Summary
Individuals suffering from substance-use disorders develop strong associations between the drug’s rewarding effects and environmental cues, creating powerful, enduring triggers for relapse. We found that dephosphorylated, nuclear histone deacetylase 5 (HDAC5) in the nucleus accumbens (NAc) reduced cocaine reward-context associations and relapse-like behaviors in a cocaine self-administration model. We also discovered that HDAC5 associates with an activity-sensitive enhancer of the Npas4 gene and negatively regulates NPAS4 expression. Exposure to cocaine and the test chamber induced rapid and transient NPAS4 expression in a small subpopulation of FOS-positive neurons in the NAc. Conditional deletion of Npas4 in the NAc significantly reduced cocaine conditioned place preference and delayed learning of the drug-reinforced action during cocaine self-administration, without affecting cue-induced reinstatement of drug seeking. These data suggest HDAC5 and NPAS4 in the NAc are critically involved in reward-relevant learning and memory processes, and that nuclear HDAC5 limits reinstatement of drug seeking independent of NPAS4.
Introduction
Drug addiction is a long-lasting behavioral disorder characterized by compulsive drug seeking and consumption despite negative consequences to the individual. The persistence of craving and high incidence of relapse following prolonged periods of abstinence in the addicted patient population is a major hurdle for therapeutic treatments (Koob and Volkow, 2010). The formation of enduring associations between the primary rewarding properties of drugs and the environmental cues linked to drug use produce powerful triggers for relapse in abstinent addicts.
Increasing evidence suggests that epigenetic regulation of gene expression, including histone acetylation and deacetylation, contributes to the development of drug reward related behaviors linked to drug addiction (Hui et al., 2010; Kenny, 2014; Nestler, 2014; Renthal et al., 2009; Renthal et al., 2007; Taniguchi et al., 2012). One major histone deacetylase enzyme, HDAC5, shuttles between the nucleus and cytoplasm, and its subcellular distribution is influenced by both calcium and cAMP signaling pathways (Belfield et al., 2006; Chawla et al., 2003; McKinsey et al., 2000a; Taniguchi et al., 2012). Published studies indicate that HDAC5 functions, at least in part, within the NAc to limit the development of cocaine reward-context associations as measured in the conditioned place preference assay, and that cocaine exposure produces a dynamic change in HDAC5 phosphorylation and nucleocytoplasmic distribution (Renthal et al., 2007; Taniguchi et al., 2012). Immediately following repeated cocaine exposure, HDAC5 is transiently hyperphosphorylated in the NAc (Renthal et al., 2007), but a dopamine D1 receptor-cAMP-PP2A signaling pathway rapidly dephosphorylates HDAC5 and promotes its delayed, transient nuclear accumulation (Taniguchi et al., 2012). Overexpression of a dephosphorylated HDAC5 mutant in the adult NAc reduced the development, but not expression, of cocaine conditioned place preference (CPP, (Taniguchi et al., 2012), suggesting that delayed, nuclear accumulation of HDAC5 serves to inactivate cocaine-stimulated target genes that promote cocaine reward-associated learning and memory. However, the relevance of HDAC5 dephosphorylation to volitional, contingent drug taking (i.e. cocaine self-administration, SA) and reinstatement of drug seeking, as well as the HDAC5 gene targets that ultimately mediate its behavioral effects, have remained unclear.
Multiple immediately early genes (IEG) have been implicated in cocaine-induced neuroplasticity (Harlan and Garcia, 1998; Piechota et al., 2010; Ye et al., 2016). Npas4 is an IEG transcription factor that is rapidly and transiently activated by strong glutamatergic synaptic activity, membrane depolarization, and by calcium influx mediated by L-type voltage-gated channels(Lin et al., 2008). NPAS4 is reported to regulate excitatory and inhibitory synapse balance by orchestrating unique and overlapping transcriptional responses in glutamatergic and GABAergic neurons (Bloodgood et al., 2013; Lin et al., 2008; Spiegel et al., 2014). In glutamatergic neurons, NPAS4 expression increases GABAergic synapses, whereas in GABAergic interneurons, it increases glutamatergic synapses. The net effect of these synaptic changes suggests a homeostatic, negative feedback mechanism that reduces network activity of strongly activated neuronal circuits (Spiegel et al., 2014). Npas4 KO mice exhibit profound deficits in fear-related contextual long-term memory, and this was shown to involve NPAS4’s role in the hippocampal CA3 region (Ramamoorthi et al., 2011). However, a functional role for NPAS4 in the NAc or drug-related behaviors has not been reported.
We show here that HDAC5 in the adult NAc reduces cocaine-conditioned behaviors, including CPP and cued reinstatement of drug seeking in the cocaine self-administration model. We also find that HDAC5 binds to and negatively regulates the expression of the Npas4 gene, and that NPAS4 in the adult NAc is required for drug reward-related learning processes, but not for cued drug seeking.
Results
Nuclear HDAC5 limits reward-context associations and reinstatement of drug seeking
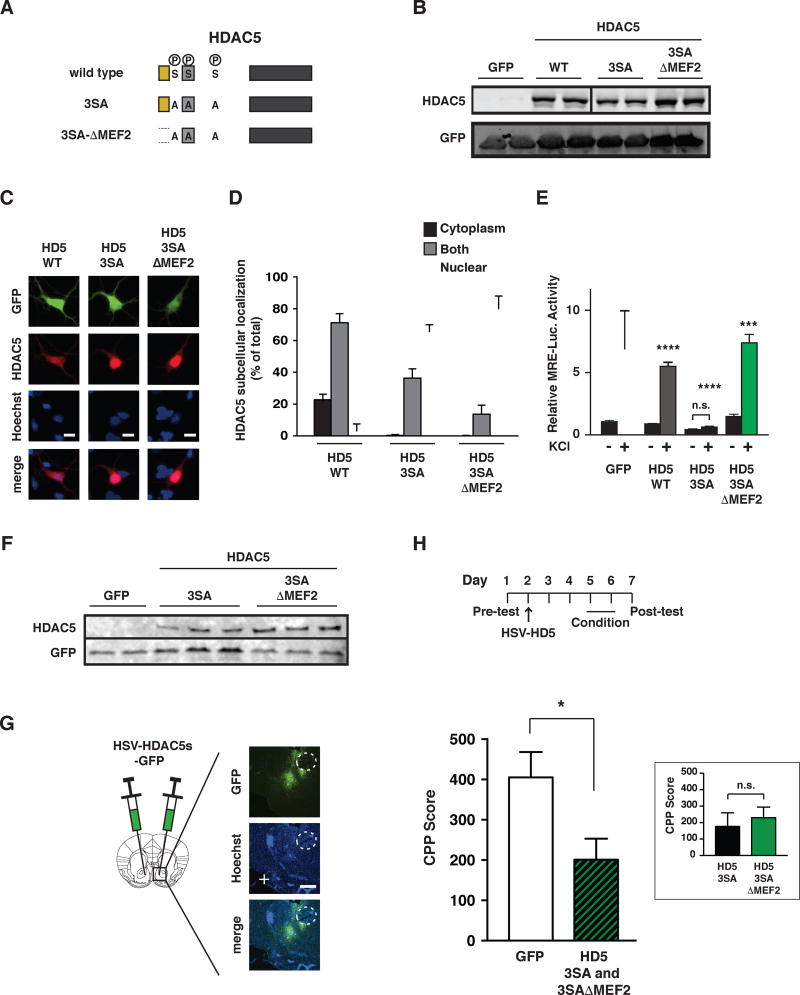
In response to cocaine administration, we observed transient dephosphorylation of three key serine residues on HDAC5 (S259, S279, S498), which promoted its nuclear accumulation. To test how dephosphorylation of these three sites impacts addiction-related behaviors, we generated neurotropic herpes simplex viruses (HSV) that expressed either wildtype HDAC5, a dephosphorylated mutant of HDAC5 (S259A/S279A/S498A or “3SA”), a dephosphorylated HDAC5 mutant lacking a critical domain needed to interact with MEF2 transcription factors (3SA-ΔMEF2) – a well-known binding partner of HDAC5 (Belfield et al., 2006; Chawla et al., 2003; McKinsey et al., 2000a; McKinsey et al., 2000b), or EGFP alone (Figure 1A–B). Compared to wildtype HDAC5, which is localized in the cytoplasm or evenly distributed in the nuclear and cytoplasmic compartments of striatal neurons, the HDAC5 3SA and 3SA-ΔMEF2 proteins were concentrated largely within the nucleus (Figure 1C–D). However, while HDAC5 3SA inhibited MEF2-dependent transcription in cultured striatal neurons, the HDAC5 3SA-ΔMEF2 failed to reduce MEF2-dependent transcription (Figure 1E).
Figure 1. Nuclear HDAC5 reduces cocaine CPP independent of MEF2 binding.
A. Domain structure of HDAC5 depicting mutated phosphorylation sites Ser259, Ser279 and Ser498 (3SA) and lacking MEF2 interaction domain (3SA-ΔMEF2). MEF2 interaction domain, nuclear localization sequence and deacetylase domain are highlighted with yellow, gray and black, respectively. B. Western blot showing similar overexpression levels of HDAC5 WT and mutants in HEK cells. C-D. Dephosphorylated HDAC5 accumulates in the nucleus of cultured striatal neurons. C-D. Representative images (Scale bar = 10 µm) and quantification of subcellular distribution of of HDAC5 WT, 3SA and 3SA-ΔMEF2 mutants (n=3 wells/condition). E. Overexpression of HDAC5 3SA, but not 3SA-ΔMEF2, blocks depolarization-induced MEF2-dependent transcription (3XMRE-luciferase reporter, n=6/condition). F. HSV-HDAC5 3SA and 3SA-ΔMEF2 mutants express at similar levels in cultured striatal neurons. G. Representative images of HSV-virus targeting in mouse NAc. aca = anterior part of anterior commissure (Scale bar = 100 µm). H. (top) Timeline of CPP assay. (Bottom) HSV-HDAC5 3SA and 3SA-ΔMEF2 reduce cocaine CPP (5 mg/kg; i.p.), but are not different from each other (inset). Data shown are mean ± SEM; *p< 0.05, ***p<0.001, ****p<0.0001, n.s. p > 0.05. See also Table S4 for detailed analyses.
We next compared the ability of HSV-HDAC5 3SA and 3SA-ΔMEF2 in the NAc to suppress CPP (Figure 1F–H) – an assay that measures an animal’s ability to learn and remember to prefer a specific environment in which the rewarding effects of drug were experienced. Compared to control, HDAC5 3SA and HDAC5 3SA-ΔMEF2 reduced the time animals spent in the previously cocaine-paired chamber (Figure 1H), but there was no difference between HDAC5 3SA and HDAC5 3SA-ΔMEF2, suggesting that dephosphorylated HDAC5 can reduce cocaine reward-context associations independent of direct binding to MEF2 transcription factors.
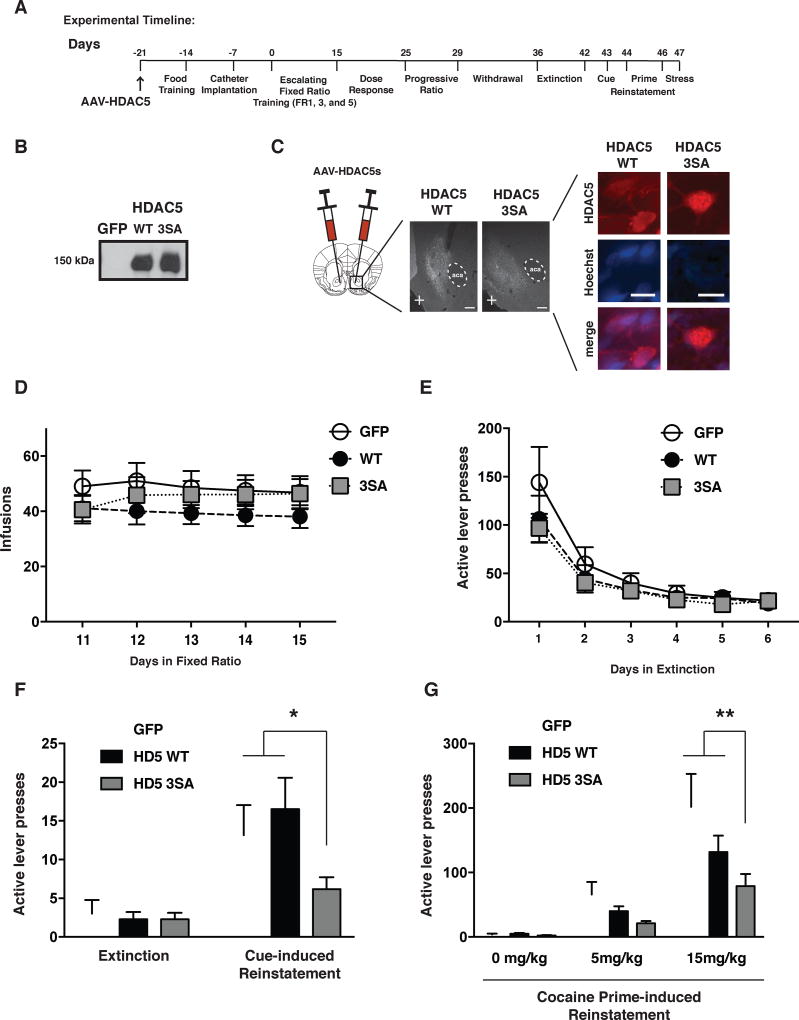
We next tested whether HDAC5 could influence volitional cocaine taking and seeking behaviors in rats trained to intravenously administer cocaine – perhaps the most relevant rodent assay for human drug addiction. Rats were initially food trained to learn operant discrimination, and then transitioned to a fixed ratio (FR) schedule of cocaine reinforcement that gradually increased from 1 (FR1) to 5 (FR5) active lever presses to receive a single infusion of cocaine. This was followed by dose response, progressive ratio, extinction training, and reinstatement test batteries as illustrated at Figure 2A and S1A. Like the effects of HSV-HDAC5 WT in cocaine CPP (Taniguchi et al., 2012), transient overexpression of HSV-HDAC5 WT, re-injected weekly in the NAc, did not alter any aspect of cocaine self-administration behavior (Figure S1A–F). To enable long-lasting, neurotropic expression of HDAC5 proteins, we generated AAV2 vectors to express HDAC5 WT, HDAC5 3SA, or GFP (control) bilaterally in the NAc (Figure 2B–C). All experimental groups exhibited comparable levels of drug intake on the FR5 schedule (Figure 2D), indicating that these vectors failed to influence the preferred level of drug intake. For all three groups, no significant changes were observed in the subsequent dose-response analysis (Figure S2A) or progressive ratio (Figure S2B) where the animal’s motivation for cocaine is assessed under an increasingly demanding response requirement. Together, these findings suggest that nuclear-enriched HDAC5 does not reduce cocaine’s reinforcing effects when voluntarily self-administered.
Figure 2. Nuclear HDAC5 in the NAc suppresses reinstatement of cocaine seeking.
A. Timeline of rat cocaine self-administration studies. B. Western blot of AAV-HDAC5 WT and 3SA expression. C. AAV2-mediated HDAC5 WT and 3SA expression in NAc. Hoechst stain (blue) = nucleus. (Scale bars = 250 µm (middle) and 10 µm (right)). D. Last 5 days of cocaine intake (0.5 mg/kg/0.05 ml infusion; FR5) from rats expressing GFP, HDAC5 WT and 3SA in the NAc (n=18–19/condition). E. Extinction training data from rats expressing GFP, HDAC5 WT and 3SA (n=17–20/condition). F. Cue-induced reinstatement of drug seeking was attenuated by overexpression of HDAC5 3SA in the NAc (n=17–20/condition). G. Cocaine prime-induced reinstatement behavior was attenuated by overexpression of HDAC5 3SA in NAc (n=18–19/condition). Data shown are mean ± SEM; * p < 0.05, ** p < 0.01. See also Figure S1, S2 and Table S4.
Since HDAC5 S279A and HDAC5 3SA/3SA-ΔMEF2 significantly reduced cocaine CPP (Figure 1H), we hypothesized that nuclear-enriched HDAC5 mutants might reduce the enduring link formed between the rewarding effects of cocaine and cues in the environment associated with drug taking. To test this idea, self-administering animals were left for a one-week withdrawal period in their home cages, and subsequently, subjected to 6-days of extinction training (Figure 2A) to reduce drug-seeking behavior under non-reinforced conditions (i.e. no cocaine delivered with an active lever press). Immediately following a final extinction session, the animals were exposed to two environmental cues linked with prior cocaine delivery – a small light above the active lever that turned on with previous cocaine infusion and the sound of the activated infusion pump. Compared to animals expressing GFP and HDAC5 WT, animals expressing HDAC5 3SA showed a significant reduction in cue-induced reinstatement of drug seeking (Figure 2F). Similarly, animals significantly increased their drug-seeking behavior following an experimenter-delivered priming dose of cocaine (15 mg/kg), but HDAC5 3SA-expressing animals showed a significant reduction in prime-induced drug seeking behavior compared to GFP-expressing animals (Figure 2G). In contrast, no group differences were detected following acute stress-induced reinstatement of drug seeking (Figure S2C), although a high degree of variance in the stress-induced reinstatement obscured our ability to detect a possible reduction produced by HDAC5 3SA. Together, our findings suggest that nuclear-enriched HDAC5 significantly reduced the ability of external (light and sound) and internal (cocaine priming) drug-associated cues to trigger reinstatement of drug seeking in extinguished animals.
HDAC5 association with the activity-regulated enhancer of the Npas4 gene
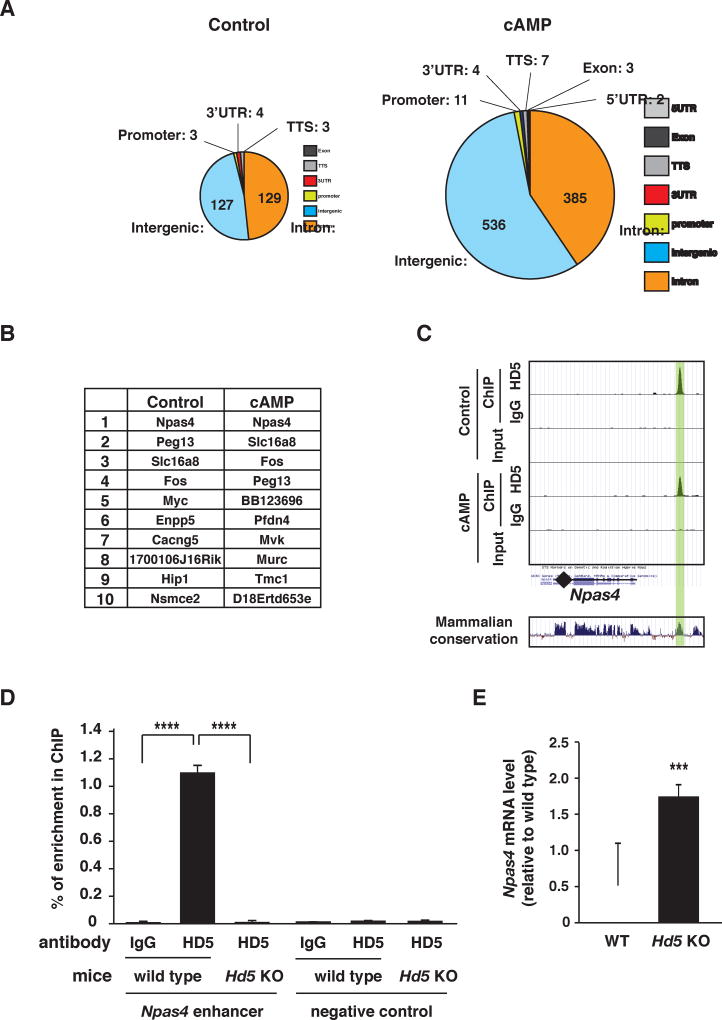
We speculated that nuclear HDAC5 modulates cocaine behaviors, at least in part, by binding to and regulating the expression of key target genes. To identify potential HDAC5 target genes, we performed an unbiased analysis of HDAC5 genomic binding sites using chromatin immunoprecipitation followed by next generation sequencing (ChIP-seq) of genomic DNA fragments associated with HDAC5. Analysis of our HDAC5 ChIP-seq data revealed that multiple genomic DNA regions were significantly enriched for HDAC5 association (Figure 3A and Table S1, S2 and S3). As expected, striatal neurons treated with forskolin, which increases cAMP levels via activation of adenylyl cyclase, produced nearly 4-fold more HDAC5-associated genomic peaks (948) than vehicle treated striatal neurons (266) (Figure 3A and Table S1), consistent with our prior observation that cAMP signaling promotes HDAC5 nuclear accumulation (Taniguchi et al., 2012). In contrast to other HDACs (Wang et al., 2009), HDAC5-associated genomic regions are abundant in intergenic and intronic regions, and only a small proportion of HDAC5 associates with putative promoter regions, 3’ UTRs, transcriptional start sites (TSS) or exonic regions (Figure 3A and Table S1). Genes located near the HDAC5-enriched binding sites included transcription factors, kinases, phosphatases, ion channels, and signaling molecules (Figure 3B and Table S1, S2 and S3). The highest level of HDAC5 genomic region enrichment was a region located ~3 kb upstream of the Npas4 gene (Figure 3C), which codes for a synaptic activity-regulated transcriptional factor (Lin et al., 2008). The HDAC5-associated genomic region at the Npas4 gene is highly-conserved across species (Figure 3C) – sharing 89% sequence homology between mouse and human, and was previously defined as an activity-regulated Npas4 enhancer region (Kim et al., 2010). Using striatal tissues from adult wild-type and Hdac5 KO mice, we confirmed strong and specific enrichment of HDAC5 association at the Npas4 enhancer region in vivo (Figure 3D). We also observed that Npas4 mRNA expression was elevated in the NAc of Hdac5 KO mice (Figure 3E). In contrast, cFos expression was unaltered (Figure S3B), suggesting that HDAC5 specifically represses Npas4 gene expression rather than reducing general activity-dependent gene expression.
Figure 3. HDAC5 binds and regulates Npas4 expression.
A. HDAC5 ChIP-seq identified 266 and 948 total peaks of significant genomic association in vehicle (left) and forskolin-treated (right) conditions. Pie charts represent the distribution of HDAC5 peak annotations within genome regions. B. List of putative target genes with largest HDAC5 association levels. C. Charts (top) represent the enrichment of genomic fragments by HDAC5 ChIP, IgG ChIP, or total DNA input around the Npas4 gene illustrated using the University of California Santa Cruz (UCSC) genome browser. The peak of HDAC5 binding is highlighted in green. This region is highly conserved across mammalian species, as shown by the histogram (bottom). D. Independent confirmation of HDAC5 binding to the Npas4 enhancer region in striatum in vivo. ChIP with normal rabbit IgG and anti-HDAC5 antibody were performed with striatal tissue from wild type and Hdac5 KO mice (n=4–5/condition). E. qPCR results show that Hdac5 KO mice exhibit higher Npas4 mRNA levels in the NAc than wild-type mice (n=8/condition). Data shown are mean ± SEM; *** p < 0.001, **** p < 0.0001, n.s.= p > 0.05. See also Figure S3 and Table S1–S4.
HDAC5 regulates activity-dependent Npas4 gene expression
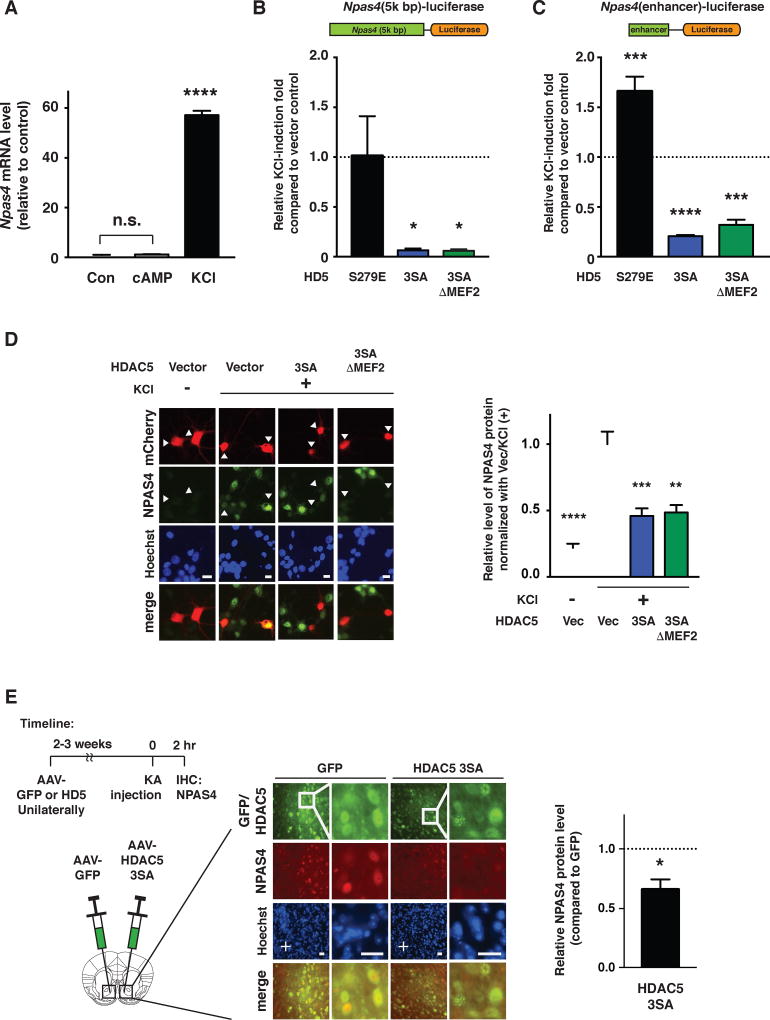
Similar to reported regulation of Npas4 gene expression in forebrain excitatory neurons, we observed that membrane depolarization (Lin et al., 2008; Ramamoorthi et al., 2011), but not elevation of cAMP (forskolin), induced transcription of the Npas4 gene (Figure 4A). We also observed that overexpression of HDAC5 3SA, but not the cytoplasm-localized phospho-mimetic (HDAC5 S279E, (Taniguchi et al., 2012)), dramatically repressed depolarization-induced expression of a Npas4-luciferase reporter plasmid containing ~5 kb of upstream genomic sequence (including the HDAC5 association region, Figure 4B). Interestingly, the HDAC5 3SA-ΔMEF2 mutant also repressed Npas4 expression to a similar extent as HDAC5 3SA (Figure 4B), suggesting that direct binding of nuclear HDAC5 to MEF2 is not required for its ability to regulate Npas4 gene expression. Using a Npas4-luciferase reporter containing only a small (~400 bp) region that also spans the HDAC5 binding site, we observed that HDAC5 3SA and HDAC5 3SA-ΔMEF2, but not cytoplasm-localized HDAC5, strongly repressed depolarization-induced Npas4 reporter activity (Figure 4C), suggesting that dephosphorylated HDAC5 associates with the activity-sensitive Npas4 enhancer and suppresses activity-dependent Npas4 expression. In addition, overexpression of HDAC5 3SA or HDAC5 3SA-ΔMEF2 proteins blocked the depolarization-induced expression of endogenous NPAS4 protein (Figure 4D), and infusion of AAV2-HDAC5 3SA in the NAc of adult mice reduced the neuronal activity-induced expression of endogenous NPAS4 protein (Figure 4E). Together, these data indicate that HDAC5 negatively regulates Npas4 gene expression in culture and in vivo, at least in part, through binding to a conserved, activity-sensitive Npas4 enhancer region.
Figure 4. HDAC5 limits neuronal activity-dependent Npas4 expression.
A. qPCR results show that endogenous Npas4 mRNA expression in cultured striatal neurons is induced by membrane depolarization (60 mM KCl, isotonic), but not by forskolin (10 µM). B and C. HDAC5 3SA and 3SA- ΔMEF2 reduce depolarization-induced Npas4-luciferase reporter gene expression in cultured primary striatal neurons. Data expressed as relative fold induction compared to vector-only control (dashed line). (B) 5kb region upstream of the Npas4 transcription start site or (C) 400 bp activity-sensitive enhancer region containing the HDAC5 binding region controlling expression of the luciferase open reading frame. (n = 4/condition). D. HDAC5 3SA and 3SA-ΔMEF2 reduces depolarization-induced expression of endogenous NPAS4 protein in cultured neurons. Left panels, representative images of transfected neurons expressing mCherry (red), endogenous NPAS4 (green) and counterstained with Hoechst nuclear dye (blue). Arrowheads indicate transfected neurons. Scale bar = 10 µm. Right panel, relative levels of endogenous NPAS4 expression in the presence of HDAC5 3SA, 3SA-ΔMEF2 or vector control (n = 3/condition). E. AAV2-HDAC5 3SA limits induction of endogenous NPAS4 expression in the NAc following Kainic acid (KA)-induced seizures. Left panel, timeline of experimental procedures. Middle, representative images showing AAV2-GFP or HDAC5 3SA expression and KA-induced NPAS4 expression. Scale bars = 50 µm. Right panel, quantification of normalized NPAS4 levels HDAC5 3SA vs. GFP control. A dashed line indicates normalized control level of NPAS4 in contralateral NAc. (n=4/condition). Data shown are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, n.s. > 0.05. See also Table S4 for statistics analyses.
Npas4 in the adult NAc is regulated by cocaine and exposure to testing chambers
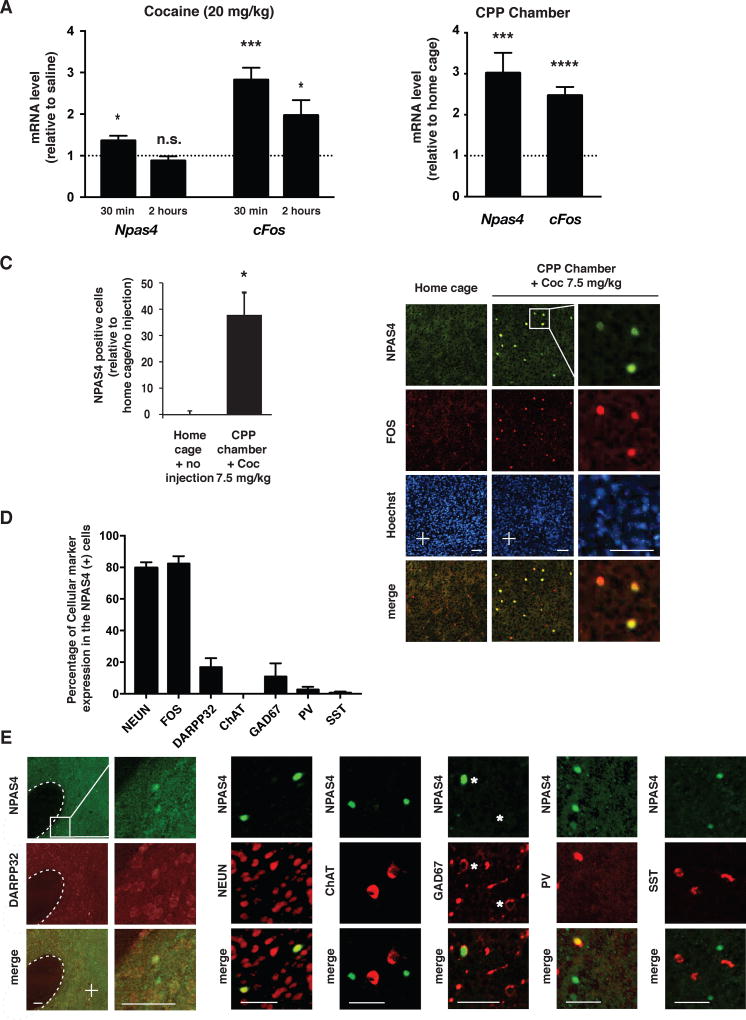
To study the possible role of NPAS4 in cocaine addiction-related behaviors, we first administered cocaine (20 mg/kg) to young adult mice and measured the expression of Npas4 mRNA from acute tissue punches. Compared to saline-injected controls, cocaine produced a rapid (30 min) and transient increase in Npas4 mRNA levels in the NAc (Figure 5A), consistent with the expression pattern of Npas4 in glutamatergic neurons (Lin et al., 2008; Ramamoorthi et al., 2011). As expected, cocaine induced a similar transient increase in cFos mRNA, although the cFos mRNA induction was larger in magnitude and longer lasting (Figure 5A). We also observed that simply placing the mice into the CPP test chambers for 15 minutes produced a ~3-fold increase in Npas4 mRNA levels in the NAc, suggesting that the novelty and/or stress experienced in the test chambers regulates Npas4 mRNA (Figures 5B and S4A). Using immunohistochemistry, we detected very few NPAS4-positive cells in the NAc in mice removed from the home cage. However, mice injected with 7.5 mg/kg cocaine and placed into the CPP chamber showed a ~40-fold increase in NPAS4-positive cells within the NAc (Figure 5C and 5E). Under those conditions, the NPAS4-positive cells in the NAc were predominantly neuronal (NEUN-positive) and co-expressed FOS (Figure 5D and 5E). In contrast, only ~20% of the FOS-expressing cells co-expressed NPAS4 (Figure S4C). A vast majority (~90%) of neurons within the NAc are DARPP-32-expressing medium spiny neurons (MSNs), but surprisingly, only ~15% of NPAS4-positive neurons co-localized with the MSN marker (Figure 5D–E). We detected no NPAS4 colocalization in cholinergic interneurons (ChAT-positive; Figure 5D–E), suggesting that NPAS4 is induced predominantly in one or more subclasses of GABAergic interneurons. Consistent with this idea, we detected colocalization of NPAS4 in the NAc with some interneuron markers analyzed, including GAD67-, somatostatin (SST)-and parvalbumin (PV)-positive interneurons (Figure 5D–E). Together, our data suggest that exposure to cocaine and the CPP test chamber induces NPAS4 expression in multiple NAc cell types, including MSNs and GABAergic interneurons.
Figure 5. NPAS4 expression in the NAc is induced by cocaine and exposure to test chambers.
A. Quantification of Npas4 and cFos mRNA expression following cocaine administration (20 mg/kg, i.p., n=4–8/condition). Data are plotted as mRNA levels relative to saline-only controls (dashed line). B. Npas4 and cFos mRNA expression in the adut NAc were induced by 15 min. exposure to the CPP testing apparatus (n=5–9/condition). Data are plotted as mRNA levels relative to homecage controls (dashed line). C. Left panel, data plot represents fold change in NPAS4-positive cell number in the NAc in mice injected with cocaine (7.5 mg/kg; i.p.) and placed in the test chamber as per the CPP conditioning conditions. (n=3 and 5, respectively). Right panel, representative images of NPAS4 and FOS protein expressions in the NAc of mice subjected to cocaine (7.5mg/kg, i.p.) and CPP chamber exposure vs. home cage controls. Arrowheads indicate NPAS4-positive cells, whereas Hoechst (blue) labels nuclei. Scale bar = 50 µm. D. Data plot shows the percentage of NPAS4-positive cells that co-express different neuronal markers in the NAc following cocaine (7.5 mg/kg; i.p.) and CPP chamber exposure (n=3–6/condition). E. Representative images of NPAS4 IHC and colocalization with cell-type specific markers, including NEUN (pan-neuronal), DARPP32 (Dopamine and cAMP-regulated phosphoprotein 32 kDa; MSNs), ChAT (Choline acetyltransferase; cholinergic interneurons), GAD67 (Glutamic Acid Decarboxylase; pan-GABAergic interneurons), PV (Parvalbumin; subclass of GABAergic fast-spiking interneurons), and SST (somatostatin; a subclass of GABAergic interneurons). White arrowheads = NPAS4-positive cells and asterisks = GAD67-positive cells. Scale bar = 50 µm. Data shown are mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001. See also Figure S4 and Table S4.
NPAS4 in the adult NAc is required for cocaine reward-context associations
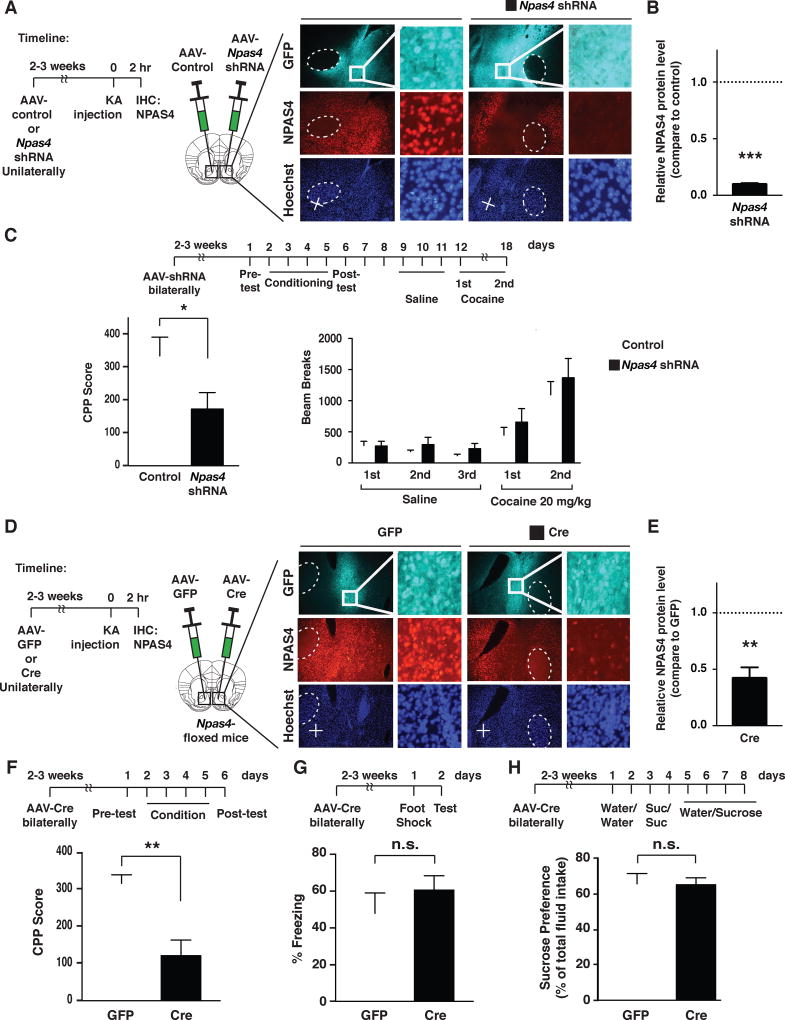
Since NPAS4 is induced in the NAc under conditions that produce CPP to cocaine, we tested whether it was required in the NAc for development of cocaine reward-context associations. To this end, we generated a viral-vector (AAV2) expressing either a validated shRNA that reduces levels of Npas4 mRNA or a scrambled shRNA control (Lin et al., 2008; Ramamoorthi et al., 2011) (Figure 6A–B). Compared to controls, bilateral NAc infusion of AAV2-Npas4 shRNA in WT mice produced a significant reduction in cocaine CPP (Figure 6C, Left), indicating an essential role for NAc NPAS4 in this drug behavior. In contrast, AAV2-Npas4 shRNA did not alter naïve locomotor responses to cocaine, nor did it alter behavioral sensitization with repeated cocaine exposure (Figure 6C, Right), suggesting that the CPP deficit was not caused by altered cocaine sensitivity. Moreover, we observed no significant TUNEL staining in NAc sections from either viral injection group, arguing against the possibility that cell death of NPAS4-expressing neurons contributed to the cocaine CPP deficit (Figure S5A).
Figure 6. NPAS4 expression in the NAc is required for cocaine conditioned place preference, but not fear-related contextual learning and memory.
A-B. AAV2-Npas4 shRNA in the adult NAc decreases NPAS4 protein expression. A. Left panel, timeline of experimental procedures. Right panel, representative images showing shRNA-mediated reduction of NPAS4 in the NAc of mice after induction of kainic acid seizures (scale bars = 100 µm and 20 µm). B. Quantification of NPAS4 expression in the NAc of mice expressing AAV2-Npas4 shRNA plotted as relative to scrambled control (dashed line, n=3/condition). C. Bilaterally expression of AAV2-Npas4 shRNA reduced cocaine CPP (7.5 mg/kg; i.p.; n=12–13/condition) without reducing subsequently tested cocaine locomotor sensitization (20 mg/kg, i.p.; n=8/condition). D. Left panel, timeline of experimental procedures in Npas4 cKONAc mice. Right panel, representative image showing KA seizure-induced NPAS4 expression in Npas4fl/fl mice injected with AAV2-GFP and AAV2-Cre in the NAc. E. Quantification of NPAS4 expression in Npas4 cKONAc plotted relative to AAV2-GFP control (dashed line) (n=5/condition). F-H. Top panels, timeline of surgeries and behavioral testing. F. Conditional deletion of Npas4 in NAc reduces cocaine CPP (7.5 mg/kg; i.p.; n=7–10/condition), without altering contextual fear conditioning (G; n=9–11/condition) preference for sucrose in the two-bottle choice test (H; n=7/condition). Data shown are mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001, n.s. p >0.05. See also Figure S5 and Table S4.
To confirm that NPAS4 expression in the NAc was required for cocaine CPP, we infused AAV2-Cre/eGFP or AAV2-eGFP control viruses into the NAc of adult mice possessing loxP sites flanking the Npas4 gene (Ramamoorthi et al., 2011), to produce conditional Npas4 knockdown in the NAc (Npas4 cKONAc,Figure 6D–H). Consistent with the Npas4 shRNA, we observed that Npas4 cKONAc mice displayed a significant reduction in cocaine CPP (Figures 6F, S5C, and S5E), confirming the importance of NPAS4 in the adult NAc for cocaine reward-context associations. We observed no evidence of neuronal apoptosis (TUNEL staining) in the NAc of Npas4 cKONAc mice (Figure S5B). In theory, altered anxiety levels could influence the cocaine reward-context associations; however, in the open field test of anxiety-like behavior, we detected no genotype differences in the time spent in the center zone, an area typically avoided by anxious mice (Figure S5D).
Conditional deletion of Npas4 in the CA3 region of the hippocampus reduces contextual fear learning and memory (Ramamoorthi et al., 2011). To determine if Npas4 cKONAc mice demonstrate general learning and memory deficits, we tested cohorts in a task that links an aversive stimulus (foot-shock) with a specific context. However, we observed that Npas4 cKONAc mice showed normal contextual freezing behaviors (Figure 6G). In addition, Npas4 cKONAc mice showed normal natural reward preference in the 2-bottle choice sucrose preference test (Figure 6H) – a natural reward assay that measures an animal’s preference to consume a highly-palatable sucrose solution over water. Taken together, our findings reveal that NPAS4 in the adult NAc is induced upon exposure to a novel environment and is required for development of cocaine reward-context associations.
NPAS4 in the NAc is required for discrimination of the drug-reinforced action during cocaine self-administration in mice
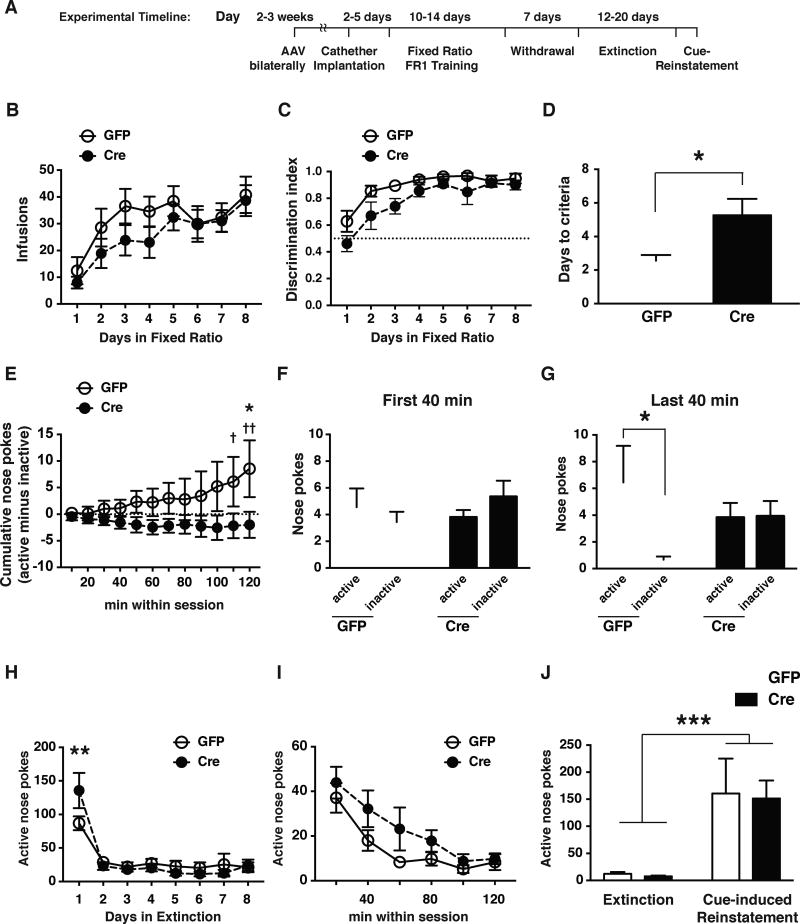
Given that NAc HDAC5 3SA overexpression blocks NPAS4 expression, cocaine CPP and reinstatement of cocaine seeking, and that Npas4 loss-of-function in the adult NAc reduces cocaine CPP, we tested whether reducing NPAS4 in the NAc also influenced cocaine self-administration behaviors. Npas4 cKONAc and control mice self-administered cocaine (0.5 mg/kg/infusion) using an FR1 schedule of reinforcement in operant chambers containing both active and inactive ports (Figure 7A). Successful nose-pokes of the active port triggered an infusion of cocaine and activation of a cue light, whereas nose pokes of the inactive port had no consequences. Interestingly, WT and Npas4 cKONAc mice both learned to SA cocaine (Figure 7B–C, S6A and S6B), the Npas4 cKONAc mice required significantly more sessions to reach criteria for operant discrimination of the drug-reinforced action (Figure 7D). The difference in operant discrimination learning was apparent within the first day of cocaine SA. During the first 2-hr cocaine SA session, control mice developed a significant increase in active vs. inactive port discrimination, whereas the Npas4 cKONAc mice continued to explore the active and inactive ports at a similar ratio (Figure 7E–G), suggesting that Npas4 cKONAc mice have a significant deficit in acquisition of operant discrimination of a drug reinforced action. Interestingly, on the first day of sucrose SA, the Npas4 cKONAc mice also showed a significant within-session reduction in the development of operant discrimination of the active vs. inactive port, suggesting that NPAS4 in the NAc is critical in naïve mice for positively-reinforced operant learning (Figure S7E). However, there was only a statistical trend for a reduction in operant discrimination in sucrose SA (Figure S7D), suggesting that NPAS4’s function in the NAc might be more pronounced during the learning of operant responding for drugs compared to natural rewards.
Figure 7. NPAS4 expression in the NAc is required for the learning the drug-reinforced action during cocaine self-administration.
A. Timeline for the analysis of Npas4 cKONAc mice in cocaine intravenous self-administration assay. B. Plot of daily cocaine infusions in Npas4 cKONAc and control mice (FR1; n=9 and 11/condition). C. Plot of the discrimination index (number of active nose poke over total number of active and inactive nose pokes) during acquisition of cocaine self-administration (n=9 and 11/condition). D. Npas4 cKONAc mice required significantly more training days than controls to reach a discrimination index ≥ 0.85 and infusions >10 per session (n=9 and 11/condition). E-G. NPAS4 in the NAc appears to regulate operant learning of the drug-reinforced action. E. Graph displays cumulative difference (in 10 min increments) between active and inactive nose pokes during the first 2hr SA session (n=9 and 11/condition, †p < 0.05, ††p < 0.01). F and G. Graphs plot the number of active and inactive nose pokes in the first 40 min (F) and last 40 min (G) of the first 2hr cocaine SA session. H. Daily active nose-pokes during all extinction sessions of Npas4 cKONAc and control mice (n=9 and 11/condition). I. Plot of the number of active nose pokes in 20 min intervals during the first 2hr extinction session. J. Cue-induced reinstatement of cocaine seeking is similar between control and Npas4 cKONAc mice (n=9 and 10/condition). Data shown are mean ± SEM; * p<0.05, **p<0.01, ***p<0.001, unless otherwise noted. See also Figure S6, S7 and Table S4.
After reaching a similar stable level of cocaine SA, mice were subjected to a one-week forced abstinence period in the home cage followed by daily extinction training sessions where nose-pokes in the active port were no longer reinforced. Interestingly, the Npas4 cKONAc mice showed a significant increase in overall active port nose-pokes on the first day of extinction (Figure 7H), reflecting either a deficit in extinction learning and/or an enhancement of context-induced cocaine seeking. Further analysis of nose-pokes during the first extinction session suggests that Npas4 cKONAc mice have a reduced rate of within-session extinction learning (Figure 7I). By contrast, after sucrose SA and forced abstinence, the Npas4 cKONAc mice showed extinction training behaviors indistinguishable from control mice (Figure S7H–I). Surprisingly, Npas4 cKONAc mice showed normal cue-induced reinstatement of cocaine seeking (Figure 7J), indicating that reduction of NPAS4 expression in the NAc by HDAC5 3SA overexpression is not sufficient to explain its ability to reduce cued reinstatement, and that additional or distinct gene targets account for HDAC5’s effects on relapse-like behaviors.
Discussion
In the present study, we found that dephosphorylated HDAC5 (3SA) in the adult NAc reduced cocaine reward-context associations in the CPP model, and that it does so independent of its ability to bind to MEF2 transcription factors – a well-studied HDAC5 genomic binding partner (Belfield et al., 2006; Chawla et al., 2003; McKinsey et al., 2000a; McKinsey et al., 2000b). Moreover, HDAC5 3SA in the NAc reduced both contingent cue- and drug prime-induced reinstatement of drug seeking in the cocaine self-administration task, a rodent preclinical model of relapse in human addicts. Using an unbiased genome-wide approach (HDAC5 ChIP-seq), we observed a significant increase in genomic binding sites after stimulation of cAMP signaling in striatal neurons. We identified ~1000 putative HDAC5 gene targets, including the neuronal activity-regulated transcription factor, Npas4. We showed that endogenous HDAC5 binds to a highly-conserved, activity-sensitive enhancer, and we found that overexpression of HDAC5 3SA blocked activity-dependent Npas4 gene expression via the ~400 bp enhancer region. We showed that cocaine and/or exposure to the test environment induced NPAS4 expression in a small subpopulation of FOS-positive neurons within the NAc, and that NPAS4 is required for cocaine reward-context associations in the CPP assay without altering cocaine sensitivity, sucrose reward sensitivity, anxiety, or aversive Pavlovian conditioning. Finally, we showed that NPAS4 in the NAc appears to be involved in operant reward learning during cocaine, and likely sucrose, self-administration, but unlike HDAC5, NPAS4 is not required for cue-induced reinstatement.
The CPP assay is often interpreted as an indirect measure of drug reward, since the impact of the drug-paired environment on preference is exhibited in a drug-free state (Renthal et al., 2007). Our results suggest that the development of this place preference requires the engagement of NAc-dependent plasticity processes involving both HDAC5 and NPAS4. Considering the Npas4 cKONAc deficits in operant learning during the early stages of cocaine SA, we speculate that the deficit in cocaine CPP observed in Npas4 cKONAc mice might reflect a similar learning process. Importantly, reducing NAc NPAS4 levels doesn’t appear to reduce cocaine or sucrose reward sensitivity (Figures 6C right and 6H, respectively). Additionally, once the animals learned the operant procedure to receive cocaine, neither HDAC5 3SA overexpression nor reduction of NAc Npas4 levels altered stable drug intake, or in the case of HDAC5 3SA, effect motivation to work for drug (progressive ratio) or sensitivity to the reinforcing effects of cocaine (dose-response). Nonetheless, HDAC5 3SA overexpression reduced both external (light and sound) cued and drug (15 mg/kg cocaine, i.p.) cued reinstatement of drug seeking, suggesting that HDAC5 3SA reduces the ability of these cues to subsequently impact motivated behavior during reinstatement. In contrast, Npas4 cKONAc mice showed no deficits in external cued reinstatement (Figure 7J), suggesting that additional, or distinct, HDAC5 target genes contribute to HDAC5’s anti-relapse-like effects. Our findings also reveal that cocaine CPP and cued reinstatement in the cocaine SA assay are dissociable processes, as previously suggested (Anderson et al., 2017; Graham et al., 2007; Graham et al., 2009).
In our HDAC5 ChIP-seq findings, we observed that cAMP signaling increased the number of significant genomic HDAC5 binding sites by ~4-fold (Figure 3A), generally consistent with the dramatic increase in nuclear accumulation of HDAC5 under the same conditions (Taniguchi et al., 2012). While HDAC5 associated with ~1000 genomic regions, the HDAC5 binding peak near Npas4 had the highest percent enrichment in both basal and cAMP-stimulated conditions, suggesting that it might be an important gene target. The fact that this Npas4 peak co-localized with the activity-sensitive enhancer region of Npas4, and its reported activity-dependent role to regulate inhibitory synapses on developing pyramidal neurons and excitatory synapses on inhibitory interneurons (Bloodgood et al., 2013; Kim et al., 2010; Lin et al., 2008) increased our interest in this candidate target gene. In the NAc, only ~15% of the NPAS4+ cells were DARPP-32-expressing MSNs, which is striking since nearly 90% of all striatal neurons are MSNs (Matamales et al., 2009). We failed to detect any NPAS4 colocalization with ChAT-positive cholinergic interneurons, but did detect partial co-localization with multiple classes of GABAergic interneurons, including GAD67-, PV- and SST-positive interneurons. Based on the reported role of NPAS4 in inhibitory interneurons, it’s tempting to speculate that in the NAc, NPAS4 induction might strengthen local GABAergic inhibitory tone, and in conjunction with synaptic potentiation on NAc spiny projection neurons recruited to the behavioral engram, this could alter the signal-to-noise ratio to promote efficient reward-related learning.
NPAS4 is robustly induced in the hippocampal CA3 region following exposure to a novel test chamber, but not home cage, and NPAS4 in the CA3 is required for fear-related long-term contextual memory (Ramamoorthi et al., 2011). Interestingly, we find that Npas4 induction in the adult NAc is sensitive to multiple emotional stimuli, including cocaine administration (Figure 5A), the experience of a novel environment (Figure 5B–C and S3A), and multiple psychological and physiological stressors (M. Taniguchi, unpublished data). However, Npas4 cKONAc mice had no deficits in contextual fear conditioning, suggesting that NAc NPAS4 might be more critical for reward-related learning and memory. In the cocaine self-administration assay, Npas4 cKONAc mice require more training sessions than control mice to learn to discriminate the drug-reinforced action (nose-poke of the active port), despite taking a similar number of drug infusions per session. The delay in operant learning was observed with both cocaine and sucrose SA, albeit to a lesser extent in sucrose SA, and could be detected during the first 2-hr SA training session (Figure 7E and S6E). NPAS4 is induced in vivo as quickly as 5–15 minutes (Ramamoorthi et al., 2011), suggesting that its downstream transcriptional functions could participate in an active learning process on the time scale of a 2-hr operant learning assay, and indeed, operant discrimination only emerged in the second hour of the SA session (Figure 7E and S6E). Alternatively, viral-mediated loss of NPAS4 in the NAc could have changed basal NAc circuitry, and this basal change, rather than the rapid NPAS4 induction during the behavioral test, might account for its role in the reward learning. Numerous studies have revealed that IEGs are stimulated within the adult NAc, including cfos and ΔfosB, and some of these genes are essential for cocaine behaviors (Kelz et al., 1999; Nestler et al., 2001; Zhang et al., 2006). Indeed, following cocaine administration, Daun02-mediated inhibition of the FOS-positive neuronal population in the NAc reduces multiple cocaine behaviors that depend upon the unique environmental context of drug administration (Cruz et al., 2014; Koya et al., 2009). In our studies here, we find that NPAS4 is induced by the novel test environment in a small subpopulation of the FOS-positive neurons in the NAc. Since only a small fraction (~20% or less) of the FOS-positive neurons are also NPAS4-positive and knockdown of Npas4 reduces cocaine CPP, it will be interesting to determine the relative contribution of the dual NPAS4-positive/FOS-positive population to previously described roles for FOS-positive engrams in drug-related behaviors.
In summary, we found that dephosphorylated HDAC5 (3SA) in the adult NAc reduced the development of cocaine CPP and external or internal cue-induced relapse-like behavior in the extinction-reinstatement test following stable cocaine self-administration. We identified a novel role for HDAC5 in the regulation of an activity-sensitive enhancer upstream of the Npas4 gene. We showed that NPAS4 is induced rapidly and transiently in the NAc in a discrete subpopulation of FOS-positive neurons following exposure to emotional stimuli, including cocaine or a novel environment. Reduction of NPAS4 levels in the adult NAc decreased cocaine CPP, without altering sucrose preference, contextual fear conditioning or the motor activating effects of cocaine. It also reduced the rate at which Npas4 cKONAc mice learned to discriminate the drug-reinforced action during acquisition of drug self-administration, but did not alter cue-induced reinstatement of drug seeking. Together our findings suggest that HDAC5 and NPAS4 regulate multiple addiction-relevant behaviors, and that these proteins (or their respective target genes) might serve as potential therapeutic targets for the treatment of drug addiction.
STAR Methods
Detailed methods are provided in the online version of this paper and including the following:
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
- EXPERIMENTAL MODELS AND SUBJECT
- Animals
- Recombinant plasmids and viral vectors
- METHOD DETAILS
- Viral-mediated gene transfer
- Cultured primary striatal neurons
- Luciferase assay in primary cultured neuron
- Immunocytochemistry and Immunohistochemistry
- Sample preparation for ChIP, mRNA and IHC
- ChIP and ChIP-seq analysis
- qRT-PCR for gene expression and ChIP
- Sucrose Pellet Training
- Rodents intravenous catheterization
- Intracranial cannulation and viral-gene transfer
- Rodents cocaine or sucrose self-administration
- Dose Response and Progressive Ratio
- Extinction and Reinstatement
- Cocaine Conditioned Place Preference
- Locomotor sensitization
- Sucrose Preference test
- Open Field test
- Contextual fear conditioning
- Gene Ontology Analysis
QUANTIFICATION AND STATISTICAL ANALYSIS
- DATA AND SOFTWARE AVAILABILITY
- Data Resources
KEY RESOURCES TABLE
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Christopher W. Cowan (cowanc@musc.edu).
EXPERIMENTAL MODELS AND SUBJECT
Animals
Sprague Dawley rats (Charles River Laboratories) weighing 250–300g were housed individually in standard cages. All C57BL/6 mice (Charles River Laboratories), Hdac5-/- and Npas4fl/fl mice (Chang et al., 2004; Ramamoorthi et al., 2011) were adult males, tested between 9–16 weeks of age, and were housed as described. Rodents were allowed access to food and water ad libitum and were kept on a 12 hr light-dark cycle. All procedures were in accordance with the Institutional Animal Care and Use (IACUC) guidelines.
Recombinant plasmids and viral vectors
HSV-expression constructs for HDAC5 3SA and 3SA- ΔMEF2 mutants were generated from the previously described HSV-HDAC5 wildtype expression construct (Taniguchi et al., 2012). In HSV vectors, HDAC5 and mutant expressions are driven by an IE4/5 promoter while GFP is driven by a CMV promoter. These plasmids were processed for packaging and purification (McGovern Institute, MIT, Viral Core). Adeno-associated virus serotype 2 (AAV2) vectors were constructed to deliver either CMV promoter driven GFP, HDAC5 WT or HDAC5 3SA expression. The HDAC5 coding regions digested from HSV construct were inserted into AAV-MCS containing a CMV promoter. For knockdown of endogenous Npas4 expression in NAc, a validated anti-Npas4 shRNA or scrambled shRNA control (Lin et al., 2008), was cloned into pAAV.shRNA vector as previously described (Pulipparacharuvil et al., 2008). The AAV2-shRNA vector consisted of a CMV promoter driving eGFP with SV40 polyadenylation signal followed downstream by a U6 polymerase III promoter, shRNA or control oligonucleotides, and polymerase III termination signal all flanked by AAV2 inverted terminal repeats. These AAV2-HDAC5 WT, -HDAC5 3SA, -scramble shRNA, and -anti-Npas4 shRNA plasmids were processed for packaging and purification (UNC Vector Core and Vector Biolabs). AAV2-eGFP control and AAV2-Cre-eGFP viruses used to generate Npas4 conditional knockout mice were obtained from Vector Biolabs. All Npas4 luciferase-encoding plasmids used the pGL4.11 backbone (luc2p, Promega). The Npas4(5kbp)-luciferase construct was generated from a previous study (Ramamoorthi et al., 2011), in which a 5kb piece of the proximal Npas4 promoter was used to drive the expression of the luciferase gene. The Npas4(Enhancer)-luciferase construct used a 400 bp piece of the enhancer which contained the elements corresponding to the HDAC5 binding peak on the endogenous Npas4 promoter.
METHOD DETAILS
Viral-mediated gene transfer
Stereotaxic surgery was performed under general anesthesia with ketamine/xylazine cocktail (120 mg/kg: 16 mg/kg in mice and 100 mg/kg: 10 mg/kg in rat) or isoflurane (induction 5% v/v, maintenance 1–2% v/v). Coordinates to target the NAc (shell and core) were +1.6 mm anterior, +1.5 mm lateral, and −4.4 mm ventral from bregma (relative to skull) at 10° angle in mice, and +1.8 mm anterior, +1.0 mm lateral and −7.7 mm ventral (relative to skull) in rat. HSV-GFP (3.0 * 108 transducing units/mL), HSV-HDAC5 WT (3.0 * 108 transducing units/mL), HSV-HDAC5 3SA (3.0 * 108 transducing units/mL), HSV-3SA-ΔMEF2 (3.0 * 108 transducing units/mL), AAV2-scramble shRNA (1.1 * 1012 GC/mL), AAV2-Npas4 shRNA (3.1 * 1012 GC/mL), AAV2-eGFP (0.4~1.0 * 1013 GC/mL), AAV2-Cre-eGFP (1.0 * 1013 GC/mL), AAV2-HDAC5 wildtype (5.0 * 1012 GC/mL) and AAV-HDAC5 3SA (5.0~7.5 * 1012 GC/mL) were delivered bilaterally using Hamilton syringes at a rate of 0.1 µL/min for a total 0.6 µL hemisphere on mice and total 1.0 µL/hemisphere on rat with an additional 7–10 minutes before needles were retracted as previously described (Taniguchi et al., 2012). Viral placements were confirmed by HDAC5 signal for AAV-mediated HDAC5 expression and GFP signal for HSV-mediated HDAC5.
Cultured primary striatal neurons
Mouse and rat primary striatal neurons were prepared from C57BL/6 and Long-Evans rat (Charles River Laboratory) at embryonic day 15 and postnatal day 0 for mouse and at embryonic day 18 for rat culture using previously described methodology with some modification (Lin et al., 2008; Taniguchi et al., 2012). Mouse postnatal striatal neurons were plated at a density of 150,000 cells/well on 24 well plates pre-coated with poly-D-lysine. Mouse postnatal cultures were plated in cold Neurobasal A Medium (NBA, Life Technologies) with horse serum and glutamine added and kept at 37°C/5% CO2. After three hours of incubation, culture media was changed to NBA with B27 (Invitrogen) supplement and GlutaMAX (Life Technologies). Mouse and rat embryonic cultures were plated at a density 100,000 cells/well on cover glass pre-coated with Laminin and poly-D-lysine on 24 well plates or 1.0*107 cells/10 cm dishes pre-coated with poly-D-lysine in pre-warmed DMEM (Invitrogen) supplemented with 10% (v/v) fetal bovine serum (Invitrogen), penicillin (50 µg/ml)- streptomycin (50 units/ml; Sigma) and L-glutamine (4 mM, Sigma) and incubated at 37°C/5% CO2 for 24 hours in a humidified incubator. 24 hr after plating, the medium was changed to Neurobasal (Invitrogen) containing B27 supplement (2% (v/v); Invitrogen), penicillin-streptomycin (1X; Sigma) and L-glutamine (4 mM; Sigma). For the Luciferase and immunocytochemistry assays, at DIV 5–6, 20–30% of the culture media was replaced with fresh media. At DIV 8, the neurons were transfected using lipofectamine 2000 (Life Technologies) transfection reagent and calcium phosphate method for mouse postnatal and rat embryonic cultures, respectively.
Luciferase assay in primary cultured neuron
MRE and Npas4 luciferase assays were performed with previously described methodology (Pulipparacharuvil et al., 2008; Ramamoorthi et al., 2011). For MRE luciferase assay, dissociated rat embryonic striatal neurons were transfected on day in vitro (DIV) 8. Cultures were stimulated at DIV 10 for 6 hr with 60 mM KCl. For Npas4-luciferase assay, cultures were stimulated on DIV 11. 2 hr prior to stimulation, TTX (1 µm) and APV (100 µm) were added to the cells. Neurons were stimulated for 4 hr with 35 mM KCl. Stimulated neurons were lysed in passive lysis buffer (Promega). The Dual-Glo Luciferase Assay System reagents were used in accordance with manufacturer’s recommendations (Promega). Firefly luciferase levels induced by MRE and Npas4 enhancer were measured and expressed relative to renilla luciferase levels. Npas4-Luciferase fold induction was calculated as the ratio between relative luciferase values for stimulated and unstimulated conditions.
Immunocytochemistry and Immunohistochemistry
Mouse brains were fixed overnight in 4% PFA in PBS and transferred to a 30% sucrose solution in PBS before slicing (40 or 50 µm) with a microtome. The slices were permeabilized and blocked in 3% BSA, 0.3% Triton-X100, 0.2 % Tween-20, 3% normal donkey or goat serum in PBS, then incubated with primary antibodies: anti-GFP (1:1000, Aves Labs, Inc; 1:1000-10,000, Invitrogen), anti-HDAC5 (1:500, abcam), anti-NPAS4 (1:1000, kindly provided by Dr. Michael Greenberg’s lab), anti-FOS (1:500, goat: Santa Cruz), anti-NEUN (1:250, Millipore), anti-DARPP32 (1:1000, Santa Cruz), anti-GAD67 (1:1000, Millipore), anti-Parvalbumin (1:1000, Millipore), anti-Somatostatin (1:500, Millipore) in blocking buffer at room temperature for 2–4 hours or at 4°C for overnight. Following a series of 1X PBS rinses, slices were incubated for 1–3 hr at room temperature with secondary antibodies (Donkey anti-Rabbit 488, Goat anti-mouse 594, Donkey anti-Rabbit 594, Donkey anti-Goat cy3, or Donkey anti-Chicken 488) while protected from light. Slices were counterstaining with Hoechst, mounted and coverslipped on glass slides and analyzed with fluorescent microscopy. Immunocytochemistry and analyses of localization of HDAC5 and expression of NPAS4 were performed with previously described methodology (Taniguchi et al., 2012). Striatal and cortical neurons (E18 rat) were transfected using calcium phosphate method. Neurons were fixed with 4% paraformaldehyde in 1X PBS for 20 min at room temperature, permeabilized, and stained with antibodies anti-HDAC5 (1:500, abcam), GFP (1:1000, Invitrogen), and NPAS4 (1:1000-10,000). Neurons were counterstained with Hoechst. The localization of HDAC5 was categorized as cytoplasmic, nuclear, or both for each neuron co-expressed fluorescent proteins under experimenter-blind conditions. The expression of NPAS4 protein was measured using ImageJ software in transfected neurons labeled with co-expressed fluorescent proteins under experimenter blind condition.
Sample preparation for ChIP, mRNA and IHC
In cocaine administration studies, C57BL/6 mice were habituated to saline injections for 3 days, and then given either a saline or cocaine injection (7.5 or 20 mg/kg, i.p.) before sacrifice at the described timepoint. To avoid potential impact on gene expression by cage-mate separation, we either singly housed animals (> 2 days) or used only the 1st animal removed from the cage during the process of sacrifice for the analysis. For novel environment studies, mice were singly housed ≥2 days, habituated with saline injections for 3 days, and then placed into an open CPP chamber (pre-test exploration session). 24 hr later, mice were confined to one side of the CPP chamber (conditioning session) before being sacrificed at the described time point. For neuronal activity-induced endogenous NPAS4 protein expression analyses in the NAc in vivo, C57BL/6 mice received AAV-Npas4-shRNA, AAV-scramble shRNA, AAV-GFP, or AAV-HDAC5 3SA, and floxed-Npas4 mice received AAV-Cre recombinase or AAV-GFP and after three weeks of expression, were administered with Kainic Acid (KA, 25 mg/kg. i.p.). Brains were collected 2 hr after KA administration for immunohistochemistry assay. For basal gene expression and ChIP assays, Hdac5 KO and littermate control mice were singly housed ≥2 days before sacrifice. Brain tissues were rapidly isolated following live decapitation. For mRNA expression, samples were frozen at −80 °C until further processing. For ChIP analyses, samples were processed for cross-linking immediately after decapitation and frozen at −80 °C until further processing.
ChIP and ChIP-seq analysis
Chromatin immunoprecipitation with HDAC5 was performed as previously described with some modifications (Kim et al., 2010; Koike et al., 2012). Cultured mouse striatal neurons (DIV6: 2.0 × 107 cells) and mouse brain tissue (dissected from 1 mm coronal section slices) were cross-linked by incubation (1% of formaldehyde, 50 mM HEPES pH 7.9, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA) for 10 min at room temperature with rotation. The reaction was quenched by adding 125 mM Glycine and incubating for 5 more min at room temperature. The samples were washed with ice-cold PBS and dissociated and homogenized in the lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X100, 0.1% deoxycholate and 1X Roche Complete EDTA-Free Protease Inhibitor Cocktail). Lysates were sonicated (Qsonica Q700), and fragmented chromatin was pre-cleared (Dynabeads Protein G, G-beads) and then incubated with anti-HDAC5 antibody (cell signaling, #2082) or an equal amount of normal rabbit IgG (control) overnight at 4 °C with rotation. Following, new G-beads, blocked with 5 mg/ml BSA, were added and incubated for 2 hours at 4 °C. The protein/DNA complex was washed twice with low salt buffer (0.1% SDS, 1% TritonX-100, 2 mM EDTA, 20 mM Tris-HCl, pH8.0, 150 mM NaCl), once with high salt buffer (0.1% SDS, 1% TritonX-100, 2 mM EDTA, 20 mM Tris-HCl, pH8.0, 500 mM NaCl), twice with LiCl buffer (250 mM LiCl, 1% IGEPAL CA 630, 1% deoxycholic acid, 1 mM EDTA, 10 mM Tris, pH8.0) and twice with TE buffer. Co-immunoprecipitated DNA fragments were eluted (10 mM Tris pH8.0, 1 mM EDTA, 1% SDS) and incubated at 65 °C overnight to reverse the cross-linking reaction. RNA was digested by adding RNase A (10 µg) and incubated at 37 °C for 1 hour, and proteins were digested by proteinase K (140 µg) incubation for two hours at 65 °C. The HDAC5-associated genomic DNA fragments were purified using phenol/chloroform extraction and QIAGEN columns. Quantitative PCR was used to confirm enrichment of DNA fragments, and HDAC5-associated DNA fragments were analyzed by high throughput sequencing. Sequencing library construction from immunoprecipitated DNA was performed as described (Kim et al., 2010; Koike et al., 2012). SOLiD sequencing of ChIP-seq libraries were performed on an Applied Biosystems SOLiD with 35-bp reads according to manufacturer’s instructions (Life Technologies) by The UT Southwestern McDermott Next Generation Sequencing Core. Sequencing reads were mapped to the mouse genome (mm9) with Bowtie. The peak findings and motif analyses were performed with the analysis package, HOMER (Heinz et al., 2010).
qRT-PCR for gene expression and ChIP
RNA isolation, reverse transcription, and quantitative real-time PCR were carried out as described previously (Pulipparacharuvil et al., 2008). The level of mRNA expression was analyzed by the fold change relative to GAPDH expression. The relative mRNA level was analyzed as the difference from experimental relative to control condition. The enrichment of ChIPed DNA fragments was analyzed by the percentage of ChIP sample DNA relative to input DNA.
Sucrose Pellet Training
To facilitate acquisition of drug self-administration, rats were first trained to lever press for sucrose pellets (45mg, BioServe) under restricted feeding conditions until they reached 100 pellets/session for three consecutive sessions. Following inclusion criteria, rats were returned to ad libitum feeding for at least three days prior to catheter implantation.
Rodents Intravenous Catheterization
Rats were anesthetized with ketamine/xylazine (100 mg/kg: 10 mg/kg, i.p.) and implanted with a chronic in-dwelling silastic catheter in the right jugular vein, as described previously (Edwards et al., 2007). Analgesia was achieved with ketoprofen (5 mg/kg s.c.) delivered at the beginning of the surgical procedure and as needed for up to 3 days post-operatively. Catheter patency was maintained with two daily catheter flushes of heparinized saline (0.2 ml; 30 IU/ml), which included gentamycin (5 mg/kg) on the first 3 days following surgery. Mice were anesthetized using isoflurane (induction 5% v/v, maintenance 1–2% v/v) and implanted with an indwelling catheter in the right jugular vein, connected to a head mounted access port as described previously (Heinsbroek et al., 2017). Mice were given cephazolin (200 mg/kg, i.v.), carprofen (5 mg/kg, s.c), and topical antibiotic ointment. Following surgery catheter patency was maintained by flushing catheters twice daily with heparinized saline (0.03 ml; 100 IU/ml). Mice were given at least three days of recovery following catheter surgery before starting cocaine self-administration.
Intracranial Cannulation and Viral-gene transfer
Intracranial cannulation and viral infusion was performed as previously described (Larson et al., 2011). After catheter implantation surgery described above, rats were implanted with chronically indwelling 26-gauge bilateral intracranial guide cannula for subsequent HSV infusions. Viral vector infusions through chronic cannula were performed by inserting a specialized injector (Plastics One). HSV infusions were targeted to the NAc, +1.7 mm anterior and +0.8 mm lateral from bregma, −6.7 mm ventral from dura. HSV viruses were infused at a rate of 0.1 µl/30 seconds for total 2 µl hemisphere, and injections were held in place for an additional 2 min to allow for local diffusion in the NAc before injector removal. Rats were allowed to recover for 5–7 d after surgery before self-administration experiments. Cannulated rats that completed study were anesthetized with chloral hydrate (400 mg/kg, i.p.) and cresyl violet (0.5 µl/side) was infused bilaterally through their indwelling cannula over a 5 min period. Coronal sections of 0.8 mm brain slices were analyzed for the location of infusion sites.
Rodent Cocaine or Sucrose Self-Administration
Following sucrose pellet training (described above), rats were returned to ad libitum feeding for at least three days prior to catheter implantation. Rat SA sessions (3 hr) were performed during the light phase at the same time each day, during which rats were placed in an operant conditioning chamber and connected to a drug line controlled by an external delivery pump. A “house” light inside the chamber signaled drug availability. All chambers contained an active (50 µL, 3 sec infusion; 0.5 mg/kg cocaine) and an inactive lever. During an infusion, a cue light above the lever was illuminated and followed by a 20-second time-out period signaled by the house light going off. Rats self-administered cocaine on a fixed ratio (FR) schedule beginning at FR1, followed by an FR3 and finally an FR5. When increasing the FR requirements, intake was analyzed for stability (< 20% variability within the last 3 sessions). Rats that completed 15–20 days of FR training and were stable on the FR5 schedule for at least 3 days, continued on to subsequent studies. Mouse self-administration sessions (2hr) were performed at the same time each day during the dark phase as described previously (Bobadilla et al., 2017). Briefly, drug or sucrose availability was signaled both by the house light and a light above the active nose poke hole. Following a poke in the active hole both availability lights went off and a cue light inside the nose poke hole was illuminated. Cocaine (12 µl, 2 sec infusion; 0.5 mg/kg,) or a sucrose pellet (15 mg) were delivered immediately upon the active nosepoke, followed by a 10-second time-out period. Nose pokes in the inactive hole were without programmed consequences. Mice that met requirements for stable cocaine self-administration (last 3 days >85% discrimination between active and inactive nose poke hole and >10 infusions) entered into a 7 day abstinence phase.
Dose Response and Progressive Ratio
A subset of animals underwent dose response and progressive ratio schedules following FR5 training. Animals were subjected to a 5 dose randomized series: 0, 0.03, 0.1, 0.3 and 1.0 mg/kg cocaine infusion. Each dose was presented for two days and the average of the days was calculated per animal. Subsequently, animals were run on a progressive ratio schedule of reinforcement. For these studies we analyzed two doses, 0.25 and 0.75 mg/kg cocaine/infusion. During a progressive ratio schedule of reinforcement, the requirements for an infusion are increased on subsequent infusions in an exponential manner. Animals are allowed to self-administer until they fail to earn an infusion in a 60 min timeframe. The last infusion achieved is reported as an indicator of how much animals consumed before reaching breakpoint.
Extinction and reinstatement
Rats were allowed a week of forced abstinence (withdrawal) undisturbed in their home cages. Then, animals were placed back into the operant chambers and lever pressing in the absence of any drug administration, cues and timeouts was measured on both levers. Extinction training continued for 6 days followed by reinstatement sessions. Each reinstatement session consisted of a 3 hr extinction session followed by a priming stimulus. Priming stimuli included presentation of the drug paired cue (light), experimenter-administrated cocaine (0, 5.0, and 15 mg/kg, i.p., counterbalanced) or foot shock. Lever pressing was measured for 1 hr following the priming stimuli. For mouse cocaine SA, animals were given one week of forced abstinence followed by an abstinence test during which nose pokes had no programmed consequences (i.e. extinction day 1). Afterwards mice were extinguished for at least 12 additional days until they reached criterion (<30% active nose pokes compared to the average of the last 3 days of self-administration). During cue-induced reinstatement, reward availability (house and active port light) and reward delivery cues (nose poke hole light) were returned to active nosepokes, but drug or sucrose delivery was omitted.
Cocaine Conditioned Place Preference
Mice were conditioned to cocaine using an unbiased paradigm described previously (Smith et al., 2016). On day 1, mice were placed in the middle chamber and allowed to explore the conditioning apparatus, which consisted of 3 distinct environment chambers (white side, grey middle, and black side). Mice that showed pre-conditioning preference (more than 30% of total time spent in either of the 3 chambers) were excluded from the study. On days 2 and 4, mice received cocaine (2.5 or 7.5 mg/kg; i.p.) and were confined to one chamber. On days 3 and 5, the mice received saline (0.9%; 1 ml/kg; i.p.) and were confined to the opposite chamber. On day 6, mice were placed again in the middle chamber with free access to all chambers, and the time spent in each side was quantified. For the animals expressing HSV-HDAC5 in the NAc were subjected to accelerated CPP schedule to accommodate the brief expression of the HSV as described previously (Taniguchi et al., 2012). On day 1, mice received a pre-conditioning test. On day 2, stereotactic surgery was performed as detailed above. On days 5 and 6, mice received two parings per day: saline (0.9 %; 1 ml/kg; i.p.) in the morning and cocaine (5 mg/kg; i.p.) in the afternoon on the opposite side of the place preference chamber. On the post-test at day 7, mice were placed again in the middle chamber with free access to all chambers, and the time spent in each side was quantified. Data are expressed as time spent on the cocaine-paired side minus the time spent on the saline-paired side (CPP score).
Locomotor Sensitization
Locomotor activity was measured via photobeam array (San Diego Instruments) before and after each injection using the two-injection protocol of sensitization (Valiente and Marin, 2010). Mice received saline injections for 3 days, then on the 4th day, mice received an injection of cocaine (20 mg/kg; i.p.). After home-cage withdrawal for 5 days, the mice received a 2nd cocaine injection (20 mg/kg; i.p.) in the locomotor apparatus. Data are shown as the sum of total beam breaks in the first 30 minutes of each session.
Sucrose Preference Test
The sucrose preference procedure was performed as previously described (Taniguchi et al., 2012). Singly housed mice were provided tap water in 2 identical double-ball-bearing sipper-style bottles for 2 days followed by 2 days of 1% (w/v) sucrose solution to allow for acclimation. Then mice were given 1 bottle containing tap water and another containing 1% sucrose solution. Consumption from each bottle was measured every 24 hours for 4 days, and bottle positions were swapped each day to avoid potential side bias.
Open Field Test
Mice were introduced into an open field box (44 cm diameter, as previously described (Renthal et al., 2007)) under dim light. The total time spent within the center field (14 cm diameter) was measured for 5 minutes.
Contextual Fear Conditioning
The procedure for fear conditioning was performed as previously described (Ramamoorthi et al., 2011). On the first day, mice were allowed to explore the chamber for 58 seconds, then were given 3 foot shocks (2 sec, 0.55 mA) with 58 sec inter-shock intervals. After the last shock, mice were left in the chamber for 1 minute and then returned to their home cage. After 24 hrs, mice were placed into the same chamber for 4 min and memory for the context was measured by recording freezing behavior, defined as the absence of movement aside from that required for respiration.
Gene Ontology Analysis
WebGestalt and HOMER were used for gene ontology and KEGG pathway analyses (Wang et al., 2013; Zhang et al., 2005).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics
One-way, two-way, and three-way analyses of variance (ANOVAs) with or without repeated measures (RM) followed by Bonferroni, Dunnett, and Tukey post hoc tests were used to analyze cocaine CPP, cocaine and sucrose SA, Npas4 mRNA, Npas4-luciferase, enrichment in the ChIP assay and cocaine locomotor assay. Student’s and paired t-tests were used to analyze the Npas4 and cfos mRNA and protein expressions, enrichment in ChIP assay, behavioral assays (CPP score in conditioned place preference assay, open field, contextual fear conditioning, sucrose preference assay, cocaine and saline SA), and NPAS4 protein expression. All statistics were performed using GraphPad Prism, except SPSS software was used to handle complex data sets (e.g., three-way ANOVAs and missing values). Statistical outliers were detected using Grubbs test and excluded from analysis. All data are presented as the mean ± SEM. Significance was shown as * = p <0.05, ** = p < 0.01, *** = p < 0.001, and not significant values were not noted or shown as n.s. Also see Table S4 for detailed statistics information.
DATA AND SOFTWARE AVAILABILITY
The accession number for the ChIP sequencing data reported in this paper is NCBI GEO:103296
Supplementary Material
Figure S1. HDAC5 WT overexpression in the NAc does not influence cocaine self-administration or reinstatement, related to Figure 2
Figure S2. HDAC5 3SA in the NAc does not alter dose response, progressive ratio, or stress-induced reinstatement in the cocaine self-administration model, related to Figure 2
Figure S3. HDAC5-associated genomic DNA sequence motifs and lack of influence of HDAC5 on cFos mRNA expression in the NAc, related to Figure 3
Figure S4. Exploration in the CPP test chambers induces expression of immediate early genes, Npas4 and cFos in vivo, related to Figure 5
Figure S5. Reduction of Npas4 expression does not produce apoptosis in the NAc. Npas4 cKONAc mice display normal anxiety-like behavior, but deficits in cocaine CPP, related to Figure 6
Figure S6. NPAS4 expression in the NAc is required for discrimination of the drug-reinforced action during cocaine self-administration in mice, related to Figure 7
Figure S7. Npas4 cKONAc mice show modest deficits in operant discrimination during sucrose self-administration, related to Figure 7
Table S1. Lists of HDAC5 genomic binding from HDAC5 ChIP-seq, related to Figure 3
Table S2. Gene ontology analysis for HDAC5 ChIP-seq experiments, related to Figure 3
Table S3. KEGG pathway analysis for HDAC5 ChIP-seq experiments, related to Figure 3
Table S4. Detailed statistics information for all experiments
Highlights.
Nuclear HDAC5 in the NAc attenuates relapse-like drug seeking behaviors
ChIP-seq revealed numerous HDAC5-associated target genes including Npas4
NPAS4 in NAc is induced in subset of FOS+ neurons during cocaine-context learning
HDAC5 and NPAS4 in NAc are involved in cocaine conditioned behaviors
Acknowledgments
The authors thank Yuhong Guo, Ben Zirlin, Kelly Girskis, Guohong Xia, and Carly F. Hale for technical assistance and members of the Cowan lab and colleagues at MUSC, McLean Hospital and UTSW for helpful discussions. We thank Dr. Michael Greenberg for sharing NPAS4 reagents. MT was supported by Fugaku Trust for Medicinal Research and a NARSAD Young Investigator Award from the Brain & Behavior Research Foundation. LNS was supported by funds from the Eleanor and Miles Shore HMS Fellowship and the Phyllis and Jerome Lyle Rappaport Foundations. MBC was supported by a NIH predoctoral fellowship (F31 DA035073) and a Rossano Family Fellowship. ACB received a postdoctoral fellowship from the French Fyssen Foundation. RDP was supported by a NIH postdoctoral fellowship (F32 DA036319). This work was supported by grants from the NIH (DA027664 and DA032708 to CWC, DA10460 to DWS, MH091220 to YL, and DA003906 to PWK). JST in an Investigator in the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, M.T, M.B.C, A.C.B., J.A.H., E.A.B., R.D.P, L.N.S., D.W.S., Y.L., and C.W.C.; Methodology, N.K., T-K.K., and J.S.T.; Formal Analysis, M.T., M.B.C., Y.A.C., N.K., E.B.L., L.N.S., D.W.S., Y.L., and C.W.C.; Investigation, M.T., M.B.C., Y.A.C., A.C.B., J.A.H., E.B.L, E.A.B., B.W.H., R.D.P., J.K., and D.G.; Writing –Original Draft, M.T., M.B.C., and C.W.C.; Writing –Review & Editing, M.T., M.B.C., Y.A.C., A.C.B., J.A.H., N.K., E.B.L., E.A.B., B.W.H., R.D.P., J.K., L.N.S., D.G., J.S.T., T-K.K., P.W.K., D.W.S., Y.L., and C.W.C.; Visualization, M.T., M.B.C., and E.B.L.; Supervision, M.T. and C.W.C.; Funding Acquisition, M.T. and C.W.C.
References
- Anderson EM, Wissman AM, Chemplanikal J, Buzin N, Guzman D, Larson EB, Neve RL, Nestler EJ, Cowan CW, Self DW. BDNF-TrkB controls cocaine-induced dendritic spines in rodent nucleus accumbens dissociated from increases in addictive behaviors. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1702441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfield JL, Whittaker C, Cader MZ, Chawla S. Differential effects of Ca2+ and cAMP on transcription mediated by MEF2D and cAMP-response element-binding protein in hippocampal neurons. J Biol Chem. 2006;281:27724–27732. doi: 10.1074/jbc.M601485200. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sharma N, Browne HA, Trepman AZ, Greenberg ME. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature. 2013;503:121–125. doi: 10.1038/nature12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla AC, Garcia-Keller C, Heinsbroek JA, Scofield MD, Chareunsouk V, Monforton C, Kalivas PW. Accumbens Mechanisms for Cued Sucrose Seeking. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, Hope BT. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- Graham DL, Hoppenot R, Hendryx A, Self DW. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology (Berl) 2007;191:719–730. doi: 10.1007/s00213-006-0473-5. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC, 3rd, Siegel GS, Bobadilla AC, Kupchik YM, Kalivas PW. Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. J Neurosci. 2017;37:757–767. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui B, Wang W, Li J. Biphasic modulation of cocaine-induced conditioned place preference through inhibition of histone acetyltransferase and histone deacetylase. Saudi Med J. 2010;31:389–393. [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues Clin Neurosci. 2014;16:335–344. doi: 10.31887/DCNS.2014.16.3/pkenny. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, et al. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, Bass CE, Neve RL, Terwilliger EF, Nestler EJ, Self DW. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Herve D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000a;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000b;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76(Pt B):259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Solecki W, Gieryk A, Slezak M, Bilecki W, Ziolkowska B, Kostrzewa E, Cymerman I, Swiech L, et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010;11:R48. doi: 10.1186/gb-2010-11-5-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, Otto T, Lin Y. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Smith LN, Penrod RD, Taniguchi M, Cowan CW. Assessment of Cocaine-induced Behavioral Sensitization and Conditioned Place Preference in Mice. J Vis Exp. 2016:53107. doi: 10.3791/53107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Mardinly AR, Gabel HW, Bazinet JE, Couch CH, Tzeng CP, Harmin DA, Greenberg ME. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell. 2014;157:1216–1229. doi: 10.1016/j.cell.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Carreira MB, Smith LN, Zirlin BC, Neve RL, Cowan CW. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron. 2012;73:108–120. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Marin O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010;20:68–78. doi: 10.1016/j.conb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, Ramakrishnan C, Wang AC, Jennings JH, Adhikari A, Halpern CH, et al. Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences. Cell. 2016;165:1776–1788. doi: 10.1016/j.cell.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HDAC5 WT overexpression in the NAc does not influence cocaine self-administration or reinstatement, related to Figure 2
Figure S2. HDAC5 3SA in the NAc does not alter dose response, progressive ratio, or stress-induced reinstatement in the cocaine self-administration model, related to Figure 2
Figure S3. HDAC5-associated genomic DNA sequence motifs and lack of influence of HDAC5 on cFos mRNA expression in the NAc, related to Figure 3
Figure S4. Exploration in the CPP test chambers induces expression of immediate early genes, Npas4 and cFos in vivo, related to Figure 5
Figure S5. Reduction of Npas4 expression does not produce apoptosis in the NAc. Npas4 cKONAc mice display normal anxiety-like behavior, but deficits in cocaine CPP, related to Figure 6
Figure S6. NPAS4 expression in the NAc is required for discrimination of the drug-reinforced action during cocaine self-administration in mice, related to Figure 7
Figure S7. Npas4 cKONAc mice show modest deficits in operant discrimination during sucrose self-administration, related to Figure 7
Table S1. Lists of HDAC5 genomic binding from HDAC5 ChIP-seq, related to Figure 3
Table S2. Gene ontology analysis for HDAC5 ChIP-seq experiments, related to Figure 3
Table S3. KEGG pathway analysis for HDAC5 ChIP-seq experiments, related to Figure 3
Table S4. Detailed statistics information for all experiments