. doi: 10.1007/s11934-016-0627-x. [DOI] [PubMed] [Google Scholar]
- 33.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mecklenburg JM, Hermann BP. Mechanisms Regulating Spermatogonial Differentiation. Results Probl Cell Differ. 2016;58:253–87. doi: 10.1007/978-3-319-31973-5_10. [DOI] [PubMed] [Google Scholar]
- 35.Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31:309–19. doi: 10.1016/j.rbmo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Xu YR, Dong HS, Yang WX. Regulators in the apoptotic pathway during spermatogenesis: Killers or guards? Gene. 2016;582:97–111. doi: 10.1016/j.gene.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Tarin JJ, Garcia-Perez MA, Hamatani T, Cano A. Infertility etiologies are genetically and clinically linked with other diseases in single meta-diseases. Reprod Biol Endocrinol. 2015;13:31. doi: 10.1186/s12958-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagirnaja L, Vigh-Conrad K, Conrad DF. How to Map the Genetic Basis for Conditions that are Comorbid with Male Infertility. Semin Reprod Med. 2017;35:225–30. doi: 10.1055/s-0037-1603567. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887–94. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 40.Little M, Wells C. A clinical overview of WT1 gene mutations. Hum Mutat. 1997;9:209–25. doi: 10.1002/(SICI)1098-1004(1997)9:3<209::AID-HUMU2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Seabra CM, Quental S, Lima AC, Carvalho F, Goncalves J, Fernandes S, et al. The mutational spectrum of WT1 in male infertility. J Urol. 2015;193:1709–15. doi: 10.1016/j.juro.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Ma W, Tessarollo L, Hong SB, Baba M, Southon E, Back TC, et al. Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res. 2003;63:5320–8. [PubMed] [Google Scholar]
- 43.Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD. Dicer1 is required for differentiation of the mouse male germline. Biol Reprod. 2008;79:696–703. doi: 10.1095/biolreprod.108.067827. [DOI] [PubMed] [Google Scholar]
- 44.Romero Y, Meikar O, Papaioannou MD, Conne B, Grey C, Weier M, et al. Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS One. 2011;6:e25241. doi: 10.1371/journal.pone.0025241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang MC, Jacobs SA, Mattiske DM, Soh YM, Graham AN, Tran A, et al. Contribution of the two genes encoding histone variant h3.3 to viability and fertility in mice. PLoS Genet. 2015;11:e1004964. doi: 10.1371/journal.pgen.1004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuen BT, Bush KM, Barrilleaux BL, Cotterman R, Knoepfler PS. Histone H3.3 regulates dynamic chromatin states during spermatogenesis. Development. 2014;141:3483–94. doi: 10.1242/dev.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang QE, Gwost I, Oatley MJ, Oatley JM. Retinoblastoma protein (RB1) controls fate determination in stem cells and progenitors of the mouse male germline. Biol Reprod. 2013;89:113. doi: 10.1095/biolreprod.113.113159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–21. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 49.Boyer A, Yeh JR, Zhang X, Paquet M, Gaudin A, Nagano MC, et al. CTNNB1 signaling in sertoli cells downregulates spermatogonial stem cell activity via WNT4. PLoS One. 2012;7:e29764. doi: 10.1371/journal.pone.0029764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82:422–32. doi: 10.1095/biolreprod.109.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Archambeault DR, Yao HH. Loss of smad4 in Sertoli and Leydig cells leads to testicular dysgenesis and hemorrhagic tumor formation in mice. Biol Reprod. 2014;90:62. doi: 10.1095/biolreprod.113.111393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Denison FC, Smith LB, Muckett PJ, O’Hara L, Carling D, Woods A. LKB1 is an essential regulator of spermatozoa release during spermiation in the mammalian testis. PLoS One. 2011;6:e28306. doi: 10.1371/journal.pone.0028306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towler MC, Fogarty S, Hawley SA, Pan DA, Martin DM, Morrice NA, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J. 2008;416:1–14. doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 54.Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23:276–92. doi: 10.1200/JCO.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 55.Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006;25:5898–905. doi: 10.1038/sj.onc.1209879. [DOI] [PubMed] [Google Scholar]
- 56.Romero-Laorden N, Castro E. Inherited mutations in DNA repair genes and cancer risk. Curr Probl Cancer. 2017;41:251–64. doi: 10.1016/j.currproblcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9:331–45. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 58.Ji G, Long Y, Zhou Y, Huang C, Gu A, Wang X. Common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. BMC Med. 2012;10:49. doi: 10.1186/1741-7015-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerykova-Bujalkova M, Krivulcik T, Bartosova Z. Novel approaches in evaluation of pathogenicity of single-base exonic germline changes involving the mismatch repair genes MLH1 and MSH2 in diagnostics of Lynch syndrome. Neoplasma. 2008;55:463–71. [PubMed] [Google Scholar]
- 60.Ji G, Gu A, Xia Y, Lu C, Liang J, Wang S, et al. ERCC1 and ERCC2 polymorphisms and risk of idiopathic azoospermia in a Chinese population. Reprod Biomed Online. 2008;17:36–41. doi: 10.1016/s1472-6483(10)60290-8. [DOI] [PubMed] [Google Scholar]
- 61.Ding YW, Gao X, Ye DX, Liu W, Wu L, Sun HY. Association of ERCC1 polymorphisms (rs3212986 and rs11615) with the risk of head and neck carcinomas based on case-control studies. Clin Transl Oncol. 2015;17:710–9. doi: 10.1007/s12094-015-1298-7. [DOI] [PubMed] [Google Scholar]
- 62.Guo XG, Wang Q, Xia Y, Zheng L. The C8092A polymorphism in the ERCC1 gene and breast carcinoma risk: a meta-analysis of case-control studies. Int J Clin Exp Med. 2015;8:3691–9. [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Z, Ma W, Gao L, Xing B. Association between ERCC1 C8092A and ERCC2 K751Q polymorphisms and risk of adult glioma: a meta-analysis. Tumour Biol. 2014;35:3211–21. doi: 10.1007/s13277-013-1420-9. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Yu J, Zhang T, Li H, Ni Y. rs189037, a functional variant in ATM gene promoter, is associated with idiopathic nonobstructive azoospermia. Fertil Steril. 2013;100:1536–41 e1. doi: 10.1016/j.fertnstert.2013.07.1995. [DOI] [PubMed] [Google Scholar]
- 65.Zhoucun A, Zhang S, Yang Y, Ma Y, Zhang W, Lin L. The common variant N372H in BRCA2 gene may be associated with idiopathic male infertility with azoospermia or severe oligozoospermia. Eur J Obstet Gynecol Reprod Biol. 2006;124:61–4. doi: 10.1016/j.ejogrb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Qin Y, Xia Y, Wu W, Han X, Lu C, Ji G, et al. Genetic variants in microRNA biogenesis pathway genes are associated with semen quality in a Han-Chinese population. Reprod Biomed Online. 2012;24:454–61. doi: 10.1016/j.rbmo.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Ge YZ, Xu LW, Jia RP, Xu Z, Li WC, Wu R, et al. Association of polymorphisms in estrogen receptors (ESR1 and ESR2) with male infertility: a meta-analysis and systematic review. J Assist Reprod Genet. 2014;31:601–11. doi: 10.1007/s10815-014-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng P, Chen H, Liu SR, Pu XY, A ZC. SNPs in KIT and KITLG genes may be associated with oligospermia in Chinese population. Biomarkers. 2013;18:650–4. doi: 10.3109/1354750X.2013.838307. [DOI] [PubMed] [Google Scholar]
- 69.Frantzen C, Klasson TD, Links TP, Giles RH. Von Hippel-Lindau Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 70.Seabra CM, Quental S, Neto AP, Carvalho F, Goncalves J, Oliveira JP, et al. A novel Alu-mediated microdeletion at 11p13 removes WT1 in a patient with cryptorchidism and azoospermia. Reprod Biomed Online. 2014;29:388–91. doi: 10.1016/j.rbmo.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 71.Xu J, Jiang L, Yu W, Guo H, Zhang H, Wei D, et al. A novel functional variant in Wilms’ Tumor 1 (WT1) is associated with idiopathic non-obstructive azoospermia. Mol Reprod Dev. 2017;84:222–8. doi: 10.1002/mrd.22768. [DOI] [PubMed] [Google Scholar]
- 72.Gallinger S, Aronson M, Shayan K, Ratcliffe EM, Gerstle JT, Parkin PC, et al. Gastrointestinal cancers and neurofibromatosis type 1 features in children with a germline homozygous MLH1 mutation. Gastroenterology. 2004;126:576–85. doi: 10.1053/j.gastro.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Alotaibi H, Ricciardone MD, Ozturk M. Homozygosity at variant MLH1 can lead to secondary mutation in NF1, neurofibromatosis type I and early onset leukemia. Mutat Res. 2008;637:209–14. doi: 10.1016/j.mrfmmm.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Xu K, Lu T, Zhou H, Bai L, Xiang Y. The role of MSH5 C85T and MLH3 C2531T polymorphisms in the risk of male infertility with azoospermia or severe oligozoospermia. Clin Chim Acta. 2010;411:49–52. doi: 10.1016/j.cca.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 75.Vymetalkova V, Pardini B, Rosa F, Di Gaetano C, Novotny J, Levy M, et al. Variations in mismatch repair genes and colorectal cancer risk and clinical outcome. Mutagenesis. 2014;29:259–65. doi: 10.1093/mutage/geu014. [DOI] [PubMed] [Google Scholar]
- 76.Duraturo F, Liccardo R, Izzo P. Coexistence of MLH3 germline variants in colon cancer patients belonging to families with Lynch syndrome-associated brain tumors. J Neurooncol. 2016;129:577–8. doi: 10.1007/s11060-016-2203-0. [DOI] [PubMed] [Google Scholar]
- 77.Saunders EJ, Dadaev T, Leongamornlert DA, Al Olama AA, Benlloch S, Giles GG, et al. Gene and pathway level analyses of germline DNA-repair gene variants and prostate cancer susceptibility using the iCOGS-genotyping array. Br J Cancer. 2016;114:945–52. doi: 10.1038/bjc.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scarbrough PM, Weber RP, Iversen ES, Brhane Y, Amos CI, Kraft P, et al. A Cross-Cancer Genetic Association Analysis of the DNA Repair and DNA Damage Signaling Pathways for Lung, Ovary, Prostate, Breast, and Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:193–200. doi: 10.1158/1055-9965.EPI-15-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramchander NC, Ryan NA, Crosbie EJ, Evans DG. Homozygous germ-line mutation of the PMS2 mismatch repair gene: a unique case report of constitutional mismatch repair deficiency (CMMRD) BMC Med Genet. 2017;18:40. doi: 10.1186/s12881-017-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clendenning M, Senter L, Hampel H, Robinson KL, Sun S, Buchanan D, et al. A frame-shift mutation of PMS2 is a widespread cause of Lynch syndrome. J Med Genet. 2008;45:340–5. doi: 10.1136/jmg.2007.056150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su Y, Swift M. Mortality rates among carriers of ataxia-telangiectasia mutant alleles. Ann Intern Med. 2000;133:770–8. doi: 10.7326/0003-4819-133-10-200011210-00009. [DOI] [PubMed] [Google Scholar]
- 82.Pilie PG, Johnson AM, Hanson KL, Dayno ME, Kapron AL, Stoffel EM, et al. Germline genetic variants in men with prostate cancer and one or more additional cancers. Cancer. 2017 doi: 10.1002/cncr.30817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang HX, Wang XY, Zhou DX, Zheng LR, Zhang J, Huo YW, et al. Effects of low-dose, long-term formaldehyde exposure on the structure and functions of the ovary in rats. Toxicol Ind Health. 2013;29:609–15. doi: 10.1177/0748233711430983. [DOI] [PubMed] [Google Scholar]
- 84.Yi L, Xiao-Feng H, Yun-Tao L, Hao L, Ye S, Song-Tao Q. Association between the XRCC1 Arg399Gln polymorphism and risk of cancer: evidence from 297 case-control studies. PLoS One. 2013;8:e78071. doi: 10.1371/journal.pone.0078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S, Peng Q, Chen Y, You J, Chen Z, Deng Y, et al. DNA repair gene XRCC1 polymorphisms, smoking, and bladder cancer risk: a meta-analysis. PLoS One. 2013;8:e73448. doi: 10.1371/journal.pone.0073448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ji G, Yan L, Liu W, Huang C, Gu A, Wang X. Polymorphisms in double-strand breaks repair genes are associated with impaired fertility in Chinese population. Reproduction. 2013;145:463–70. doi: 10.1530/REP-12-0370. [DOI] [PubMed] [Google Scholar]
- 87.Tseng RC, Hsieh FJ, Shih CM, Hsu HS, Chen CY, Wang YC. Lung cancer susceptibility and prognosis associated with polymorphisms in the nonhomologous end-joining pathway genes: a multiple genotype-phenotype study. Cancer. 2009;115:2939–48. doi: 10.1002/cncr.24327. [DOI] [PubMed] [Google Scholar]
- 88.Roddam PL, Rollinson S, O’Driscoll M, Jeggo PA, Jack A, Morgan GJ. Genetic variants of NHEJ DNA ligase IV can affect the risk of developing multiple myeloma, a tumour characterised by aberrant class switch recombination. J Med Genet. 2002;39:900–5. doi: 10.1136/jmg.39.12.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gu A, Ji G, Zhou Y, Long Y, Shi X, Fu G, et al. Polymorphisms of nucleotide-excision repair genes may contribute to sperm DNA fragmentation and male infertility. Reprod Biomed Online. 2010;21:602–9. doi: 10.1016/j.rbmo.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 90.Gao C, Wang J, Li C, Zhang W, Liu G. A Functional Polymorphism (rs10817938) in the XPA Promoter Region Is Associated with Poor Prognosis of Oral Squamous Cell Carcinoma in a Chinese Han Population. PLoS One. 2016;11:e0160801. doi: 10.1371/journal.pone.0160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding D, Zhang Y, Yu H, Guo Y, Jiang L, He X, et al. Genetic variation of XPA gene and risk of cancer: a systematic review and pooled analysis. Int J Cancer. 2012;131:488–96. doi: 10.1002/ijc.26391. [DOI] [PubMed] [Google Scholar]
- 92.Hill DA, Wang SS, Cerhan JR, Davis S, Cozen W, Severson RK, et al. Risk of non-Hodgkin lymphoma (NHL) in relation to germline variation in DNA repair and related genes. Blood. 2006;108:3161–7. doi: 10.1182/blood-2005-01-026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu X, Gu J, Grossman HB, Amos CI, Etzel C, Huang M, et al. Bladder cancer predisposition: a multigenic approach to DNA-repair and cell-cycle-control genes. Am J Hum Genet. 2006;78:464–79. doi: 10.1086/500848. [DOI] [PMC free article] [PubMed] [Google Scholar]


