Abstract
Background
Reproducible and repeatable assessment of right heart function is vital for monitoring congenital and acquired heart disease. There is increasing evidence for the additional value of myocardial deformation (strain and strain rate) in determining prognosis. This study aims to determine the reproducibility of deformation analyses in the right heart using cardiovascular magnetic resonance feature tracking (FT-CMR); and to establish normal ranges within an adult population.
Methods
A cohort of 100 healthy subjects containing 10 males and 10 females from each decade of life between the ages of 20 and 70 without known congenital or acquired cardiovascular disease, hypertension, diabetes, dyslipidaemia or renal, hepatic, haematologic and systemic inflammatory disorders underwent FT-CMR assessment of right ventricular (RV) and right atrial (RA) myocardial strain and strain rate.
Results
RV longitudinal strain (Ell) was − 21.9 ± 3.24% (FW + S Ell) and − 24.2 ± 3.59% (FW-Ell). Peak systolic strain rate (S′) was − 1.45 ± 0.39 s− 1 (FW + S) and − 1.54 ± 0.41 s− 1 (FW). Early diastolic strain rate (E′) was 1.04 ± 0.26 s− 1 (FW + S) and 1.04 ± 0.33 s− 1 (FW). Late diastolic strain rate (A′) was 0.94 ± 0.33 s− 1 (FW + S) and 1.08 ± 0.33 s− 1 (FW). RA peak strain was − 21.1 ± 3.76%. The intra- and inter-observer ICC for RV Ell (FW + S) was 0.92 and 0.80 respectively, while for RA peak strain was 0.92 and 0.89 respectively.
Conclusions
Normal values of RV & RA deformation for healthy individuals using FT-CMR are provided with good RV Ell and RA peak strain reproducibility. Strain rate suffered from sub-optimal reproducibility and may not be satisfactory for clinical use.
Keywords: Right ventricle, Right atrium, Feature tracking, Cardiovascular magnetic resonance imaging, Strain, Strain rate
1. Introduction
Reproducible and repeatable quantification of right heart function is vital for monitoring patients, yet volume-based measurements are time-consuming, difficult and require meticulous care even in the era of semi-automated boundary detection [1]. Although there is extensive evidence that left ventricular (LV) and right ventricular (RV) ejection fraction are of prognostic importance, there is increasing evidence for the incremental value of myocardial deformation (strain and strain rate) imaging in both congenital [2], [3] and acquired cardiovascular disease [4]. Strain measured by cardiovascular magnetic resonance imaging (CMR) has been shown to be both a more sensitive and earlier marker of contractile dysfunction in multiple studies but until the development of feature-tracking, has not gained wide popularity in routine clinical practice due to the time needed for additional acquisition and time required for analysis [5]. Feature-tracking CMR (FT-CMR) of the RV and right atrium (RA) offers the potential for rapid and sensitive quantification of function, with the advantage of high image quality unlimited by the availability of an adequate acoustic window. Normal ranges are available for LV strain and strain rate by FT-CMR [6]. Data for the right heart are limited and knowledge of reproducibility is particularly important in the RV due to the potential adverse effects of through plane motion, with loss of features leaving the image plane, and needs to be known to ensure clinical utility [7]. Therefore, the aims of this study were: firstly, to establish normal ranges for RV and RA peak longitudinal strain and strain rate within an adult population that can be used for future comparative studies; secondly, to determine reproducibility of analysis of deformation of the RV and RA using FT-CMR.
2. Methods
2.1. Study population
A cohort of 100 normal healthy subjects, with 10 men and 10 women in each of 5 age deciles from 20 to 70 years was constructed. Subjects were recruited from control participants of an on-going research study within the Department of Cardiology, University Hospital Birmingham and the Institute of Cardiovascular Science, University of Birmingham (CRIB-Donor NCT01769924). Self-report history was taken to exclude subjects with chest pain, breathlessness or other cardiac symptoms, and those with a history of hypertension, diabetes, dyslipidaemia, or any cardiovascular, renal, hepatic, haematological and systemic inflammatory disease. Clinical examination was performed, including office blood pressure (normal < 140/90 mm Hg). Blood samples were taken to confirm normal range full blood count, serum electrolytes, and random glucose. All subjects had a normal resting 12‑lead ECG and either negative (maximal > 85% maximum predicted heart rate) exercise stress echocardiography or exercise ECG. Demographic data were collected and informed consent was obtained from each patient but ethnicity was not recorded. The QRISK®2 score is widely used within the National Health Service to predict the risk of an individual developing cardiovascular disease over the next 10 years [8]; this was calculated for each patient using the QRISK®2-2017 calculator. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
2.2. CMR acquisition
CMR imaging was conducted using a 1.5-T scanner (Magnetom Avanto, Siemens, Germany). Cardiac morphology and function were studied using standard CMR protocols, with steady state free precession (SSFP) cine images (typical parameters: resolution 40–50 ms, repetition time 3.2 ms, echo time 1.7 ms, flip angle 60, field of view 300 mm, in-plane resolution 1.5 × 1.5 mm2, slice thickness 7 mm with 3 mm gap, minimum 25 phases per cardiac cycle) in accordance with previously validated methodology [9].
2.3. CMR analysis
Image analysis was performed off-line using a standardized approach by trained cardiologists (AD; BL) with delineation of papillary muscles and trabeculations using thresholding (version 5.3.4 cvi42, Circle Vascular Imaging, Canada).
2.4. CMR RV feature tracking
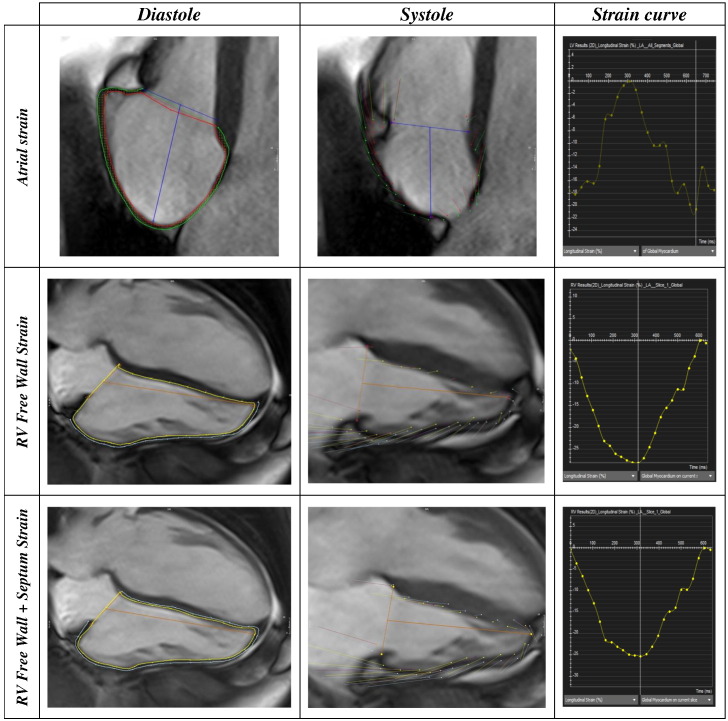
Cvi42 is based upon an incompressible volume-based algorithm, which has been previously validated to produce accurate biventricular anatomical tracking [10]. From the horizontal long axis view, right ventricular 2D longitudinal (Ell) strain as well as strain rates (peak systolic strain rate SRS′; peak early diastolic strain rate SRE′; peak late diastolic strain rate SRA′) were defined in the region of interest between the endocardial and epicardial borders (Fig. 1) drawn in the end-diastolic frame (defined as phase 1 out of 25). Beginning of systole is defined as phase 2 out of 25; end-systole is defined as peak RV contraction with the smallest RV area. RV peak strain was taken as the highest strain irrespective of time during systole. Two datasets were produced with inclusion (free wall plus septum Ell, FW + S Ell) [11] and exclusion (free wall Ell, FW-Ell) [12] of the septum to reflect different approaches to the contribution of the septum to RV function. The accuracy of feature tracking for both endocardial and epicardial RV borders was visually checked following automated strain analysis on the CMR model, and good quality tracking was obtained in all subjects following a maximum of two user adjustments. Endocardial and epicardial borders were drawn around the largest RA area to coincide with the RV end-systolic phase.
Fig. 1.
Contouring for RA and RV strain, with the timing of peak strain interpretation on the strain curve. RA Ell was defined as the peak in the strain curve irrespective of the time in the cardiac cycle. RV Ell was defined as the peak in the strain curve irrespective of the time in the cardiac cycle.
2.5. Reproducibility
All CMR studies were anonymized prior to strain analysis. For intra-observer variability, observer 1 (AD) performed tissue tracking analysis for all 100 subjects, with a second analysis repeated in a randomly generated subset of 10 patients after a 1-month interval. For inter-observer variability, observer 2 (BL) independently feature tracked the randomly generated set of 10 scans.
2.6. Statistical analysis
Data are presented as mean ± standard deviation. Data distribution for continuous variables was assessed using normality plots and the Kolmogorov–Smirnov test. Independent t-tests were used to compare inter-gender differences. Correlations were assessed with Pearson's correlation coefficient. Intra- and inter-observer agreement was tested by calculating mean absolute bias and 95% limits of agreement (confidence intervals, CI) from Bland–Altman analyses and intraclass correlation coefficient (ICC). A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v24.0 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Baseline demographics
Full demographic data are available within the ‘data in brief’ article [13]. All participants had a 10-year QRISK-2 score of < 20% [8]. There were 8 current smokers and 19 ex-smokers. No patient had a clinical history of COPD. Indexed cardiac volumes, mass, and ejection fraction were within normal limits for all participants for both the LV (LVEF 70.6 ± 6.6%) and RV (RVEF 66.9 ± 7.7%) [14]. There was a weak correlation between age and increasing LVEF (r = 0.4, P < 0.001), RVEF (r = 0.2, P = 0.03), and decreasing indexed biventricular volumes (LVEDVi r = − 0.4, P < 0.001; LVESVi r = − 0.45, P < 0.001; RVEDVi r = − 0.3, P = 0.001; RVESVi r = − 0.3, P = 0.001).
3.2. Reference values for RV strain and strain rate
Good quality tracking was obtained in all subjects following a maximum of two editions. Normal RV strain and strain rates are shown in Table 1. FW-Ell (− 24.2 ± 3.59) was significantly higher than FW + S Ell (− 21.9 ± 3.24). There were no gender differences in strain (Table 3, 'data in brief') [13]. For both FW and FW + S techniques, there were no clear relationships between strain or strain rates and increasing age on linear regression analysis. However, with inclusion of the septum, increasing age was associated with a linear decrease in early diastolic strain rate (SRE, falling from a mean of 1.18 ± 0.24 s− 1 to 0.92 ± 0.25 s− 1 with advancing age from 20 to 70 years of age, r = − 0.30, P = 0.002) and an increase in late diastolic strain rate (SRA, r = 0.24, P = 0.018) [13]. Considering the absence of a consistent impact of age and gender on strain and strain rate, the normal range values of the overall cohort are presented (Table 1) with individual decile values presented in the associated ‘data in brief’ article [13].
Table 1.
Normal ranges for RV deformation.
| Free wall plus septum (FW + S) | Free wall (FW) | P | |
|---|---|---|---|
| RV Ell (%) | − 21.9 ± 3.24 | − 24.2 ± 3.59 | < 0.001 |
| SRS′ (s− 1) | − 1.45 ± 0.39 | − 1.54 ± 0.41 | 0.03 |
| SRE′ (s− 1) | 1.04 ± 0.26 | 1.04 ± 0.33 | 0.95 |
| SRA′ (s− 1) | 0.94 ± 0.33 | 1.08 ± 0.33 | < 0.001 |
Values are mean ± SD; P values derived from paired T-test. RV Ell = right ventricular peak longitudinal strain; SRS′ = peak systolic strain rate; SRE′ = peak early diastolic strain rate, SRA′ = peak late diastolic strain rate.
3.3. Reference values for RA strain
Mean RA Ell was − 21.1 ± 3.76 and was unaffected by age or gender [13].
3.4. Reproducibility
Intra- and inter-observer reproducibility for RV and RA Ell was consistently high (ICC range 0.80–0.92) but worse for strain rate whether analyzed with inclusion or exclusion of the septum. Full reproducibility data are presented in the associated ‘data in brief’ article [13].
4. Discussion
This is the first study to report reference values for RV and RA myocardial Ell using FT-CMR from a group of healthy volunteers across a balanced stratification of age and sex. No consistent relationship between RV Ell, RA Ell or strain rate parameters and either age or sex was found, despite the relationship between baseline biventricular volumes and age being similar to that observed in the general population [14]. The reproducibility of RV and RA Ell was satisfactory but poor for RV strain rate, which are differential quantities that are derivatives of the former.
In contrast to studies that have demonstrated an association between age and LV Ell and strain rate [6], no such relationship was found in our study between age and RV Ell. It is interesting to note that our study found the same relationship between increasing age and conventional measures of chamber size and function as previous research, demonstrating decreasing RV volumes with increasing RV ejection fraction [14]. There is one other small study of longitudinal free wall RV Ell using FT-CMR [12], and one study of 219 healthy subjects using speckle tracking echocardiography that found no relationship with age [15]. While results using pulsed Tissue Doppler are discordant, the largest study identified a fall in systolic velocity with age as might be expected from the gradual increase in pulmonary artery systolic pressure [16]. In contrast, another speckle tracking study of 276 healthy volunteers found a statistically significant decline in RV Ell with age that was felt too small to be of clinical importance [17] but did find a difference in values between genders. It is interesting to speculate whether FT-CMR RV Ell does not change with age due to greater load-independence but unclear why there are differences in the results relating to gender. The values obtained in the cohort studied by Muraru are much higher than those in our study and it is possible these are due to differences in the procedures used for calculation of the physical quantities, not only in terms of algorithms. Such issues have bedeviled echocardiography and are likely to be greater between modalities [18].
Our study confirms that different results are obtained when measuring RV Ell and strain rate when including and excluding the septum, with lower FW + S Ell than FW-Ell as reported using speckle tracking echocardiography [15]. The lower limit of normal RV FW-Ell in our study (− 17.8%) was below that obtained in the previous smaller study with a lower mean age (− 21.11%) [12] but similar to the values from a small control cohort in a study of arrhythmogenic RV cardiomyopathy [19]. It is not known however, whether FW + S or FW-Ell may be more useful as a marker of RV contractility. It is equally possible that the difference between these values is technical — in order to maximize accuracy, the FT-CMR algorithm uses all available information to track a point between consecutive frames, including tissue–cavity interface and spatial coherence, and the addition of the septum may affect the tracking adaptation. Further work is needed using both values across a range of disease states and in subjects of different ethnicity.
4.1. Limitations
The FT-CMR algorithm has been adapted here for analysis of the RA, and while there is work validating this algorithm for biventricular strains, there is no validation for the RA. No measurement of time to peak strain was recorded within this study, although it is a recognized limitation of deformation analyses of cine studies on CMR that temporal resolution is limited by multi-beat acquisitions.
4.2. Conclusions
FT-CMR for RV and RA Ell is a novel technique that is reproducible and allows rapid measurement of myocardial deformation without the limitation of acoustic window or need for additional sequence acquisition. We provide here age-stratified normal range values for right heart FT-CMR, which will have important consequences for the prognostic stratification of congenital and acquired cardiovascular diseases. RV strain rate by FT-CMR however, has poorer reproducibility and at present, may not provide repeatable quantification for clinical use.
Conflict of interest
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. The authors declare no conflict of interest.
Footnotes
Funding from British Heart Foundation grant no: PG/14/74/31056 and FS/11/17/28700.
References
- 1.Blalock S.E., Banka P., Geva T., Powell A.J., Zhou J., Prakash A. Interstudy variability in cardiac magnetic resonance imaging measurements of ventricular volume, mass, and ejection fraction in repaired tetralogy of Fallot: a prospective observational study. J. Magn. Reson. Imaging. 2013;38(4):829–835. doi: 10.1002/jmri.24050. [DOI] [PubMed] [Google Scholar]
- 2.Orwat S., Diller G.P., Kempny A., Radke R., Peters B., Kuhne T. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart. 2016;102(3):209–215. doi: 10.1136/heartjnl-2015-308569. [DOI] [PubMed] [Google Scholar]
- 3.Moon T.J., Choueiter N., Geva T., Valente A.M., Gauvreau K., Harrild D.M. Relation of biventricular strain and dyssynchrony in repaired tetralogy of Fallot measured by cardiac magnetic resonance to death and sustained ventricular tachycardia. Am. J. Cardiol. 2015;115(5):676–680. doi: 10.1016/j.amjcard.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Fine N.M., Chen L., Bastiansen P.M., Frantz R.P., Pellikka P.A., Oh J.K. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ. Cardiovasc. Imaging. 2013;6(5):711–721. doi: 10.1161/CIRCIMAGING.113.000640. [DOI] [PubMed] [Google Scholar]
- 5.Hor K.N., Gottliebson W.M., Carson C., Wash E., Cnota J., Fleck R. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. J. Am. Coll. Cardiol. Img. 2010;3(2):144–151. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Taylor R.J., Moody W.E., Umar F., Edwards N.C., Taylor T.J., Stegemann B. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur. Heart J. Cardiovasc. Imaging. 2015;16(8):871–881. doi: 10.1093/ehjci/jev006. [DOI] [PubMed] [Google Scholar]
- 7.Morais P., Marchi A., Bogaert J.A., Dresselaers T., Heyde B., D'hooge J. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: assessment of variability in a real-life clinical setting. J. Cardiovasc. Magn. Reson. 2017;19(1):24. doi: 10.1186/s12968-017-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hippisley-Cox J., Coupland C., Vinogradova Y., Robson J., Minhas R., Sheikh A. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. Br. Med. J. 2008;336(7659):1475–1482. doi: 10.1136/bmj.39609.449676.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maceira A.M., Prasad S.K., Khan M., Pennell D.J. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur. Heart J. 2006;27(23):2879–2888. doi: 10.1093/eurheartj/ehl336. [DOI] [PubMed] [Google Scholar]
- 10.Bistoquet A., Oshinski J., Skrinjar O. Myocardial deformation recovery from cine MRI using a nearly incompressible biventricular model. Med. Image Anal. 2008;12(1):69–85. doi: 10.1016/j.media.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Morton G., Schuster A., Jogiya R., Kutty S., Beerbaum P., Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J. Cardiovasc. Magn. Reson. 2012;14(1):43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Truong V.T., Safdar K.S., Kalra D.K., Gao X., Ambach S., Taylor M.D. Cardiac magnetic resonance tissue tracking in right ventricle: feasibility and normal values. Magn. Reson. Imaging. 2017;38:189–195. doi: 10.1016/j.mri.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu B., Dardeer A.M., Moody W.E., Edwards N.C., Hudsmith L.E., Steeds R.P. Reference ranges and reproducibility studies for right heart myocardial deformation by feature tracking cardiovascular magnetic resonance imaging. Int. J. Cardiol. 2017 doi: 10.1016/j.dib.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen S.E., Aung N., Sanghvi M.M., Zemrak F., Fung K., Paiva J.M. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 2017;19(1):18. doi: 10.1186/s12968-017-0327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ermacora D.B.L., Muraru D., Gentian D., Dal Bianco L., Casablanca S., Peluso D., Zoppellaro G., Cucchini U., Iliceto S. Reference values of right ventricular longitudinalstrain by speckle tracking echocardiographyin 219 healthy volunteers. Eur. Heart J. 2013;34(Supplement 1):684. [Google Scholar]
- 16.Innelli P., Esposito R., Olibet M., Nistri S., Galderisi M. The impact of ageing on right ventricular longitudinal function in healthy subjects: a pulsed tissue Doppler study. Eur. J. Echocardiogr. 2009;10(4):491–498. doi: 10.1093/ejechocard/jen313. [DOI] [PubMed] [Google Scholar]
- 17.Muraru D., Onciul S., Peluso D., Soriani N., Cucchini U., Aruta P. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ. Cardiovasc. Imaging. 2016;9(2):3866. doi: 10.1161/CIRCIMAGING.115.003866. [DOI] [PubMed] [Google Scholar]
- 18.Voigt J.U., Pedrizzetti G., Lysyansky P., Marwick T.H., Houle H., Baumann R. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015;28(2):183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Heermann P., Hedderich D.M., Paul M., Schülke C., Kroeger J.R., Baeßler B. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J. Cardiovasc. Magn. Reson. 2014;16(1):75. doi: 10.1186/s12968-014-0075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]