Abstract
The life cycle of Taenia solium, the pork tapeworm, is continuously closed in many rural settings in developing countries when free roaming pigs ingest human stools containing T. solium eggs and develop cysticercosis, and humans ingest pork infected with cystic larvae and develop intestinal taeniasis, or may also accidentally acquire cysticercosis by fecal-oral contamination. Cysticercosis of the human nervous system, neurocysticercosis, is a major cause of seizures and other neurological morbidity in most of the world. The dynamics of exposure, infection and disease as well as the location of parasites result in a complex interaction which involves immune evasion mechanisms and involutive or progressive disease along time. Moreover, existing data is limited by the relative lack of animal models. This manuscript manuscript revises the available information on the immunology of human taeniasis and cysticercosis.
Keywords: Parasitic infections, Cysticercosis, Neurocysticercosis, Taenia solium, seizures, Peru
LIFE CYCLE, EPIDEMIOLOGY, AND CLINICAL MANIFESTATIONS
Life Cycle
Taenia solium, the pork tapeworm, is a cestode (flatworm) parasite whose life cycle is continuously closed in many rural settings in developing countries when free roaming pigs ingest human stools containing T. solium eggs and develop the larval infection, becoming intermediate hosts, and humans ingest pork infected with cystic larvae and develop intestinal taeniasis.(1, 2)
When pork contaminated with parasitic cysts is ingested by a human, the cyst’s scolex evaginates by action of bile and intestinal enzymes, and affixes to the mucosa of the human small intestine. Here the worm grows by reproducing its cells at the neck level, developing segments or proglottids which mature as they become more distal to the scolex. The adult T. solium measures between 2 to 4 meters in length.(1, 3) The tapeworm is hermaphroditic and after fertilization the final segments are gravid and full of mature eggs. These infective eggs are expelled to the environment with the feces of the tapeworm carrier. Once ingested by a suitable host (usually the pig), the embryos contained in the eggs hatch, cross the intestinal wall, and are carried by the bloodstream to all body tissues where they establish as the larval stage or cysticercus. Humans get infected with cysticercosis via fecal oral contamination. Thus humans may have adult intestinal tapeworm (taeniasis), or larval (human cysticercosis) infections, while pigs only act as intermediate hosts (porcine cysticercosis).(4)
Geographical Distribution
Taeniasis/cysticercosis is endemic in Latin America, Sub-Saharan Africa, India, vast parts of China, and South East Asia.(5–7) Cysticercosis cases are also seen with some frequency in non endemic countries in North America, Europe and Muslim regions because of travel and immigration from endemic countries, as clearly demonstrated in an outbreak of cysticercosis in Orthodox Jews in New York city.(8) The infection and subsequent disease result in significant costs both from health and other costs related to symptomatic disease and from losses to farmers because of porcine cysticercosis.(9–11)
The large and very similar tapeworms Taenia solium, Taenia saginata, and Taenia asiatica may coexist in some geographical areas.(12, 13) It has been suggested that the co-existence of other close taenid species may somehow reduce or restrict Taenia solium transmission.(14)
Clinical manifestations
While intestinal taeniasis is basically asymptomatic,(15) cysticercosis cysts in the nervous system produce neurocysticercosis (NCC), which is responsible for most of the burden of human disease. Seizures are the commonest clinical manifestation and in fact NCC is considered the major cause of adult onset seizures worldwide. The overall contribution of NCC to the prevalence of epilepsy in endemic regions is estimated to be around 30% of all epilepsy cases.(16, 17) Surveys in endemic regions using serology or CT consistently demonstrate two or three times more evidence of infection in individuals with epilepsy than in comparable asymptomatic populations.(17–23) Risk factors for cysticercosis include a history of intestinal taeniasis, pig raising, and poverty-related factors including living in a rural area and poor sanitation.(24)
The clinical manifestations of symptomatic human NCC reflect the number, location, size and evolutionary stage of the parasites, as well as the presence and degree of the inflammatory response of the host. Parasitic larvae located in the parenchyma of the brain most frequently manifest with seizures. They establish as viable cysts, and after an extremely variable period (which may be decades) follow an involutive process, driven by the attack of the immune response of the host. Whether this process is always a consequence of the death of the parasite is unlikely since in a placebo-controlled study of antiparasitic treatment of patients with viable NCC cysts 87% of the cysts were still viable 6 months later.(25) Initially the viable cysts are rounded vesicles of parasitic membrane filled with clear fluid, containing a scolex or tapeworm head. Following the host’s attack the cysts contents become turbid, the membrane and scolex degenerate by the action of the cellular response, and the cyst structures shrink and are replaced by hyaline and fibrotic tissue to later disappear or leave a residual calcified scar.(26, 27) Parasites in the subarachnoid space follow a different course and tend to grow and infiltrate, becoming mass occupying lesions and blocking the circulation of the cerebrospinal fluid with subsequent hydrocephalus. Unlike intraparenchymal NCC; subarachnoid disease is progressive and associated with significant mortality.(28–30)
IMMUNOLOGY OF HUMAN CYSTICERCOSIS
Exposure to the parasite
In Taenia solium endemic regions, exposure of the human population to the parasite is a very frequent event. Between 10 and 25% of all villagers show specific anti T. solium serum antibody responses in the very specific enzyme-linked immunoelectrotransfer blot assay using lentil-lectin-purified glycoprotein antigens (LLGP-EITB).(5, 31) This is clearly an underestimation of the proportion ever exposed since a sizable group of people, up to 50% of those seropositive, will turn seronegative in a short term (transient antibodies).(32–34) It follows that at population level T. solium human infection is a very dynamic process. Most if not all seroepidemiological studies have used antibodies to the cyst stage. Antibodies to the oncospheral stage, which should arise much early in the infection process, have been identified.(35) However, no population studies of transmission dynamics using antibodies to the oncospheral stage are available. How previous exposures to the parasite affect the likelihood of successful infection in further challenges is not known.
Established infection
Very likely most of the invading oncospheres are destroyed while passing through the liver, or early at arrival in non-immunologically privileged sites.(4, 36) Some oncospheres survive, preferentially in protected places like the central nervous system or the eye. Still, human infection is a frequent event which in most cases courses and resolves without obvious symptoms, as proven by the sizable proportion of people showing residual brain calcifications in endemic populations. Consistently, epidemiological studies using brain CT (or more rarely using MRI), finding that 10 to 20% of villagers have brain cysticercosis, most of them with only one or a few calcified scars and no clinical disease.(37–41) Most cases of human infection in population based studies correspond to these calcified lesions. The proportion of people who show viable NCC in non-symptomatic individuals in population-based studies is much smaller, usually below 1%.(40) An Indian study using MRI in a highly endemic population reported 5% of asymptomatic individuals having one or two small brain cysts.(42) So far, no other studies have replicated these findings. Also there is basically no information on how many people could have cysticercosis in sites other than the nervous system in a population setting.
Exposure, infection, established viable infection, and symptomatic disease
While exposure and resolved infections are a frequent event in human populations, infections with viable parasites are present in smaller proportions in asymptomatic individuals (<10% of all imaging positive).(40) In clinical series, conversely, viable NCC cases comprise the majority of cases,(24) suggesting that established viable infections are associated with a higher likelihood of symptoms and more severe disease.
Evolution of human neurologic and extraneural infection
Available information on the evolution of human infections comes from large case series described more than a century ago.(43–45) These case series are from neurological patients so neurocysticercosis is the dominant presentation, although exhaustive search identified residual calcifications in muscles or in subcutaneous tissues in many cases.(45) From the few patients with concomitant subcutaneous cysticercosis it is apparent that subcutaneous nodules are noticeable months or years before neurological symptoms appear.(45) Also necropsy series demonstrate that in most cases cysts in tissues other than the nervous system had resolved while brain cysts are still viable.(43, 44) Patients may host viable parenchymal cysts for many years, even decades. Patients with subarachnoid neurocysticercosis manifest with mass effects or intracranial hypertension at older ages. Overall, the fragmentary available information suggests that embryos get distributed by the circulatory system to all tissues, and survive preferentially in the nervous system where they can be alive for many years or even be the cause of progressive disease as it commonly occurs in subarahnoid NCC.
Immune response by type of NCC
In general, extraparenchymal NCC (cysts in the ventricles or subarachnoid space) is associated with high parasite antigen levels, an exuberant immune response expressed as very strong antibody reactions.(30, 46) and marked local inflammation with mononuclear CSF pleocytosis and increased proteins. Conversely, the degree of immune response in patients with only intraparenchymal lesions is dependent on the number (and likely the volume) of the lesions as well as their stage of involution. Antigen levels and antibody responses may not be detectable in up to 40% of individuals with a single degenerating parasite, while individuals with multiple viable cysts are consistently seropositive and their antigen levels correlate with the numbers of parasites.(47, 48)
Immunopathogenesis
The T. solium cysticercus uses a series of active immune evasion mechanisms which include protection by local barriers (such as the blood brain barrier or the hemato-ocular barrier), blockage of the complement system, secretion of cytokines affecting the cellular response, safe degradation of host immunoglobulins, or masking itself with host immunoglobulins to evade immune surveillance.(49–55)
Intraparenchymal NCC
Most available information refers to intraparenchymal NCC. Once the cysts establish in the nervous system, it was originally felt that a Th2 type response was established with low IFN g and IgG2a antibodies and increased IgG1, IgE, IL 4, IL 13 and IL 15.(39, 56–62). However, it rapidly became apparent that the acquired T cell response was mixed in the murine model of infection using T. crassiceps with a mixed Th1 and Th2 phenotype.(63) A similar mixed picture was reported in pigs vaccinated against T. solium with the suggestion that the Th1 cytokines were associated with a post-vaccination inflammatory response.(64) In man, where a mixed Th1/Th2 response is also found,(65, 66) the presence of Th2 cytokines have been associated with asymptomatic disease suggesting that these cytokines reduce inflammation whatever the anatomical location of cysts.(67) Protective induction of Th2 responses has been shown in other tissue larval helminth infections including schistosomiasis,(68) Echinococcus multilocularis,(69) and others. Osteopontin may have a role in down-regulating the inflammatory Th1 response(70) although other endocrine mediators including sex hormones may also be involved.(71) Little is known about other T cell subsets in neurocysticercosis although IL-17, the prototypic Th17 cytokine, has been reported in infection (71) and recently Tregs have been found in the CNS and appeared to be important in limiting inflammation.(72)
More recently, alternatively activated macrophages have been proposed to contribute in maintaining this stage.(73, 74) Immune modulation is lost once the host discovers the parasitic cyst naturally(75) or more markedly after antiparasitic treatment. At this point the chemokine profile switches back to Th1-like and the host’s inflammatory response attacks and destroy the parasite. Perilesional inflammation is a major contributor to seizures and other symptoms in intraparenchymal NCC.(76, 77) The pro-inflammatory response is in part mediated via MyD88 pathway a key regulator of cytokines such as TNF, IL-1B and IL-6 gene expression and secretion in monocyte-derived cells including microglia.(78) We demonstrated that in human monocytes chemokine secretion in response to cyst antigens is dependent on the transcription factor NF-kB but independent of TLR-4.(79) Monocyte-astrocyte networks may be key in amplifying the pro-inflammatory response.(80) However, whether this pathway is directly associated with seizure activity has been questioned in the murine model.(81)
In addition to cytokines, immune mediators involved in migration and adhesion of inflammatory leukocytes are also up-regulated in neurocysticercosis although data are relatively sparse. Soluble intercellular adhesion molecule (sICAM)-1 was elevated in patients with symptomatic neurocysticercosis.(82, 83) There are no data on other adhesion molecules or on conformational changes in their ligands, the integrins.
Although cytokines and other immune mediators are important in driving the pro-inflammatory response, tissue damage observed on CT scans and increased permeability of the blood brain barrier implies the involvement of enzymes driving tissue destruction. The blood brain barrier is rich in type 4 collagen which is one of the substrates of matrix metalloproteinase (MMP)-9. The MMPs are a family of zinc-containing enzymes potentially able to degrade all components of the extra-cellular matrix.(84) They are secreted usually as pro-forms by a large variety of cells including those of the monocyte lineage and stromal cells. MMPs are tightly transcriptionally regulated and also blocked by specific Tissue Inhibitors of Metalloproteinases (TIMPs). MMP-9 or gelatinase B has been associated with both breakdown of the blood brain barrier in the murine model of neurocysticercosis(85, 86) and there are data consistent with this observation from patient studies(87, 88) Of particular interest was the observation that the non-specific MMP inhibitor doxycycline reduced leukocyte-dependent inflammation in the murine model of neurocysticercosis.(89) In addition to its known antibiotic activity, doxycycline is licensed as a non-specific MMP inhibitor for use in periodontal disease in the USA and these emerging data suggest that anti-MMP therapy or possibly blockade of the upstream signaling pathways may be an effective way to control inflammatory tissue damage in neurocysticercosis.
Extraparenchymal NCC
There two major types of extraparenchymal NCC. In intraventricular disease, parasites are usually cystic in nature (spherical, most times with a visible scolex), and most of the clinical manifestations are caused by direct blockage of the CSF circulation and the resulting obstructive hydrocephalus. There is an associated inflammatory component and high dose steroids frequently contribute to improve the patient situation. Steroids should always be administered in the settings of appropriate control of intracranial hypertension and shunt placement where indicated. We have found that cyst fluid contains an anti-inflammatory IL-10-like mediator which is lost during inflammation associated with anti-parasitic therapy which results in increased pro-inflammatory chemokine secretion in response to scolex and membrane antigens.(90)
In subarachnoid NCC (basal or of the Sylvian fissure), the lesions demonstrate an exuberant growth of the cystic membrane, many times forming clumps of vesicles (hence the old name of recemose cysticercosis, because of its similitude to a bunch of grapes) in which many times no scolex is visible. This type of NCC occurs in patients quite older than those with intraparenchymal NCC.(28)
Coinfections with NCC and other agents
The coexistence of NCC and HIV has been reported in multiple occasions.(91, 92) While some of these patients had severe forms of NCC, and some authors hypothesized that HIV infection could be associated with more severe NCC, no controlled data exist to support this affirmation.(93, 94) Another co-infection reported multiple times is the coexistence of NCC with Japanese encephalitis. Lesions of both diseases tend to occur in the same hemisphere, and it is also assumed that NCC predisposes to more severe disease in Japanese encephalitis.(95–97)
IMMUNOLOGY OF THE INTESTINAL TAPEWORM STAGE
Adult tapeworm infections are present in a small proportion of individuals in endemic regions, usually between 0.5% to 2% of the population.(7, 98) The enormous biotic potential of T. solium allows this small population of tapeworms to infect many pigs and thus ensure the survival of the species.
Life span of the tapeworm
It was initially believed that the adult Taenia solium tapeworm lived for many years. This seems to have been based on anecdotal case reports most likely corresponding to other tapeworms. Clinical evidence do not support this claim. The tapeworm carrier is the person most exposed to infection, however only a few patients with NCC carry a tapeworm by the time of diagnosis.(99) In the classic reports of the British military “outbreak” (in which 454 British soldiers or immediate relatives developed neurological disease due to cysticercosis after returning from their tour of duty in India, data which nicely demonstrated that a significant majority of cases presented symptoms between 2 to 5 years after exposure),(44, 45, 100) very few tapeworm carriers were detected, suggesting that most tapeworms would live less than 5 years. Epidemiological data also supports this concept. In the largest series of Taenia solium taeniasis infections published, Allan et al presented a curve of taeniasis prevalence by age which sharply decreases after age 30,(101) where a sharp decrease in prevalence at a given age period is unlikely to occur in a long lived infection. Also, seizure cases do not cluster around tapeworm carriers(102) reflecting a changing tapeworm population.
Interaction with the host
The T. solium tapeworm lodges in the upper small intestine and uses its four suckers and its double crown of hooks to anchor in the intestinal mucosa.(103) Once the suckers attach to the villi, the rostellum projects into the mucosa and the hooks extend, fixing the scolex to the intestine. There is local damage and an inflammatory response which involves mast cells and goblet cells, and varied cell populations including plasma cells, lymphocytes, neutrophils and eosinophils.(55)
Stage-specific antibody responses have been identified in serum from tapeworm carriers (see below under diagnosis of taeniasis). T. solium calreticulin, a calcium binding protein which preferentially localizes in the rostellum and suckers, has been explored as a potential oral vaccine to increase the local intestinal mucosal response to an incoming tapeworm.(104) After immunization with this protein, evidences of partial protection have been obtained in two different rodent models, hamsters and gerbils.(105, 106) In the related tapeworm Echinococcus granulosus, two different research groups have demonstrated significant protection after vaccination of the definitive host, the dog.(107–109)
In summary, human taeniasis/cysticercosis is a very complex example of host-parasite interaction where the immune response of the host and the extent and nature of the inflammatory reaction, with active immunomodulatory mechanisms enacted by the parasite, determine the symptomatic expression of clinical disease. Our understanding of the specific mechanisms involved in this interaction is limited by the inherent variability of infections in terms of infective dose, number, size and location of the parasites (with the consequent extreme variability of its clinical expression), the very high frequency of exposure of individuals and animals to the parasite in endemic regions, the long period between infection and disease, and also by the lack of appropriate animals models.
Figure 1.
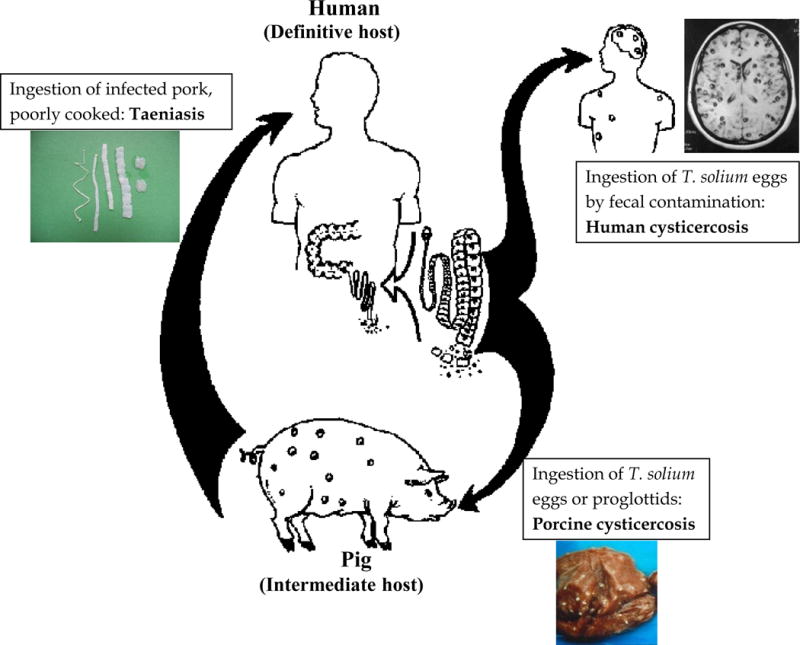
Life cycle of Taenia solium (adapted with permission from Garcia HH and Martinez SM. Taenia solium taeniasis/cysticercosis, Lima, Ed. Universo; 1999: 360 p.)
Figure 2.
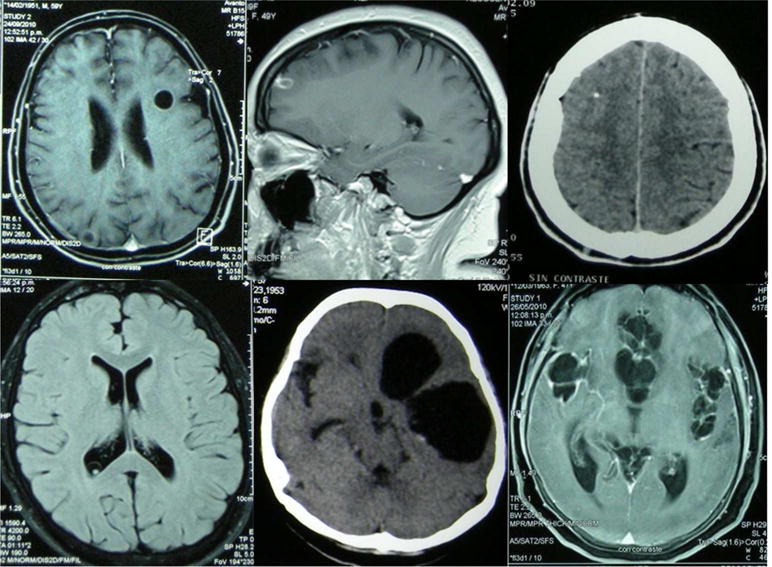
Intraparenchymal neurocysticercosis: typical images of viable (top left), degenerating (top center), and calcified (top right). Extraparenchymal neurocysticercosis: intraventricular cyst (bottom left), a cyst mass in the Sylvian fissure (bottom center), and basal subarachnoid cysticercosis (bottom right).
Acknowledgments
H.G. is supported by a Wellcome Trust Senior International Research Fellowship in Public Health and Tropical Medicine. S. R. is supported by the Fogarty International Center/NIH (training grant D43 TW001140). The funders had no role in study design; data collection, analysis, or interpretation; in writing the report, or in the decision to submit the article for publication.
References
- 1.Garcia HH, Del Brutto OH. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4(10):653–61. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 2.Praet N, Kanobana K, Kabwe C, Maketa V, Lukanu P, Lutumba P, et al. Taenia solium cysticercosis in the Democratic Republic of Congo: how does pork trade affect the transmission of the parasite? PLoS neglected tropical diseases. 2010;4(9):e817. doi: 10.1371/journal.pntd.0000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flisser A. State of the art of Taenia solium as compared to Taenia asiatica. The Korean journal of parasitology. 2013;51(1):43–9. doi: 10.3347/kjp.2013.51.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flisser A. Taeniasis and cysticercosis due to Taenia solium. Prog Clin Parasitol. 1994;4:77–116. [PubMed] [Google Scholar]
- 5.Schantz PM. Taenia solium cysticercosis: an overview of global distribution and transmission. In: Singh G, Prabhakar S, editors. Taenia solium cysticercosis From basic to clinical science. Oxon, UK: CABI Publishing; 2002. [Google Scholar]
- 6.Winkler AS. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathogens and global health. 2012;106(5):261–74. doi: 10.1179/2047773212Y.0000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, Gavidia C, et al. Hyperendemic human and porcine Taenia solium infection in Peru. The American journal of tropical medicine and hygiene. 2003;68(3):268–75. [PubMed] [Google Scholar]
- 8.Schantz PM, Moore AC, Munoz JL, Hartman BJ, Schaefer JA, Aron AM, et al. Neurocysticercosis in an Orthodox Jewish community in New York City. The New England journal of medicine. 1992;327(10):692–5. doi: 10.1056/NEJM199209033271004. [DOI] [PubMed] [Google Scholar]
- 9.Rajkotia Y, Lescano AG, Gilman RH, Cornejo C, Garcia HH. Economic burden of neurocysticercosis: results from Peru. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101(8):840–6. doi: 10.1016/j.trstmh.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Praet N, Speybroeck N, Manzanedo R, Berkvens D, Nsame Nforninwe D, Zoli A, et al. The disease burden of Taenia solium cysticercosis in Cameroon. PLoS neglected tropical diseases. 2009;3(3):e406. doi: 10.1371/journal.pntd.0000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croker C, Reporter R, Mascola L. Use of statewide hospital discharge data to evaluate the economic burden of neurocysticercosis in Los Angeles County (1991–2008) The American journal of tropical medicine and hygiene. 2010;83(1):106–10. doi: 10.4269/ajtmh.2010.09-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon HK, Kim KH, Chai JY, Yang HJ, Rim HJ, Eom KS. Sympatric distribution of three human Taenia tapeworms collected between 1935 and 2005 in Korea. The Korean journal of parasitology. 2008;46(4):235–41. doi: 10.3347/kjp.2008.46.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anantaphruti MT, Yamasaki H, Nakao M, Waikagul J, Watthanakulpanich D, Nuamtanong S, et al. Sympatric occurrence of Taenia solium, T. saginata, and T. asiatica, Thailand. Emerging infectious diseases. 2007;13(9):1413–6. doi: 10.3201/eid1309.061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conlan JV, Vongxay K, Fenwick S, Blacksell SD, Thompson RC. Does interspecific competition have a moderating effect on Taenia solium transmission dynamics in Southeast Asia? Trends in parasitology. 2009;25(9):398–403. doi: 10.1016/j.pt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Garcia HH, Gonzalez AE, Gilman RH, for The Cysticercosis Working Group in Peru Diagnosis, treatment and control of Taenia solium cysticercosis. Curr Opin Inf Dis. 2003;(16):411–9. doi: 10.1097/00001432-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet. 2012;380(9848):1193–201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- 17.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, et al. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS neglected tropical diseases. 2010;4(11):e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chopra JS, Kaur U, Mahajan RC. Cysticerciasis and epilepsy: a clinical and serological study. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75(4):518–20. doi: 10.1016/0035-9203(81)90189-9. [DOI] [PubMed] [Google Scholar]
- 19.Garcia HH, Gilman R, Martinez M, Tsang VC, Pilcher JB, Herrera G, et al. Cysticercosis as a major cause of epilepsy in Peru. The Cysticercosis Working Group in Peru (CWG) Lancet. 1993;341(8839):197–200. doi: 10.1016/0140-6736(93)90064-n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottbeck R, Nshimiyimana JF, Tugirimana P, Dull UE, Sattler J, Hategekimana JC, et al. High prevalence of cysticercosis in people with epilepsy in southern Rwanda. PLoS neglected tropical diseases. 2013;7(11):e2558. doi: 10.1371/journal.pntd.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, Schmutzhard E. Epilepsy and neurocysticercosis in rural Tanzania-An imaging study. Epilepsia. 2009;50(5):987–93. doi: 10.1111/j.1528-1167.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh G, Bawa J, Chinna D, Chaudhary A, Saggar K, Modi M, et al. Association between epilepsy and cysticercosis and toxocariasis: a population-based case-control study in a slum in India. Epilepsia. 2012;53(12):2203–8. doi: 10.1111/epi.12005. [DOI] [PubMed] [Google Scholar]
- 23.Quet F, Guerchet M, Pion SD, Ngoungou EB, Nicoletti A, Preux PM. Meta-analysis of the association between cysticercosis and epilepsy in Africa. Epilepsia. 2010;51(5):830–7. doi: 10.1111/j.1528-1167.2009.02401.x. [DOI] [PubMed] [Google Scholar]
- 24.Del Brutto OH, Garcia HH. Cysticercosis of the human nervous system. Viena: Springer; 2014. p. 135. [Google Scholar]
- 25.Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, et al. A trial of antiparasitic treatment to reduce the rate of seizures due to cerebral cysticercosis. The New England journal of medicine. 2004;350(3):249–58. doi: 10.1056/NEJMoa031294. [DOI] [PubMed] [Google Scholar]
- 26.Escobar A. The pathology of neurocysticercosis. In: Palacios E, Rodriguez-Carbajal J, Taveras JM, editors. Cysticercosis of the central nervous system. Springfield: Charles C. Thomas; 1983. pp. 27–54. [Google Scholar]
- 27.Garcia HH, Gonzalez AE, Rodriguez S, Tsang VC, Pretell EJ, Gonzales I, et al. Neurocysticercosis: unraveling the nature of the single cysticercal granuloma. Neurology. 2010;75(7):654–8. doi: 10.1212/WNL.0b013e3181ed9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bickerstaff ER, Cloake PCP, Hughes B, Smith WT. The racemose form of cerebral cysticercosis. Brain. 1952;75:1–16. doi: 10.1093/brain/75.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Bandres JC, White AC, Jr, Samo T, Murphy EC, Harris RL. Extraparenchymal neurocysticercosis: report of five cases and review of management. Clinical infectious diseases. 1992;15(5):799–811. doi: 10.1093/clind/15.5.799. [DOI] [PubMed] [Google Scholar]
- 30.Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: a focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011;9(1):123–33. doi: 10.1586/eri.10.150. [DOI] [PubMed] [Google Scholar]
- 31.Tsang V, Wilson M. Taenia solium cysticercosis, an under-recognized but serious public health problem. Parasitol Today. 1995;(11):124–6. [Google Scholar]
- 32.Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, et al. Short report: transient antibody response in Taenia solium infection in field conditions-a major contributor to high seroprevalence. The American journal of tropical medicine and hygiene. 2001;65(1):31–2. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 33.Mwape KE, Phiri IK, Praet N, Speybroeck N, Muma JB, Dorny P, et al. The incidence of human cysticercosis in a rural community of Eastern Zambia. PLoS neglected tropical diseases. 2013;7(3):e2142. doi: 10.1371/journal.pntd.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meza-Lucas A, Carmona-Miranda L, Garcia-Jeronimo RC, Torrero-Miranda A, Gonzalez-Hidalgo G, Lopez-Castellanos G, et al. Short report: limited and short-lasting humoral response in Taenia solium: seropositive households compared with patients with neurocysticercosis. The American journal of tropical medicine and hygiene. 2003;69(2):223–7. [PubMed] [Google Scholar]
- 35.Verastegui M, Gilman RH, Gonzales A, Garcia HH, Gavidia C, Falcon N, et al. Taenia solium oncosphere antigens induce immunity in pigs against experimental cysticercosis. Veterinary parasitology. 2002;108(1):49–62. doi: 10.1016/s0304-4017(02)00182-6. [DOI] [PubMed] [Google Scholar]
- 36.de Aluja AS, Martinez MJ, Villalobos AN. Taenia solium cysticercosis in young pigs: age at first infection and histological characteristics. Veterinary parasitology. 1998;76(1–2):71–9. doi: 10.1016/s0304-4017(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Noval J, Allan JC, Fletes C, Moreno E, DeMata F, Torres-Alvarez R, et al. Epidemiology of Taenia solium taeniasis and cysticercosis in two rural Guatemalan communities. The American journal of tropical medicine and hygiene. 1996;55(3):282–9. doi: 10.4269/ajtmh.1996.55.282. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez AL, Lindback J, Schantz PM, Sone M, Sakai H, Medina MT, et al. A population-based, case-control study of Taenia solium taeniasis and cysticercosis. Ann Trop Med Parasitol. 1999;93(3):247–58. [PubMed] [Google Scholar]
- 39.Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22(2):139–45. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 40.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65(2):229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 41.Del Brutto OH, Santibanez R, Idrovo L, Rodriguez S, Diaz-Calderon E, Navas C, et al. Epilepsy and neurocysticercosis in Atahualpa: a door-to-door survey in rural coastal Ecuador. Epilepsia. 2005;46(4):583–7. doi: 10.1111/j.0013-9580.2005.36504.x. [DOI] [PubMed] [Google Scholar]
- 42.Prasad A, Gupta RK, Pradhan S, Tripathi M, Pandey CM, Prasad KN. What triggers seizures in neurocysticercosis? A MRI-based study in pig farming community from a district of North India. Parasitology international. 2008;57(2):166–71. doi: 10.1016/j.parint.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Henneberg R. Die tierischen parasiten des zentralnervensystem. In: Lewandowsky M, editor. Handbuch der neurologie. Berlin: Verlag Von Julius Springer; 1912. pp. 643–712. [Google Scholar]
- 44.McArthur WP. Cysticercosis as seen in the British army with special reference to the production of epilepsy. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1934;27:343–63. [Google Scholar]
- 45.Dixon HB, Lipscomb FM. In: Cysticercosis: an Analysis and Follow-up of 450 cases. Office HMsS, editor. London: Medical Research Council; 1961. [Google Scholar]
- 46.Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathogens and global health. 2012;106(5):286–98. doi: 10.1179/2047773212Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson M, Bryan RT, Fried JA, Ware DA, Schantz PM, Pilcher JB, et al. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164(5):1007–9. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]
- 48.Zini D, Farrell VJ, Wadee AA. The relationship of antibody levels to the clinical spectrum of human neurocysticercosis. J Neurol Neurosurg Psychiatry. 1990;53(8):656–61. doi: 10.1136/jnnp.53.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan NA, Sotelo J. Presentation of a membrane cysticercus antigen and its homology with excretory–secretory antigen. Acta Leiden. 1989;57(2):123–9. [PubMed] [Google Scholar]
- 50.Evans CA, Garcia HH, Hartnell A, Gilman RH, Jose PJ, Martinez M, et al. Elevated concentrations of eotaxin and interleukin-5 in human neurocysticercosis. Infect Immun. 1998;66(9):4522–5. doi: 10.1128/iai.66.9.4522-4525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sciutto E, Chavarria A, Fragoso G, Fleury A, Larralde C. The immune response in Taenia solium cysticercosis: protection and injury. Parasite Immunol. 2007;29(12):621–36. doi: 10.1111/j.1365-3024.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 52.Damian RT. The exploitation of host immune responses by parasites. J Parasitol. 1987;73(1):3–13. [PubMed] [Google Scholar]
- 53.Correa D, Dalma D, Espinoza B, Plancarte A, Rabiela MT, Madrazo I, et al. Heterogeneity of humoral immune components in human cysticercosis. J Parasitol. 1985;71(5):535–41. [PubMed] [Google Scholar]
- 54.Flisser A. Taenia solium cysticercosis: some mechanisms of parasite survival in immunocompetent hosts. Acta Leiden. 1989;57(2):259–63. [PubMed] [Google Scholar]
- 55.Willms K. Morphology and biochemistry of the pork tapeworm, Taenia solium. Curr Top Med Chem. 2008;8(5):375–82. doi: 10.2174/156802608783790875. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez JI, Colegial CH, Castano CA, Trujillo J, Teale JM, Restrepo BI. The human nervous tissue in proximity to granulomatous lesions induced by Taenia solium metacestodes displays an active response. J Neuroimmunol. 2002;127(1–2):139–44. doi: 10.1016/s0165-5728(02)00101-7. [DOI] [PubMed] [Google Scholar]
- 57.Bueno EC, dos Ramos Machado L, Livramento JA, Vaz AJ. Cellular immune response of patients with neurocysticercosis (inflammatory and non-inflammatory phases) Acta tropica. 2004;91(2):205–13. doi: 10.1016/j.actatropica.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 58.Chavarria A, Roger B, Fragoso G, Tapia G, Fleury A, Dumas M, et al. TH2 profile in asymptomatic Taenia solium human neurocysticercosis. Microbes Infect. 2003;5(12):1109–15. doi: 10.1016/s1286-4579(03)00206-5. [DOI] [PubMed] [Google Scholar]
- 59.Restrepo BI, Aguilar MI, Melby PC, Teale JM. Analysis of the peripheral immune response in patients with neurocysticercosis: evidence for T cell reactivity to parasite glycoprotein and vesicular fluid antigens. The American journal of tropical medicine and hygiene. 2001;65(4):366–70. doi: 10.4269/ajtmh.2001.65.366. [DOI] [PubMed] [Google Scholar]
- 60.Rodriguez-Sosa M, David JR, Bojalil R, Satoskar AR, Terrazas LI. Cutting edge: susceptibility to the larval stage of the helminth parasite Taenia crassiceps is mediated by Th2 response induced via STAT6 signaling. J Immunol. 2002;168(7):3135–9. doi: 10.4049/jimmunol.168.7.3135. [DOI] [PubMed] [Google Scholar]
- 61.Terrazas LI, Bojalil R, Govezensky T, Larralde C. Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps) J Parasitol. 1998;84(1):74–81. [PubMed] [Google Scholar]
- 62.Villa OF, Kuhn RE. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112(Pt 6):561–70. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 63.Toenjes SA, Kuhn RE. The initial immune response during experimental cysticercosis is of the mixed Th1/Th2 type. Parasitol Res. 2003;89(5):407–13. doi: 10.1007/s00436-002-0788-z. [DOI] [PubMed] [Google Scholar]
- 64.Diaz MA, Villalobos N, de Aluja A, Rosas G, Gomez-Conde E, Hernandez P, et al. Th1 and Th2 indices of the immune response in pigs vaccinated against Taenia solium cysticercosis suggest various host immune strategies against the parasite. Vet Immunol Immunopathol. 2003;93(3–4):81–90. doi: 10.1016/s0165-2427(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 65.Amit P, Prasad KN, Kumar GR, Shweta T, Sanjeev J, Kumar PV, et al. Immune response to different fractions of Taenia solium cyst fluid antigens in patients with neurocysticercosis. Exp Parasitol. 2011;127(3):687–92. doi: 10.1016/j.exppara.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Kashyap B, Das S, Jain S, Agarwal A, Kaushik JS, Kaur IR. Correlation between the clinico radiological heterogeneity and the immune-inflammatory profiles in pediatric patients with neurocysticercosis from a tertiary referral centre. J Trop Pediatr J Trop Pediatr. 2012;58:320–3. doi: 10.1093/tropej/fmr093. [DOI] [PubMed] [Google Scholar]
- 67.Chavarria A, Fleury A, Bobes RJ, Morales J, Fragoso G, Sciutto E. A depressed peripheral cellular immune response is related to symptomatic neurocysticercosis. Microbes Infect. 2006;8(4):1082–9. doi: 10.1016/j.micinf.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Pillars article: downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 1991. 173: 159-166. J Immunol. 2012;189(3):1104–11. [PubMed] [Google Scholar]
- 69.Vuitton DA, Gottstein B. Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay. Journal of biomedicine & biotechnology. 2010;2010:923193. doi: 10.1155/2010/923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang IC, Fan PC, Lu SC, Fan CK, Su KE. Suppression of host Th1-type granulomatous inflammation by Taenia solium metacestodes is related to down-regulation of osteopontin gene expression. Int J Parasitol. 2008;38(2):239–48. doi: 10.1016/j.ijpara.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Cardenas G, Valdez R, Saenz B, Bottasso O, Fragoso G, Sciutto E, et al. Impact of Taenia solium neurocysticercosis upon endocrine status and its relation with immuno-inflammatory parameters. Int J Parasitol. 2012;42(2):171–6. doi: 10.1016/j.ijpara.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Adalid-Peralta L, Fleury A, Garcia-Ibarra T, Hernandez M, Parkhouse M, Crispin JC, et al. Human Neurocysticercosis: in Vivo Expansion of Peripheral Regulatory T Cells and Their Recruitment in the Central Nervous System. J Parasitol. 2012;98(1):142–8. doi: 10.1645/GE-2839.1. [DOI] [PubMed] [Google Scholar]
- 73.Gundra UM, Mishra BB, Wong K, Teale JM. Increased disease severity of parasite-infected TLR2−/− mice is correlated with decreased central nervous system inflammation and reduced numbers of cells with alternatively activated macrophage phenotypes in a murine model of neurocysticercosis. Infect Immun. 2011;79(7):2586–96. doi: 10.1128/IAI.00920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol. 2005;35(13):1349–58. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Guerra-Giraldez C, Marzal M, Cangalaya C, Balboa D, Orrego MA, Paredes A, et al. Disruption of the blood-brain barrier in pigs naturally infected with Taenia solium, untreated and after anthelmintic treatment. Exp Parasitol. 2013;134(4):443–6. doi: 10.1016/j.exppara.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White AC, Jr, Robinson P, Kuhn R. Taenia solium cysticercosis: host-parasite interactions and the immune response. Chem Immunol. 1997;66:209–30. [PubMed] [Google Scholar]
- 77.Nash TE, Singh G, White AC, Rajshekhar V, Loeb JA, Proano JV, et al. Treatment of neurocysticercosis: current status and future research needs. Neurology. 2006;67(7):1120–7. doi: 10.1212/01.wnl.0000238514.51747.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mishra BB, Gundra UM, Wong K, Teale JM. MyD88-deficient mice exhibit decreased parasite-induced immune responses but reduced disease severity in a murine model of neurocysticercosis. Infect Immun. 2009;77(12):5369–79. doi: 10.1128/IAI.00455-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uddin J, Gonzalez AE, Gilman RH, Garcia HH, Verastegui M, Moore LJ, et al. Neurocysticercal antigens stimulate chemokine secretion from human monocytes via an NF-kappaB-dependent pathway. Microbes Infect. 2006;8(7):1732–40. doi: 10.1016/j.micinf.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uddin J, Garcia HH, Gilman RH, Gonzalez AE, Friedland JS. Monocyte-astrocyte networks and the regulation of chemokine secretion in neurocysticercosis. J Immunol. 2005;175(5):3273–81. doi: 10.4049/jimmunol.175.5.3273. [DOI] [PubMed] [Google Scholar]
- 81.Patil S, Robinson P, Actor JK, Baig S, White AC., Jr Proinflammatory cytokines in granulomas associated with murine cysticercosis are not the cause of seizures. J Parasitol. 2006;92(4):738–41. doi: 10.1645/GE-676R1.1. [DOI] [PubMed] [Google Scholar]
- 82.Prasad A, Prasad KN, Gupta RK, Pradhan S. Increased expression of ICAM-1 among symptomatic neurocysticercosis. J Neuroimmunol. 2009;206(1–2):118–20. doi: 10.1016/j.jneuroim.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Verma A, Prasad KN, Cheekatla SS, Nyati KK, Paliwal VK, Gupta RK. Immune response in symptomatic and asymptomatic neurocysticercosis. Med Microbiol Immunol. 2011;200(4):255–61. doi: 10.1007/s00430-011-0198-x. [DOI] [PubMed] [Google Scholar]
- 84.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 85.Alvarez JI, Teale JM. Differential changes in junctional complex proteins suggest the ependymal lining as the main source of leukocyte infiltration into ventricles in murine neurocysticercosis. J Neuroimmunol. 2007;187(1–2):102–13. doi: 10.1016/j.jneuroim.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alvarez JI, Teale JM. Multiple expression of matrix metalloproteinases in murine neurocysticercosis: Implications for leukocyte migration through multiple central nervous system barriers. Brain Res. 2008;1214:145–58. doi: 10.1016/j.brainres.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verma A, Prasad KN, Nyati KK, Singh SK, Singh AK, Paliwal VK, et al. Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology. 2011;138(11):1423–8. doi: 10.1017/S0031182011001259. [DOI] [PubMed] [Google Scholar]
- 88.Gupta RK, Awasthi R, Garg RK, Kumar N, Gupta PK, Singh AK, et al. T1-weighted dynamic contrast-enhanced MR evaluation of different stages of neurocysticercosis and its relationship with serum MMP-9 expression. AJNR Am J Neuroradiol. 2013;34(5):997–1003. doi: 10.3174/ajnr.A3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alvarez JI, Krishnamurthy J, Teale JM. Doxycycline treatment decreases morbidity and mortality of murine neurocysticercosis: evidence for reduction of apoptosis and matrix metalloproteinase activity. Am J Pathol. 2009;175(2):685–95. doi: 10.2353/ajpath.2009.081073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uddin J, Gonzalez AE, Gilman RH, Thomas LH, Rodriguez S, Evans CA, et al. Mechanisms regulating monocyte CXCL8 secretion in neurocysticercosis and the effect of antiparasitic therapy. J Immunol. 2010;185(7):4478–84. doi: 10.4049/jimmunol.0904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serpa JA, Moran A, Goodman JC, Giordano TP, White AC., Jr Neurocysticercosis in the HIV era: a case report and review of the literature. The American journal of tropical medicine and hygiene. 2007;77(1):113–7. [PubMed] [Google Scholar]
- 92.Soto Hernandez JL, Ostrosky Zeichner L, Tavera G, Gomez Avina A. Neurocysticercosis and HIV infection: report of two cases and review. Surgical neurology. 1996;45(1):57–61. doi: 10.1016/0090-3019(95)00259-6. [DOI] [PubMed] [Google Scholar]
- 93.Delobel P, Signate A, El Guedj M, Couppie P, Gueye M, Smadja D, et al. Unusual form of neurocysticercosis associated with HIV infection. European journal of neurology. 2004;11(1):55–8. doi: 10.1046/j.1351-5101.2003.00696.x. [DOI] [PubMed] [Google Scholar]
- 94.Ramos JM, Masia M, Padilla S, Bernal E, Martin-Hidalgo A, Gutierrez F. Fatal infection due to larval cysts of cestodes (neurocysticercosis and hydatid disease) in human immunodeficiency virus (HIV) infected patients in Spain: report of two cases. Scandinavian journal of infectious diseases. 2007;39(8):719–23. doi: 10.1080/00365540701242392. [DOI] [PubMed] [Google Scholar]
- 95.Handique SK, Das RR, Saharia B, Das P, Buragohain R, Saikia P. Coinfection of Japanese encephalitis with neurocysticercosis: an imaging study. AJNR American journal of neuroradiology. 2008;29(1):170–5. doi: 10.3174/ajnr.A0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh P, Kalra N, Ratho RK, Shankar S, Khandelwal N, Suri S. Coexistent neurocysticercosis and Japanese B encephalitis: MR imaging correlation. AJNR American journal of neuroradiology. 2001;22(6):1131–6. [PMC free article] [PubMed] [Google Scholar]
- 97.Das SK, Nityanand S, Sood K, Agarwal A, Kapoor R, Pant MC, et al. Japanese B encephalitis with neurocysticercosis. The Journal of the Association of Physicians of India. 1991;39(8):643–4. [PubMed] [Google Scholar]
- 98.Allan JC, Avila G, Garcia Noval J, Flisser A, Craig PS. Immunodiagnosis of taeniasis by coproantigen detection. Parasitology. 1990;101(Pt 3):473–7. doi: 10.1017/s0031182000060686. [DOI] [PubMed] [Google Scholar]
- 99.Gilman RH, Del Brutto OH, Garcia HH, Martinez M. Prevalence of taeniosis among patients with neurocysticercosis is related to severity of infection. The Cysticercosis Working Group in Peru. Neurology. 2000;55(7):1062. doi: 10.1212/wnl.55.7.1062. [DOI] [PubMed] [Google Scholar]
- 100.Dixon HBF, Hargreaves WH. Cysticercosis (Taenia solium): a further ten years’ clinical study, covering 284 cases. Quart J Med. 1944;13:107–21. [Google Scholar]
- 101.Allan JC, Velasquez-Tohom M, Garcia-Noval J, Torres-Alvarez R, Yurrita P, Fletes C, et al. Epidemiology of intestinal taeniasis in four, rural, Guatemalan communities. Ann Trop Med Parasitol. 1996;90(2):157–65. doi: 10.1080/00034983.1996.11813039. [DOI] [PubMed] [Google Scholar]
- 102.Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Rodriguez S, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS neglected tropical diseases. 2009;3(1):e371. doi: 10.1371/journal.pntd.0000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flisser A, Viniegra AE, Aguilar-Vega L, Garza-Rodriguez A, Maravilla P, Avila G. Portrait of human tapeworms. J Parasitol. 2004;90(4):914–6. doi: 10.1645/GE-3354CC. [DOI] [PubMed] [Google Scholar]
- 104.Mendlovic F, Carrillo-Farga J, Torres J, Laclette JP, Flisser A. Differential expression of calreticulin in developmental stages of Taenia solium. J Parasitol. 2006;92(4):789–95. doi: 10.1645/GE-724R1.1. [DOI] [PubMed] [Google Scholar]
- 105.Leon-Cabrera S, Cruz-Rivera M, Mendlovic F, Avila-Ramirez G, Carrero JC, Laclette JP, et al. Standardization of an experimental model of human taeniosis for oral vaccination. Methods. 2009;49(4):346–50. doi: 10.1016/j.ymeth.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 106.Leon-Cabrera S, Cruz-Rivera M, Mendlovic F, Romero-Valdovinos M, Vaughan G, Salazar AM, et al. Immunological mechanisms involved in the protection against intestinal taeniosis elicited by oral immunization with Taenia solium calreticulin. Exp Parasitol. 2012;132(3):334–40. doi: 10.1016/j.exppara.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 107.Zhang W, McManus DP. Vaccination of dogs against Echinococcus granulosus: a means to control hydatid disease? Trends in parasitology. 2008;24(9):419–24. doi: 10.1016/j.pt.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Zhang W, Zhang Z, Shi B, Li J, You H, Tulson G, et al. Vaccination of dogs against Echinococcus granulosus, the cause of cystic hydatid disease in humans. J Infect Dis. 2006;194(7):966–74. doi: 10.1086/506622. [DOI] [PubMed] [Google Scholar]
- 109.Petavy AF, Hormaeche C, Lahmar S, Ouhelli H, Chabalgoity A, Marchal T, et al. An oral recombinant vaccine in dogs against Echinococcus granulosus, the causative agent of human hydatid disease: a pilot study. PLoS neglected tropical diseases. 2008;2(1):e125. doi: 10.1371/journal.pntd.0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]


